Abstract
Polar cell expansion in differentiating tissues is critical for the development and morphogenesis of plant organs and is modulated by hormonal and developmental signals, yet little is known about signaling in this fundamental process in plants. In contrast to tip-growing cells, such as pollen tubes and root hairs, cells in developing tissues are thought to expand by diffuse growth. In this study, we provide evidence that these cells expand in two phases with distinct mechanisms. In the early phase, cell expansion can occur in both longitudinal and radial or lateral directions and is mediated by Rop GTPase signaling, a mechanism known to control tip growth. The expression of a dominant-negative mutant for ROP2 (DN-rop2) inhibited polar cell expansion, whereas the expression of a constitutively active mutant (CA-rop2) caused isotropic expansion in the early phase. In the late phase, expansion occurs only in the longitudinal direction and is not affected by DN-rop2 or CA-rop2 expression. The transition from the early to the late phase coincides with the reorientation of cortical microtubules from random to transverse arrangements. Thus, cell expansion in the late phase is consistent with polar diffuse growth, in which polarity probably is defined by transverse cortical microtubules. We show that the direction of cell expansion in the early phase is associated with the localization of diffuse fine cortical F-actin in leaf epidermal cells. DN-rop2 expression specifically inhibited the formation of this F-actin, but not actin cables, whereas CA-rop2 expression caused delocalized distribution of this fine F-actin throughout the cell cortex. Furthermore, green fluorescent protein–ROP2 was localized preferentially to the cortical region of the cell, where expansion apparently occurs. These observations suggest that ROP2 control of the polar expansion of cells within tissues is analogous to the Rop control of tip growth and of tip-localized F-actin in pollen tubes and root hairs and that the tip growth mechanism also may modulate polar cell expansion in differentiating tissues.
INTRODUCTION
Cell shape formation and regulation are crucial for cell functions, tissue and organ development, and morphogenesis in plants. Because of the constraint by the cell wall, plant cells develop specific shapes almost exclusively by directional cell expansion. Directional cell expansion in plants can be achieved either by tip growth or by diffuse polar growth, depending on the cell type (Kropf et al., 1998; Waller et al., 2000). Mechanisms for this directional control are thought to be distinct for these two types of growth. In tip growth, post-Golgi vesicles are targeted to and fused to a defined region of the plasma membrane (PM), leading to a unidirectional extension of the PM (derived from the vesicle membrane) and the cell wall (derived from the contents of the vesicle). Only pollen tubes and root hairs have been shown clearly to rely on tip growth for their morphogenesis. Most cell types in plants are thought to expand by diffuse growth. In diffuse growth, expansion presumably is driven by turgor and occurs diffusely throughout the entire cell surface, whereas the direction (or polarity) of expansion is thought to be determined by the pattern of deposition and loosening of cell wall polymers, especially cellulose microfibrils (Kropf et al., 1998; Martin et al., 2001).
The cytoskeleton plays a critical role in cell shape formation. In diffusely growing cells, cortical microtubules (MTs) have been shown to modulate the direction of expansion (Lloyd and Wells, 1985; Quader, 1986; Shibaoka, 1994; Baskin and Wilson, 1997; Fisher and Cyr, 1998; Kropf et al., 1998; Mathur et al., 1999; Szymanski et al., 1999; Whittington et al., 2001). The mechanism whereby MTs control the polarity of diffuse expansion is not clear, although it has been suggested that they direct the deposition of cellulose microfibrils (Kropf et al., 1998; Wasteneys, 2000; Martin et al., 2001). A MT-dependent mechanism also is involved in the maintenance of tip growth direction in root hairs (Blancaflor et al., 1998; Bibikova et al., 1999). F-actin plays an essential role in tip growth (Miller et al., 1999; Baluska et al., 2000; Fu et al., 2001). At least two forms of F-actin have been observed in tip-growing cells: actin cables arranged along the growth axis, and dynamic fine F-actin localized to the tip (Gibbon et al., 1999; Miller et al., 1999; Baluska et al., 2000; Fu et al., 2001).
Longitudinal actin cables are thought to act as tracks for cytoplasmic streaming and thus have been assumed to be required for tip growth by delivering vesicles to the tip (for reviews, see Taylor and Hepler, 1997; Kost et al., 1998; Gibbon et al., 1999). Evidence suggests that the dynamic of polar fine F-actin at the tip is essential for growth (Gibbon et al., 1999; Miller et al., 1999; Baluska et al., 2000; Fu et al., 2001). The mode of action for this form of actin is not understood, but it might target Golgi vesicles to the region of the PM for localized exocytosis (Miller et al., 1999; Fu et al., 2001). Studies involving actin-disrupting drugs or altered levels of actin binding proteins suggest that F-actin also plays a role in the polar expansion of diffusely growing cells (Thimann et al., 1992; Baskin and Bivens, 1995; Mathur et al., 1999; Szymanski et al., 1999; Ramachandran et al., 2000; Dong et al., 2001). However, these studies have not identified a specific form of actin filaments for the control of polar diffuse expansion.
In differentiating tissues and organs, cell-to-cell communication and signal transduction are expected to play a major role in the regulation of polar cell expansion. Many studies have linked altered cell expansion to changes in the organization of MTs and F-actin induced by hormone treatments (Shibaoka, 1994; Wasteneys, 2000; Catterou et al., 2001). Thus, it is important to elucidate the signal transduction that controls the organization and dynamics of specific cytoskeletal arrays involved in cell expansion, yet our knowledge of this process is very limited (Kropf et al., 1998; Staiger, 2000; Wasteneys, 2000).
Recent studies on the function of Rop GTPases in plants have begun to elucidate signaling in tip growth and the dynamics of F-actin involved in tip growth (Lin et al., 1996; Lin and Yang, 1997; Kost et al., 1999a; Li et al., 1999; Fu et al., 2001; Molendijk et al., 2001; Jones et al., 2002). Rop is a plant-specific subfamily of the Rho family of small GTPases (Li and Yang, 2000; Zheng and Yang, 2000b). Rho GTPases are conserved molecular switches that link signal transduction to actin organization in various eukaryotic organisms (Li and Yang, 2000; Zheng and Yang, 2000b). In Arabidopsis, three closely related ROP genes, ROP1, ROP3, and ROP5, are expressed in pollen (Li et al., 1998; Zheng and Yang, 2000a). Rop GTPases are localized to the apical PM region in pollen tubes and play an essential role in the control of pollen tube tip growth (Lin et al., 1996; Lin and Yang, 1997; Kost et al., 1999a; Li et al., 1999). The localization of Rop GTPases to the tip defines the apical PM region of the pollen tubes for tip growth (Li et al., 1999; for reviews, see Fu and Yang, 2001; Yang, 2002). Our studies suggest that Rop controls tip growth by modulating the formation of both the dynamic fine tip F-actin and a tip-focused cytosolic calcium gradient in pollen tubes (Li et al., 1999; Fu et al., 2001). In pollen tubes, the dynamics of tip F-actin is critical for the maintenance of polar growth, because the stabilization of tip F-actin by ROP1 overexpression causes depolarized growth in pollen tubes (Fu et al., 2001).
Because of the aforementioned general role for F-actin in cell expansion and because Rop GTPases are expressed ubiquitously in various plant cells (Li et al., 2001), we sought to determine whether Rop serves as a universal signaling switch to modulate polar cell expansion. We have investigated the function of the Arabidopsis ROP2 gene, which is expressed constitutively in vegetative tissues (Li et al., 2001). Using transgenic Arabidopsis plants overexpressing ROP2 and its dominant-negative (DN-rop2) or its constitutively active (CA-rop2) mutant genes under the control of the 35S promoter of Cauliflower mosaic virus (Li et al., 2001), we have shown that ROP2 controls root hair initiation and tip growth, similar to the ROP1 control of tip growth in pollen tubes (Jones et al., 2002). A similar conclusion was reported by Palme's group using inducible expression of constitutively active mutants for the Arabidopsis ROP4 and ROP6 genes (Molendijk et al., 2001).
In this study, we demonstrate that ROP2 modulates polar expansion in various cells within differentiating tissues, which generally are thought to expand by diffuse growth. Our results suggest two distinct mechanisms for polar expansion of these cells: a Rop-dependent mechanism for multidimensional expansion in the early phase, and a Rop-independent mechanism for longitudinal expansion in the late phase. We have shown that ROP2 spatially regulates the formation of a diffuse form of cortical fine F-actin that is associated tightly with cell expansion in the early phase. These observations suggest that cell expansion in the early phase in developing tissues may be modulated by a mechanism involving the Rop-dependent formation of cortical F-actin, analogous to the Rop control of tip growth in pollen tubes and root hairs.
RESULTS
Rop2 Dominant Mutants Affect Cell Morphogenesis in Different Cell Types in Arabidopsis Plants
Using transgenic Arabidopsis plants expressing CA-rop2 or DN-rop2 genes (Li et al., 2001), we first determined the effect of CA-rop2 and DN-rop2 expression on trichome development. Unlike root hairs and pollen tubes, however, which grow by tip growth, trichomes probably expand by diffuse growth (Mathur et al., 1999; Mathur and Chua, 2000). Arabidopsis trichomes usually form three branches, and branch initiation is MT dependent (Szymanski et al., 1999; Mathur and Chua, 2000). As shown in Figure 1, although DN-rop2 expression did not alter trichome morphology dramatically (Figures 1A and 1C), CA-rop2 expression caused two major changes (Figure 1B). First, trichome stalks and branches were much thicker than those of the wild type. Second, branch position was altered, although the number of branches was not affected. The stalk was longer, and the first two branches were much farther apart compared with those in the wild type. These results suggest that ROP2 may play a role in the morphogenesis of cells undergoing diffuse growth.
