Abstract
Suc represents the major transport form for carbohydrates in plants. Suc is loaded actively against a concentration gradient into sieve elements, which constitute the conduit for assimilate export out of leaves. Three members of the Suc transporter family with different properties were identified: SUT1, a high-affinity Suc proton cotransporter; SUT4, a low-affinity transporter; and SUT2, which in yeast is only weakly active and shows features similar to those of the yeast sugar sensors RGT2 and SNF3. Immunolocalization demonstrated that all three SUT proteins are localized in the same enucleate sieve element. Thus, the potential of Suc transporters to form homooligomers was tested by the yeast-based split-ubiquitin system. The results show that both SUT1 and SUT2 have the potential to form homooligomers. Moreover, all three Suc transporters have the potential to interact with each other. As controls, a potassium channel and a monosaccharide transporter, expressed in the plasma membrane, did not interact with the SUTs. The in vivo interaction between the functionally different Suc transporters indicates that the membrane proteins are capable of forming oligomeric structures that, like mammalian Glc transporter complexes, might be of functional significance for the regulation of transport.
INTRODUCTION
Suc transport activity is essential for the distribution of photoassimilates between source and sink tissues. Members of a family of proton-coupled Suc uptake transporters within the major facilitator superfamily play essential roles in long-distance transport of Suc of plants. In Solanaceae, the Suc transporter SUT1, and in Arabidopsis, its counterpart AtSUC2, are required for phloem transport of sugars (Riesmeier et al., 1993; Kühn et al., 1996; Bürkle et al., 1998; Gottwald et al., 2000). SUT1 from potato was characterized as a high-affinity Suc/proton cotransporter (Riesmeier et al., 1994). In tomato, potato, and Arabidopsis, two distinct paralogs, SUT2 and SUT4 (Lalonde et al., 1999), exist in addition to SUT1/SUC2, one of which may be responsible for the second kinetic component of Suc transport found in leaves (Delrot and Bonnemain, 1981).
SUT4 has been suggested to represent the low-affinity/high-capacity Suc transport system, playing a role in phloem loading in minor veins (Weise et al., 2000). The third paralog, SUT2, differs structurally from the other SUTs (Lalonde et al., 1999; Barker et al., 2000) as a result of extended domains at the N terminus (∼30 amino acids) and central cytoplasmic loop (∼40 amino acids). Thus, SUT2 shows structural analogies to the yeast Glc sensors Rgt2 and Snf3 (Özcan et al., 1996), and like these, it has a low codon bias. SUT2 is expressed at low levels and is active to a low extent when expressed in yeast. Furthermore, SUT2 maps to a major quantitative trait locus for starch content of potato tubers (Barker et al., 2000). Therefore, it was speculated whether SUT2 may function as a Suc sensor (Barker et al., 2000). The N-terminal domain of AtSUT2 was shown to play a role in determining affinity for Suc (Schulze et al., 2000).
The activity of many proteins is affected or regulated in macromolecular complexes. These often consist of homooligomers or heterooligomers, in some cases even as dimers of dimers (Wes et al., 1999; Veenhoff et al., 2002). Yeast two-hybrid systems have allowed rapid progress in this field, and oligomers of soluble proteins have been studied systematically (Ito et al., 2000, 2001; Schwikowski et al., 2000). However, because of the difficult biochemistry and because classic two-hybrid systems are not suitable for the study of membrane protein interactions, very little is known about the oligomerization of transporters. Large parts of the work on this topic has been performed on channels, such as chloride and potassium channels that exist as dimers and tetramers, respectively (Ludewig et al., 1996). Recently, oligomerization has been shown for seven bacterial solute transporters (Heuberger et al., 2002). One of the few examples in metabolite transport is the human Glc transporter that exists as dimers of dimers, in which the transport status of individual subunits affects the status of all of the others (Hebert and Carruthers, 1992; Hamill et al., 1999).
Early biochemical efforts to purify a sugar beet Suc transporter protein with a molecular mass of 42 kD indicate that under nondenaturing conditions, Suc transporter activity is present in a high molecular mass complex (Li et al., 1991). This may indicate that the regulation of Suc transport may occur through the oligomerization of Suc transporters. The yeast split-ubiquitin system provides access to membrane protein interactions (Johnsson and Varshavsky, 1994; Stagljar et al., 1998). In a previous study using an improved system less sensitive to artifacts, it was shown that separately expressed halves of the potato Suc transporter SUT1 interact with each other and reconstitute a functional Suc transporter in yeast (Reinders et al., 2002).
