Abstract
Root hairs provide a model system for the study of cell polarity. We examined the possibility that one or more members of the distinct plant subfamily of RHO monomeric GTPases, termed Rop, may function as molecular switches regulating root hair growth. Specific Rops are known to control polar growth in pollen tubes. Overexpressing Rop2 (Rop2 OX) resulted in a strong root hair phenotype, whereas overexpressing Rop7 appeared to inhibit root hair tip growth. Overexpressing Rops from other phylogenetic subgroups of Rop did not give a root hair phenotype. We confirmed that Rop2 was expressed throughout hair development. Rop2 OX and constitutively active GTP-bound rop2 (CA-rop2) led to additional and misplaced hairs on the cell surface as well as longer hairs. Furthermore, CA-rop2 depolarized root hair tip growth, whereas Rop2 OX resulted in hairs with multiple tips. Dominant negative GDP-bound Rop2 reduced the number of hair-forming sites and led to shorter and wavy hairs. Green fluorescent protein–Rop2 localized to the future site of hair formation well before swelling formation and to the tip throughout hair development. We conclude that the Arabidopsis Rop2 GTPase acts as a positive regulatory switch in the earliest visible stage in hair development, swelling formation, and in tip growth.
INTRODUCTION
Root hairs have important roles in water and nutrient uptake (Clarkson, 1985), anchorage of the plant in the soil, and interactions with microorganisms (Bauer, 1981). They are exceptionally polarized structures resulting from tubular outgrowth at a defined site of single root epidermal cells. Consequently, root hair development provides an attractive model for studying the establishment of cell polarity and polar growth.
The root epidermis is composed of adjacent files of hair and nonhair cells. Root epidermal patterning is controlled by a specific set of transcription factors (Schiefelbein, 2000). Hair cell development can be subdivided into stages: selection of a site for hair formation, swelling formation, the transition to tip growth (Dolan et al., 1994), and tip growth proper. The molecular mechanisms that control these processes remain poorly understood.
Swelling formation, the earliest visible stage of hair cell development, is characterized by changes in cytoplasmic and cell wall pH at the site of hair development (Bibikova et al., 1998). To date, few genes have been described that have roles in swelling formation (e.g., TIP1 and RHD1) (Schiefelbein and Somerville, 1990; Ryan et al., 1998). Root hair tip growth has been shown to involve both the actin cytoskeleton (Miller et al., 1999; Baluska et al., 2000; Parker et al., 2000) and microtubules (Bibikova et al., 1999) and is dependent on a tip-focused influx of calcium ions (Schiefelbein et al., 1992; Bibikova et al., 1997; Wymer et al., 1997; Very and Davies, 2000). Wild-type plants rarely form branched hairs, but mutants such as tip1, cow1, cen2, cen3, and scn1 have an increased number of branched hairs (Schiefelbein et al., 1993; Grierson et al., 1997, 2001; Ryan et al., 1998). Tip branching also can be induced by actin and microtubule antagonists and by drugs that disrupt myosin ATPases and exocytosis (Bibikova et al., 1999; Ovecka et al., 2000). Altogether, some 40 different genes have been identified that affect one or more stages of hair development (Grierson et al., 2001). A complex network of gene activity has been characterized that controls hair morphogenesis (Parker et al., 2000).
The RHO small GTPases are a ubiquitous eukaryotic family of molecular switches within the RAS superfamily of monomeric GTPases. The RHO family in animals and yeast comprises the Cdc42, Rac, and Rho subfamilies. In budding yeast, three distinct small GTPases (a Ras-like GTPase, Cdc42, and Rho1) form a cascade of signaling networks and control the selection of bud sites, the establishment of bud sites, and subsequent polar growth in budding yeast, respectively (Chant, 1994; Chant and Stowers, 1995). In mammalian cells, a similar cascade involving Cdc42, Rac, and Rho controls the actin cytoskeleton during cell movement (Hall, 1998). Two members of the RHO family in Drosophila, Rac1 and Cdc42, are important in a developmental process analogous to root hair development, wing hair formation (Eaton et al., 1996). Rac1 acts to restrict the site of hair emergence. Cdc42 localizes actin polymerization in the extending hair. Interestingly, disrupting Rac1 activity by expressing a dominant negative form of the protein led to multiple hairs arising from a single wing epithelial cell.
Plant members of the RHO family are strong candidates for proteins involved very early in root hair development. No homologs of Cdc42, Rac, or Rho GTPases have been identified in plants. Instead, plants possess a large family of genes encoding Rop (Rho-related GTPase from plants). Rops form a distinct subfamily of the RHO family (Yang and Watson, 1993; Delmer et al., 1995; Winge et al., 1997, 2000; Li et al., 1998; Zheng and Yang, 2000). Rops have an important role in pollen tube growth and development. Tip-localized, pollen-specific Rop1 (Arac11) has been shown to control pollen tube elongation (Lin et al., 1996; Lin and Yang, 1997; Li et al., 1999). Transgenic lines overexpressing wild-type Rop1 and constitutively active (CA) rop1 mutants in Arabidopsis reveal a role for Rop1 in the control of cell polarity development in pollen tubes (Li et al., 1999). The transient expression of rop5 mutants in tobacco pollen tubes suggests a similar role for Rop5 (Arac6/At-Rac2) (Kost et al., 1999). Rop1 has been shown to direct polar growth in pollen tubes by controlling the formation of high calcium gradients in the tip (Li et al., 1999), similar to those observed in growing root hairs, and tip-localized actin (Fu et al., 2001). Apart from the Rops that control pollen tube tip growth, the functions for the majority of the 11 Arabidopsis Rop genes remain poorly characterized. Analysis of transgenic Arabidopsis plants expressing CA and dominant negative (DN) forms of Rop2 (Arac4) has implicated Rop in modulating multiple distinct signaling pathways in plant growth and development (Li et al., 2001).
Recently, Rops also have been implicated in root hair development (Molendijk et al., 2001). Rop protein was detected in developing root hair cells, and green fluorescent protein (GFP)–CA-rop4 (Arac5) and GFP–CA-rop6 (Arac3) expression led to depolarized root hair growth. It was not shown whether Rop is usually expressed in developing root hair cells. Here, we show that a specific Rop, Rop2, is expressed during root hair development and localizes in a pattern that matches that observed by Molendijk et al. (2001). We also show that overexpression of native Rop2, or CA- and DN-rop2, leads to root hair phenotypes at all stages of hair formation, including dramatic effects on root hair initiation and tip growth. Our results provide convincing evidence that the Rop2 GTPase controls multiple stages of root hair development.
RESULTS
Overexpression of Rop2 Affects Root Hair Morphogenesis
The Arabidopsis pollen-specific Rop1 gene and a closely related Rop (Rop5/At-Rac2) have been shown to control polar growth in pollen tubes. We wished to determine whether any Rops expressed in vegetative tissues (Winge et al., 1997; Li et al., 1998) have an analogous role in root hair cells. We generated transgenic plants overexpressing several members of the Arabidopsis Rop family, including Rop2 (Arac4), Rop7 (Arac2), Rop8 (Arac9), and Rop11 (Arac1D). Each of these Rops represents one of the four different phylogenetic Rop groups (Zheng and Yang, 2000). As shown in Figures 1C and 1D, overexpression of Rop8 or Rop11 had no effect on root hair development. In Rop7-overexpressing roots (Rop7 OX; Cauliflower mosaic virus [CaMV] 35S transgenic line), a significant portion of root hairs apparently did not make the transition from swelling to tip growth, producing thick short hairs (Figure 1E). Rop2 belongs to the same phylogenetic group as Rop1. When overexpressed (CaMV 35S transgenic line), Rop2 (Rop2 OX) led to multiple hairs forming on individual cells and hairs with multiple tips (Figures 1F and 1G). Similar phenotypes were observed in all other Rop2-overexpressing lines (data not shown), indicating that the phenotype is caused by Rop2 overexpression. Interestingly, Rop2 overexpression caused root hair phenotypes distinct from the depolarized growth induced in pollen tubes by Rop1 overexpression (i.e., multiple initiation and tip branching). Semiquantitative reverse transcriptase–mediated polymerase chain reaction (RT-PCR) analysis showed that all of these Rop transgenes were overexpressed (Figure 1H).