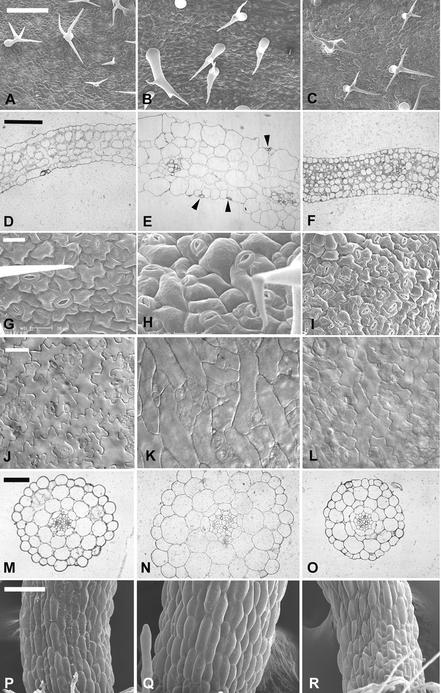
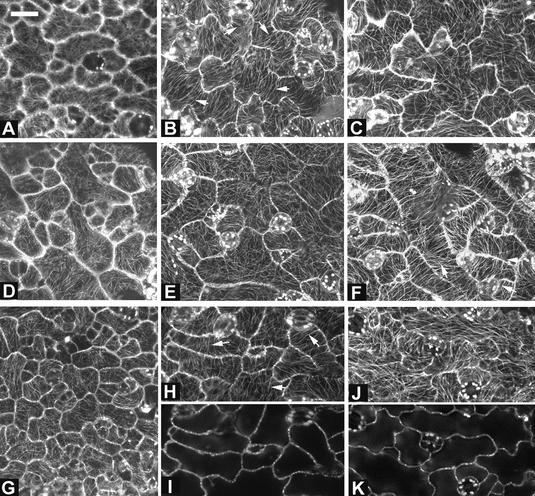
Figure 1.
Rop2 Controls the Morphogenesis of Various Cell Types in Arabidopsis.
The effects of the transgenic expression of 35S:CA-rop2 and 35S:DN-rop2 fusion genes on cell shape in Arabidopsis plants were analyzed using different methods as described in the text. Images at left show wild-type plants, images in the middle show CA-rop2 transgenic plants, and images at right show DN-rop2 transgenic plants.
(A) to (C) Scanning electron microscopy images of leaf trichomes. Bar = 200 μm.
(D) to (F) Leaf cross-sections. Bar = 250 μm.
(G) to (I) Scanning electron microscopy images of leaf pavement cells. Bar = 20 μm.
(J) to (L) Pavement cells in cleared leaves. Bar = 40 μm.
(M) to (O) Hypocotyl cross-sections. Bar = 50 μm.
(P) to (R) Scanning electron microscopy images of hypocotyls. Bar = 100 μm.
We have shown that ROP2 is expressed constitutively in different vegetative tissues and modulates organ morphogenesis in Arabidopsis (Li et al., 2001). Thus, we were interested in whether ROP2 played a general role in the morphogenesis of cells that are thought to expand by diffuse growth during organogenesis. We first examined the effect of CA-rop2 and DN-rop2 expression on cell shape in fully mature leaves. Figures 1D to 1F show cell shapes in leaf cross-sections. Different cell types in wild-type leaves display distinct morphology. CA-rop2 expression did not alter cell differentiation significantly, because guard cells, trichomes, xylem, and phloem cells all were present in CA-rop2 leaves. CA-rop2 expression increased radial expansion of these highly differentiated cells slightly, but it did not affect their overall cell shapes dramatically (data not shown). However, CA-rop2 expression increased cell sizes dramatically and altered cell shapes of those cells that had undergone rapid radial or lateral expansion, including epidermal pavement cells and mesophyll cells (Figure 1E). In wild-type leaves, both palisade and sponge mesophyll cells are more or less oval in sections, with the longitudinal axes of palisade and sponge cells oriented vertically and horizontally, respectively, with respect to the leaf surface. The mesophyll cells are connected loosely to each other, leaving extensive intercellular space (Figure 1D). In CA-rop2 leaves, the two types of mesophyll cells were hardly distinguishable, and mesophyll cells were tightly associated with each other and with epidermal cells, leaving little or no intercellular space (Figure 1E).
CA-rop2 expression induced the most dramatic changes in the morphology of epidermal pavement cells. In wild-type leaves, these cells have lobes intercalated with “necks” of adjacent cells and display wavy cross-walls (Figures 1G and 1J). Thus, epidermal pavement cells display various sizes and are either nearly rectangular or circular in cross-sections (Figure 1D), depending on the position and the orientation of the cell in the cross-section. In contrast, CA-rop2 pavement cells were much fatter than wild-type cells, nearly circular or squared, and similar to each other in size (Figure 1E). These results suggest that pavement cells have lost their lateral polarity. The lack of lateral polarity in these cells is further supported by the misplacement of guard cells on the top of (Figure 1E, arrowheads) or beneath (data not shown) pavement cells. In the wild type, a pair of guard cells is bordered by two pavement cells with a large air space underneath the guard cells in cross-sections (Figure 1D). Interestingly, the surface view of epidermal cells reveals that CA-rop2 expression completely eliminated lobes found in wild-type cells, but apparently it did not affect cell elongation along the longitudinal axis of the leaf, causing nearly rectangular or spindly cells with straight-sided walls (Figures 1H and 1K). In general, the radial diameter of CA-rop2 pavement cells was much greater than that of the neck of wavy pavement cells in wild-type leaves. This finding suggests that rectangular or spindly cell shapes were not attributable to the CA-rop2 inhibition of lobe extension but instead were caused by the abnormal expansion of the necks of the cell.
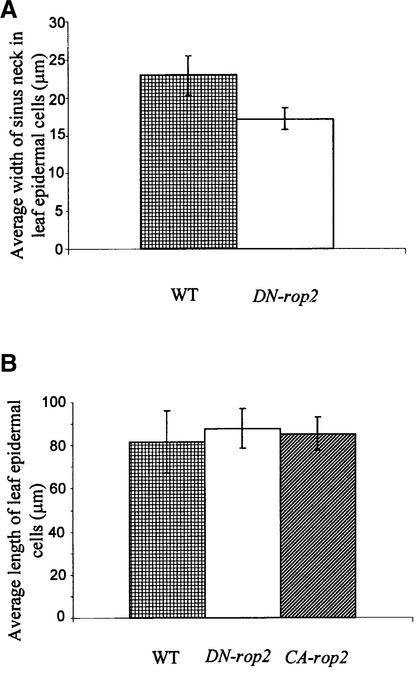
The effect of DN-rop2 expression on leaf cell shape was distinct from that of CA-rop2. Leaf cross-sections show that DN-rop2 cells were less expanded in the lateral and radial directions than wild-type cells (Figure 1F). In both palisade and spongy mesophyll cells, DN-rop2 expression apparently caused greater inhibition of lateral cell expansion in the long axis than in the short axis, resulting in nearly rounded cells. This finding suggests that DN-rop2 inhibited polar cell expansion. This is further confirmed by the effect of DN-rop2 on the morphology of epidermal pavement cells (Figures 1I and 1L). DN-rop2 cells were less wavy, because lobe extension was inhibited, although lobes were formed. In addition, quantitative analysis shows that the necks of wavy DN-rop2 cells were significantly narrower than the necks of wild-type cells (Figure 2A). However, the length of DN-rop2 pavement cells was not significantly different from that of wild-type or CA-rop2 leaves (Figure 2B), suggesting that DN-rop2 expression specifically affected cell expansion in the radial and localized lateral dimensions. Together, these results suggest that ROP2 is involved in the regulation of polar expansion in localized regions of the cell (e.g., during lobe formation) and in the radial or lateral direction.
Figure 2.
CA-rop2 and DN-rop2 Expression Alters Radial Expansion but Not Longitudinal Expansion in Mature Pavement Cells.
(A) To determine whether Rop is involved in radial cell expansion, the average width of the neck region of wavy pavement cells was measured. Statistical analysis (t test) showed that pavement cells expressing DN-rop2 display significantly narrower necks than wild-type cells (n = 200; P ≤ 0.05).
(B) To determine whether Rop is involved in longitudinal cell expansion, the maximum length was measured from randomly chosen pavement cells of cleared leaves (see Figure 1). Statistical analysis (t test) showed no significant difference between wild-type cells and cells expressing CA-rop2 or DN-rop2 (n = 200, P > 0.05).
WT, wild type.
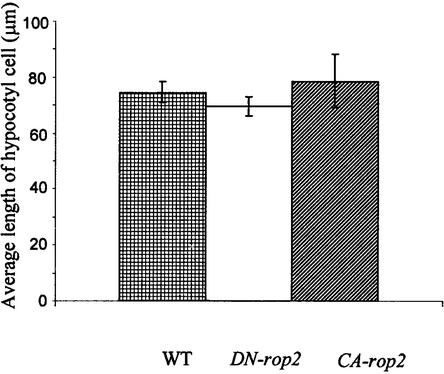
To further investigate whether ROP2 has a general role in the control of radial cell expansion, we examined cell shapes in the hypocotyl. As shown in Figure 1N, CA-rop2 expression increased radial expansion of cells in every layer compared with the wild type (Figure 1M), whereas DN-rop2 expression reduced radial expansion in most cell layers (Figure 1O). Interestingly, the effect of rop2 mutants was most dramatic in cortex and endodermis cells, the two cell types that display the greatest radial expansion in wild-type plants (Figure 1M). As in leaf pavement cells, however, the length of hypocotyl epidermal cells was not affected by the expression of either rop2 mutant gene (Figures 1P to 1R and 3). These observations confirm that Rop signaling regulates cell expansion in the radial dimension but does not affect the longitudinal dimension of mature cells.