Using this novel membrane interaction trap, it was possible to demonstrate the capability of Suc transporters to interact with each other as homooligomers or heterooligomers. All three proteins were found to be present in the same sieve elements of potato, indicating that in planta, all three proteins may be able to form oligomeric complexes.
RESULTS
Colocalization of Three Suc Transporter Paralogs in the Same Sieve Element
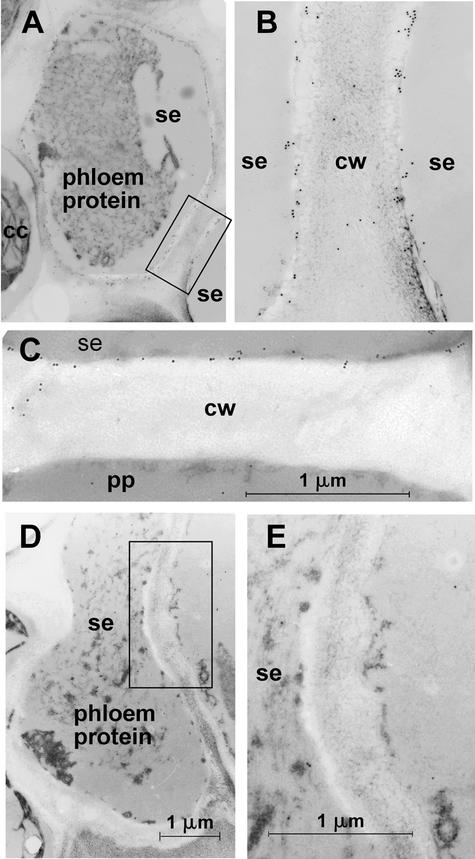
The main phloem-loading Suc transporter in solanaceous plants, SUT1, is localized in sieve elements (Kühn et al., 1997). In a previous study of the subcellular localization of different paralogs of the Suc transporter family, it was shown by immunogold labeling and electron microscopic analyses that the Suc transporter SUT1 and the putative Suc sensor SUT2 are localized at the plasma membrane of enucleate sieve elements in tomato and potato (Barker et al., 2000; Weise et al., 2000). Here, it is shown by immunogold labeling and transmission electron microscopy analysis that the low-affinity Suc transporter SUT4 also is localized at the plasma membrane of sieve elements in potato and tomato (Figures 1A to 1C).
Figure 1.
Electron Microscopic Localization of LeSUT4.
(A) Immunogold labeling of the sieve element plasma membrane in a potato petiole transverse section using anti-SUT4 antiserum.
(B) Magnification of the plasma membranes of two adjacent sieve elements (boxed in [A]).
(C) Immunogold labeling of a sieve element plasma membrane of tomato adjacent to a phloem parenchyma cell.
(D) Control with IgG-enriched preimmune serum on a transverse section of a sieve element in a tomato petiole.
(E) Magnification of the region boxed in (D) indicating no label of the plasma membrane.
Note the nonspecific cross-reaction of preimmune and immune serum with phloem protein aggregates. cc, companion cells; cw, cell wall; pp, phloem parenchyma cell; se, sieve element.
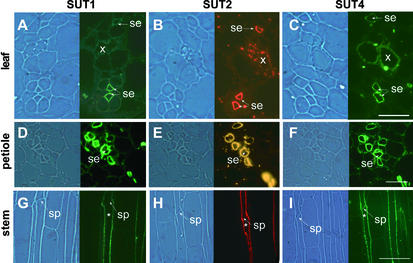
Background immunogold labeling of phloem protein also was observed in the control sections labeled with preimmune serum (Figure 1D). However, plasma membrane labeling was not observed in sections treated with preimmune serum (Figures 1D and 1E). The identification of three Suc transporters with differing properties (Boorer et al., 1996; Schulze et al., 2000; Weise et al., 2000) localized to the plasma membrane of sieve elements raised the question of whether the three Suc transporters colocalize in the same cell. Therefore, immunolocalization was performed on consecutive sections using specific antisera directed against nonconserved peptides of each of the three proteins. All three proteins were detected in the same enucleate sieve elements in petioles, minor veins of source leaves, and stems of potato (Figure 2).