Figure 1.
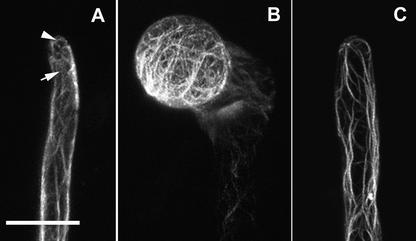
Root Hair Phenotypes of Wild-Type Arabidopsis and Rop7, Rop8, Rop11, and Rop2 OX Transgenic Plants.
(A) and (B) Wild-type root hairs.
(C) and (D) Overexpression of Rop8 and Rop11, respectively, did not affect hair cell development.
(E) Overexpression of Rop7 led to some very short root hairs.
(F) and (G) Overexpression of Rop2 led to a dramatic root hair phenotype.
In (B) and (G), roots were grown through semisolid medium. In (A) and (C) to (F), roots were grown in contact with the air. (A), (B), (F), and (G) are bright-field images, and (C) to (E) are phase contrast images. Bars = 200 μm.
(H) RT-PCR analysis of Rop transgene expression. The relative intensity of different transcripts was similar when either 20 or 30 cycles of PCR amplification were performed. The top band shows actin2 as an internal PCR amplification and template control. The bottom band shows Rop transcript specific for each construct shown. Lane 1, wild-type control*; lane 2, Rop2 OX*; lane 3, CA1-rop2 (CA1-1)*; lane 4, CA2-rop2 (CA2-2); lane 5, Rop7-2; lane 6, Rop7-34*; lane 7, Rop8-25*; lane 8, Rop8-32*; lane 9, Rop11-4*; lane 10, Rop11-7*. Asterisks indicate lines used for the characterization of phenotypes shown in this figure, although >20 independent lines with similar phenotypes were obtained for each construct. The CA-rop2 mutants (CA1-1 and CA2-2) were described previously (Li et al., 2001).
Rop2 Is Expressed in Root Hair Cells
Because of the interesting Rop2 OX root hair phenotypes, we focused on detailed analysis of the roles of Rop2 in root hair development. To determine whether the root hair phenotype of Rop2 OX plants reflects the function of the endogenous Rop2, we examined the expression of Rop2 in wild-type root hairs by RT-PCR and whole mount in situ hybridization. First, we aspirated the contents of 16 wild-type root hair cells that were at or just before the stage of swelling, reverse transcribed the mRNA (M.A. Jones and C.S. Grierson, unpublished data), and used the cDNA as a template for PCR using Rop2 primers. The results in Figure 2A show that the Rop2 transcript was present in hair cells during swelling formation. DNA sequencing confirmed that it was identical to the published Rop2 cDNA sequence and different from other Arabidopsis Rop sequences. We showed expression of Rop2 in growing root hairs by whole mount in situ hybridization. Rop2-specific sense probes (Figure 2B) gave faint background staining in the root epidermis, whereas antisense probes (Figure 2C) gave much more intense staining of the epidermis, including root hair cells. Analysis of transgenic plants expressing promoter:β-glucuronidase (GUS) fusions confirmed that both the Rop2 promoter (Figure 2D) and the 35S promoter of CaMV (Figure 2E) are active in developing root hairs.
Figure 2.
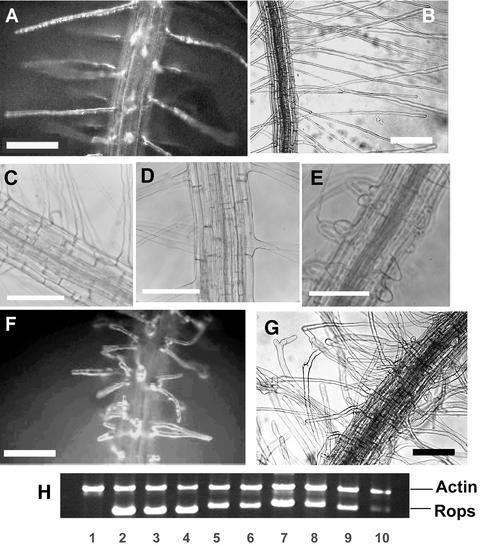
Rop2 Is Expressed in Root Hair Cells.
(A) Nested RT-PCR using primers specific for the 5′ and 3′ untranslated regions of the native Rop2 transcript and mRNA from 16 root hair cells at the stage of swelling formation.
(B) and (C) Whole mount in situ hybridization using Rop2-specific sense (B) and antisense (C) probes.
(D) and (E) β-Glucuronidase staining of Rop2 promoter:GUS and CaMV 35S promoter:GUS transgenic lines, respectively, confirming that both were expressed in growing root hairs.
Bars in (B) and (C) = 10 μm; bar in (E) = 100 μm for (D) and (E).
Rop2 Overexpression Affects the Number and the Positions of Swellings within a Hair-Forming Epidermal Cell
Rop2 OX plants had multiple hairs on single hair-forming epidermal cells (Figure 1G, Table 1). To determine whether Rop2 OX induces multiple hairs by altering the number of hair-forming swellings or the number of tip-growth sites per swelling, we used light microscopy to determine the sites of root hair formation in roots grown through semisolid medium. This phenotype also was observed on Rop2 OX roots grown in contact with the air (data not shown). As shown in Figure 3A, wild-type Arabidopsis plants usually produce one hair from each hair-forming cell, and this hair usually is close to the end of the cell nearest the root tip (Masucci and Schiefelbein, 1994). Ninety percent of Rop2 OX hair-forming cells had more than one hair formation event (Table 1); these were either swellings or swellings that had developed into growing hairs. Half of these cells had two or more hairs or swellings arising from the root tip end of the cell. The other half also had at least two hairs or swellings, but these occurred at separate sites along the cell surface (i.e., at least one of these hairs or swellings was located basal to the root tip end of the cell) (Figures 3B and 3C). These results show that Rop2 overexpression affects both the frequency and the position of swelling formation within the hair-forming epidermal cell.
Table 1.
Rop2 Affects the Number of Hair-Forming Events on Root Hair Cells
| Type of Plant |
Multiple Hair-Forming Events (%)a
|
||
|---|---|---|---|
| Same Site | Separate Sites | Total | |
| Wild type | 0.8 | 0 | 0.8 |
| Rop2 OX | 45.9 | 43.2 | 89.2 |
| CA-rop2 | 14.0 | 4.9 | 18.9 |
| DN-rop2 | 0 | 0 | 0 |
Percentage of hair cells with more than one hair or swelling counted on a 1-mm portion of each of 15 primary roots.
Figure 3.
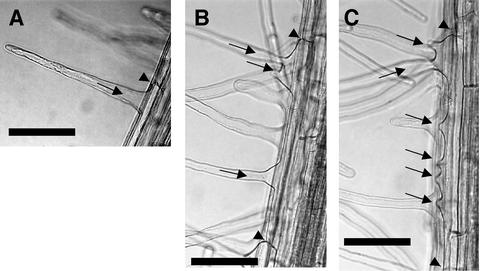
Rop2 Overexpression Affects the Number and Position of Hair-Forming Events in Root Hair Cells.