Figure 3.
DN-rop2 and CA-rop2 Expression Does Not Alter the Length of Hypocotyl Epidermal Cells.
The average length of 150 epidermal cells located within 200 μm of the root-hypocotyl junction was determined from scanning electron microscopy images (see Figure 1). No significant difference was found between wild-type cells and cells expressing DN-rop2 or CA-rop2. WT, wild type.
Rop2 Dominant Mutants Affect the Early but Not the Late Stages of Cell Expansion in Growing Tissues
The effect of CA-rop2 and DN-rop2 expression on the radial or lateral dimension but not the longitudinal dimension of mature cells can be explained by two different models. Rop signaling could specifically control radial or lateral cell expansion but not longitudinal expansion. Alternatively, Rop signaling could specifically regulate the early phase of cell expansion, in which expansion occurs in both the radial/lateral and longitudinal dimensions, but does not affect the late phase of expansion, in which cells expand only longitudinally. To distinguish these two models, we examined the effect of CA-rop2 and DN-rop2 expression on different stages of the development of epidermal pavement cells and hypocotyl cortex cells. The base of the leaf contained undifferentiated dividing cells, whereas the tip of the leaf contained the most expanded mature cells. Thus, the degree of cell expansion forms a base-to-tip gradient. To facilitate the analysis of cell shapes, we used transgenic lines expressing green fluorescent protein (GFP)–tagged α-tubulin (see below), because cortical localization of GFP-tubulin provides a convenient means for visualizing cell shapes.
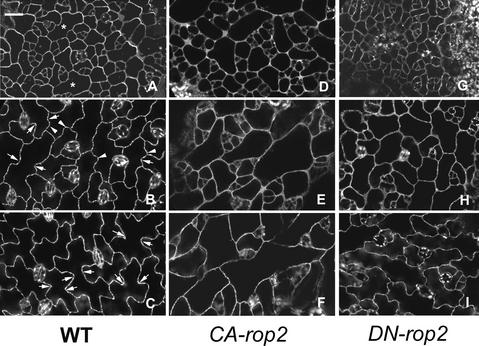
Because the development of epidermal pavement cells has not been described in Arabidopsis, we first characterized this process in wild-type plants expressing GFP-tubulin. We used expanding leaves ∼0.5 cm long for our analysis and divided the development of pavement cells into three stages, which are predictable according to the positions of cells along the long axis of the leaf. Stage I cells, localized at the base of the leaf, have just begun to expand (Figure 4A). Their morphology is more or less regular, mostly square, rectangular, or pentagonal. Some cells in this region have already expanded (asterisks) and are bordered by several smaller cells. These larger cells are at the transition to stage II cells. Localized around the midpoint of the leaf, stage II cells have clearly expanded and established the long axis of expansion (Figure 4B). Lobes (arrowheads) are clearly initiated along their cross-walls bordering adjacent elongated cells. Thus, stage II cells show a slightly wavy appearance of the cell outline and have one or more apparent necks (arrows) along the long axis of the cell. The neck region apparently has stopped expanding. After stage II, cell expansion occurs primarily in the lobes, leading to the formation of stage III cells (Figure 4C). Stage III cells, which are localized at the tip of the leaf, contain multiple extended lobes and highly wavy cell outlines.
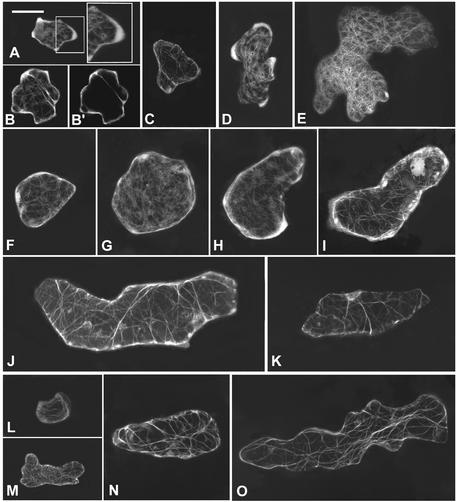
Figure 4.
Effect of CA-rop2 and DN-rop2 Expression on the Development of Different Stages of Leaf Epidermal Pavement Cells.
Expanding leaves (∼0.5 cm in length) from wild-type plants and transgenic plants expressing CA-rop2 or DN-rop2 were used for this analysis. Cell shapes were imaged by confocal microscopy using plants expressing the GFP-tubulin fusion gene as described in the text.
(A) to (C) Wild-type epidermal cells expressing GFP-tubulin.
(D) to (F) CA-rop2 epidermal cells expressing GFP-tubulin.
(G) to (I) DN-rop2 epidermal cells expressing GFP-tubulin.
(A), (D), and (G) show pavement cells in stage I; (B), (E), and (H) show pavement cells in stage II; and (C), (F), and (I) show pavement cells in stage III. WT, wild type. Bar in (A) = 20 μm for (A) to (I).
The different stages of pavement cells in transgenic plants were identified based on their leaf positions with respect to those in wild-type leaves. In CA-rop2 plants, stage I cells are similar to wild-type cells in the same stage but are slightly more expanded (Figure 4D). At stage II, no lobes are visible, but cells undergo dramatic expansion, apparently in random orientations, leading to the formation of irregularly shaped or nearly spherical cells (Figure 4E). Stage II cells expand largely in the direction of the leaf long axis to form elongated stage III cells (Figure 4F). In DN-rop2 plants, stage I cells show normal cell shapes, but they are smaller than wild-type cells (Figure 4G). At stage II, the long axis of cells appears to have been established as in the wild type, but overall, they are smaller than wild-type cells at the same stage. Furthermore, the site of lobe initiation is apparent but much less obvious than in the wild type (Figure 4H). At stage III, lobes were formed in DN-rop2 cells but were much shallower; thus, the outline of DN-rop2 cells was much less wavy than that of wild-type cells at the same stage (Figure 4I). However, dramatic cell elongation had occurred in these cells; thus, they were as elongated as wild-type and CA-rop2 cells (Figure 2B). These results show that both CA-rop2 and DN-rop2 expression had a strong effect on the early stages (stages I and II) but had little or no effect on the late stage (after stage II) of cell expansion in leaf epidermal pavement cells.
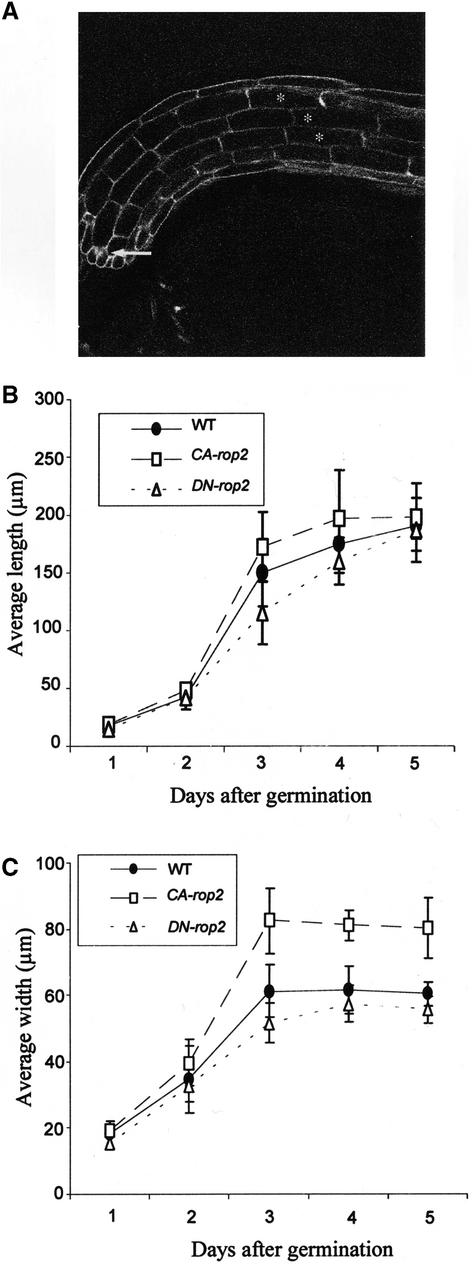
Next, we assessed whether the differential effect of ROP2 on different stages of cell expansion represents a general mechanism for different cell types. We performed a time-course analysis of the effect of CA-rop2 and DN-rop2 expression on the elongation and radial expansion of hypocotyl cortex cells. Because it is extremely difficult to follow the same cell for changes in cell shape in growing tissues, we measured temporal changes in the average length and width of the fifth cortex cells from the root-hypocotyl junction (Figure 5A). The length (longitudinal expansion) and width (radial expansion) of these cells were measured in seedlings every 24 hr after germination. In wild-type seedlings, rapid longitudinal and radial expansion occurred within 2 to 3 days after germination, although the longitudinal expansion was two to three times faster than the radial expansion (Figures 5B and 5C). After day 3, no radial expansion was observed, whereas cell elongation continued. These observations are consistent with the hypothesis that cell expansion is divided into two phases in anisotropically growing cells: the first phase involves both longitudinal and radial expansion, and the second phase involves only longitudinal expansion (Schindelman et al., 2001).
Figure 5.
Distinct Effects of CA-rop2 and DN-rop2 Expression Distinguish the Rop-Dependent and Rop-Independent Phases of Anisotropic Cell Expansion.
