Abstract
Trimming of N-linked oligosaccharides by endoplasmic reticulum (ER) glucosidase II is implicated in quality control of protein folding. An alternate glucosidase II-independent deglucosylation pathway exists, in which endo-α-mannosidase cleaves internally the glucose-substituted mannose residue of oligosaccharides. By immunogold labeling, we detected most endomannosidase in cis/medial Golgi cisternae (83.8% of immunogold labeling) and less in the intermediate compartment (15.1%), but none in the trans-Golgi apparatus and ER, including its transitional elements. This dual localization became more pronounced under 15°C conditions indicative of two endomannosidase locations. Under experimental conditions when the intermediate compartment marker p58 was retained in peripheral sites, endomannosidase was redistributed to the Golgi apparatus. Double immunogold labeling established a mutually exclusive distribution of endomannosidase and glucosidase II, whereas calreticulin was observed in endomannosidase-reactive sites (17.3% in intermediate compartment, 5.7% in Golgi apparatus) in addition to the ER (77%). Our results demonstrate that glucose trimming of N-linked oligosaccharides is not limited to the ER and that protein deglucosylation by endomannosidase in the Golgi apparatus and intermediate compartment additionally ensures that processing to mature oligosaccharides can continue. Thus, endomannosidase localization suggests that a quality control of N-glycosylation exists in the Golgi apparatus.
INTRODUCTION
A common posttranslational modification on proteins, while being present in the endoplasmic reticulum (ER), is the addition of asparagine-linked oligosaccharides. Immediately after the transfer of the lipid-linked preassembled Glc3Man9GlcNAc2 oligosaccharide to asparagine, the glucose residues are trimmed by the sequential action of the ER residents glucosidase I and II (reviewed in Moremen et al., 1994; Roth, 1995). Although it has been known for some time that the glucose residues are essential determinants for N-glycosylation (Spiro et al., 1979; Turco and Robbins, 1979; Murphy and Spiro, 1981) and that subsequent excision of these sugars is required for the formation of complex carbohydrate units, it is only recently that the monoglucosylated oligosaccharide has been implicated in quality control of ER-situated protein folding (reviewed in Ellgaard et al., 1999). Monoglucosylated oligosaccharide intermediate involved in this process can be generated either by glucosidase II trimming (Hammond et al., 1994; Hebert et al., 1995; Jakob et al., 1998b) or by reglucosylation through the action of UDP-Glc:glycoprotein glucosyltransferase (Trombetta and Parodi, 1992; Sousa and Parodi, 1995; Fernandez et al., 1996; Fanchiotti et al., 1998). Current evidence points to an ER control mechanism monitoring the folding state of proteins by the concerted action of UDP-Glc:glycoprotein glucosyltransferase, glucosidase II, and various chaperones, including calnexin and calreticulin (Zapun et al., 1988; Hammond and Helenius, 1994; Oliver et al., 1997; Zhang et al., 1997; Jakob et al., 1998b; Trombetta and Helenius, 1998). Proteins failing to become correctly folded may be degraded via the ubiquitin–proteasome pathway (Kopito, 1997; Sommer and Wolf, 1997; Bonifacino and Weissman, 1998) and the involvement of specific oligosaccharides in the degradation process has been shown (Knop et al., 1996; Jakob et al., 1998a; Liu et al., 1999).
An alternate glucosidase II-independent processing route involving an endo-α-mannosidase has been discovered by Spiro and coworkers (Lubas and Spiro, 1987; Lubas and Spiro, 1988). This enzyme is unique among all other known trimming glycosidases (Moremen et al., 1994) in that it cleaves internally between the glucose substituted mannose and the remaining oligosaccharide to release a Glcα1,3Man disaccharide (Lubas and Spiro, 1987; Lubas and Spiro, 1988; Rabouille and Spiro, 1992). In glucosidase II-deficient mouse lymphoma cells (Moore and Spiro, 1992) and in the presence of glucosidase inhibitors (Moore and Spiro, 1990; Karaivanova et al., 1998), endomannosidase provides an alternate pathway for the formation of complex asparagine-linked oligosaccharides because it can act on tri- and di- as well as monoglucosylated N-linked oligosaccharides. The recent cloning of endomannosidase revealed no homology with other known proteins (Spiro et al., 1997) and contrasts with the situation of the other trimming mannosidases, which have been grouped into two classes based on protein sequence homologies (Moremen et al., 1994). Furthermore, and in contrast to glucosidase I and II and α1,2 mannosidase, endomannosidase seems to have arisen late during evolution starting with the chordate phylum (Dairaku and Spiro, 1997). The meaning for the late evolutionary appearance of this special trimming enzyme is unclear, although it may reflect the more prominent biological role that complex N-linked oligosaccharides take in higher organisms.
The recent observation of the copurification of calreticulin with endomannosidase, and the striking similarities of their saccharide specificities, has led to the proposal that endomannosidase, like glucosidase II, by its glucose-trimming function is involved in the dissociation of calreticulin–glycoprotein complexes (Spiro et al., 1996). It was furthermore proposed that this dissociation could occur at a location distal to glucosidase II (Spiro et al., 1996). In this context, it is noteworthy that endomannosidase, in contrast to glucosidase II (Grinna and Robbins, 1980), has the capacity to act on monoglucosylated oligosaccharides in which mannose trimming has occurred (Lubas and Spiro, 1988).
To date, the exact subcellular distribution of endomannosidase, as determined by high-resolution in situ immunogold labeling, is unknown. We have used a specific antibody against endomannosidase to investigate its subcellular distribution and its relation in situ to ER glucosidase II, the intermediate compartment marker p58, the cis/medial Golgi apparatus marker Golgi mannosidase II, and calreticulin. We found endomannosidase in a dual localization with 83.8% of immunolabeling in cis- and medial Golgi apparatus, and 15.1% in the intermediate compartment. Under conditions of 15°C transport blockade, endomannosidase localization in peripheral sites became more prominent as visualized by confocal immunofluorescence. After release of ER-to-Golgi apparatus transport blockades, endomannosidase behaved like the Golgi resident mannosidase II but unlike intermediate compartment marker p58. Notably, endomannosidase and glucosidase II exhibited a mutually exclusive distribution. The subcellular distribution of endomannosidase protein and activity suggests that trimming of glucose residues of asparagine-linked oligosaccharides is not limited to the ER and can occur in the Golgi apparatus and intermediate compartment. Because oligosaccharide deglucosylation is indispensable for the synthesis of mature oligosaccharide side chains, both the unique localization and substrate specificity of endomannosidase, compared with the ER glucosidases, makes it a candidate for post-ER quality control of N-glycosylation.
MATERIALS AND METHODS
Antibodies
Details of the preparation and specificity of a polyclonal rabbit anti-endomannosidase antiserum raised against highly purified endomannosidase from transfected JM109 Escherichia coli lysates have been described previously (Spiro et al., 1997). The antiserum reacted with a single protein band on blots of rat liver Golgi membranes. In the present study, an IgG fraction prepared by protein A-Sepharose chromatography from this antiserum was used. Furthermore, polyclonal rabbit antibodies against pig and rat glucosidase II (Lucocq et al., 1986; Brada et al., 1990), calreticulin (Peter et al., 1992; kindly provided by Dr. H. D. Söling, Göttingen, Germany), rat p58 (Saraste and Svensson, 1991; affinity-purified and kindly provided by Dr. J. Saraste, University of Bergen, Norway), and Golgi mannosidase II (Velasco et al., 1993; kindly provided by Dr. K. Moremen, University of Georgia, Athens, GA) were used. A mouse monoclonal anti-Golgi mannosidase II antibody (ascites form) was purchased from Babco (Richmond, CA). Affinity-purified Fab fragments of goat anti-rabbit IgG and goat anti-mouse IgG antibodies, as well as rhodamine red-X–conjugated affinity-purified Fab fragments of goat anti-rabbit IgG, were from Jackson ImmunoResearch Laboratories (West Grove, PA), Alexa 488-conjugated (Fab)2 fragments of goat anti-mouse IgG from Molecular Probes (Eugene, OR), and staphylococcal protein A from Amersham Pharmacia Biotech (Zurich, Switzerland). An Alexa 488 labeling kit was purchased from Molecular Probes and was used to prepare Alexa 488-conjugated Fab fragments of goat anti-rabbit IgG (color to protein ration 4:1) according to the manufacturer's instructions. Secondary antibodies and staphylococcal protein A were complexed with 6-, 8-, 10-, and 12-nm gold particles according to standard procedures (Roth et al., 1978; Roth, 1983).