(A) Single wild-type root hair.
(B) and (C) Rop2 OX hair cells showing multiple swellings and hairs located away from the root tip end of the cell.
Arrowheads show end cell walls, and arrows show root hairs or swellings. Bars = 50 μm.
Effect of Rop2 Overexpression on Tip Growth
Rop1 has been implicated in both the control of growth and the specification of the polar site for tip growth in pollen tubes (Li et al., 1999). The formation of multiple hairs from the same swelling in Rop2 OX plants suggests that Rop2 OX also affects the site of tip-growth initiation. A role for Rop2 in specifying tip-growth sites is further supported by the branched hair tip phenotype of Rop2 OX plants (Figures 4B and 4C). Once hairs on Rop2 OX plants reached ∼500 to 600 μm in length, their tips branched repeatedly (mean number of hairs with branched tips per 2 mm of root tissue 2 cm from root tip: wild type, 0; Rop2 OX, 3.0 ± 2.4; n = 9 roots). Unlike in wild-type hairs, there was no observable cessation of tip growth in Rop2 OX hairs, with the number of new tips increasing as hairs grew older. This tip-branching phenotype also occurred on roots grown in contact with the air (data not shown). The Rop2 OX tip-branching phenotype is very similar to the effects of microtubule-disrupting drugs on wild-type hairs (see Figure 2 of Bibikova et al., 1999). Wild-type hairs are thought to complete the transition to tip growth before they reach 40 μm in length (Dolan et al., 1994). Thus, the elongation of root hairs is attributable primarily to tip growth. Rop2 OX plants had much longer hairs than did wild-type plants (Figures 4D and 4E; Table 2). These results suggest that Rop2 regulates both tip growth and the specification of tip-growth sites in root hairs, as does Rop1 in pollen tubes.
Figure 4.
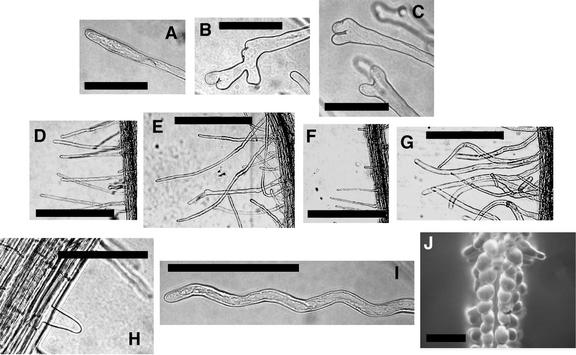
Effects of Rop2 Transgene Expression on Tip Growth.
(A) Tip of growing wild-type root hair.
(B) and (C) Tips of growing Rop2 OX hairs showing different degrees of branching.
(D) to (G) Root hair density and length on wild-type (D), Rop2 OX (E), DN-rop2 (F), and CA-rop2 (G) roots.
(H) DN-rop2 expression led to an increased number of hairs that stopped growing before reaching 40 μm in length (i.e., they did not make the transition to tip growth).
(I) Wavy DN-rop2 hair.
(J) CA-rop2 expression led to depolarization of hairs grown in contact with the air.
Bars in (A) to (C), (H), and (I) = 100 μm; bars in (D) to (G) = 500 μm; bar in (J) = 200 μm.
Table 2.
Rop2 Affects Root Hair Length
| Type of Plant |
No. of Hairs <40 μma |
Length of Mature Root Hairs (μm)b
|
||
|---|---|---|---|---|
| Minimum | Maximum | Mean | ||
| Wild type | 3.0 ± 2.3 | 63 | 625 | 351 ± 127 |
| Rop2 OX | 2.5 ± 1.7 | 200 | 1050 | 577 ± 188 |
| CA-rop2 | 1.2 ± 1.5 | 213 | 850 | 516 ± 126 |
| DN-rop2 | 10.1 ± 4.3 | 50 | 588 | 197 ± 118 |
Per millimeter of primary root. Hairs were counted on a 2-mm portion of each of nine primary roots. For DN-rop2, hairs were counted on a 1-mm portion of each of 27 primary roots.
Hairs <40 μm long were excluded. Ten hairs were measured on a 2-mm portion of each of nine primary roots. For DN-rop2, 10 hairs were measured on a 1-mm portion of each of 27 primary roots. Mature hairs were measured on regions of root approximately the same distance from the root tip. The longest hairs in these regions were all of similar length. This indicated that the hairs had stopped elongating.
DN-rop2 and CA-rop2 Expression Caused Opposite Effects on Swelling Formation and Tip Growth
To determine whether the function of Rop2 in the control of swelling formation and tip growth involves the signaling activity of Rop2, we used transgenic plants expressing CA and DN mutants of Rop2. CA-rop2 and DN-rop2 mutants activate and inhibit Rop signaling, respectively (Kost et al., 1999; Li et al., 1999, 2001). The effects of CA-rop2 and DN-rop2 transgenic lines on primary root elongation and lateral root formation have been described (Li et al., 2001). Rop2 OX did not affect the primary root length of 4-day-old seedlings (data not shown).
The total number of hairs per millimeter (including those <40 μm in length) was lower in DN-rop2 plants than in wild-type plants (Figure 4F, Table 3). These results imply that Rop2 activity either is required for the formation of swellings or affects hair cell length. DN-rop2 plants had dramatically shorter root hairs and also had a significant increase in the number of hairs <40 μm in length (Figure 4H, Table 2). This latter phenotype is similar to the effect of DN-rop1 expression on pollen tube elongation and suggests that Rop2 activity is crucial for tip growth. DN-rop2 hairs often were wavy (Figure 4I) and are remarkably reminiscent of the wavy hair phenotype of wild-type plants treated with microtubule-disrupting drugs (see Figure 2 of Bibikova et al., 1999).
Table 3.
Rop2 Affects the Number of Root Hairs per Millimeter of Root
| Type of Plant |
No. of Root Hairs per Millimeter a
|
||
|---|---|---|---|
| Minimum | Maximum | Mean | |
| Wild type | 22 | 36 | 28 ± 5b |
| Rop2 OX | 40 | 63 | 51 ± 9 |
| CA-rop2 | 28 | 43 | 35 ± 5 |
| DN-rop2 | 13 | 30 | 21 ± 5b |
Hairs were counted on a 2-mm portion of each of nine primary roots. For DN-rop2, hairs were counted on a 1-mm portion of each of 27 primary roots.
Standard error of the difference between two means = 2.078, t = 3.532, P < 0.01.
The number of hairs per millimeter of root increased in CA-rop2 plants relative to wild-type plants (Table 3). CA-rop2 plants also had many more cells with multiple hairs than did wild-type plants but fewer than did Rop2 OX plants (Table 1). Furthermore, these multiple hairs most frequently were found at the root tip end of the cell. Rarely, hairs were observed at the extreme opposite end of the cell in CA-rop2 plants (data not shown). We calculated the frequency of mature hairs <40 μm in length on CA-rop2 plants (Table 2). There was no significant difference between wild-type and CA-rop2 plants. CA-rop2 plants grown through semisolid medium had much longer hairs than did wild-type plants, although not as long as did Rop2 OX plants (Table 2). Unlike Rop2 OX plants, hair branching was not observed in CA-rop2 plants. Root hairs from both CA-rop2 and Rop2 OX plants had greater diameters than did wild-type hairs (mean root hair diameter [μm]: CA-rop2, 19.54 ± 2.72; Rop2 OX, 16.62 ± 4.16; wild type, 9.31 ± 1.36; n = 20). Both tended to be curved (Figures 4E and 4G), but CA-rop2 hairs were particularly so. The phenotype of depolarized growth in CA-rop2 root hairs was much more dramatic when roots were grown in contact with the air (Figure 4J). Hairs in this condition were bulbous, resembling the depolarized pollen tube growth induced by CA-rop1 or CA-rop5 expression (Kost et al., 1999; Li et al., 1999). We did not detect any noticeable differences in the level of transgene expression by RT-PCR between CA-rop2 and Rop2 OX (Figure 1H).