(A) A “pseudo-time-course” analysis of radial and longitudinal expansion was conducted on hypocotyl cortex cells. The fifth cortex cells from three adjacent files were used in this analysis. Changes in cell expansion on these cortex cells were followed from days 1 to 5 after germination.
(B) and (C) Changes in average lengths and widths of hypocotyl cortex cells from days 1 to 5 after germination (n = 32). The cortex cells displayed their most rapid cell expansion in both the radial and longitudinal directions during days 2 and 3, although preferential expansion occurred in the longitudinal direction. Statistical analysis (t test) showed that during this period, longitudinal expansion was inhibited significantly by DN-rop2 expression, whereas radial expansion was promoted significantly by CA-rop2 expression (P ≤ 0.05). After day 3, only longitudinal expansion was observed, and neither radial nor longitudinal expansion was affected significantly by CA-rop2 or DN-rop2 expression.
WT, wild type.
Interestingly, CA-rop2 expression greatly increased radial expansion within 2 to 3 days after germination but only weakly affected longitudinal expansion. In CA-rop2 cells, radial expansion ceased and elongation increased gradually by day 3 after germination, as in wild-type seedlings (Figures 5B and 5C). These results suggest that CA-rop2 expression promotes radial expansion in the first phase but has little effect on the second phase. In contrast, DN-rop2 expression reduced radial expansion slightly but had a dramatic effect on cell elongation on days 2 and 3 after germination. Therefore, DN-rop2 and CA-rop2 had opposite effects on polar cell expansion (i.e., preferential expansion in the longitudinal dimension in wild-type cells). CA-rop2 promoted nonpolar cell expansion by increasing radial expansion, whereas DN-rop2 inhibited polar expansion in the longitudinal direction. After day 3, however, DN-rop2 had no effect on cell elongation. In fact, by day 5, cell length in DN-rop2 cells was approximately the same as that in wild-type cells, suggesting ROP2-independent developmental control of cell elongation in the second phase. Together, these results further confirm that ROP2 regulates the first phase of cell expansion (both radial and longitudinal expansion) but is not involved in the second phase (cell elongation only).
The Transition to ROP2-Independent Cell Expansion Is Correlated with the Appearance of Transverse MTs in Leaf Epidermal Cells
Many studies have revealed a crucial role for MTs in modulating the direction of cell expansion, and it is proposed that transverse cortical MTs define the longitudinal direction of diffuse polar expansion (for reviews, see Kropf et al., 1998; Wasteneys, 2000). To determine whether the ROP2-independent expansion corresponds to polar diffuse expansion requiring transverse MTs, we analyzed the organization of MTs during different stages of cell morphogenesis in leaf pavement cells. To visualize MT organization, we used transgenic lines expressing GFP-tagged α-tubulin (Ueda et al., 1999). In control wild-type pavement cells expressing GFP-tubulin, the organization of MTs varied with the stages of pavement cells. In young pavement cells before the formation of obvious lobes, cortical MTs were oriented randomly (Figure 6A). When pavement cells expanded to the size with a maximum neck and began lobe extension (soon after stage II; see Figure 4), they generally contained transversely arranged cortical MTs (Figure 6B). These transverse MTs were most prominent in the neck region of elongating cells, but sometimes they were found between a neck region and a lobe shoulder opposite the neck region in these cells. However, no transverse MTs were found in the tip region of lobes, which presumably were extending.
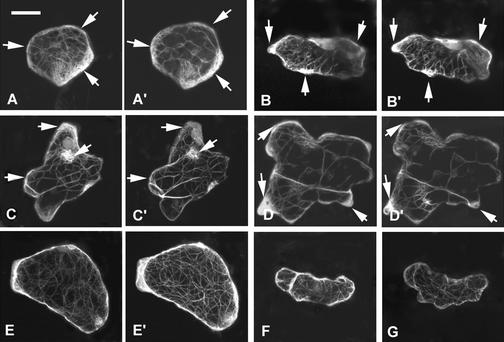
Figure 6.
Transition from Early to Late Phase of Cell Expansion Is Correlated with the Formation of Transverse Cortical MTs.
MT organization was analyzed in different stages of wild-type, DN-rop2, and CA-rop2 pavement cells using GFP-tagged α-tubulin as described in the text. In the early phase of cell expansion (between stages I and II), cortical MTs were oriented largely randomly, and DN-rop2 and CA-rop2 expression did not affect the organization of MTs. (A), (D), and (G) show late stage I cells from wild-type, DN-rop2, and CA-rop2 plants, respectively. During the transition from stage II to stage III, transverse MTs were found in certain regions of the cell in both wild-type plants (B) and DN-rop2 plants (H). CA-rop2 expression delayed the formation of transverse MTs slightly (E). Extensive transverse MTs remained even in fully expanded CA-rop2 cells (F). In contrast, in mature wild-type and DN-rop2 cells, cortical MTs were oriented randomly or longitudinally ([C] and [J]). All images shown except for (I) and (K) are projections of serial sections; (I) and (K) are medial sections showing cell shapes for (H) and (J), respectively. Bar in (A) = 20 μm for (A) to (K).
In most mature pavement cells (stage III), cortical MTs formed a network with randomly oriented arrays (Figure 6C). CA-rop2 pavement cells expressing GFP-tubulin retained randomly oriented cortical MTs through stage II (Figures 6D and 6E). However, transverse MTs were found throughout the length of expanded cells at late stage II and sometimes remained in fully expanded cells expressing CA-rop2 (Figure 6F). In DN-rop2 pavement cells, random MTs were found in cells before stage II, as in wild-type cells (Figure 6G). Also as in wild-type cells, DN-rop2 cells formed transverse MTs soon after stage II that were prominent only in some regions of the cell (Figures 6H and 6I). Interestingly, in fully expanded wavy DN-rop2 cells, MT arrays were situated parallel to the long axis of the pavement cell. Such longitudinally oriented MTs (Figure 6J) were rare in wild-type and CA-rop2 pavement cells. Together, these results suggest that transverse cortical MTs are apparent only during the transition from Rop-dependent to Rop-independent cell expansion. This finding is consistent with previous observations showing that transverse cortical MTs are found only during specific stages of anisotropically growing cells in which cells shift to predominantly longitudinal expansion (see Baskin et al., 1999, and references therein).
Expression of Dominant rop2 Mutant Genes Specifically Alters Cortical Fine F-Actin That Is Associated with the Early Phase of Cell Expansion
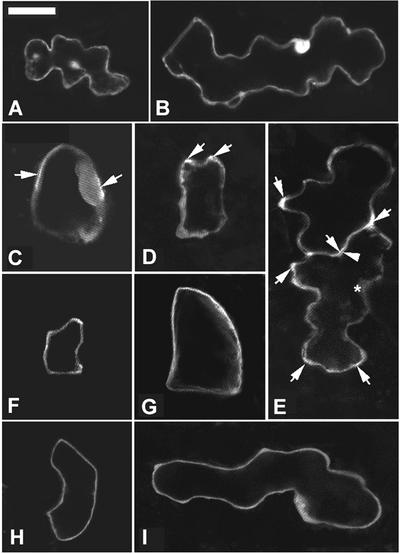
Because polar cell expansion in the early phase is controlled by Rop signaling and because of the role for Rop in the regulation of tip F-actin dynamics during pollen tube and root hair tip growth, we tested whether Rop controls polar cell expansion via the organization of F-actin. We examined F-actin organization in pavement cells from wild-type, CA-rop2, and DN-rop2 plants. F-actin in live leaf epidermal cells at different developmental stages was visualized using transient expression of the GFP-mTalin fusion (Kost et al., 1998; Fu et al., 2001). Pavement cells were used in this experiment for two reasons. First, the distinct morphology of different stages of pavement cells allowed us to correlate cell morphology with actin organization. Second, projectile-mediated transient expression could be performed most efficiently in leaf pavement cells and was difficult in other cell types in intact tissues.
In wild-type young cells (stage I), diffuse fluorescence was found throughout the cell cortex, with strong fluorescence associated with the apparent sites of lobe initiation (Figures 7A and 7B). A few thin cables also were observed in these cells. In cells with developing lobes (stage II), the cortical diffuse fluorescence became more intense in apparently expanding lobes, but it disappeared in the neck region of the cortex (Figure 7D). At this stage, F-actin cables became fine actin networks. In cells with extended lobes, the cortical diffuse fluorescence disappeared completely, and a network of actin cables was formed (Figure 7D).
Figure 7.
Formation of Diffuse Cortical F-Actin Is Associated with Cell Expansion and Is Altered by CA-rop2 and DN-rop2 Expression.
To visualize F-actin in live cells, GFP-mTalin was expressed transiently in leaf pavement cells of wild-type, CA-rop2, and DN-rop2 plants, and different stages of cells expressing GFP-mTalin were imaged using confocal microscopy as described in the text. All images shown are projections of serial laser sections except for (B′). Bar in (A) = 25 μm for (A) to (O).
(A) and (B) Wild-type stage I cells contained diffuse F-actin localized throughout the cell cortex.
(B′) Mid-plane section of the cell shown in (B) showing the cortical localization of diffuse actin and a cytoplasmic strand containing F-actin.
(C) A stage I cell showed no diffuse cortical actin when AtPFN1 is coexpressed with GFP-mTalin.
(D) A stage II cell contained strong diffuse F-actin in the cortical region of the lobe primordia.
(E) No diffuse cortical actin was found in a stage III cell, but an extensive network of actin cables was seen.