Figure 2.
Colocalization of Different Suc Transporters in Sieve Elements on Serial Sections of Potato Source Leaves, Petioles, and Stems.
(A) to (C) Source leaves.
(D) to (F) Petioles.
(G) to (I) Stems.
SUT1 detection ([A], [D], and [G]) was performed using StSUT1-specific antibodies as described previously (Kühn et al., 1997). SUT2 detection ([B], [E], and [H]) and SUT4 detection ([C], [F], and [I]) were performed using LeSUT2 and LeSUT4 antibodies able to recognize their potato orthologs StSUT2 and StSUT4, respectively (Barker et al., 2000; Weise et al., 2000). SUT1 and SUT4 detection was visualized with a fluorescein isothiocyanate–coupled secondary antiserum, and SUT2 detection was visualized with a cy3-coupled secondary antiserum that was excited either via a broad-spectrum filter set (source leaves and stem longitudinal sections) or via a cy3-specific filter set (petiole cross-sections). The same sieve element in the longitudinal serial section is indicated by asterisks. se, sieve elements; sp, sieve plate; x, xylem. Magnification was ×250. Bars = 20 μm in (A) to (C), 40 μm in (D) to (F), and 100 μm in (G) to (H).
Homooligomerization of Suc Transporters
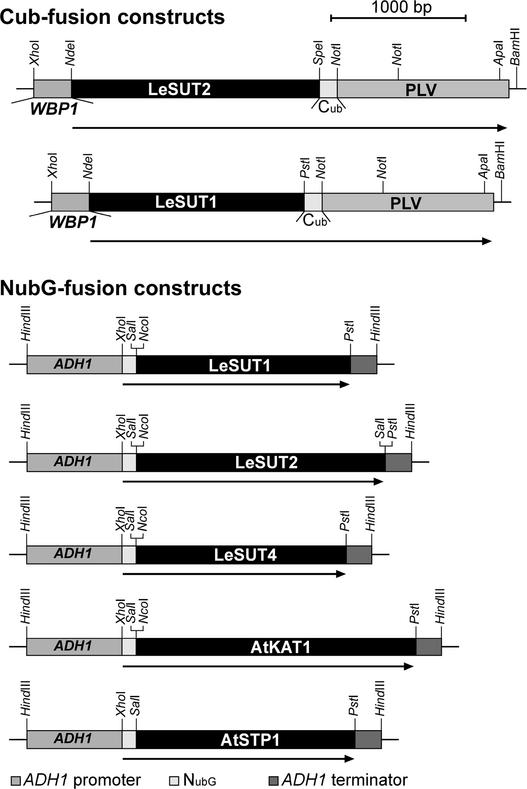
To determine whether Suc transporters can form homodimers, a yeast two-hybrid system that allowed detection of interactions between plasma membrane proteins was established. For this, a modified split-ubiquitin system was used in which interactions between two membrane-bound fusion proteins can be monitored by the release of an artificial transcription factor consisting of protein A, LexA, and VP16 (PLV). SUT open reading frames were fused to either the C-terminal part of ubiquitin (Cub) followed by PLV or the N-terminal domain of ubiquitin (NubG, I13G) (Stagljar et al., 1998).
To reduce signals caused by autoactivation or colocalization in the same compartment, an improved system was developed using the weak wheat germ agglutinin binding protein (WBP1) promoter driving the Cub fusion (Reinders et al., 2002). In vivo interaction between the two fusion proteins reconstituted NubG with CubPLV, leading to the cleavage and release of PLV by ubiquitin-specific proteases and thus to expression of the lacZ and HIS3 reporter genes integrated in the yeast genome.