Endomannosidase Assay
Enzyme activity was determined on postnuclear membranes that were prepared as previously described (Weng and Spiro, 1993). The endomannosidase assay (Lubas and Spiro, 1987) used 14C-labeled Glc1Man9GlcNAc (10,000 dpm) as substrate and the release of the Glcα1,3Man component was quantitated after separation by thin layer chromatography. Enzyme activity was expressed in units (1000 dpm Glcα1,3Man released) per milligram of protein per hour as previously defined (Hiraizumi et al., 1993).
Cell Culture
Clone 9 and BRL3A cell lines were from American Type Culture Collection (Rockville, MD). The RL-19 cell line was established from the liver of newborn rats (Karsten et al., 1976). Clone 9 rat hepatoma cells were grown in F-12 medium, and BRL3A buffalo rat liver and RL-19 rat liver cells in RPMI medium supplemented with 10% fetal calf serum. Primary cultures from freshly isolated rat liver hepatocytes were kindly provided by Dr. B. Stieger (Division of Clinical Pharmacology and Toxicology, University Hospital Zurich, Switzerland).
For brefeldin A treatment and temperature shift experiments, cell monolayers grown on glass coverslips were incubated in Na2CO3-free medium buffered with 20 mM HEPES (pH 7.2) on a water bath. One protocol consisted in culturing cells for up to 3 h at 15°C followed by fixation at 15°C or by fixation after different periods of time (2, 5, 10, 30, and 60 min) temperature shift to 37°C. The other was performed as follows. In a first step, cells were incubated with 1.5 μg/ml brefeldin A for 90 min at 37°C. In a second step, they were shifted to 15°C, washed three times with brefeldin A-free medium, and kept at 15°C for 3 h. In a third step, cells were incubated at 20°C in presence or absence of 20 mM caffeine for different periods of time (10, 30, and 60 min). Finally, cells were warmed to 37°C. At the end of each of the incubation steps, cells were processed for immunofluorescence as described below.
Immunofluorescence Staining and Confocal Laser Scanning Microscopy
Cells were grown on glass coverslips and fresh medium was added to the cells 16 h before fixation in 2% formaldehyde (freshly prepared from paraformaldehyde; Fluka, Buchs, Switzerland) in Hanks' salt solution buffered with HEPES (10–20 mM, pH 7.0). The coverslips were rinsed briefly with prewarmed (37°C) fixative and fixed in newly added fixative for 5 min at 37°C, followed by 25 min at room temperature. After two rinses in phosphate-buffered saline (PBS), coverslips were transferred to 50 mM NH4Cl in PBS for 30 min at 4°C, followed by two rinses in PBS. The coverslips were then immediately processed for immunofluorescence.
For immunofluorescence staining, the fixed cells were permeabilized in PBS containing 0.15% saponin and 1% bovine serum albumin (BSA) for 15 min at room temperature. All washing steps were performed with PBS containing 0.1% BSA (BPBS). The following antibody dilutions were prepared in PBS containing 1% BSA, 0.45% saponin, 0.003% Triton X-100, and 0.003% Tween 20: mouse monoclonal anti-rat liver mannosidase II (5000-fold diluted ascites), rabbit anti-rat endomannosidase (0.4 μg/ml IgG), affinity-purified rabbit anti-rat p58 (200-fold dilution). Cells and frozen sections from rat liver (see below for fixation conditions) were incubated for 2 h at room temperature in the respective primary antibodies, rinsed twice in BPBS for 2 min, and then incubated either with rhodamine red-X–conjugated Fab fragments of goat anti-rabbit IgG (250-fold diluted in BPBS) or Alexa 488-conjugated (Fab)2 fragments of goat anti-mouse IgG (2000-fold diluted in BPBS) for 45 min at room temperature. After two rinses in BPBS for 5 min and one in double distilled water for 30 s, coverslips were embedded in Moviol.
For double immunofluorescence staining, rabbit anti-endomannosidase and mouse monoclonal anti-Golgi mannosidase II antibodies (dilutions and incubation time as described above) were applied simultaneously, followed by rinses with BPBS and simultaneous incubation with rhodamine red-X–conjugated Fab fragments of goat anti-rabbit IgG and Alexa 488-conjugated (Fab)2 fragments of goat anti-mouse IgG (dilutions and incubation time as described above). Double immunofluorescence staining, with the use of two primary antibodies raised in the same animal species, was performed as follows. After incubation with a primary antibody and secondary rhodamine red-X– or Alexa 488-conjugated affinity-purified Fab fragments of goat anti-rabbit IgG antibodies, slides were incubated for 30 min with unlabeled goat anti-rabbit Fab (12 μg/ml in BPBS) to block residual rabbit IgG. Two rinses in BPBS for 5 min each followed this blocking. Afterward, a second antibody incubation sequence applying another primary antibody and secondary anti-species IgG antibody was performed, including an additional conditioning step with 0.15% saponin and 1% BSA containing PBS.
Immunofluorescence was observed and recorded with a Leica confocal laser scanning microscope by using the 100× objective (1.4). In double immunofluorescence overlays, effects of pixel shift were excluded. The z-axis resolution of this equipment was at maximum 300 nm/voxel and the x;y settings were between 50 and 250 nm/voxel.
Immunoelectron Microscopy
Male adult Wistar rats (150–200 g body weight) were fasted overnight with free access to drinking water. They were anesthetized by an intraperitoneal injection of Nembutal (50 mg/kg body weight) and perfused via the left cardiac ventricle with oxygenated Hanks' buffered salt solution (pH 7.4) containing 3% polyvinyl pyrrolidone (30 kDa; Fluka) and 70 mM NaNO2 (Merck, Darmstadt, Germany) for 2 min at 37°C followed by the same solution additionally containing either 3% formaldehyde (freshly depolymerized from paraformaldehyde; Fluka) plus 0.1% glutaraldehyde (vacuum distilled; Fluka) or 3% formaldehyde for 15 min at 37°C. Afterward, thin slices of liver were immersion-fixed in the same fixatives for 15 min at ambient temperature, rinsed with PBS, immersed in PBS (10 mM phosphate buffer, pH 7.4, 0.15 M NaCl) containing 50 mM NH4Cl for 60 min, and stored in PBS at 4°C until use. In addition, monolayer cultures of freshly isolated rat liver hepatocytes, clone 9, BRL3A, and RL-19 cells were immersion-fixed in the above-described fixatives for 5 min at 37°C, followed by fixation for 25 min at ambient temperature. After brief rinses with PBS, they were immersed with PBS containing 50 mM NH4Cl for 30 min and stored in PBS at 4°C until use.
For electron microscopy, small pieces of rat liver or cell pellets prepared from the monolayer cultures were immersed in 2 M sucrose containing 15% polyvinol pyrrolidone (10 kDa), enclosed in ∼2% agarose (FMC Bioproducts, Rockland, ME), mounted on aluminum pins, and frozen and stored in liquid nitrogen. Frozen ultrathin sections were prepared according to Tokuyasu (1978, 1980) by using a Reichert ultracut S ultramicrotome equipped with a Reichert FCS cryochamber, picked up on nickel grids and stored overnight on gelatin at 4°C. Before immunolabeling, gelatin was liquefied at 37°C, and nickels grids removed and washed by floating them on droplets of PBS (pH 7.4).
For single immunolabeling, grids with the attached thin sections were conditioned on droplets of PBS containing 1% BSA, 0.01% Triton X-100, and 0.01% Tween 20 for 10 min at ambient temperature. Grids were then transferred to droplets of primary antibodies diluted in conditioning buffer for 2 h at ambient temperature, rinsed on droplets of PBS, and incubated with 8- or 10-nm labeled protein A-gold (Roth et al., 1978) or gold-labeled secondary antibodies (diluted to an absorbance of 0.06 and 0.1, respectively, in conditioning buffer containing 10% normal goat serum). Finally, grids with the attached thin sections were rinsed in PBS, fixed with 2% glutaraldehyde in PBS for 10–20 min, rinsed with PBS and distilled water, and embedded and stained with methylcellulose and uranyl acetate according to Tokuyasu (1978, 1980). For double immunolabeling, the sequential protein A-gold method was applied.
Controls for specificity of endo-α-mannosidase immunolabeling included the use of IgG from preimmune serum, and incubation only in protein A-gold and gold-labeled goat anti-rabbit IgG.