(F) to (J) CA-rop2 cells showed diffuse cortical actin at all stages: stage I (F), stage II ([G] to [I]), and stage III (J).
(K) Stage II CA-rop2 cell showed no diffuse cortical actin when AtPFN1 is coexpressed with GFP-mTalin.
(L) to (N) DN-rop2 cells contained little diffuse cortical actin in stages I ([L] and [M]) and II (N) and no diffuse cortical actin at stage III (O). Actin cables were present in all stages of pavement cells and were unaffected by CA-rop2 or DN-rop2 expression.
The specific association of strong diffuse fluorescence with the region of lobe formation suggests that this diffuse fluorescence is composed of very fine or short F-actin, but not G-actin or unincorporated GFP-mTalin, consistent with the fact that mTalin binds F-actin and that GFP-mTalin–decorated actin patterns are identical to those resulting from phalloidin staining (Kost et al., 1998). The expression of GFP alone did not produce strong localized diffuse fluorescence associated with lobe initiation or formation sites, further supporting the notion that the diffuse fluorescence was not caused by unincorporated GFP-mTalin. However, the diffuse form of F-actin did not appear as a filamentous structure under confocal microscopy.
To confirm that the diffuse fluorescence was in fact caused by F-actin, a GFP-mTalin construct was cotransformed with a construct encoding an Arabidopsis profilin (AtPFN1) that sequesters G-actin (Gibbon and Staiger, 2000; Ramachandran et al., 2000). AtPFN1 expression eliminated the diffuse cortical fluorescence but did not reduce the cytoplasmic actin cables dramatically (Figure 7C). Of 11 stage I and II cells coexpressing GFP-mTalin and AtPFN1 that were examined, none had diffuse cortical fluorescence. These results clearly demonstrate the presence of a diffuse form of cortical fine F-actin that is particularly sensitive to profilin and is associated with the early phase of cell expansion. Using either GFP-mTalin expression or staining of freeze-fixed cells with phalloidin, diffuse F-actin was reported in both root hair bulges or tips and certain stages of developing trichomes (Mathur et al., 1999; Szymanski et al., 1999; Baluska et al., 2000; Jones et al., 2002). It is not clear whether this diffuse F-actin belongs to the same population of fine F-actin bundles found just behind the extreme apex of root hairs by phalloidin staining (Miller et al., 1999). To our knowledge, however, no such diffuse form of F-actin had been demonstrated previously in cells within intact tissues in plants.
Interestingly, in CA-rop2 plants, the diffuse fluorescence was found throughout the cortex of the whole cell in all stages of epidermal pavement cells (Figures 7F to 7J). It is striking that even in older CA-rop2 cells that had stopped radial expansion (Figure 7H) or were fully mature (Figure 7I), the cortical diffuse fluorescence remained prominent and uniformly distributed throughout the cortex. However, actin cables formed in these CA-rop2 cells as in wild-type cells at equivalent stages. Coexpression with AtPFN1 eliminated the cortical diffuse fluorescence but did not affect the actin cables, confirming that the diffuse fluorescence represented fine F-actin (Figure 7K). In contrast to wild-type and CA-rop2 cells, different stages of epidermal pavement cells expressing DN-rop2 contained little or dramatically less diffuse cortical fluorescence (Figures 7L to 7O). In younger cells, only weak diffuse fluorescence was found in regions that apparently were forming lobes (Figure 7M). Again, no detectable changes in actin cables were obvious in DN-rop2 cells, as in CA-rop2 cells, with the exception that actin cables appeared to be thicker in the later stages (Figures 7N and 7O) compared with wild-type cells at similar stages (Figure 7E). These results demonstrate that Rop signaling directly or indirectly regulates the formation and distribution of a specific population of F-actin that is AtPFN1-hypersensitive cortical fine F-actin and is tightly associated with the early phase of cell expansion in leaf pavement cells.
ROP2 GTPase Signaling Directly Regulates the Formation of Cortical Fine F-Actin
Because the cortical fine F-actin was found in mature pavement cells expressing CA-rop2 but not in wild-type mature cells, the alteration of this actin by rop2 mutants is most likely explained by a direct role for Rop signaling in regulating this form of actin rather than by an indirect effect of morphological changes induced by the rop2 mutant genes. To test this possibility further, we investigated the effect of the transient expression of rop2 mutant genes on the organization of F-actin in wild-type pavement cells. We cotransformed CA-rop2 or DN-rop2 genes and the GFP:mTalin fusion gene into wild-type Arabidopsis epidermal cells by a particle-bombardment method. We then analyzed F-actin localization of the same cell at two different times: 8 and 18 hr after bombardment. Within this 10-hr period, no significant changes in cell morphology were observed. In control cells expressing GFP-mTalin alone, cortical fine F-actin increased slightly in certain regions of the cell where slight cell expansion was apparent during the 10-hr period (Figures 8A and 8B).
Figure 8.
Transient Expression of CA-rop2 and DN-rop2 Generated an Opposite Effect on Cortical F-Actin.
GFP-mTalin was coexpressed with CA-rop2 or DN-rop2 in wild-type pavement cells and visualized by confocal microscopy as described for Figure 7 at two different times after particle bombardment (8 and 18 hr, respectively). Arrows point to loci of cortical F-actin at the first time point (8 hr after bombardment) and corresponding loci at the second time point (18 hr after bombardment). Two cells expressing GFP-mTalin alone showed no significant differences in F-actin distribution when visualized at 8 hr ([A] and [B]) and 18 hr ([A′] and [B′]), respectively. In cells expressing DN-rop2, localized diffuse cortical F-actin was reduced greatly at 18 hr ([C′] and [D′]) compared with 8 hr ([C] and [D]). On the contrary, CA-rop2 expression increased diffuse cortical F-actin dramatically during the same period ([E] versus [E′]). In contrast to the strong cortical F-actin in cells expressing CA-rop2 (F), no cortical F-actin was found in cells coexpressing AtPFN1 with CA-rop2, although normal actin cables were seen (G), showing that the cortical actin induced by transient expression of CA-rop2 was the same population of cortical F-actin induced by stable CA-rop2 expression, as shown in Figure 7. Bar in (A) = 15 μm for (A) to (G).
In the mean time, actin cables appeared slightly thicker, probably reflecting a slight increase in GFP-mTalin expression during this period. In contrast, during the same interval, DN-rop2 expression dramatically reduced cortical fine F-actin in all cells examined, whereas certain actin cables appeared to be slightly thicker than those in control cells (Figures 8C and 8D). Most cells (∼80%) expressing CA-rop2 showed significant increases in cortical fine F-actin throughout the entire cell cortex (Figures 8E and 8E′). Compared with the effects of expressing GFP:mTalin alone (Figures 8A and 8B), the CA-rop2–induced increase in cortical fine F-actin is much more extensive and global. As in transgenic plants expressing CA-rop2, coexpression of AtPFN1 eliminated cortical fine F-actin (Figures 8G and 8H) in cells transiently expressing CA-rop2.
GFP-ROP2 Is Preferentially Localized in Specific Regions of the PM That Presumably Are Expanding
The ROP2 regulation of localized cortical fine F-actin during the early phase of polar cell expansion in pavement cells is similar to the Rop regulation of fine F-actin localized to the tip in both root hairs and pollen tubes (Fu and Yang, 2001; Fu et al., 2001; Jones et al., 2002). In pollen tubes, the polar localization and activation of Rop1 at the apical PM region is critical for the maintenance of polar growth (Yang, 2002). Thus, the formation of localized fine F-actin in pavement cells also may involve the proper localization of ROP2 to the PM region, where growth takes place. To localize ROP2, we transiently expressed GFP-ROP2 in leaf pavement cells. As shown in Figure 9, different stages of wild-type cells expressing GFP alone show accumulation of GFP in the nucleus and throughout the cytoplasm (Figures 9A and 9B). In contrast, GFP-ROP2 was stronger in certain regions of the PM or the cell cortex in wild-type cells before the formation of lobes (Figure 9C, arrows). During lobe initiation, stronger GFP-ROP2 fluorescence was detected at the site of lobe initiation (Figure 9D).
Figure 9.
GFP-Tagged Rop2 Is Localized Preferentially to the Apparently Expanding Region of the PM.
To assess the subcellular localization of ROP2, GFP-ROP2 was expressed transiently in pavement cells and visualized by confocal microscopy. (A) and (B) show cells expressing GFP alone as a control. (C) to (I) show localization of GFP-ROP2. Arrows indicate strong GFP-ROP2 localization to specific cortical regions. Images shown are 1-μm laser sections. Bar in (A) = 20 μm for (A) to (I).
(A) and (B) Stage II and stage III cells, respectively. GFP was localized in the nucleus and throughout the cytoplasm.
(C) and (D) Two wild-type cells at stage I expressing GFP-ROP2.
(E) Two adjacent stage II cells expressing GFP-Rop2. Note a sinus region (arrowhead) in the bottom cell showing weak fluorescence, whereas its opposing lobe region in the neighboring cell showed strong PM fluorescence. Some regions of the cell were rich in cytoplasm, as indicated by the asterisk in the bottom cell, but contained weak fluorescence, indicating that the strong localized fluorescence was not attributable to dense cytoplasm in those regions.
(F) and (G) GFP-ROP2 localization in CA-rop2 pavement cells. GFP-ROP2 was distributed much more evenly throughout the PM in CA-rop2 cells at the equivalent stage (stage I) than in wild-type cells.
(H) and (I) PM localization of GFP-ROP2 in DN-rop2 pavement cells was very weak and was indistinguishable from its cytoplasmic localization.