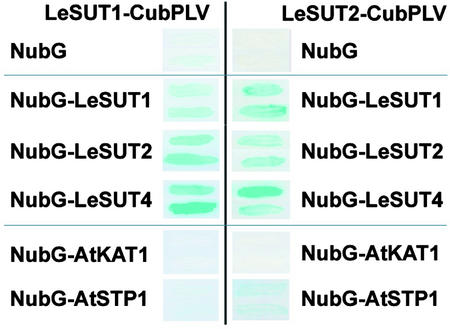
The Suc transporters LeSUT1 and LeSUT2 were cloned as CubPLV fusions for use as bait and as NubG fusions for use as prey (Figure 3). The bait constructs were integrated into the chromosome of yeast strain L40 (Stagljar et al., 1998). Subsequently, the strain was transformed with prey constructs on episomal plasmids. Potential interactions were detected by LacZ expression. The combination of LeSUT1-CubPLV or LeSUT2-CubPLV and soluble NubG did not induce β-galactosidase activity (Figure 4). Coexpression of the Cub and NubG fusion of LeSUT1 and of the Cub and NubG fusion of LeSUT2 allowed release of the artificial transcription factor PLV and activated the transcription of β-galactosidase, indicating a strong interaction between the two fusion constructs. This result demonstrates the potential of the LeSUT1 and LeSUT2 fusion proteins to form homooligomers.
Figure 3.
Fusion Protein Constructs for the Split-Ubiquitin System.
Interactions of LeSUT2-CubPLV were determined with all of the other proteins shown, and interactions of LeSUT1-CubPLV were determined with the other Suc transporter fusion constructs. For the constructs, open reading frames were amplified by PCR and cloned into the expression vectors in frame with the C-terminal (Cub) or the N-terminal (Nub) half of ubiquitin. Restriction sites important for cloning are indicated.
Figure 4.
Interaction of Two Suc Transporters, LeSUT1 and LeSUT2, with Different NubG Fusion Proteins.
No interaction with the ubiquitin domain NubG, strong interaction with LeSUT1, LeSUT2, and LeSUT4, no interaction with the control protein KAT1, and weak interaction of LeSUT2 with the related protein STP1 were detected. Positive interaction was visualized as β-galactosidase activity as determined by filter assays.
Heterooligomerization of Suc Transporters
Because Suc transporters have the capacity to form homodimers or even oligomers, and SUT1, SUT2, and SUT4 colocalize in the same cell, the question arose whether all three proteins are capable of forming heterooligomers. To test this, LeSUT1-CubPLV and LeSUT2-CubPLV were used as bait and NubG fusions of LeSUT1, LeSUT2, and LeSUT4 were used as prey (Figure 3). Yeast cells expressing LeSUT1 or LeSUT2 in combination with either of the two other Suc transporters as prey, growth on His-free medium (data not shown), and β-galactosidase filter assays demonstrated the potential for both Suc transporters to interact with their paralogs, indicating the potential of Suc transporters to form heterooligomeric complexes (Figure 4).
However, this finding does not exclude the possibility that as a result of the presence of bait and prey proteins within the secretory system or at the plasma membrane, or as a result of lateral diffusion, random collisions cause positive signals. Therefore, an unrelated plasma membrane protein (i.e., a potassium channel) was used as a control (Figure 3). It had been shown previously that the NubG fusion of AtKAT1 complements a potassium uptake–deficient yeast mutant, demonstrating its presence at the plasma membrane (Reinders et al., 2002). AtKAT1 as a prey did not interact with LeSUT1 and LeSUT2 as a bait in the split-ubiquitin analysis, supporting the specificity of the interaction of LeSUT1 or LeSUT2 with itself and with other Suc transporters (Figure 4). However, because of the channel activity, few AtKAT1 molecules are required to allow efficient potassium uptake (Reinders et al., 2002). Thus, we cannot exclude the possibility that the lack of a positive interaction with AtKAT1 may be attributable to the low abundance of the protein.
Hexose transporters belong to the same major facilitator superfamily as Suc transporters. They are related structurally to Suc transporters, although the primary sequence is not conserved. To test the specificity of the observed interaction among Suc transporters, a plant hexose transporter, AtSTP1, was fused to NubG and used as prey (Figure 3). No interaction was detected between LeSUT1 and AtSTP1, and only low β-galactosidase activity was found when coexpressing LeSUT2 with AtSTP1 (Figure 4).
Quantitative β-galactosidase assays show that the interaction of LeSUT2 with Suc transporters was three to four times stronger than the interaction of LeSUT2 with AtSTP1 (Table 1). However, the interaction of LeSUT2 with AtSTP1 was significantly stronger than the interactions of LeSUT2 with KAT1 and NubG (Table 1). Although the functional relevance of the potential of Suc transporters to form oligomers remains to be shown, the findings reported here indicate that Suc transporters can undergo oligomeric protein–protein interactions.