Quantification of Immunolabeling
Micrographs were taken at the original magnification of 25,000× and the percentage of gold particle labeling for endomannosidase, glucosidase II, and calreticulin was estimated over the ER, intermediate compartment, and Golgi apparatus as well as mitochondria. A total of 48 micrographs containing these three structures was evaluated.
RESULTS
Detection of Endomannosidase Activity in Cell Lines Derived from Rat Liver
Assay of the postnuclear membranes from the rat liver cell lines used in this study, namely, clone 9, BRL3A, and RL-19, gave the following values, respectively: 9.3, 5.4, and 8.2, all expressed in units per milligram of protein. These values represent high enzyme activity compared with other cells that have been tested (Weng and Spiro, 1993; Karaivanova et al., 1998).
Endomannosidase Is Present in cis- and Medial Golgi Apparatus Cisternae and the Intermediate Compartment
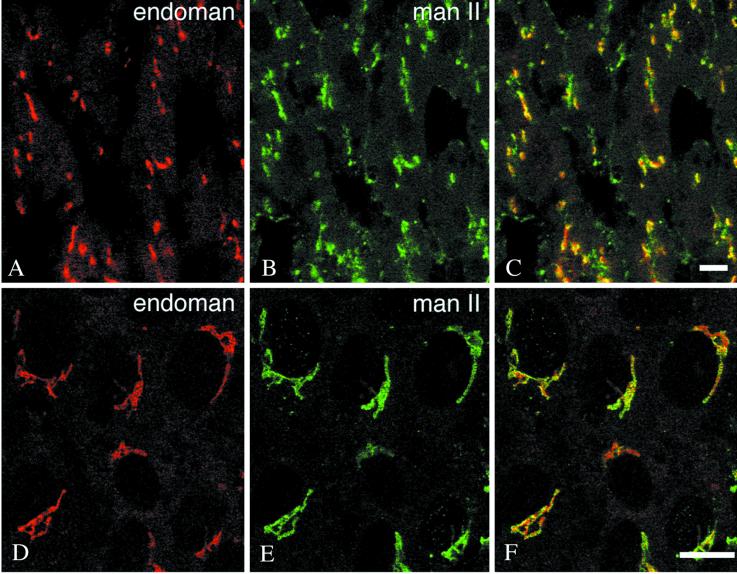
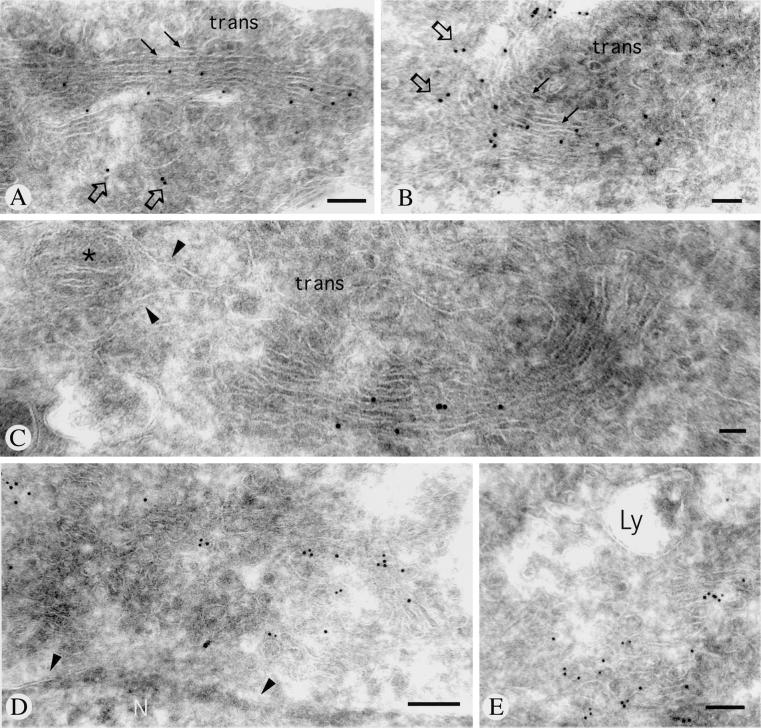
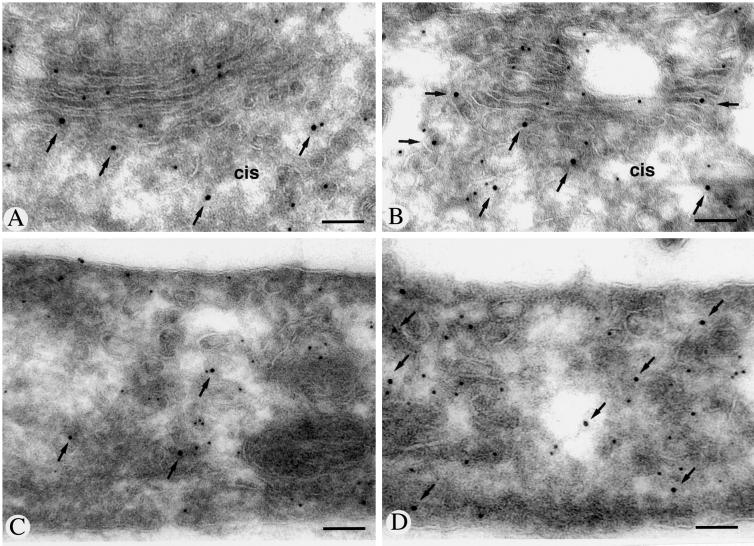
The polyclonal anti-endomannosidase antibody raised against the enzyme expressed in E. coli (Spiro et al., 1997) was used to study by confocal laser scanning immunofluorescence frozen sections of rat liver and primary cultures of rat liver hepatocytes, as well as the clone 9, BRL3A, and RL-19 rat liver cell lines. In all these materials, a distinct perinuclear, crescent-shaped or ring-like fluorescence could be observed (Figure 1, A and D). Depending on the conditions used to permeabilize the cell monolayers, additional punctate cytoplasmic fluorescence was apparent. The relationship of the immunofluorescence pattern of endomannosidase with the Golgi apparatus marker mannosidase II (Moremen and Touster, 1988; Velasco et al., 1993) was investigated by double immunofluorescence. As shown in Figure 1, A and B and D and E, both antibodies produced a perinuclear, crescent-shaped or ring-like fluorescence. To disclose the fine localization of endomannosidase, ultrathin frozen sections were processed for immunogold labeling. We noticed that use of fixatives containing low concentrations (0.1%) of glutaraldehyde was deleterious for endomannosidase detection, and this effect could not be overcome by low pH antigen retrieval (Guhl et al., 1998). In ultrathin frozen sections from only formaldehyde perfusion-fixed rat liver, specific immunogold labeling was detectable in the Golgi apparatus and consistently absent over nuclear envelope, as well as the rough and smooth endoplasmic reticulum. Despite various technical efforts (Liou et al., 1996), a detailed analysis of the endomannosidase distribution in the Golgi apparatus could not be accomplished, due to limited fine structural preservation. Nonetheless, good fine structural preservation of the Golgi apparatus could be achieved in formaldehyde-fixed clone 9, BRL3A, and RL-19 cells. In all three cell types, gold particle labeling indicating immunoreactivity for endomannosidase was detectable in the Golgi apparatus (Figure 2, A–C) and the intermediate compartment (Figure 2, D and E, and open arrows in A and B), but not the nuclear envelope and the ER, including its transitional elements. Quantitative evaluation of the gold particle labeling revealed that 83.8% of the gold particles were over the Golgi apparatus, 15.1% over the intermediate compartment, and 1.1% over the rough ER, which corresponded to labeling estimated over mitochondria. In control incubations, use of an IgG fraction prepared from the preimmune serum, or of fluorescent and gold-labeled goat anti-rabbit IgG and protein A-gold alone, gave no immunolabeling, neither did antigen-preabsorbed specific IgG (our unpublished results). In the Golgi apparatus, immunolabeling was present in cis- and medial cisternae with two trans-cisternae and the trans-Golgi network being unlabeled (Figure 2, A–C). The endomannosidase unreactive trans-Golgi apparatus corresponds to the α2,6-sialyltransferase reactive trans-cisternae and trans-Golgi network (Roth et al., 1985). To prove the intermediate compartment localization of endomannosidase, double immunogold labeling was performed by using an antibody against the intermediate compartment marker protein p58 (Saraste et al., 1987; Saraste and Kuismanen, 1992). As shown in Figure 3, A and B, immunolabeling for both endomannosidase (small gold particles) and p58 (large gold particles marked by arrows) was present in vesiculotubular elements at the cis-side of the Golgi apparatus and in a cis-cisterna. However, colocalization was only occasionally observed. In addition, p58 has been shown to be present in peripheral sites (Saraste and Svensson, 1991), which represent part of peripheral ER export complexes (Bannykh et al., 1996; Presley et al., 1997). In the cell lines studied here, vesiculotubulo clusters in the peripheral cytoplasm were found to be labeled for both endomannosidase (small gold particles) and p58 (large gold particles marked by arrows) (Figure 3, C and D)
Figure 1.