In stage II cells, GFP-ROP2 was concentrated at the tip of newly formed lobe primordia and was much stronger at some lobe tips than others (Figure 9E). The stronger GFP-ROP2 localization in these specific loci was not caused by the presence of the dense cytoplasm, because some lobe tips contained dense cytoplasm but showed only background fluorescence. Occasionally, dense cytoplasm was found in the region of sinuses that did not contain strong GFP-ROP2 (Figure 9E, asterisk). This further confirms that GFP-ROP2 is localized preferentially to specific regions of the cell cortex or the PM. The C terminus of ROP2 contains a geranylgeranylation motif and a polybasic region that enable ROP2 to localize to the PM. We have shown that GFP-ROP2 also is localized preferentially to the root hair initiation site of the PM in root hair–forming cells (Jones et al., 2002). Thus, we conclude that GFP-ROP2 apparently is localized to the regions of the PM that presumably are growing.
If localized ROP distribution is involved in localized actin organization, how can we explain CA-rop2 induction of the even distribution of F-actin throughout the cortex? One possible explanation is that CA-rop2 caused ectopic localization of ROP2 to the entire PM. In CA-rop2 pavement cells, GFP-ROP2 was localized to almost the entire PM in both younger and older cells, indicating a correlation between ectopic ROP2 localization and depolarized pavement cell expansion (Figures 9F and 9G). In DN-rop2 epidermal cells, GFP-ROP2 was distributed evenly throughout the PM and the cytoplasm (Figures 9H and 9I).
DISCUSSION
This study has provided new insights into the mechanism underlying polar cell expansion within growing plant tissues. We have demonstrated that Rop signaling modulates polar growth in the early phase of expanding cells, most likely by promoting the assembly of a diffuse form of cortical fine F-actin.
Localized Rop GTPase Signaling Provides a Common Mechanism for Directional Cell Expansion
Cell expansion is regulated by various environmental, hormonal, and developmental signals, and it is believed that the cytoskeleton is an important target of signal transduction that regulates cell growth and shape formation. Recent studies have linked Rop GTPase signaling to actin-dependent tip growth in pollen tubes and root hairs and probably anisotropic growth in hypocotyl and root epidermal cells (Kost et al., 1999; Li et al., 1999; Fu et al., 2001; Molendijk et al., 2001; Jones et al., 2002). The data presented here show that Rop signaling regulates shape formation in various cells from different growing tissues, including those that expand in a complex spatial manner (e.g., leaf epidermal cells) and those that display typical anisotropic expansion (e.g., hypocotyl cortex cells). In both cases, DN-rop2 expression inhibits cell expansion in specific directions, whereas CA-rop2 expression tends to randomize the direction of cell expansion.
This is similar to the effect of rop1 and rop5 or rop2 mutants on tip growth in pollen tubes or root hairs, respectively (Kost et al., 1999; Li et al., 1999; Fu et al., 2001; Jones et al., 2002). For example, DN-rop1 inhibited pollen tube elongation, whereas CA-rop1 induced nonpolar isotropic tube growth. The ability of Rop signaling to promote cell expansion in specific directions is emphasized by the localization of Rop to specific PM regions in which cells expand (Fu et al., 2001). As shown for the localization of ROPs to the apical PM region in tip-growing cells (Kost et al., 1999; Li et al., 1999; Fu et al., 2001; Molendijk et al., 2001; Jones et al., 2002), GFP-ROP2 was localized preferentially to the expanding region of the PM in leaf epidermal cells. As shown for Rop control of tip growth (Fu et al., 2001), ROP2 evidently also signals to a dynamic form of F-actin associated with the site of cell expansion in developing organs (see below). Therefore, localized Rop signaling appears to provide a common mechanism for the regulation of cell expansion and cell shape formation in plants.
Rop GTPase Controls Cell Expansion through the Spatial Regulation of a Diffuse Form of Cortical F-Actin
Pharmacological studies and transgenic expression of actin binding proteins have suggested an important role for F-actin in cell growth or morphogenesis in these tissues (Thimann et al., 1992; Baskin and Bivens, 1995; Ramachandran et al., 2000; Baluska et al., 2001; Dong et al., 2001). However, gross disruption of F-actin in these studies has not been informative regarding the role of specific forms of F-actin. Using GFP-mTalin as a live marker for F-actin, we have identified a diffuse form of actin localized in the cell cortex of epidermal pavement cells and have shown that the distribution of this fine cortical F-actin is associated with the region of cell expansion in pavement cells.
Several lines of evidence suggest that Rop signaling specifically regulates the formation and distribution of this fine cortical F-actin during the development of pavement cells. First, GFP-ROP2 was localized preferentially to the apparently growing region of the PM at which the cortical F-actin was present in early stages of pavement cells. Second, transgenic DN-rop2 expression inhibited the formation of this F-actin and cell expansion, whereas CA-rop2 expression caused random distribution of this F-actin throughout the cortex. However, actin cables were not altered by the expression of CA-rop2 or DN-rop2. Finally, transient expression of these rop mutant genes caused similar changes in actin organization in pavement cells, suggesting that Rop signaling modulates the organization of cortical fine F-actin directly but not indirectly through changes in cell expansion.
Together, these observations suggest that ROP2 regulates polar cell expansion at least in part by activating the assembly of the fine cortical F-actin at the site of growth in pavement cells. Although this form of cortical F-actin has not been shown directly for other cells within differentiating tissues, the effect of CA-rop2 and DN-rop2 on the polar expansion of these cells is consistent with a general role for this Rop-dependent F-actin in their morphogenesis. Interestingly, the ROP2-mediated polar accumulation of cortical F-actin in pavement cells is similar to the Rop promotion of the assembly of a dynamic form of tip F-actin in its control of pollen tube and root hair tip growth (Fu and Yang, 2001; Fu et al., 2001; Jones et al., 2002). Therefore, the Rop modulation of cortical F-actin formation appears to be a universal mechanism for the control of cell expansion in plants.
Cell Shape Formation in Developing Tissues Involves Two Phases of Cell Expansion: Rop-Dependent Early Phase and Rop-Independent Late Phase
From the temporal analysis of the impact of CA-rop2 and DN-rop2 expression on the expansion of leaf pavement cells and hypocotyl cortex cells, we conclude that shape formation in these cells involves two phases of mechanistically distinct cell expansion. In the early phase, expansion is multidirectional and dependent on Rop signaling. During this phase, anisotropic hypocotyl cortex cells undergo both radial and longitudinal expansion, whereas leaf epidermal cells undergo radial, longitudinal, and local lateral expansion to form cells with wavy cross-walls. In the late phase, expansion ceases in certain directions or regions of the cell and is independent of Rop signaling. In hypocotyl cortex cells, expansion is restricted to the longitudinal direction, whereas in leaf pavement cells, radial expansion in the neck region of the cell ceases but longitudinal expansion and lobe expansion can continue.
A two-phase model also was proposed recently by Peter Nick and his colleagues to explain the role of F-actin in the control of auxin-mediated elongation of coleoptile epidermal cells (Waller et al., 2000). Similarly, cell expansion in various cell files of Arabidopsis roots is divided into two distinct phases (Schindelman et al., 2001), as in the hypocotyl cortex described above. In this study, we also have shown that CA-rop2 and DN-rop2 expression affected only the radius but not the length of other mature cells (e.g., hypocotyl epidermal cells). Hence, the expansion of various cells within developing tissues generally can be divided into the Rop-dependent early phase and the Rop-independent late phase.
Based on these observations, we propose that the separation of cell expansion into two distinct phases provides an important mechanism for the regulation of cell shape formation during organogenesis. The Rop-dependent cell expansion allows the establishment of the cell radius in anisotropically growing cells in stems and roots as well as in cells with more complex shapes, such as mesophyll and pavement cells. The timing of the transition from the early to the late phase appears to be controlled developmentally and is expected to be critical for the regulation of the radial dimension of the cell. Cells with a small radius (e.g., those in vascular tissues and in the stele) are expected to make this transition very early during development. This explains why CA-rop2 and DN-rop2 expression had a much weaker effect on the morphogenesis of these cells than in cortex cells with a much greater radius.
These observations also raise an important and intriguing question: is there a distinct cytoskeletal basis for each of the two different phases of cell expansion? Our results suggest that the transition to the Rop-independent late phase of cell expansion is associated with the reorganization of cortical MTs from seemingly random patterns to a transverse array in leaf epidermal cells. In lobed wild-type pavement cells, transverse MT hoops are present in the region of the cells that has ceased expansion. In anisotropically expanding cells, the formation of transverse cortical MTs also has been shown to coincide with the cessation of radial cell expansion and the beginning of rapid longitudinal expansion (Sugimoto et al., 2000; Bichet et al., 2001). Transverse MTs likely guide the formation of transverse microfibrils. The neck region of wavy pavement cells has been shown to contain transverse cellulose microfibrils aligned with transverse cortical MTs (Panteris et al., 1994). Many drug experiments have shown that the disruption of MTs causes changes in microfibril order (for reviews, see Kropf et al., 1998; Wasteneys, 2000). If transverse cortical MTs guide microfibril deposition, by definition, cells then expand by diffuse growth during the Rop-independent late phase, when the direction of cell expansion is determined by the constraint of cell walls and not by targeted exocytosis.
We show that the early phase of cell expansion in leaf epidermal cells is tightly associated with a diffuse form of cortical F-actin. As discussed above, the formation and localization of this form of F-actin are controlled by Rop signaling. Thus, the diffuse form of cortical actin most likely plays a key role in the regulation of the early phase of cell expansion. This actin-dependent mechanism could explain several recent studies suggesting that the direction of cell expansion is not always correlated with transverse cortical MTs and microfibrils (Baskin et al., 1999; Wasteneys, 2000; Wenzel et al., 2000). However, this does not exclude a role for MTs in the modulation of the first phase of cell morphogenesis.