Table 1.
Quantitative β-Galactosidase Assays for the Two Bait Constructs LeSUT1-CubPLV and LeSUT2-CubPLV
| β-Galactosidase activity
|
||
|---|---|---|
| Prey construct | LeSUT1-CubPLV | LeSUT2-CubPLV |
| LeSUT2 | 0.29 ± 0.03ab | 0.17 ± 0.03a |
| LeSUT4 | 0.66 ± 0.15ab | 0.19 ± 0.02a |
| LeSUT1 | 1.10 ± 0.23a | 0.23 ± 0.04a |
| AtSTP1 | 0.08 ± 0.01c | 0.06 ± 0.03b |
| AtKAT1 | 0.04 ± 0.02c | 0.03 ± 0.01c |
| NubG | 0.12 ± 0.03b | 0.04 ± 0.01c |
β-Galactosidase activity is shown as pmol o-nitrophenyl β-d-galactopyranoside·min-1·μg protein. Values are means ±sd of four independent measurements. Different letters indicate significant differences (P < 0.05) within one column, as determined by one-way analysis of variance using SPSS statistical software.
Plasma Membrane Localization of NubG Fusion Proteins in Yeast
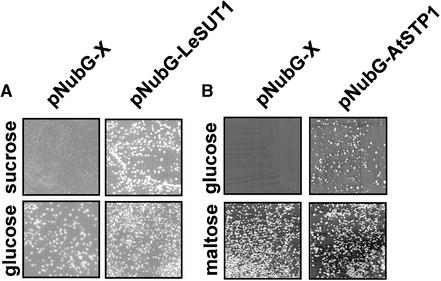
The fusion of LeSUT1 with NubG enabled the Suc uptake and invertase-deficient yeast strain SUSY7/ura3 (Riesmeier et al., 1992) to grow on Suc as the sole carbon source, demonstrating that the fusion protein is functional and thus must be localized at the plasma membrane of yeast (Figure 5A). The NubG-AtSTP1 fusion enabled the yeast strain 18gas, which is deficient in hexose uptake (Wieczorke et al., 1999), to grow on Glc as the sole carbon source, demonstrating that the AtSTP1 fusion protein is functional and localized at the plasma membrane (Figure 5B).
Figure 5.
Determination of the Functionality of the NubG Fusion of LeSUT1 and AtSTP1.
(A) The SUSY7/ura3 yeast strain was transformed with either the empty pNubG-X vector or with the same vector containing the full-length LeSUT1 open reading frame. The growth of SUSY7/ura3 on Suc depends on the presence of a functional Suc transporter in the plasma membrane. Transformed cells were tested for growth on medium containing either Glc (nonselective) or Suc (selective) as the sole carbon source.
(B) The 18gas yeast strain was transformed with either the empty pNubG-X vector or with the same vector containing the full-length AtSTP1 open reading frame. The growth of 18gas on Glc depends on the presence of a functional Glc transporter in the plasma membrane. Transformed cells were tested for growth on medium containing either maltose (nonselective) or Glc (selective) as the sole carbon source.
Yeast cells expressing NubG alone showed similar growth on maltose as cells expressing the fusion protein. Although equal cell densities were plated on maltose and Glc, fewer cells expressing NubG-AtSTP1 grew on Glc compared with maltose, and growth was more variable (Figure 5B). This finding indicates that plasmid copy number, which affects NubG-AtSTP1 expression and thus uptake capacity, limits growth on Glc.