Immunofluorescence localization of endomannosidase and Golgi mannosidase II. In single optical sections of ∼0.3 μm thickness from rat liver (A–C) and clone 9 cells (D–F), both endomannosidase (endoman) and Golgi mannosidase II (man II) give a perinuclear crescent-shaped staining. Bars, 10 μm.
Figure 2.
Immunogold labeling of endomannosidase in ultrathin frozen sections of clone 9 cells. Gold particle labeling is present over cis- and medial Golgi apparatus cisternae (A–C). Unlabeled trans-cisternae are indicated by arrows in A and B. Immunolabeling is additionally observed in part of the intermediate compartment (open arrows in A and B). Grazing sections through the intermediate compartment are shown in D and E. No immunolabeling is observed in the nuclear envelope (arrowheads in D), endoplasmic reticulum (arrowheads in C), nucleus (N), lysosomes (Ly), and mitochondrium (asterisk in C). Bars, 200 nm (A and C), 100 nm (B), and 150 nm (D and E).
Figure 3.
Immunogold double labeling of endomannosidase and p58 in ultrathin frozen sections of clone 9 cells. Immunolabeling for p58 (large gold particles marked by arrows) is present in the intermediate compartment and one or two cis-cisternae, whereas endomannosidase (small gold particles) is additionally observed in medial cisternae (A and B). In the peripheral cytoplasm shown in C and D, vesiculotubulo clusters exhibit labeling for both p58 (large gold particles marked by arrows) and endomannosidase (small gold particles). Bars, 120 nm (A), 130 nm (B), 150 nm (C), and 100 nm (D).
Endomannosidase Immunolabeling Does Not Overlap with Glucosidase II
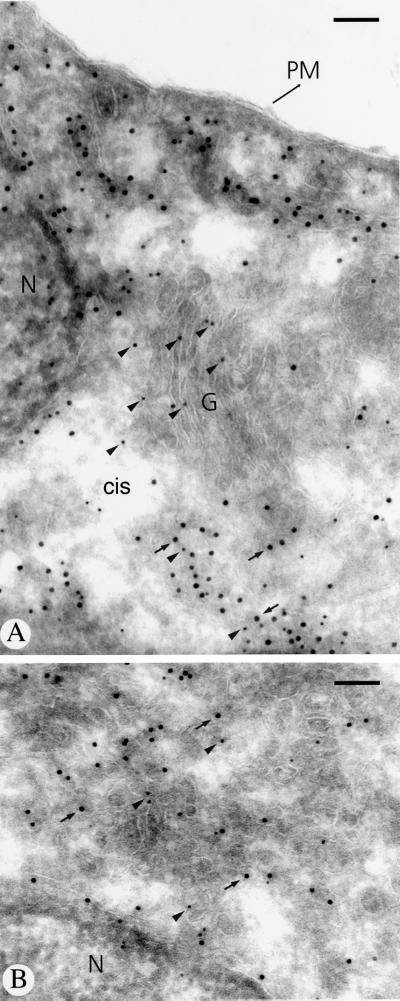
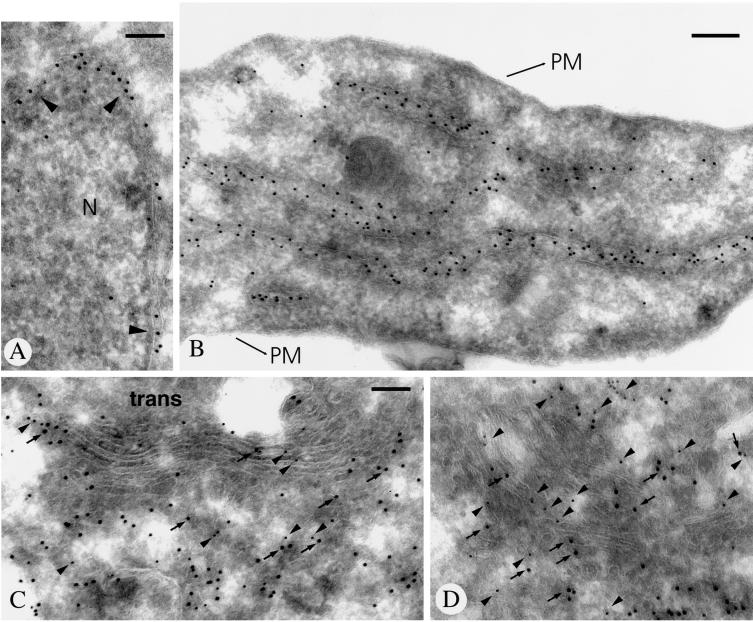
Because both glucosidase II (Burns and Touster, 1982) and endomannosidase (Lubas and Spiro, 1987, 1988) can act on monoglucosylated oligosaccharides, their subcellular distributions relative to each other were studied by double immunolabeling. At the resolution of confocal immunofluorescence, a nonoverlapping staining pattern was observed (our unpublished results). By immunogold double labeling, glucosidase II immunoreactivity in the studied rat hepatocytes was detectable in the nuclear envelope, the ER, and the intermediate compartment, and not in the Golgi apparatus (Figure 4, large gold particles), as reported for pig liver (Lucocq et al., 1986), which was in strong contrast with the distribution of endomannosidase (Figure 4, small gold particles). Quantitative evaluation of the immunolabeling for glucosidase II revealed that 81.6% of the gold particles were over the ER, 17.9% over the intermediate compartment, and 0.5% over the Golgi apparatus, corresponding to levels of nonspecific labeling over mitochondria. Although immunolabeling for both glucosidase II (Figure 4B, large gold particles marked by arrows) and endomannosidase (Figure 4B, small gold particles marked by arrowheads) was detectable in the intermediate compartment, colocalization was rarely found in the same vesiculotubulo clusters.
Figure 4.
Immunogold double labeling of endomannosidase and glucosidase II in ultrathin frozen sections of clone 9 cells. Immunolabeling for glucosidase II (large gold particles, some marked by arrows) extends through the nuclear envelope and endoplasmic reticulum and is also observed in the intermediate compartment, but not in the Golgi apparatus (G). Endomannosidase (small gold particles, arrowheads) and glucosidase II immunolabeling are both present in the intermediate compartment and a grazing section through it is shown in B. In the Golgi apparatus, only small gold particles (arrowheads) are present. Bars, 100 nm.
Redistribution Patterns of Endomannosidase and p58
The intermediate compartment marker protein p58/ERGIC-53 exhibits a dual localization by being present in both the intermediate compartment and a cis-Golgi cisterna (Saraste et al., 1987; Saraste and Svensson, 1991; Klumperman et al., 1998). It has been previously shown that p58 cycles between the ER, intermediate compartment, and the cis-Golgi apparatus (Saraste and Kuismanen, 1984; Saraste and Svensson, 1991). However, the major recycling route of ERGIC-53 seems to bypass the Golgi apparatus (Klumperman et al., 1998). As demonstrated in the present study, endomannosidase exhibits a dual localization under steady-state conditions such as polypeptide-GalNAc transferase (Roth et al., 1994): both are concentrated in the Golgi apparatus and to a lesser, still substantial amount detectable in the intermediate compartment.