There are at least two possible means by which MTs could be involved in the early phase of cell expansion. First, a specific form of MTs may act to regulate Rop and the subsequent formation of cortical F-actin. Second, MTs and F-actin could interact in a Rop-independent or dependent manner to spatially regulate cell expansion. Evidence suggests that F-actin and MTs interact with each other (for review, see Kost et al., 1999). Interestingly, a recent study suggests that the spk1 mutation, which eliminates trichome branches and the waviness of the pavement cell wall, alters the distribution of both cortical F-actin and MT clusters, which normally are interdigitizing (Qiu et al., 2002). A future effort should be directed at determining if and how MTs and Rop-dependent F-actin work together to control the early phase of cell expansion.
Do Cells in Developing Tissues Expand by Diffuse or Tip Growth?
It is thought generally that cells in developing tissues form their shapes by diffuse growth (see Introduction). This hypothesis needs to be reevaluated given our demonstration of Rop-dependent and Rop-independent cell expansion. As discussed above, diffuse growth can explain the Rop-independent expansion associated with transverse cortical MTs. However, it cannot satisfactorily explain the Rop-dependent expansion, during which cells can expand in a complex spatial pattern. Pharmacological studies suggest that anisotropic cell expansion in Arabidopsis roots actually may involve actin-dependent polar secretion (Baskin and Bivens, 1995; Kost et al., 1999b). In this study, we show that Rop signaling promotes the assembly of cortical fine F-actin during Rop-dependent cell expansion, analogous to the Rop regulation of dynamic tip-localized F-actin during tip growth.
This observation raises the intriguing possibility that the Rop-dependent expansion in tissues is controlled by a mechanism similar to that for tip growth, whereby the direction of expansion is determined by actin-based targeted exocytosis. The notion that Rop-dependent expansion does not involve diffuse growth also is consistent with the inability of CA-rop2 and DN-rop2 expression to alter the late phase of cell expansion. Clearly, it will be important to understand how Rop signaling is regulated and how Rop-dependent cortical F-actin controls cell expansion. Answers to these questions will not only help to clarify the mode of Rop action in cell expansion but also will provide important insights into the regulatory mechanism underlying cell shape formation and associated developmental processes.
METHODS
Arabidopsis Plants and Growth Conditions
All experiments described in this study involve Arabidopsis thaliana ecotype Columbia. Transgenic plants expressing CA-rop2 and DN-rop2 mutant genes were described previously (Li et al., 2001). Transgenic plants expressing green fluorescent protein (GFP)–tagged α-tubulin were provided by Dr. T. Hashimoto (Nara Institute of Science and Technology, Nara, Japan; Ueda et al., 1999). For particle bombardment, plants were grown at 22°C in soil in a growth room with 16-hr-light/8-hr-dark cycles, and specific stages of rosette leaves were used. For light or scanning electron microscopy analysis of cell morphology, seedlings were grown on Murashige and Skoog (1962) agar plates under the same conditions.
Scanning Electron Microscopy
Two-week-old seedlings were fixed in 37% formaldehyde, glacial acetic acid, 70% ethanol (5:5:90) solution overnight and dehydrated in an ethanol series (70, 80, 90, and 100%). Fixed seedlings were subjected to critical point drying and coated with gold. Samples were observed with a Philips XL30-FEG scanning electron microscope (FEI Company, Hillsboro, OR). Images were taken using a microscope control program and processed using Adobe Photoshop 5.5 (Mountain View, CA).
Light Microscopy Analysis of Cell Shapes in Tissues
To examine cell morphology in tissue sections, seedlings were fixed in 2.5% glutaraldehyde overnight. Fixed tissues went through serial dehydration in ethanol and were infiltrated and embedded in Spurr's embedding medium. Tissues were sectioned into 5-μm-thick sections using a microtome with glass knives. Sections were stained in 0.1% toluidine blue before observation with a light microscope.
The morphology of leaf epidermal cells also was analyzed using cleared intact leaves. Leaves were immersed in 5% NaOH, boiled for 1 min, washed with distilled water, and incubated in bleach (pH adjusted to 7.5 using HCl) until they became clear (Platt and Thomson, 1992). Cleared leaves were observed with a Nikon Eclipse TE300 inverted microscope (Tokyo, Japan) equipped with a cooled charge-coupled device camera (C4742-95; Hamamatsu, Hamamatsu City, Japan). The measurement of cell sizes of pavement cells was conducted using cleared leaves.
To analyze the shapes of different stages of leaf pavement cells and temporal changes in hypocotyl cortex cells, we used confocal laser scanning microscopy analysis of tissues expressing GFP-tagged α-tubulin (see below). Expanding leaves of ∼0.5 cm in length were placed under a cover slip, and 1-μm horizontal medial laser sections were taken from different parts of leaves using a Bio-Rad 600 confocal system. To follow developmental changes in hypocotyl cortex cell shapes, 1-μm vertical laser sections of three adjacent files of cortex cells were taken every 24 hr. We measured the average width and length of the fifth cortex cells from the root-hypocotyl junction, which is marked by a layer of cells much smaller than hypocotyl cells. Similar results were obtained from three independent experiments, and one representative set of data is presented here. All measurements were conducted using MetaMorph version 4.5 software (Universal Imaging, West Chester, PA).
DNA Manipulation and Plasmid Constructs
All plasmids used for transient expression in leaves were constructed in the pBI221 vector (Clontech, Palo Alto, CA). The chimeric gene encoding an enhanced GFP mutant fused to the actin binding domain of the mouse talin (GFP-mTalin) was described previously (Fu et al., 2001). GFP-mTalin was subcloned behind the 35S promoter of Cauliflower mosaic virus in pBI221 using BamHI and SstI sites, and the resulting plasmid was named pBI221:GFP-mTalin. The coding sequence of profilin (AtPFN1) cDNA was amplified by reverse transcriptase–mediated polymerase chain reaction using primers 5′-CCATGGGATCCATGTCTTGGCAATCATACGTC and 3′-ACCTGAAAT-GGTACCTTGGTTTTAGAGTTC. The polymerase chain reaction product was subcloned into BamHI and KpnI sites of the pZero vector and subsequently cloned into BamHI and SstI sites behind the 35S promoter of Cauliflower mosaic virus in pBI221, and the resulting plasmid was named pBI221:PFN1. CA-rop2 and DN-rop2 coding sequences (Li et al., 1999) were first subcloned into pZero vector using BamHI-KpnI. The inserts then were isolated using BglII-SstI digestion and fused into BamHI-SstI sites in pBI221. To determine the localization of Rop2, we fused the Rop2 coding sequence (Li et al., 1998) with the C terminus of GFP in the pBI221:GFP vector using BglII-SstI sites. These rop constructs were named pBI221:CA-rop2, pBI221:DN-rop2, and pBI221:GFP-Rop2, respectively.
Particle Bombardment–Mediated Transient Expression in Arabidopsis Leaves
For particle bombardment, all plasmids were amplified in Escherichia coli strain Top 10 and purified using plasmid midi or mini kits according to the manufacturer's instructions (Qiagen, Valencia, CA). Expanding rosette leaves of 0.8 to 1.2 cm in length were collected from 3- to 6-week-old plants and were bombarded with gold particles coated with plasmids using a Bio-Rad PDS-1000/He particle delivery system. In all experiments, 0.8 μg of pBI221:GFP-mTalin, 0.5 μg of pBI221:CA-rop2 or pBI221:DN-rop2, and 1.0 μg of pBI221:PFN1 constructs were used. The bombardment procedure was described previously for pollen (Fu et al., 2001). Bombarded leaves were incubated in water before observation with a confocal microscope.
Visualization of F-Actin and Microtubules, and Confocal Microscopy
To visualize F-actin in leaf epidermal cells, the GFP-mTalin chimeric gene was expressed transiently as described above. Six to 8 hr after bombardment, epidermal cells expressing GFP-mTalin were identified using epifluorescence microscopy and observed with a Nikon Optiphot upright microscope equipped with a Bio-Rad MRC 600 confocal laser scanning device. One-micrometer optical sections were scanned and captured using Comos software (Bio-Rad, Hercules, CA). For three-dimensional reconstruction, serial optical sections were taken for each cell. To follow the effect of transiently expressed DN-rop2 and CA-rop2 on F-actin, the same cells were imaged at 10- to 12-hr intervals. Confocal images were analyzed using MetaMorph 4.5 software and processed using Adobe Photoshop 5.5.
To visualize microtubules in wild-type leaf epidermal cells, we used the same transgenic line expressing GFP-tagged α-tubulin described previously (Ueda et al., 1999). To determine the effect of CA-rop2 and DN-rop2 expression on microtubule organization, the same line was crossed to transgenic plants expressing CA-rop2 or DN-rop2. F1 or F2 plants were used for the analysis of microtubules. Images were collected using a Zeiss LSM 510 confocal microscope (Jena, Germany) and processed with LSM5 and Adobe Photoshop 5.5 software.
Acknowledgments
We thank Dr. T. Hashimoto for the gift of seed from transgenic Arabidopsis plants expressing GFP-tagged α-tubulin. This work was supported by grants from the National Science Foundation (IBN-0115078 and MCB-0111082).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.001537.