Potential Effect of Oligomerization on Transport Properties in Yeast
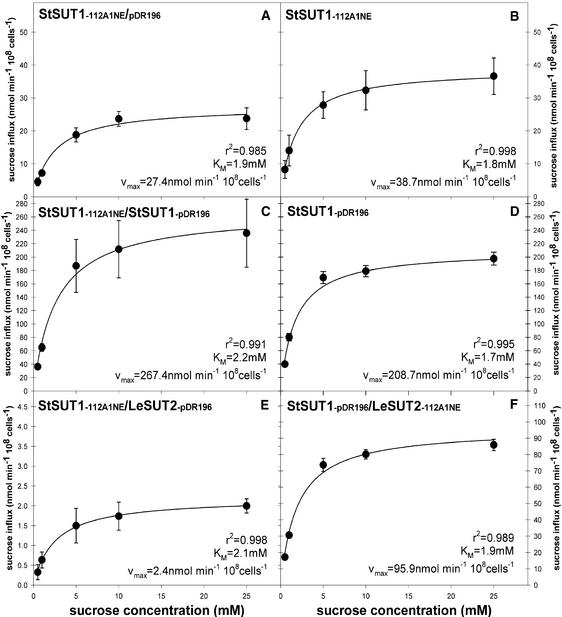
Biochemical studies provided evidence that erythrocytic Glc transporters form tetramers in which the activity of individual subunits affects the status of the other subunits (Hamill et al., 1999). To determine whether oligomerization affects the transport properties of Suc transporters, SUT1 and the putative Suc sensor SUT2 were coexpressed in yeast strain SUSY7/ura3 (Figure 6). Analysis of the Suc influx kinetics revealed no significant change in the apparent affinity; however, a 10-fold reduction in Vmax was observed when SUT1 and SUT2 were coexpressed (Figures 6A and 6E), provided that SUT2 was expressed under a strong promoter (PMA1 promoter). A twofold reduction in Suc influx was observed when SUT2 was expressed from a weaker promoter (ADH1 promoter) (Figures 6D and 6F).
Figure 6.
Suc Uptake into Yeast Cells (SUSY7/ura3) Coexpressing Different Suc Transporters.
(A) StSUT1 under the control of the ADH1 promoter (112A1NE) and empty vector pDR196.
(B) StSUT1 under the control of the ADH1 promoter and no other plasmid.
(C) StSUT1 under the control of the ADH1 promoter (112A1NE) and the PMA1 promoter (112A1NE).
(D) StSUT1 under the control of the PMA1 promoter (pDR196) and no other plasmid.
(E) StSUT1 under the control of the ADH1 promoter, and LeSUT2 under the control of the PMA1 promoter.
(F) StSUT1 under the control of the PMA1 promoter, and LeSUT2 under the control of the ADH1 promoter.
Values presented are means of three measurements ± se.
Effects caused by the competition of plasmids or promoters for trans factors were excluded by coexpression with empty vectors (Figures 6A and 6B) and coexpression of SUT1 from two different plasmids (Figures 6C and 6D). Coexpression did not affect the viability of the cells, as determined by a viability stain with propidium iodine (data not shown).
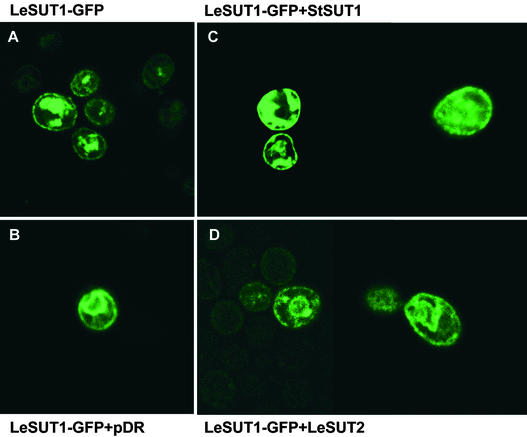
Alternatively, the differences in yeast Suc influx when SUT1 and SUT2 were coexpressed may reflect differences in targeting to the plasma membrane. Microscopic analysis of SUT1–green fluorescent protein (GFP) fusions expressed in yeast showed that the fusion protein was present both at intracellular membranes and at the plasma membrane (Figure 7A). Coexpression with the empty vector, StSUT1, or LeSUT2 did not affect SUT1-GFP localization significantly (Figures 7B and 7D). Together with the transport studies, these results indicate that coexpression of SUT2 with SUT1 does not affect the affinity of SUT1 for Suc but may downregulate the transport activity. Further studies in planta are required to prove directly that SUT2 can regulate SUT1 activity under physiological conditions.
Figure 7.
Targeting of SUT1 to the Plasma Membrane in Yeast (SUSY7/ura3) as Visualized by SUT1-GFP Fusions.
(A) Yeast cells expressing only SUT1-GFP.
(B) Yeast cells coexpressing SUT1-GFP and the empty vector pDR196.
(C) Yeast cells coexpressing SUT1-GFP and SUT1.
(D) Yeast cells coexpressing SUT1-GFP and SUT2.