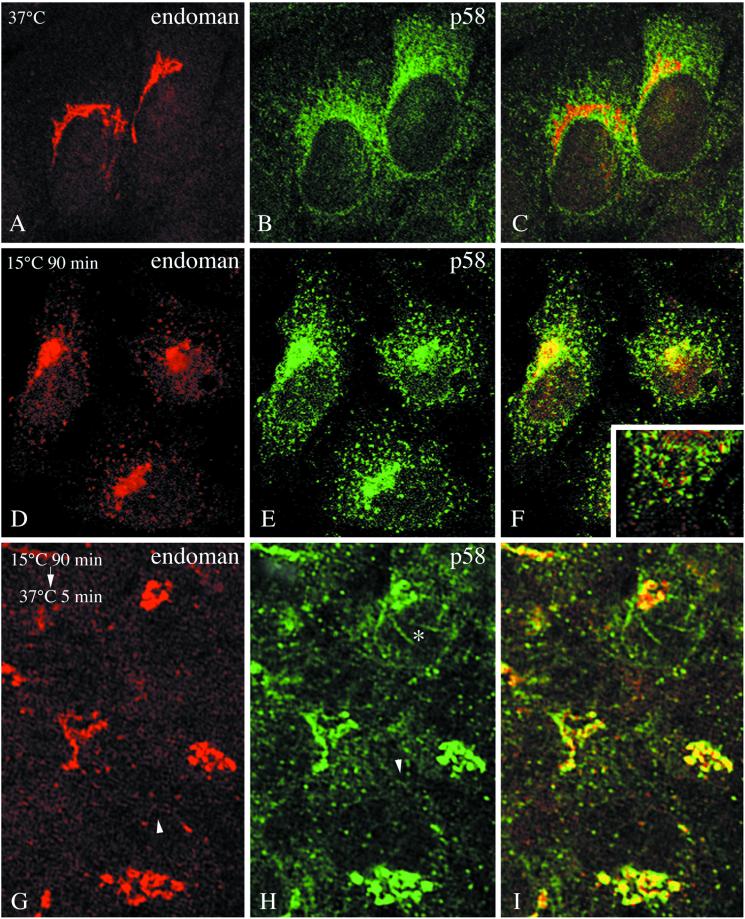
To determine the behavior of endomannosidase, and to compare it with that of p58 under conditions of inhibition of ER-to-Golgi transport, cells were exposed to 15°C for 90 min. This resulted in redistribution of p58 (compare Figure 5, B and E) as reported previously by Saraste and Svensson (1991) and Saraste and Kuismanen (1992). Likewise, endomannosidase was observed in peripheral sites in addition to its presence in the compacted Golgi region (compare Figure 5, A and D). However, colocalization of endomannosidase and p58 was only occassionally observed in the peripheral sites (Figure 5F, inset). When cells maintained at 15°C for 90 min were warmed to 37°C for 5 and 10 min, p58-positive tubules, often exhibiting a necklace appearance, emananted from the Golgi region (Figure 5H), as previously described for ERGIC-53 in HepG2 cells (Klumperman et al., 1998). By confocal immunofluorescence, these p58-positive tubules were unreactive for endomannosidase (asterisk in Figure 5I), although endomannosidase-reactive tubules did exist in the Golgi region. Furthermore, tubules emanating from peripheral sites either positive for endomannosidase (Figure 5G, arrowhead) or p58 (Figure 5H, arrowhead) could be observed. After 5- and 10-min rewarming, a fine reticular network positive for endomannosidase (Figure 5G) and p58 (Figure 5H) indicative of the ER was evident. After 10 min of rewarming, cells were observed in which only p58 exhibited ER-like staining and endomannosidase staining was concentrated perinuclearly. It should be noted that the intensity of fluorescence for endomannosidase in the Golgi region remained constant over the entire rewarming period of 60 min. This contrasts the reported behavior of ERGIC-53/p58 (Klumperman et al., 1998; present study). After 60 min at 37°C the inherent endomannosidase immunofluorescence pattern was observed.
Figure 5.
Temperature-dependent distribution pattern of endomannosidase and p58. In clone 9 cells fixed at 37°C the typical immunofluorescence pattern for endomannosidase (A) and p58 (B) is evident. When cells were maintained at 15°C for 90 min, both endomannosidase (D) and p58 (E) were present in a compacted Golgi region and peripheral sites. Endomannosidase and p58 were only occasionally confined to the same peripheral sites (F and inset showing a detail from the lower part of the left cell). After rewarming to 37°C for 5 min, the number of peripheral sites positive for endomannosidase (G) and p58 (H) is reduced and the Golgi region starts to decompact. Tubules emanating from peripheral sites are either positive for endomannosidase (arrowhead in G) or p58 (arrowhead in H). Note that p58-positive tubules emanating from the Golgi region (asterisk in H) are unreactive for endomannosidase (I). Bar, 10 μm.
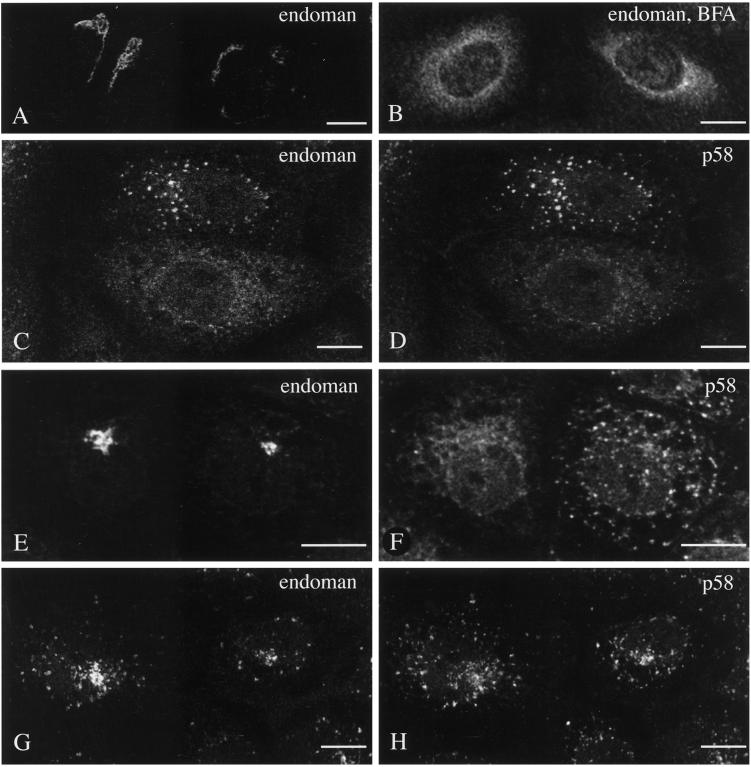
Jäntti and Kuismanen (1993) and Jäntti et al. (1997) reported that a Golgi protein and intermediate compartment proteins segregate after brefeldin A redistribution at the level of the 15°C peripheral sites. When subsequently exposed to caffeine at 20°C, p58 remained in the peripheral sites, whereas Golgi mannosidase II became centralized perinuclearly. Therefore, we decided to study the behavior of endomannosidase and to compared it with p58 under such specific experimental conditions. When cells were exposed to brefeldin A at 37°C, washed free of brefeldin A at 15°C, and kept at 15°C followed by incubation for various periods of time at 20°C in the presence of caffeine, endomannosidase distribution showed characteristic changes. After brefeldin A treatment, endomannosidase rapidly assumed an ER-like distribution (compare Figure 6, B and A), as reported for other Golgi membrane proteins (Klausner et al., 1992). In cells subsequently incubated at 15°C in the absence of brefeldin A, endomannosidase and p58 exhibited an overlapping pattern in peripheral sites (Figure 6, C and D). Subsequent culturing at 20°C in the presence of caffeine resulted in a time-dependent endomannosidase relocation to the perinuclear Golgi region (Figure 6E), as reported for Golgi mannosidase II (Jäntti et al., 1997). In contrast, p58 retained its localization in peripheral sites (Figure 6F; Jäntti et al., 1997). However, at 20°C in the absence of caffeine, both endomannosidase (Figure 6G) and p58 (Figure 6H) exhibited a similar behavior because both were relocated to the perinuclear Golgi region, and when shifted to 37°C reassumed their intrinsic distribution (our unpublished results). The temperature shift effects were determined by evaluating the staining pattern in at least 250 cells for each experimental condition. At 20°C in presence of caffeine 31% (after 30 min) and 29% (after 60 min) of the cells showed prominent perinuclear Golgi-like staining for endomannosidase, whereas such a pattern was observed for p58 in 7 to 8% of the cells only. This difference was not observed when the cells were exposed to 20°C in the absence of caffeine. After 60 min, a perinuclear localization for endomannosidase and p58 was found in 47 and 37% of the cells, respectively. Collectively, this indicates that endomannosidase redistributed to peripheral sites behaves like Golgi mannosidase II under the effect of caffeine.