Growth media and conditions are known to influence molecular events in plants (Chung and Feri, 1999). Although we observed that CA-rop2 hairs had a more extreme form of depolarized growth when grown in contact with air than when grown through medium, we did not observe any differences in wild-type root hair morphology between these two growth systems.
Localization of GFP::Rop2 in Root Hair Cells
To further understand how Rop2 is involved in the regulation of both swelling formation and tip growth, we performed confocal laser scanning microscopy of growing root hair cells of transgenic Arabidopsis expressing a fusion of Rop2 and GFP (GFP::Rop2). The results are shown in Figure 5. We used a line that had a low level of GFP::Rop2 and nearly wild-type root hair development. We were unable to obtain lines expressing high levels of GFP::Rop2, possibly as a result of the toxicity of high GFP levels. GFP::Rop2 localized to the future site of hair formation well before any visible swelling formation (Figures 5A to 5D). This GFP localization pattern was seen in every cell at this stage of growth that we observed (n = 15 cells, 5 cell files). GFP::Rop2 persisted in the same location throughout swelling formation (Figure 5E), became focused at the hair tip during the transition to tip growth (Figure 5F), and remained there throughout tip growth (Figures 5G to 5I). No GFP::Rop2 was observed at the tips of hairs that had stopped growing (Figure 5J).
Figure 5.
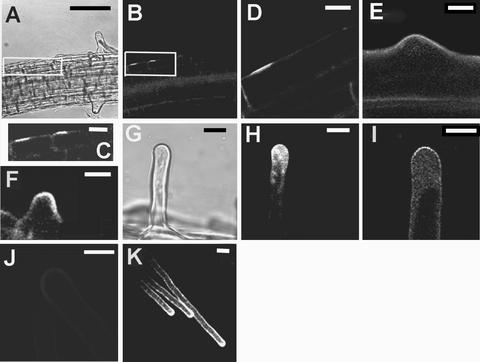
GFP::Rop2 Localizes to the Future Site of Hair Formation, Remains during Swelling Formation, and Localizes to the Tips of Growing Hairs throughout Hair Development.
Confocal laser scanning microscopy of root hair cells on transgenic Arabidopsis plants expressing a fusion of Rop2 and GFP.
(A) Bright-field image showing four cells in the same cell file, all at different stages of hair development. A box surrounds the youngest two cells. The youngest cell (far left) has not begun hair formation. The next youngest (center left) has just begun to form a swelling. The older two hairs (at right) have stopped growing.
(B) GFP::Rop2 fluorescence of the cells shown in (A).
(C) Enlargement of the boxed area in (B).
(D) A cell that is about to make a hair. GFP::Rop2 is localized to the site of future hair formation.
(E) to (K) GFP::Rop2 is localized at the hair tip during swelling formation (E), the transition to tip growth (F), and tip growth ([G] to [I] and [K]). (G) is a bright-field image of the hair shown in (H). In (J), GFP-Rop2 is absent from the tips of hairs that have stopped growing.
Bars in (A) and (B) = 40 μm; bars in (C) to (K) = 10 μm.
DN-rop2 Expression Disrupted Fine F-Actin at the Tips of Root Hairs
Fine actin meshwork or bundles localized to the tip are thought to be essential for tip growth in root hairs (Miller et al., 1999; Baluska et al., 2000). We sought to determine whether Rop2 regulates the formation of tip F-actin in root hairs as Rop1 does in pollen tubes (Fu et al., 2001). Because Arabidopsis root hair actin is preserved poorly during fixation of tissues (Molendijk et al., 2001; Y. Fu and Z. Yang, unpublished data), we visualized F-actin in live cells transiently expressing GFP–mouse talin (mTalin) (Fu et al., 2001, 2002). As shown in Figure 6, the distribution of GFP-mTalin suggests that growing root hairs in wild-type plants contained axial actin cables reaching to the subapical region and fine F-actin in the apical region. In CA-rop2 plants, bulbous root hairs contained an extensive actin network. In contrast, axial actin cables extended all the way to the extreme apex and no fine F-actin was observed in root hairs expressing DN-rop2. Similar actin organization was observed in root hairs or pollen tubes treated with low concentrations of cytochalasin D or latrunculin D (Miller et al., 1999; Baluska et al., 2000; Fu et al., 2001). These observations strongly support a role for Rop2 in the regulation of the formation of fine F-actin at the tips of root hairs.
Figure 6.
Effects of CA-rop2 and DN-rop2 Expression on F-Actin Localization in Root Hairs.
F-actin was visualized in live cells using transiently expressed GFP-mTalin as described in the text. Root hairs expressing GFP-mTalin were observed using confocal microscopy. Images shown are projections of scanning laser sections (1 μm) along the axes of root hairs.
(A) In wild-type hairs, axial actin cables end at the subapical region (arrow), whereas the apex contains fine F-actin (arrowhead).
(B) In CA-rop2 hairs, an extensive actin network was found throughout the cortex.
(C) In DN-rop2 hairs, axial actin cables protruded to the extreme apex and no fine F-actin was found in the apex.
For each line, more than six hairs were observed and showed identical or very similar staining patterns. Bar in (A) = 20 μm for (A) to (C).
DISCUSSION
Cell polarity is fundamental to many aspects of plant development, such as polar auxin transport, early embryogenesis, cell differentiation (e.g., guard cell differentiation), root hair development, and pollen tube growth. The molecular basis of cell polarity establishment in plants is poorly understood. Genetic studies in budding yeast show that cell polarity development involves a cascade of different GTPase switches. A Ras GTPase-mediated process selects a polar site for budding. This, in turn, activates a Cdc42 GTPase-dependent switch that establishes the polar site. The polar site then is stabilized or maintained via polar secretion that is mediated by the Rho1 GTPase. Arabidopsis lacks orthologs of the yeast Ras, Cdc42, and Rho1 GTPases but possesses 11 members of the Cdc42-related Rop subfamily of RHO GTPases. An intriguing question is whether different Rop members might perform functions analogous to those of Ras, Cdc42, and Rho1 in yeast.
While we were preparing this report, Molendijk et al. (2001) reported a study establishing a role for Rops in root hair development. They performed immunolocalization with a general anti-Rop antibody and detected Rop protein at the future site of hair formation and at the root hair tip throughout hair development. Here, we show that the subcellular localization of GFP::Rop2 in growing root hair cells matches the pattern of immunolocalized Rop protein observed by Molendijk et al. (2001). That study also showed that GFP–CA-rop4 and GFP–CA-rop6 expression led to depolarized root hair growth, but it did not report any root hair phenotype for GFP–DN-rop4 transgenic lines. Here, we show that overexpression of native Rop2, or CA- and DN-rop2, leads to root hair phenotypes at all stages of hair formation, including dramatic effects on root hair initiation and tip growth not observed by Molendijk et al. (2001). Finally, Molendijk et al. (2001) did not show any evidence that either Rop4 or Rop6 is usually expressed in developing root hair cells. Here, we present convincing evidence that Rop2 is expressed during root hair development.
Recent studies have shown that Rop1 and another closely related Rop, Rop5, control polar growth in pollen tubes. The results we describe here show that Rop2, which belongs to the same Rop phylogenetic group as Rop1, has a similar role in the polar growth of root hairs. Our results also provide strong evidence that the same Rop GTPase affects the establishment of polar sites for root hair formation. It is striking that a single type of monomeric GTPase may regulate many different stages in polarity development in root hair cells. This contrasts with the well-established model, described above, of a cascade of multiple GTPases each controlling a different stage in cell polarity development.