References
- Baluska, F., Salaj, J., Mathur, J., Braun, M., Jasper, F., Samaj, J., Chua, N.H., Barlow, P.W., and Volkmann, D. (2000). Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol. 227, 618–632. [DOI] [PubMed] [Google Scholar]
- Baluska, F., Jasik, J., Edelmann, H.G., Salajova, T., and Volkmann, D. (2001). Latrunculin B-induced plant dwarfism: Plant cell elongation is F-actin-dependent. Dev. Biol. 231, 113–124. [DOI] [PubMed] [Google Scholar]
- Baskin, T.I., and Bivens, N.J. (1995). Stimulation of radial expansion in Arabidopsis roots by inhibitors of actomyosin and vesicle secretion but not by various inhibitors of metabolism. Planta 197, 514–521. [DOI] [PubMed] [Google Scholar]
- Baskin, T.I., and Wilson, J.E. (1997). Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 113, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin, T.I., Meekes, H.T., Liang, B.M., and Sharp, R.E. (1999). Regulation of growth anisotropy in well-watered and water-stressed maize roots. II. Role of cortical microtubules and cellulose microfibrils. Plant Physiol. 119, 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova, T.N., Blancaflor, E.B., and Gilroy, S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17, 657–665. [DOI] [PubMed] [Google Scholar]
- Bichet, A., Desnos, T., Turner, S., Grandjean, O., and Hofte, H. (2001). BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 25, 137–148. [DOI] [PubMed] [Google Scholar]
- Blancaflor, E.B., Jones, D.L., and Gilroy, S. (1998). Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol. 118, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterou, M., Dubois, F., Schaller, H., Aubanelle, L., Vilcot, B., Sangwan-Norreel, B.S., and Sangwan, R.S. (2001). Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. II. Effects of brassinosteroids on microtubules and cell elongation in the bul1 mutant. Planta 212, 673–683. [DOI] [PubMed] [Google Scholar]
- Dong, C.H., Xia, G.X., Hong, Y., Ramachandran, S., Kost, B., and Chua, N.H. (2001). ADF proteins are involved in the control of flowering and regulate F-actin organization, cell expansion, and organ growth in Arabidopsis. Plant Cell 13, 1333–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D.D., and Cyr, R.J. (1998). Extending the microtubule/microfibril paradigm: Cellulose synthesis is required for normal cortical microtubule alignment in elongating cells. Plant Physiol. 116, 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y., and Yang, Z. (2001). Rop GTPase: A master switch of cell polarity development in plants. Trends Plant Sci. 6, 545–547. [DOI] [PubMed] [Google Scholar]
- Fu, Y., Wu, G., and Yang, Z. (2001). Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 152, 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon, B.C., and Staiger, C.J. (2000). Profilin. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Press), pp. 45–65.
- Gibbon, B.C., Kovar, D.R., and Staiger, C.J. (1999). Latrunculin B has different effects on pollen germination and tube growth. Plant Cell 11, 2349–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.A., Shen, J.J., Fu, Y., Li, H., Yang, Z., and Grierson, C.S. (2002). The Arabidopsis Rop2 GTPase is a positive regulator of both root-hair initiation and tip growth. Plant Cell 14, 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost, B., Spielhofer, P., and Chua, N.H. (1998). A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 16, 393–401. [DOI] [PubMed] [Google Scholar]
- Kost, B., Lemichez, E., Spielhofer, P., Hong, Y., Tolias, K., Carpenter, C., and Chua, N.H. (1999. a). Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost, B., Mathur, J., and Chua, N.H. (1999. b). Cytoskeleton in plant development. Curr. Opin. Plant Biol. 2, 462–470. [DOI] [PubMed] [Google Scholar]
- Kropf, D.L., Bisgrove, S.R., and Hable, W.E. (1998). Cytoskeletal control of polar growth in plant cells. Curr. Opin. Cell Biol. 10, 112–122. [DOI] [PubMed] [Google Scholar]
- Li, H., and Yang, Z. (2000). Rho GTPase and the actin cytoskeleton. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Press), pp. 301–321.
- Li, H., Wu, G., Ware, D., Davis, K.R., and Yang, Z. (1998). Arabidopsis Rho-related GTPases: Differential gene expression in pollen and polar localization in fission yeast. Plant Physiol. 118, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Lin, Y., Heath, R.M., Zhu, M.X., and Yang, Z. (1999). Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to the tip-localized calcium influx. Plant Cell 11, 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Shen, J., Zheng, Z., Lin, Y., and Yang, Z. (2001). The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 126, 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., and Yang, Z. (1997). Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell 9, 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., Wang, Y., Zhu, J., and Yang, Z. (1996). Localization of a rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 8, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, C.W., and Wells, B. (1985). Microtubules are at the tips of root hairs and form helical patterns corresponding to inner wall fibrils. J. Cell Sci. 75, 225–238. [DOI] [PubMed] [Google Scholar]
- Martin, C., Bhatt, K., and Baumann, K. (2001). Shaping in plant cells. Curr. Opin. Plant Biol. 4, 540–549. [DOI] [PubMed] [Google Scholar]
- Mathur, J., and Chua, N.H. (2000). Microtubule stabilization leads to growth reorientation in Arabidopsis trichomes. Plant Cell 12, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur, J., Spielhofer, P., Kost, B., and Chua, N. (1999). The actin cytoskeleton is required to elaborate and maintain spatial patterning during trichome cell morphogenesis in Arabidopsis thaliana. Development 126, 5559–5568. [DOI] [PubMed] [Google Scholar]
- Miller, D.D., de Ruijter, N.C.A., Bisseling, T., and Emons, A.M.C. (1999). The role of actin in root hair morphogenesis: Studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 17, 141–154. [Google Scholar]
- Molendijk, A.J., Bischoff, F., Rajendrakumar, C.S., Friml, J., Braun, M., Gilroy, S., and Palme, K. (2001). Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20, 2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Panteris, E., Apostolakos, P., and Galatis, B. (1994). Sinuous ordinary epidermal cells: Behind several patterns of waviness, a common morphogenetic mechanism. New Phytol. 127, 771–780. [DOI] [PubMed] [Google Scholar]
- Platt, K.A., and Thomson, W.W. (1992). Idioblast oil cells of avocado: Distribution, isolation, ultrastructure, histochemistry, and biochemistry. Int. J. Plant Sci. 153, 301–310. [Google Scholar]
- Qiu, J.-L., Jilk, R., Marks, M.D., and Szymanski, D.B. (2002). The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell 14, 101–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader, H. (1986). Cellulose microfibril orientation in Oocystis solitaria: Proof that microtubules control the alignment of the terminal complexes. J. Cell Sci. 83, 223–234. [DOI] [PubMed] [Google Scholar]
- Ramachandran, S., Christensen, H.E., Ishimaru, Y., Dong, C.H., Chao-Ming, W., Cleary, A.L., and Chua, N.H. (2000). Profilin plays a role in cell elongation, cell shape maintenance, and flowering in Arabidopsis. Plant Physiol. 124, 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelman, G., Morikami, A., Jung, J., Baskin, T.I., Carpita, N.C., Derbyshire, P., McCann, M.C., and Benfey, P.N. (2001). COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 15, 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibaoka, H. (1994). Plant hormone-induced changes in the orientation of cortical microtubules: Alterations in the cross-linking between microtubules and the plasma membrane. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 527–544. [Google Scholar]
- Staiger, C.J. (2000). Signaling to the actin cytoskeleton in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 257–288. [DOI] [PubMed] [Google Scholar]
- Sugimoto, K., Williamson, R.E., and Wasteneys, G.O. (2000). New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis. Plant Physiol. 124, 1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski, D.B., Marks, M.D., and Wick, S.M. (1999). Organized F-actin is essential for normal trichome morphogenesis in Arabidopsis. Plant Cell 11, 2331–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, L.P., and Hepler, P.K. (1997). Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 461–491. [DOI] [PubMed] [Google Scholar]
- Thimann, K.V., Reese, K., and Nachmias, V.T. (1992). Actin and the elongation of plant cells. Protoplasma 171, 153–166. [Google Scholar]
- Ueda, K., Matsuyama, T., and Hashimoto, T. (1999). Visualization of microtubules in living cells of transgenic Arabidopsis thaliana. Protoplasma 206, 201–206. [Google Scholar]
- Waller, F., Wang, Q.Y., and Nick, P. (2000). Actin and signal-controlled cell elongation in coleoptiles. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 477–496.
- Wasteneys, G.O. (2000). The cytoskeleton and growth polarity. Curr. Opin. Plant Biol. 3, 503–511. [DOI] [PubMed] [Google Scholar]
- Wenzel, C.L., Williamson, R.E., and Wasteneys, G.O. (2000). Gibberellin-induced changes in growth anisotropy precede gibberellin-dependent changes in cortical microtubule orientation in developing epidermal cells of barley leaves: Kinematic and cytological studies on a gibberellin-responsive dwarf mutant, M489. Plant Physiol. 124, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington, A.T., Vugrek, O., Wei, K.J., Hasenbein, N.G., Sugimoto, K., Rashbrooke, M.C., and Wasteneys, G.O. (2001). MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610–613. [DOI] [PubMed] [Google Scholar]
- Yang, Z. (2002). Small GTPases: Versatile signaling switches in plants. Plant Cell, in press. [DOI] [PMC free article] [PubMed]
- Zheng, Z.-L., and Yang, Z. (2000. a). The Rop GTPase switch turns on polar growth in pollen. Trends Plant Sci. 5, 298–303. [DOI] [PubMed] [Google Scholar]
- Zheng, Z.-L., and Yang, Z. (2000. b). The Rop GTPase: An emerging signaling switch in plants. Plant Mol. Biol. 44, 1–9. [DOI] [PubMed] [Google Scholar]