Figure 6.
Brefeldin A and reduced temperature-induced pattern of endomannosidase and p58. Brefeldin A treatment results in redistribution of endomannosidase from a Golgi-like pattern (A) into an ER-like pattern (B). When subsequently incubated at 15°C in the absence of brefeldin A, both endomannosidase (C) and p58 (D) staining was predominantly globular. After temperature shift from 15°C to 20°C in the presence of caffeine for 60 min, endomannosidase staining changed to the perinuclear Golgi region (E), whereas p58 maintained a globular pattern (F). At 20°C but in the absence of caffeine, both endomannosidase (G) and p58 (H) exhibited staining in the perinuclear Golgi region. Cells shown in C–H are from double immunofluorescence incubations. Bars, 10 μm.
Endomannosidase and Calreticulin Are Detectable in the Golgi Apparatus
Previous studies on rat liver Golgi membrane fractions have demonstrated copurification of endomannosidase and calreticulin by chromatography on Glcα1,3Man affinity matrix (Spiro et al., 1996). To determine the in situ relation between calreticulin and endomannosidase, double immunogold labeling was performed. In addition to intense calreticulin immunolabeling of the nuclear envelope (Figure 7A, arrowheads) and the ER (Figure 7B), the intermediate compartment and the Golgi apparatus cisternal stack were positive for both (Figure 7, C and D; calreticulin, large gold particles and arrows; endomannosidase, small gold particles and arrowheads). Quantitative evaluation of the immunolabeling for calreticulin revealed that 77% of the gold particles were over the rough ER, 17.3% over the intermediate compartment, and 5.7% over the Golgi apparatus. The Golgi localization of calreticulin agrees with data that rat liver calreticulin contains oligosaccharides terminated by galactose, demonstrating that the calreticulin was exposed to Golgi apparatus galactosyltransferase (Peter et al., 1992).
Figure 7.
Immunogold double labeling of endomannosidase and calreticulin in ultrathin frozen sections of clone 9 cells. Calreticulin labeling (large gold particles) is present in the nuclear envelope (arrowheads in A) and throughout the endoplasmic reticulum (B). In the intermediate compartment and the Golgi apparatus, immunolabeling for both calreticulin (large gold particles marked by arrows) and endomannosidase (small gold particles marked by arrowheads) is evident (C and D). N, nucleus; PM, plasma membrane. Bars, 150 nm (A, C, and D) and 200 nm (B).
DISCUSSION
Endomannosidase and Glucosidase II Reside in Different Subcellular Compartments
In the present study we have used a specific antibody to establish by immunofluorescence and immunogold labeling the subcellular distribution of endomannosidase in rat hepatocytes that all contain substantial endomannosidase activity. In addition, we have compared the localization of endomannosidase with the sites of immunoreactivity for glucosidase II by double immunogold labeling because both enzymes can modify monoglucosylated oligosaccharides. Although endomannosidase immunolabeling was found to be present predominantly in cis- and medial Golgi cisternae and less in the intermediate compartment, glucosidase II, being present in the nuclear envelope and the ER, was undetectable in the Golgi apparatus, and although present in the intermediate compartment (Lucocq et al., 1986; present study) rarely showed overlap with endomannosidase. This significantly advances data obtained on centrifugally prepared rat liver membrane fractions, indicating that the specific activity of the endomannosidase in the Golgi was 70-fold that in the ER (Lubas and Spiro, 1987). We recognize that distribution pattern of immunoreactive proteins may indicate only sites of maximum concentration of the respective proteins, but would like to emphasize that the used fixation protocol and immunolabeling techniques provide highest currently available sensitivity for this kind of study (Griffiths, 1993). Thus, the low levels of enzyme activity in rough ER fractions (Lubas and Spiro, 1987) and the negligible level of immunolabeling indicate absence of endomannosidase in the ER. The background level of endomannosidase immunolabeling in the ER also suggests that the immunolabeling in the intermediate compartment is not solely due to de novo synthesized endomannosidase en route to the Golgi apparatus.
The broad distribution of endomannosidase in the Golgi apparatus contributes further to the concept of overlapping distributions of trimming glycosidases and glycosyltransferases in the Golgi apparatus (Velasco et al., 1993; Roth et al., 1994; Rabouille et al., 1995; Rottger et al., 1998). In rat liver hepatocytes, Golgi α1,2 mannosidase I has been detected by immunogold labeling throughout the cisternal stack (Velasco et al., 1993). From the work of the latter authors and the present study, it can be concluded that both Golgi mannosidase I and endomannosidase overlap in the cis- and medial Golgi apparatus of hepatocytes. Furthermore, under steady-state conditions the boundaries between the intermediate compartment and the Golgi apparatus as well as the ER seem not to be sharp because Golgi apparatus proteins (Roth et al., 1994; present study) and ER proteins (Lucocq et al., 1986; Cannon and Helenius, 1999; Greenfield and High, 1999) extend in the intermediate compartment, and intermediate compartment marker proteins p58/ERGIC-53 into the cis-Golgi apparatus (Saraste et al., 1987; Schweizer et al., 1988). This would be in agreement with the highly dynamic nature of these structures and their involvement in transport processes.
Endomannosidase and p58 Exhibit Different Dynamics
Because endomannosidase exhibits a dual localization by being present in the Golgi apparatus and in the intermediate compartment, we compared its behavior with that of p58, which also has a dual localization (Saraste et al., 1987; Saraste and Svensson, 1991). To study the recycling behavior of endomannosidase and to compare it with that of p58, we have applied various established experimental protocols.
Our data on brefeldin A-induced redistribution of endomannosidase indirectly indicate that endomannosidase may have the potential to cycle through the ER. The present data obtained with the 15°C/37°C rewarming experiments show that although endomannosidase and p58 accumulate in peripheral sites and compacted Golgi regions at 15°C, they seem to follow different routes after rewarming to 37°C. Endomannosidase associated with compacted Golgi regions, in contrast to ERGIC-53/p58 (Klumperman et al., 1998; present study), seems not to relocalize to the ER because the intensity of immunofluorescence of the compacted Golgi region remained over the entire 37°C rewarming period. Concomitant to the disappearance of strongly fluorescent peripheral sites, an endomannosidase-positive fine reticular structure reminiscent of the ER appeared. This can be interpreted as evidence that part of endomannosidase is temporarily present in the ER. It should be noted that prolonged presence of functional endomannosidase in the ER would interfer with the action of glucosyltransferase in reglucosylating misfolded glycoproteins.
Jäntti et al. (1997) have demonstrated that p58 and Golgi mannosidase II, when segregated in 15°C peripheral sites, behaved strikingly different when exposed to caffeine at 20°C. Our observations clearly show that endomannosidase, like Golgi mannosidase II, becomes centralized perinuclearly, demonstrating that it behaves like a Golgi protein under these conditions.
Post-ER Localization of Endomannosidase and Quality Control
Because endomannosidase and calreticulin have been shown to copurify from rat liver Golgi membranes, the intriguing possibility that they can be involved in protein quality control had been proposed (Spiro et al., 1996). In the present study, we found calreticulin immunolabeling not only in the nuclear envelope and ER but also in substantial amounts in the intermediate compartment and the Golgi apparatus. The soluble, calcium-binding protein calreticulin shares high sequence homology with calnexin, a transmembrane protein (Helenius et al., 1997; Coppolino and Dedhar, 1998; Trombetta and Helenius, 1998). Calreticulin, like calnexin, associates transiently with numerous newly synthesized proteins in the ER and it is well established that both interact lectin-like with monoglucosylated asparagine-linked oligosaccharides (Peterson et al., 1995; Spiro et al., 1996; Vassilakos et al., 1998). The dissociation of calreticulin–glycoprotein complexes can be achieved in vitro by enzymatic removal of the glucose by glucosidase II (Peterson et al., 1995; Rodan et al., 1996; Van Leeuwen and Kearse, 1996). The function of endomannosidase in the intermediate compartment and Golgi apparatus could be the dissociation of calreticulin–glycoprotein complexes as proposed by Spiro et al. (1996), and it is reasonable to assume that calreticulin-bound monoglucosylated glycoproteins may be transported out of the ER into the Golgi apparatus. Because the present study did not explore the dynamics of such an interaction of endomannosidase with calreticulin–glycoprotein complexes, the role of endomannosidase in a final stage of protein quality control remains hypothetical.