Rop2 Controls the Establishment of Polar Sites for Hair Formation in the Hair-Forming Epidermal Cell
In the wild-type root, a single hair forms close to the root tip end of the hair-forming cell, suggesting that mechanisms exist that control the selection and establishment of a hair-forming site and subsequent swelling formation (Masucci and Schiefelbein, 1994; Drubin and Nelson, 1996). Recent work suggests that the potassium transporter TRH1 may prevent multiple hairs arising from a single cell (Rigas et al., 2001). Here, we have presented evidence that suggests that Rop2 may be involved in the selection and/or establishment of the hair-forming site. First, Rop2 OX and CA-rop2 expression led to additional, and often misplaced, swellings and hairs on individual hair-forming cells. Second, DN-rop2 reduced the number of hairs. Measurements of the length of hair-forming cells on DN-rop2 plants are required to confirm whether this reflects a reduction in the number of hair-forming sites.
It is surprising that the root hair initiation phenotype of DN-rop2 is not more severe. One possible explanation for this is that stronger DN-rop2 transgenic lines may be lethal, because constitutive expression of DN-rop2 may disrupt multiple distinct signaling pathways (Li et al., 2001). A strong DN-rop2 transgenic line that directs expression specifically in root hair cells could be used to test this possibility. An alternate hypothesis is that the weak initiation phenotype of DN-rop2 is the result of it inhibiting the function of another Rop that regulates initiation. Finally, GFP-tagged Rop2 localized to the future site of hair formation well before swelling formation. The GFP-Rop2 localization we observed is supported by the earlier immunolocalization work using an antibody raised against Rop4 (Molendijk et al., 2001). However, the antibody used did not distinguish whether the localization was caused by Rop4, Rop2, or another Rop (Rop2 and Rop4 are nearly identical at the amino acid level).
The reduced number of hairs in DN-rop2 plants and the localization of GFP-Rop2 to the future site of hair formation strongly suggest a role for Rop2 in the establishment of the polar growth site. However, the presence of misplaced hairs on the Rop2 OX and CA-rop2 lines suggests that Rop2 also could play a role in polar site selection. Loss-of-function Rop2 mutations could be used to confirm this possibility. We are currently seeking a knockout line that could be used to investigate this point. Because the loss-of-function mutants rhd6, axr2, etr1, and eto1 all affect polar site selection (Masucci and Schiefelbein, 1994), the relationship between Rop2, RHD6, and auxin and ethylene signaling could be explored using double mutants and treatments that alter auxin and ethylene signaling.
There is an interesting parallel between our Rop2 OX results and the role of Rac1 in controlling the number of wing hairs formed by wing epithelial cells in Drosophila (Eaton et al., 1996). However, it was a DN-rac1 mutant, and not overexpression, that gave rise to a multiple hair phenotype in the fruit fly, suggesting that Rac1 acts as a negative, and not a positive, regulator in the establishment of hair-forming sites in Drosophila. It is possible that another Rop plays a role in root hair development similar to the role of Rac1 in wing hair development.
We propose that the role for Rop2 in the establishment of hair-forming sites may be analogous to that of Cdc42 in yeast. Cdc42 overexpression caused a random budding pattern, whereas CA-cdc42 expression resulted in enlarged mother cells with abnormal buds (Johnson and Pringle, 1990; Ziman et al., 1991). This suggests that cycling between GDP-Cdc42 and GTP-Cdc42 forms is crucial for the budding process. Similarly, cycling between GDP-Rop2 and GTP-Rop2 may be required for swelling formation. This hypothesis is consistent with the observation that CA-rop2 caused fewer swellings per cell than did Rop2 OX, despite apparently being expressed to a similar level. Unlike Rop2 OX, CA-rop2 cannot be inactivated and return to the cytoplasmic pool of inactive Rop2 from the periphery of a plasma membrane Rop localization site. The multiple swellings and misplacement of hairs caused by Rop2 OX, therefore, may be explained by mislocalization of additional Rop2 to sites in which hairs usually would not form. Interestingly, Molendijk et al. (2001) showed that the ADP ribosylation factor guanine nucleotide exchange factor inhibitor brefeldin A delocalizes Rop from the future site of hair formation, suggesting that vesicle trafficking is essential for Rop localization to these sites.
An intriguing question is how Rop2 establishes hair-forming sites. In yeast, Cdc42 controls the establishment of budding sites, at least in part by regulating the polar localization of cortical actin patches. Evidence suggests that Rop also regulates the dynamics of localized cortical actin in both tip-growing pollen tubes and vegetative cells (Fu et al., 2001, 2002). However, previous studies suggest that the actin cytoskeleton becomes polarized only after swelling formation has begun (Braun et al., 1999; Miller et al., 1999; Baluska et al., 2000). Also, actin-perturbing drugs do not inhibit swelling formation (Miller et al., 1999; Baluska et al., 2000), suggesting that swelling formation is independent of actin. Furthermore, the localization of Rop to the future site of hair formation is not dependent on actin (Molendijk et al., 2001). Our finding that Rop2 OX and CA-rop2 have multiple hairs on single hair-forming cells suggests that swelling formation may be dependent on localized Rop2. The possible roles of activated Rop at the future site of hair formation before swelling formation remain to be determined.
Rop2 Controls Tip Growth during Root Hair Development
Rop2 OX often induced more than one hair on each swelling and had hairs that were branched at their tips, implicating Rop2 in controlling the site for tip-growth initiation. Interestingly, Rop1 OX in Arabidopsis pollen tubes caused swelling of the tip but not branching (Li et al., 1999). The difference in Rop OX tip-growth phenotypes suggests that Rop2, but not Rop1, may have a distinct function in the control of tip-growth initiation. This role could be analogous to that of Rop2 in regulating the establishment of hair-forming sites, as discussed above. Similarly, CA-rop2 did not cause hair branching and only rarely caused misplacement of hair-forming sites. Thus, the proper localization of Rop2 may be critical for defining tip-growth sites and probably requires normal cycling of Rop2 between the GDP and GTP forms. We did not find any differences in the expression level of CA-rop2 and Rop2 OX. Therefore, it is possible that ectopically localized CA-rop2 contributed to the observed differences in phenotype with Rop2 OX.
An alternate, but not mutually exclusive, explanation for the root hair–specific tip-branching phenotype caused by Rop OX concerns the differences in tip-growth mechanisms between root hairs and pollen tubes. Tip growth in root hairs and pollen tubes is directed by tip-to-base calcium ion gradients (Malhó and Trewavas, 1996; Bibikova et al., 1997). However, root hair growth also is directed by an endogenous polarity that is not found in pollen tubes (Bibikova et al., 1997). Similarly, root hair growth polarity is mediated by microtubules (Bibikova et al., 1999), whereas that of pollen tubes is independent of microtubules (Joos et al., 1994). Microtubule-disrupting drugs produce wavy root hairs and hairs with multiple branches at their tips (Bibikova et al., 1999; Ovecka et al., 2000). These effects are phenocopied by DN-rop2 and Rop2 OX, respectively. Rop2 and microtubules may act either in the same pathway or in two distinct pathways to control the polarity of root hair tip growth.
When CA-rop2 and Rop2 OX roots are grown in semisolid medium, hairs are thicker and, especially in the case of CA-rop2, tend to be curved. When grown in air, CA-rop2 causes severe or complete loss of polarity. These observations suggest that activating and/or overexpressing Rop2 partly or completely overrides the endogenous polarity that directs root hair growth (Bibikova et al., 1997). This endogenous directional cue could either regulate the localization of Rop2 to the site of tip-growth initiation or act independently of Rop2. At present, we are unable to determine if Rop2 acts independently or downstream of the endogenous polarity. On the basis of these observations, we speculate that a proper regulation of Rop2 localization plays a crucial role in the formation of highly polarized unbranched root hairs in wild-type plants.
Despite the well-established differences between pollen tubes and root hairs, many aspects of the role of Rop2 in the control of root hair tip growth are similar to those of Rop1 in pollen tubes. We have shown that both Rop2 OX and CA-rop2 had longer root hairs, whereas DN-rop2 had much shorter hairs. Similarly, the tip-localized Rop1 promotes pollen tube elongation when overexpressed to a low level (G. Wu, V. Vernoud, and Z. Yang, unpublished data), whereas DN-rop1 inhibits tube elongation (Li et al., 1999). Because hair growth rates were not measured here, differences in length could be attributable to growth continuing for a longer period of time, in the case of Rop2 OX and CA-rop2, or for a shorter period of time, in the case of DN-rop2. Furthermore, it is possible that Rop2 OX hairs are unable to stop growing but instead form more and more new tips after a hair has reached ∼500 to 600 μm in length.
Evidence suggests that Rop1 controls tip growth in pollen tubes through its effects on tip-localized F-actin and tip-focused calcium gradients (Li et al., 1999; Fu et al., 2001). Rop2 may regulate tip growth in root hairs through similar mechanisms. Both apical fine F-actin and tip-focused calcium gradients are involved in root hair tip growth (Bibikova et al., 1997; Wymer et al., 1997; Miller et al., 1999; Baluska et al., 2000). In this study, we showed that DN-rop2 expression disrupted fine tip F-actin, whereas CA-rop2 expression caused the formation of an extensive actin network in Arabidopsis root hairs, suggesting that the formation of tip actin in root hairs is controlled by localized Rop activity, as in pollen tubes. Molendijk et al. (2001) showed that CA-rop4 or CA-rop6 expression delocalized tip-focused calcium gradients in root hairs. However, they suggested that Rop activity may not be sufficient to establish a calcium gradient in root hairs because GFP–CA-rop2 did not colocalize with the high calcium gradient in the tip. It is possible that a Rop-independent pathway controls the polarity of tip growth in root hairs, as discussed above. Future studies should determine whether Rop2 activity is required for the formation of the tip-focused calcium gradient in root hairs.
Potential Targets of Rop2 Signaling
Genes carrying loss-of-function mutations that phenocopy our Rop2 transgenic lines might encode positive or negative regulators of Rop2 activity. To investigate this possibility, we are currently performing crosses with several mutants with root hair phenotypes that are remarkably similar to those of the Rop2 transgenic lines described here (Parker et al., 2000).
Two likely candidates for tip-growth factors regulated by Rop2 are profilin and ADF3. Profilin accumulates in swellings and, like ADF3 (Jiang et al., 1997; L. Herrera-Estrella, A.C. Kemp, and C.S. Grierson, unpublished results), growing root hair tips (Braun et al., 1999; Baluska et al., 2000). Plants overexpressing profilin (PFN-1) have longer root hairs (Ramachandran et al., 2000). Overexpression of ADF1 reduces root hair length and increases radial expansion, apparently induced by the depolymerization of actin cables, whereas antisense ADF1 increases root hair length by promoting the formation of actin cables (Dong et al., 2001). Both profilin and ADF3 are known to bind phosphatidylinositol-4,5-bisphosphate (PIP2), which forms a tip-to-base gradient in growing hairs (Gungabissoon et al., 1998; Braun et al., 1999) and localizes to the initiation site during swelling formation (Braun et al., 1999). Significantly, Rop5 associates physically with a phosphatidylinositol monophosphate kinase that produces PIP2 and colocalizes with PIP2 (Kost et al., 1999). It is tempting to speculate that Rop-induced PIP2 generation might regulate the localization and/or activity of profilin and/or ADF3 in root hairs.
Are Multiple Rop GTPases Involved in the Control of Root Hair Development?
Rop2 affects all stages of hair cell development. First, we have shown that Rop2 was expressed during swelling formation and tip growth. Second, it localized to the future site of hair development and throughout hair development at the hair tip. Finally, the phenotypes of Rop2 OX, DN-rop2, and CA-rop2 suggest roles for Rop2 throughout hair development. This does not exclude a potential role for other Rops in the control of hair development. Overexpression of Rop8 and Rop11 did not cause root hair phenotypes (Figure 1). Furthermore, CA-rop10 expression did not cause any root hair phenotypes (data not shown). These results suggest that Rop8/Rop10/Rop11 may be functionally distinct from Rop2, but it remains possible that they are involved in root hair development.
Rop7 OX appeared to inhibit specifically the transition from swelling to tip growth. We intend to fully characterize the root hair phenotype of Rop7 OX once it is established whether the native Rop7 gene is expressed in root hairs. Similarly, Molendijk et al. (2001) examined the effects of Rop4 and Rop6 mutant proteins on root hair development, but they did not show whether the native Rop genes were expressed in root hair cells. Both Rop4 and Rop6 are from the same phylogenetic subgroup of Rop as Rop2. Thus, if Rop4 and Rop6 are expressed and localized in root hair cells in a manner similar to Rop2, they may have redundant functions with Rop2 in the control of different stages of hair development. Alternatively, it is possible that Rop2 is either the sole Rop, or the sole member of this Rop group, expressed during hair cell development. We are working at present to identify which of the other 10 Arabidopsis Rops also might be expressed in root hairs and to determine their localization patterns. Furthermore, knockout mutants for single Rops and multiple Rops should provide us with a better understanding of the precise function of individual Rop GTPases in the control of root hair development.
Higher plants lack Ras, Cdc42, Rac, and Rho GTPases of the Ras superfamily, but the Rop subfamily has an emerging importance in plant signaling and development. It would be exciting to determine the precise role of Rop2, and perhaps other Rops, as key regulatory switches in different stages of root hair development.
METHODS
Plant Material and Growth Conditions
Sterilized Arabidopsis thaliana seed (4 min in 10% household bleach, 4 min in ethanol:water:bleach mixture [7:2:1], and 2 × 2 min in sterile water) were plated beneath the surface of semisolid medium (0.5% phytagel; adapted from Wymer et al. [1997]). Plated seed were vernalized for 48 hr at 4°C and grown in vertical orientation at 21°C with 24 hr of light. Selected seedlings were transferred to soil after 2 weeks. For single-cell aspiration, the wild-type Columbia ecotype was used. The photographs in Figures 1A, 1C to 1G, and 4J were taken of seedlings grown in air as described (Li et al., 2001).
Generation of Transgenic Lines Overexpressing Rop Genes and Analysis of Rop Overexpression
Rop7, Rop8, and Rop11 cDNAs were amplified from an Arabidopsis cDNA library by polymerase chain reaction (PCR) using gene-specific primers flanking Rop-encoding sequences (Rop7 sense, 5′-GGCCATGGGCATGAGCACAGCAAGATT-3′; Rop7 antisense, 5′-GGGTCGACTTATAGGAAAAAGCATATTC-3′; Rop8 sense, 5′-GGCCATGGGCATGTCAGCTTCAATGGCT-3′, Rop8 antisense, 5′-GGG-TCGACTCAAAGAACATGGCATAAAC-3′; Rop11 [formerly Arac10] sense, 5′-GGCCATGGGCATGGCTTCAAGTGCTTCA-3′; and Rop11 antisense, 5′-GGGTCGACTCAATGCCGAGTCACTATCCT-3′). These cDNAs were cloned into the pZERO vector (Invitrogen, Carlsbad, CA) by blunt-ended cloning, and their identities were confirmed by DNA sequencing. Rop cDNAs were subcloned into the binary pKYLX vector (Li et al., 2001). XhoI and SstI sites were used to subclone ROP8 and Arac8, BamHI and SstI sites were used to subclone ROP7, and HindIII and XhoI sites were used to subclone Rop11. The binary constructs were introduced into Agrobacterium tumefaciens strain GV3101 and transformed into Arabidopsis ecotype Columbia.