The subcellular localization of enzymatic activity and immunoreactivity for endomannosidase together with its substrate specificity demonstrate that glucose trimming occurs not only in the ER by glucosidases I and II and therefore assigns an additional trimming function to the intermediate compartment and Golgi apparatus. The finding that endomannosidase is situated in a more distal locale than glucosidase II fits well with the fact that endomannosidase is known to act effectively on oligosaccharides that have an extensively trimmed 6′-pentamannosyl branch (Lubas and Spiro, 1988). This is in contrast to glucosidase II, which acts very poorly on carbohydrate units smaller than Glc1Man9GlcNAc2 (Grinna and Robbins, 1980). In vitro endomannosidase has a preference for monoglucosylated oligosaccharides to release a Glcα1,3Man disaccharide (Lubas and Spiro, 1987) and the resulting Man8GlcNAc2 (isomer A) trimming intermediate can act as a substrate for Golgi α1,2 mannosidase I (Lubas and Spiro, 1988), which is present together with endomannosidase in the cis- and medial Golgi apparatus. As mentioned above, functionally, the presence of endomannosidase in the ER would interfere with the action of glucosyltransferase by preventing reglucosylation of misfolded glycoproteins.
It has been pointed out that the presence of an alternate glucose trimming pathway parallel to the highly conserved glucosidase route would ensure that no incompletely deglucosylated oligosaccharides would appear on the cell surface, and indeed this sugar has never been observed in mature N-linked oligosaccharides of cultured cells and tissues. More importantly, glucose trimming is indispensable for the synthesis of mature oligosaccharide side chains in the Golgi apparatus and their various biological functions in health and disease are now well recognized (Paulson, 1989; Varki, 1993; Hakomori, 1996; Varki, 1997; Dennis et al., 1999; Ellgaard et al., 1999). We therefore propose that endomannosidase functions in quality control of N-glycosylation and that this represents a mechanism in addition to those for control of DNA replication, translation and protein folding to ensure the fidelity of synthetic processes and the proper biological function of their products.
ACKNOWLEDGMENTS
We thank Drs. K. Moremen (University of Georgia, Athens, GA), J. Saraste (University of Bergen, Norway), and H.D. Söling (Max-Planck-Institute Göttingen, Germany) for kindly providing antibodies. We also thank P. Stoeckel for help with the cell culture and immunofluorescence, and Dr. Th. Bächi and the team from the Central Laboratory for Electron Microscopy of the University of Zurich for providing access for confocal laser microscopy. This work was supported by the Swiss National Science Foundation Grant 31-50835.97 (to J.R.) and Grant DK17477 from the National Institutes of Health (to R.G.S.).
REFERENCES
- Bannykh SI, Rowe T, Balch WE. The organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brada D, Kerjaschki D, Roth J. Cell type-specific post-Golgi apparatus localization of a “resident” endoplasmic reticulum glycoprotein, glucosidase II. J Cell Biol. 1990;110:309–318. doi: 10.1083/jcb.110.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DM, Touster O. Purification and characterization of glucosidase II, an endoplasmic reticulum hydrolase involved in glycoprotein biosynthesis. J Biol Chem. 1982;257:9990–10000. [PubMed] [Google Scholar]
- Cannon KS, Helenius A. Trimming and readdition of glucose to N-linked oligosaccharides determines calnexin association of a substrate glycoprotein in living cells. J Biol Chem. 1999;274:7537–7544. doi: 10.1074/jbc.274.11.7537. [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Dedhar S. Calreticulin. Int J Biochem Cell Biol. 1998;30:553–558. doi: 10.1016/s1357-2725(97)00153-2. [DOI] [PubMed] [Google Scholar]
- Dairaku K, Spiro RG. Phylogenetic survey of endomannosidase indicates late evolutionary appearance of this N-linked oligosaccharide processing enzyme. Glycobiology. 1997;7:579–586. doi: 10.1093/glycob/7.4.579. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- Fanchiotti S, Fernandez F, DAlessio C, Parodi AJ. The UDP-Glc:glycoprotein glucosyltransferase is essential for Schizosaccharomyces pombe viability under conditions of extreme endoplasmic reticulum stress. J Cell Biol. 1998;143:625–635. doi: 10.1083/jcb.143.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Jannatipour M, Hellman U, Rokeach LA, Parodi AJ. A new stress protein: synthesis of Schizosaccharomyces pombe UDP-Glc:glycoprotein glucosyltransferase mRNA is induced by stress conditions but the enzyme is not essential for cell viability. EMBO J. 1996;15:705–713. [PMC free article] [PubMed] [Google Scholar]
- Greenfield JJA, High S. The Sec61 complex is located in both the ER and the ER-Golgi intermediate compartment. J Cell Sci. 1999;112:1477–1486. doi: 10.1242/jcs.112.10.1477. [DOI] [PubMed] [Google Scholar]
- Griffiths G. Fine Structure Immunocytochemistry. Berlin: Springer Verlag; 1993. [Google Scholar]
- Grinna LS, Robbins PW. Substrate specificities of rat liver microsomal glucosidases which process glycoproteins. J Biol Chem. 1980;255:2255–2258. [PubMed] [Google Scholar]
- Guhl B, Ziak M, Roth J. Unconventional antigen retrieval for carbohydrate and protein antigens. Histochem Cell Biol. 1998;110:603–611. doi: 10.1007/s004180050323. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A, Trombetta ES, Hebert DN, Simons JF. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997;7:193–200. doi: 10.1016/S0962-8924(97)01032-5. [DOI] [PubMed] [Google Scholar]
- Hiraizumi S, Spohr U, Spiro RG. Characterization of endomannosidase inhibitors and evaluation of their effect on N-linked oligosaccharide processing during glycoprotein biosynthesis. J Biol Chem. 1993;268:9927–9935. [PubMed] [Google Scholar]
- Jakob CA, Burda P, Roth J, Aebi M. Degradation of misfolded endoplasmic reticulum glycoproteins in Saccharomyces cerevisiae is determined by a specific oligosaccharide structure. J Cell Biol. 1998a;142:1223–1233. doi: 10.1083/jcb.142.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob CA, Burda P, teHeesen S, Aebi M, Roth J. Genetic tailoring of N-linked oligosaccharides: the role of glucose residues in glycoprotein processing of Saccharomyces cerevisiae in vivo. Glycobiology. 1998b;8:155–164. doi: 10.1093/glycob/8.2.155. [DOI] [PubMed] [Google Scholar]
- Jäntti J, Kuismanen E. Effect of caffeine and reduced temperature (20oC) on the organization of the pre-Golgi and the Golgi stack membranes. J Cell Biol. 1993;120:1321–1335. doi: 10.1083/jcb.120.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäntti J, Saraste J, Kuismanen E. Protein segregation in peripheral 15oC intermediates in response to caffeine treatment. Eur J Cell Biol. 1997;74:150–164. [PubMed] [Google Scholar]
- Karaivanova VK, Luan P, Spiro RG. Processing of viral envelope glycoprotein by the endomannosidase pathway: evaluation of host cell specificity. Glycobiology. 1998;8:725–730. doi: 10.1093/glycob/8.7.725. [DOI] [PubMed] [Google Scholar]
- Karsten, Neupert U, Thust R. Characteristics of an established epithelial cell line (RL-19) from rat liver. Exp Pathol. 1976;12:88–99. [Google Scholar]
- Klausner R, Donaldson J, Lippincott-Schwartz J. Brefeldin A: insights into control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J, Schweizer A, Clausen H, Tang BL, Hong W, Oorschot V, Hauri H-P. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J Cell Sci. 1998;111:3411–3425. doi: 10.1242/jcs.111.22.3411. [DOI] [PubMed] [Google Scholar]
- Knop M, Hauser N, Wolf DH. N-glycosylation affects endoplasmic reticulum degradation of a mutated derivative of carboxypeptidase Y in yeast. Yeast. 1996;12:1229–1238. doi: 10.1002/(sici)1097-0061(19960930)12:12<1229::aid-yea15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kopito RR. ER quality control: the cytoplasmic connection. Cell. 1997;88:427–430. doi: 10.1016/s0092-8674(00)81881-4. [DOI] [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Slot JW. Improving structural integrity of immunogold labeled cryosections. Histochem Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- Liu Y, Choudhury P, Cabral CM, Sifers RN. Oligosaccharide modification in the early secretory pathway directs the selection of a misfolded glycoprotein for degradation by the proteasome. J Biol Chem. 1999;274:5861–5867. doi: 10.1074/jbc.274.9.5861. [DOI] [PubMed] [Google Scholar]