Transgenic plants were selected by kanamycin. The number of T-DNA insertions was estimated using the ratio of kanamycin-resistant to kanamycin-sensitive plants in the T2 generation. Homozygous transgenic plants with a single T-DNA insertion were selected from the T3 generation and used for phenotype characterization. The relative level of transgene expression was determined by reverse transcriptase–mediated PCR as described previously (Li et al. 2001). To construct the green fluorescent protein (GFP)::Rop2 fusion, the 3′ end of the mGFP-4 coding sequence was fused in frame with the 5′ end of the Rop2 coding sequence through a BglII site. The fusion gene was subcloned behind the 35S promoter in pBI121 by replacing β-glucuronidase using BamHI and SstI sites. The resulting plasmid was transformed into Arabidopsis plants as described above. Several independent lines expressing low levels of green fluorescence were obtained.
Cloning and Sequencing of Rop2 Full-Length cDNA from Initiating Root Hair Cells
cDNA preparation will be published separately (M.A. Jones and C.S. Grierson, unpublished data). Nested pairs of oligonucleotide primers were specific for the Rop2 3′ (forward) and 5′ (reverse) untranslated regions (Rop2 forward, 5′-CAAAGTCATCTATCAACCGC-3′; Rop2 reverse, 5′-CTGTGGACTCGAAAGATTCA-3′; Rop2 forward nested, 5′-CGCGGATCCCGAATTTGGGTGATTCTCAG-3′; and Rop2 reverse nested, 5′-CCGGAATTCTAGAAATGTCTCCCTTCACGTC-3′). Nested primers contained specific restriction enzyme sites at their 5′ ends for subcloning. Standard molecular biology techniques were used (Sambrook et al., 1989). The full-length Rop2 cDNA was ligated into pGEM-T Vector System I (Promega) according to the manufacturer's instructions. For sequencing, minipreparations were prepared using a QIAprep spin column kit (Qiagen, Valencia, CA). Sequencing was by the dideoxy chain termination method (BigDye; Perkin-Elmer) and was analyzed on an ABI 377 DNA sequencer (Applied Biosystems, Forster City, CA).
Histochemistry
Whole-mount in situ hybridization was performed as described (Friml, 2000). A 196-bp region of the Rop2 3′ untranslated region was amplified from genomic DNA by PCR using a specific pair of oligonucleotide primers (Rop2 probe forward, 5′-ACCAACTAAAGAAAGAAGCAG-3′; and Rop2 probe reverse, 5′-TACAACACACGGTCTC- TTAGTC-3′). The PCR product was cloned and sequenced as described above for the full-length Rop2 cDNA. The orientation of the insert was confirmed by sequence analysis and PCR. One microgram of cut vector was used as template DNA for in vitro transcription: 40.5 μL of DNA, 6 μL of digoxigenin RNA labeling mix (Roche Diagnostic, Mannheim, Germany), 6 μL of RNA polymerase buffer, 1.5 μL of SuperaseIn (Ambion, Austin, TX), and 6 μL of T7 or SP6 RNA Polymerase (Roche). Probe concentration was assessed by preparing dilution series of labeled RNA and digoxigenin-labeled control nucleic acid of known concentration. Histochemical β-glucuronidase activity assay of a Rop2 promoter:β-glucuronidase fusion was performed as described (Li et al., 2001).
Microscopy
Bright-field light microscopy (Axiovert 135 inverted microscope; Zeiss, Jena, Germany) was used to observe seedlings growing through semisolid medium. Images were captured using a JVC 3-CCD color video camera (JVC, Yokohama, Japan) and Leica (Wetzlar, Germany) Q500IW personal computer with Leica QWin Standard software (Y2.3a). Captured images were printed using a high-quality laser printer and used for quantitative analyses. Individual hairs were sampled at random either by capturing an image of the first hair to fall into the field of view (at high magnification) when moving the microscope stage from root to root or overlaying the image with an acetate sheet marked with a random pattern of dots. The hairs positioned closest to each dot were measured on each section. For confocal laser scanning microscopy, 3-day-old GFP-Rop2 seedlings, selected for bright fluorescence, were grown through semisolid medium on a glass-bottomed Petri dish (Willco dish; Willco Wells BV, Amsterdam). Growing root hairs were identified by the accumulated cytoplasm at their tips. Hairs were checked for growth before and after imaging. Nongrowing hairs were identified by vacuolation at the tip. Confocal laser scanning microscopy was performed using a Leica DM IRBE microscope with Leica TCS NT software (version 1.6.587). Images shown in the figures are projections of 1-μm optical sections.
Actin Visualization in Root Hairs
To visualize F-actin in root hairs, we transiently expressed a GFP-tagged actin binding domain of mouse talin using projectile-mediated transformation. The 35S:GFP–mouse talin construct in Cauliflower mosaic virus was described previously (Fu et al., 2002). Plasmid was amplified in Escherichia coli strain Top10 and purified using Qiagen plasmid mini kits according to the manufacturer's instructions. Arabidopsis ecotype Columbia, CA-rop2, or DN-rop2 seed were aligned and germinated on vertical Murashige and Skoog (1962) agar plates to allow uniform root growth on the surface of agar. Seven-day-old seedlings were bombarded with gold particles coated with plasmids (0.8 μg) using a PDS-1000/He particle delivery system (Bio-Rad, Hercules, CA) and a previously described procedure (Fu et al., 2001). Transformed root hairs were identified using epifluorescence microscopy and observed with either a Nikon (Tokyo, Japan) Optiphot upright microscope equipped with a Bio-Rad MRC 600 confocal laser scanning device or a Zeiss Axioplan microscope equipped with a Zeiss LSM 510 laser scanning confocal system (Fu et al., 2002). Confocal images were processed using Photoshop 5.5 (Adobe Systems, Mountain View, CA).
Acknowledgments
We thank Dr. Alison Kemp for useful comments, Dr. Paul Bowyer for help with Figure 5, and Dr. Ranjan Swarup and Prof. Malcolm Bennett for their kind assistance with in situ hybridization. This work was supported by U.S. Department of Agriculture, National Science Foundation, and Department of Energy grants (to Z.Y.), a Biotechnology and Biological Science Research Council studentship (to M.A.J.), and a Royal Society Dorothy Hodgkin Fellowship (to C.S.G.).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010359.