- Lubas W, Spiro R. Golgi endo-α-D-mannosidase from rat liver, a novel N-linked carbohydrate unit processing enzyme. J Biol Chem. 1987;262:3775–3781. [PubMed] [Google Scholar]
- Lubas WA, Spiro RG. Evaluation of the role of rat liver endo-α-mannosidase in processing N-linked oligosaccharides. J Biol Chem. 1988;263:3990–3998. [PubMed] [Google Scholar]
- Lucocq JM, Brada D, Roth J. Immunolocalization of the oligosaccharide trimming enzyme glucosidase II. J Cell Biol. 1986;102:2137–2146. doi: 10.1083/jcb.102.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SE, Spiro RG. Demonstration that Golgi endo-α-mannosidase provides a glucosidase-independent pathway for the formation of complex N-linked oligosaccharides of glycoproteins. J Biol Chem. 1990;265:13104–13112. [PubMed] [Google Scholar]
- Moore SE, Spiro RG. Characterization of the endomannosidase pathway for the processing of N-linked oligosaccharides in glucosidase II-deficient and parent mouse lymphoma cells. J Biol Chem. 1992;267:8443–8451. [PubMed] [Google Scholar]
- Moremen K, Touster O. Mannosidases in mammalian glycoprotein processing. In: Das R, Robbins P, editors. Protein Transfer and Organelle Biosynthesis. San Diego: Academic Press; 1988. pp. 209–240. [Google Scholar]
- Moremen KW, Trimble RB, Herscovics A. Glycosidases of the asparagine-linked oligosaccharide processing pathway. Glycobiology. 1994;4:113–125. doi: 10.1093/glycob/4.2.113. [DOI] [PubMed] [Google Scholar]
- Murphy L, Spiro R. Transfer of glucose to oligosaccharide-lipid intermediates by thyroid microsomal enzymes and its relationship to the N-glycosylation of proteins. J Biol Chem. 1981;256:7487–7494. [PubMed] [Google Scholar]
- Oliver JD, vanderWal FJ, Bulleid NJ, High S. Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science. 1997;275:86–88. doi: 10.1126/science.275.5296.86. [DOI] [PubMed] [Google Scholar]
- Paulson JC. Glycoproteins: what are the sugar chains for? Trends Biochem Sci. 1989;14:272–276. doi: 10.1016/0968-0004(89)90062-5. [DOI] [PubMed] [Google Scholar]
- Peter F, Nguyen Van P, Söling H-D. Different sorting of Lys-Asp-Glu-Leu proteins in rat liver. J Biol Chem. 1992;267:10631–10637. [PubMed] [Google Scholar]
- Peterson JR, Ora A, Van PN, Helenius A. Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Mol Biol Cell. 1995;6:1173–1184. doi: 10.1091/mbc.6.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley J, Cole N, Schroer T, Hirschberg K, Zaal K, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Spiro RG. Nonselective utilization of the endomannosidase pathway for processing glycoproteins by human hepatoma (HepG2) cells. J Biol Chem. 1992;267:11573–11578. [PubMed] [Google Scholar]
- Rodan AR, Simons JF, Trombetta ES, Helenius A. N-linked oligosaccharides are necessary and sufficient for association of glycosylated forms of bovine RNase with calnexin and calreticulin. EMBO J. 1996;15:6921–6930. [PMC free article] [PubMed] [Google Scholar]
- Roth J. The colloidal gold marker system for light and electron microscopic cytochemistry. In: Bullock GR, Petrusz P, editors. Techniques in Immunocytochemistry. London: Academic Press; 1983. pp. 217–284. [Google Scholar]
- Roth J. Biosynthesis. Compartmentation of Glycoprotein Biosynthesis. In: Montreuil J, Schachter H, Vliegenhart JFG, editors. Glycoproteins. Amsterdam: Elsevier; 1995. pp. 287–312. [Google Scholar]
- Roth J, Bendayan M, Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978;26:1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Roth J, Taatjes D, Lucocq J, Weinstein J, Paulson J. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus cisternal stack that may function in glycosylation. Cell. 1985;43:287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Roth J, Wang Y, Eckhardt AE, Hill RL. Subcellular localization of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase mediated O-glycosylation reaction in the submaxillary gland. Proc Natl Acad Sci USA. 1994;91:8935–8939. doi: 10.1073/pnas.91.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottger S, White J, Wandall HH, Olivo JC, Stark A, Bennett EP, Whitehouse C, Berger EG, Clausen H, Nilsson T. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J Cell Sci. 1998;111:45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984;38:535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin Cell Biol. 1992;3:343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Palade G, Farquhar M. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kD cis-Golgi protein. J Cell Biol. 1987;105:2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Svensson K. Distribution of intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen J, Bächi T, Ginsel L, Hauri H. Identification, by a monoclonal antibody, of a 53 kD protein associated with tubulovescular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Wolf DH. Endoplasmic reticulum degradation: reverse protein flow of no return. FASEB J. 1997;11:1227–1233. doi: 10.1096/fasebj.11.14.9409541. [DOI] [PubMed] [Google Scholar]
- Sousa M, Parodi AJ. The molecular basis for the recognition of misfolded glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. EMBO J. 1995;14:4196–4203. doi: 10.1002/j.1460-2075.1995.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro MJ, Bhoyroo VD, Spiro RG. Molecular cloning and expression of rat liver endo-alpha-mannosidase, an N-linked oligosaccharide processing enzyme. J Biol Chem. 1997;272:29356–29363. doi: 10.1074/jbc.272.46.29356. [DOI] [PubMed] [Google Scholar]
- Spiro MJ, Spiro RG, Bhoyroo VD. Glycosylation of protein by oligosaccharide-lipids. Studies on a thyroid enzyme involved in oligosaccharide transfer and the role of glucose in this reaction. J Biol Chem. 1979;254:7668–7674. [PubMed] [Google Scholar]
- Spiro RG, Zhu Q, Bhoyroo V, Söling HD. Definition of the lectin-like properties of the molecular chaperone, calreticulin, and demonstration of its copurification with endomannosidase from rat liver Golgi. J Biol Chem. 1996;271:11588–11594. doi: 10.1074/jbc.271.19.11588. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. A study of positive staining of ultrathin frozen sections. J Ultrastruct Res. 1978;63:287–307. doi: 10.1016/s0022-5320(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. Immunochemistry on ultrathin frozen sections. Histochem J. 1980;12:381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol. 1998;8:587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- Trombetta SE, Parodi AJ. Purification to apparent homogeneity and partial characterization of rat liver UDP-glucose:glycoprotein glucosyltransferase. J Biol Chem. 1992;267:9236–9240. [PubMed] [Google Scholar]
- Turco S, Robbins P. The initial stages of processing of protein-bound oligosaccharides in vitro. J Biol Chem. 1979;254:4560–4567. [PubMed] [Google Scholar]
- Van Leeuwen JEM, Kearse KP. Deglucosylation of N-linked glycans is an important step in the dissociation of calreticulin class I TAP complexes. Proc Natl Acad Sci USA. 1996;93:13997–14001. doi: 10.1073/pnas.93.24.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of the oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakos A, Michalak M, Lehrman MA, Williams DB. Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry. 1998;37:3480–3490. doi: 10.1021/bi972465g. [DOI] [PubMed] [Google Scholar]
- Velasco A, Hendricks L, Moreman KW, Tulsiani DRP, Touster O, Farquhar MG. Cell type-dependent variations in the subcellular distribution of α-mannosidases I and II. J Cell Biol. 1993;122:39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S, Spiro R. Demonstration that a kifunensine-resistant α-mannosidase with a unique processing action on N-linked oligoasaccharides occurs in rat liver endoplasmic reticulum and various cultured cells. J Biol Chem. 1993;268:25656–25663. [PubMed] [Google Scholar]
- Zapun A, Darby NJ, Tessier DC, Michalak M, Bergeron JJ, Thomas DY. Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J Biol Chem. 1988;273:6009–6012. doi: 10.1074/jbc.273.11.6009. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Braakman I, Matlack KES, Helenius A. Quality control in the secretory pathway: the role of calreticulin, calnexin and BiP in the retention of glycoproteins with C-terminal truncations. Mol Biol Cell. 1997;8:1943–1954. doi: 10.1091/mbc.8.10.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]