References
- Baluska, F., Salaj, J., Mathur, J., Braun, M., Jasper, F., Samaj, J., Chua, N.-H., Barlow, P.W., and Volkmann, D. (2000). Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol. 227, 618–632. [DOI] [PubMed] [Google Scholar]
- Bauer, W.D. (1981). Infection of legumes by rhizobia. Annu. Rev. Plant Physiol. 32, 407–449. [Google Scholar]
- Bibikova, T.N., Zhigilei, A., and Gilroy, S. (1997). Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta 203, 495–505. [DOI] [PubMed] [Google Scholar]
- Bibikova, T.N., Jacob, T., Dahse, I., and Gilroy, S. (1998). Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development 125, 2925–2934. [DOI] [PubMed] [Google Scholar]
- Bibikova, T.N., Blancaflor, E.B., and Gilroy, S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17, 657–665. [DOI] [PubMed] [Google Scholar]
- Braun, M., Baluska, F., von Witsch, M., and Menzel, D. (1999). Redistribution of actin, profilin and phosphatidylinositol-4,5-bisphosphate in growing and maturing root hairs. Planta 209, 435–443. [DOI] [PubMed] [Google Scholar]
- Chant, J. (1994). Cell polarity in yeast. Trends Genet. 10, 328–333. [DOI] [PubMed] [Google Scholar]
- Chant, J., and Stowers, L. (1995). GTPase cascades choreographing cellular behavior: Movement, morphogenesis, and more. Cell 81, 1–4. [DOI] [PubMed] [Google Scholar]
- Chung, H.-J., and Feri, R.J. (1999). Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiol. 121, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson, D. (1985). Factors affecting mineral nutrient acquisition by plants. Annu. Rev. Plant Physiol. 36, 77–115. [Google Scholar]
- Delmer, D.P., Pear, J.R., Andrawis, A., and Stalker, D.M. (1995). Genes encoding small GTP-binding proteins analogous to mammalian rac are preferentially expressed in developing cotton fibers. Mol. Gen. Genet. 248, 43–51. [DOI] [PubMed] [Google Scholar]
- Dolan, L., Duckett, C.M., Grierson, C., Linstead, P., Schneider, K., Lawson, E., Dean, C., Poethig, S., and Roberts, K. (1994). Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development 120, 2465–2474. [Google Scholar]
- Dong, C.-H., Xia, G.-X., Hong, Y., Ramachandran, S., Kost, B., and Chua, N.-H. (2001). ADF proteins are involved in the control of flowering and regulate F-actin organization, cell expansion, and organ growth in Arabidopsis. Plant Cell 13, 1333–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin, D.G., and Nelson, W.J. (1996). Origins of cell polarity. Cell 84, 335–344. [DOI] [PubMed] [Google Scholar]
- Eaton, S., Wepf, R., and Simons, K. (1996). Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J. Cell Biol. 135, 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml, J. (2000). The Analysis of Novel Members of PIN Gene Family in Arabidopsis. PhD Dissertation (Cologne, Germany: University of Cologne).
- Fu, Y., Wu, G., and Yang, Z. (2001). Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 152, 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y., Li, H., and Yang, Z. (2002). The Rop2 GTPase controls the formation of a novel cortical F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14, 777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson, C.S., Roberts, K., Feldmann, K.A., and Dolan, L. (1997). The COW1 locus of Arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation. Plant Physiol. 115, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson, C.S., Parker, J.S., and Kemp, A.C. (2001). Arabidopsis genes with roles in root hair development. J. Plant Nutr. Soil Sci. 164, 131–140. [Google Scholar]
- Gungabissoon, R.A., Jiang, C.-J., Drobak, B.K., Maciver, S.K., and Hussey, P.J. (1998). Interaction of maize actin-depolymerising factor with actin and phosphoinositides and its inhibition of plant phospholipase C. Plant J. 16, 689–696. [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Jiang, C.-J., Weeds, A.G., and Hussey, P.J. (1997). The maize actin-depolymerising factor, ZmADF3, redistributes to the growing tip of elongating root hairs and can be induced to translocate into the nucleus with actin. Plant J. 12, 1035–1043. [DOI] [PubMed] [Google Scholar]
- Johnson, D.I., and Pringle, J.R. (1990). Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 111, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos, U., Vanaken, J., and Kristen, U. (1994). Microtubules are involved in maintaining the cellular polarity in pollen tubes of Nicotiana sylvestris. Protoplasma 179, 5–15. [Google Scholar]
- Kost, B., Lemichez, E., Spielhofer, P., Hong, Y., Tolias, K., Carpenter, C., and Chua, N.-H. (1999). Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Wu, G., Ware, D., Davis, K.R., and Yang, Z. (1998). Arabidopsis Rho-related GTPases: Differential gene expression in pollen and polar localization in fission yeast. Plant Physiol. 118, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Lin, Y., Heath, R.M., Zhu, M.X., and Yang, Z. (1999). Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11, 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Shen, J.-J., Zheng, Z.-L., Lin, Y., and Yang, Z. (2001). The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 126, 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., and Yang, Z. (1997). Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell 9, 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., Wang, Y., Zhu, J.-K., and Yang, Z. (1996). Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 8, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhó, R., and Trewavas, A.J. (1996). Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 8, 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci, J.D., and Schiefelbein, J.W. (1994). The RHD6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol. 106, 1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, D.D., de Ruijter, N.C.A., Bisseling, T., and Emons, A.M.C. (1999). The role of actin in root hair morphogenesis: Studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 17, 141–154. [Google Scholar]
- Molendijk, A.J., Bischoff, F., Rajendrakumar, C.S.V., Friml, J., Braun, M., Gilroy, S., and Palme, K. (2001). Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20, 2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473.–497. [Google Scholar]
- Ovecka, M., Nadubinska, M., Volkmann, D., and Baluska, F. (2000). Actomyosin and exocytosis inhibitors alter root hair morphology in Poa annua. Biol. Bratislava 55, 105–114. [Google Scholar]
- Parker, J.S., Cavell, A.C., Dolan, L., Roberts, K., and Grierson, C.S. (2000). Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell 12, 1961–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran, S., Christensen, H.E.M., Ishimaru, Y., Dong, C.-H., Chao-Ming, W., Cleary, A.L., and Chua, N.-H. (2000). Profilin plays a role in cell elongation, cell shape maintenance and flowering in Arabidopsis. Plant Physiol. 124, 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas, S., Debrosses, G., Haralampidis, K., Vicente-Agullo, F., Feldmann, K.A., Grabov, A., Dolan, L., and Hatzopoulos, P. (2001). TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 13, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, E., Grierson, C.S., Cavell, A., Steer, M., and Dolan, L. (1998). TIP1 is required for both tip growth and non-tip growth in Arabidopsis. New Phytol. 138, 49–58. [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schiefelbein, J.W. (2000). Constructing a plant cell: The genetic control of root hair development. Plant Physiol. 124, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein, J.W., and Somerville, C. (1990). Genetic control of root hair development in Arabidopsis thaliana. Plant Cell 2, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein, J.W., Shipley, A., and Rowse, P. (1992). Calcium influx at the tip of growing root-hair cells of Arabidopsis thaliana. Planta 187, 455–459. [DOI] [PubMed] [Google Scholar]
- Schiefelbein, J., Galway, M., Masucci, J., and Ford, S. (1993). Pollen-tube and root-hair tip growth is disrupted in a mutant of Arabidopsis thaliana. Plant Physiol. 103, 979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Very, A.-A., and Davies, J.M. (2000). Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 97, 9801–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge, P., Brembu, T., and Bones, A.M. (1997). Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol. Biol. 35, 483–495. [DOI] [PubMed] [Google Scholar]
- Winge, P., Brembu, T., Kristensen, R., and Bones, A. (2000). Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics 156, 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymer, C.L., Bibikova, T.N., and Gilroy, S. (1997). Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 12, 427–439. [DOI] [PubMed] [Google Scholar]
- Yang, Z., and Watson, J.C. (1993). Molecular cloning and characterisation of rho, a ras-related small GTP-binding protein from the garden pea. Proc. Natl. Acad. Sci. USA 90, 8732–8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z.-L., and Yang, Z. (2000). The Rop GTPase: An emerging signaling switch in plants. Plant Mol. Biol. 44, 1–9. [DOI] [PubMed] [Google Scholar]
- Ziman, M., O'Brien, J.M., Ouellette, L.A., Church, W.R., and Johnson, D.I. (1991). Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 11, 3537–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]