DISCUSSION
The functionality of many proteins is affected or regulated in complexes, which can exist as homooligomers or heterooligomers. Like the distantly related Glc transporters from human (Hamill et al., 1999), plant Suc transporters can form complexes, as shown here by a membrane protein interaction trap. The data presented here indicate that the biochemically defined high molecular mass complex of Suc transporters may be composed of a heterooligomer consisting of three different Suc transporter paralogs (Li et al., 1991). The exact composition and structure of this complex in planta and its physiological role remain to be shown.
Complexes of Suc Transport Proteins
The first in planta biochemical indication that Suc transporters exist in oligomeric complexes derived from gel filtration experiments of plasma membrane fractions (Li et al., 1991). Under nondenaturing conditions, a 120-kD fraction containing Suc transport activity was identified by an antibody against a protein fraction containing Suc transport activity from sugar beet leaves (Lemoine et al., 1996). Upon treatment of the 120-kD fraction with SDS, the complex dissolved and a single polypeptide with an apparent molecular mass of 42 kD was identified by the antiserum. These data indicated that Suc transporters exist in a complex that can be composed of an oligomer of the 42-kD protein but did not exclude the possibility that other proteins are associated in the complex.
The presence of detergents precludes prediction of the composition of a putative oligomeric complex. The molecular mass of the monomer may be consistent with the molecular mass of the Suc transporter from potato (46 kD; Kühn et al., 1997). Therefore, it was hypothesized that SUT1 forms homooligomers (at least dimers) in the plasma membrane of sieve elements in solanaceous plants. To test this possibility, a novel interaction trap for membrane proteins (i.e., the yeast-based split-ubiquitin system) allowed us to demonstrate that the Suc transporter SUT1 and the putative Suc sensor SUT2 are capable of forming homodimers or higher order homooligomeric structures.
The colocalization of three different Suc transporter paralogs at the plasma membrane of the same enucleate sieve element suggested that it may be possible that Suc transporters exist as heterooligomers in planta. A more detailed analysis using the split-ubiquitin system allowed the demonstration that all three Suc transporters can interact with each other and form complexes composed of different members of the transporter family. These results were confirmed for the Arabidopsis orthologs (W. Schulze, unpublished results). However, on the basis of these interaction trap experiments, it is not possible at present to determine the exact structure of this complex. Studies using biochemical approaches will be required to solve this question.
Potential Role of the Oligomeric State
Mammalian Glc transporters belonging to the same superfamily as yeast hexose and plant Suc transporters confer regulatory effects within a complex of dimers of dimers (Hebert and Carruthers, 1992; Zottola et al., 1995; Hamill et al., 1999). GLUT1 is a uniporter that facilitates Glc transport and functions as a fixed site carrier in which four homooligomeric subunits coexist in import and export states. The presence and binding of substrate on the outside of one subunit or the inside of the other subunit can affect the activity status of the complex through cooperative conformational changes (Hamill et al., 1999).
A similar phenomenon was observed in the case of ammonium transporters, in which an amino acid exchange in the C terminus in mep1-1 has a dominant-negative effect on MEP3 activity (Marini et al., 2000). The oligomerization is thought to play a role in the regulation of hexose transport in animal cells. For the Suc transporters, we have to assume that the role of oligomerization may be different from that of the GLUTs, because at least SUT1 and probably SUT4 as well function as proton cotransporters (Boorer et al., 1996). In case of the Streptococcus proton lactose cotransporter LacS, it has been suggested that the dimeric state of LacS is required for Δp-driven lactose uptake (Veenhoff et al., 2001). The two subunits of LacS thus seem to be functionally coupled in the step associated with the conformational reorientation of the empty binding site, a step which is unique for the proton-coupled transport. It remains to be shown, whether a similar mechanism operates in case of the proton coupled SUTs.
The presence of the Suc transporter complexes in the plasma membrane of sieve elements needs to be verified in planta, as do the exact structure and the physiological function of such complexes. Kinetic analysis after coexpression in yeast indicates that SUT2 may exert negative effects on SUT1 activity, although from the data presented here we cannot fully exclude the possibility that competition between membrane proteins for targeting in yeast may mimic or mask the effects. Further analysis of the potential effects of SUT2 on SUT1 is necessary using expression systems other than yeast. It is possible that the formation of Suc transporter complexes in the plasma membrane allows the precise adjustment of transport capacities through changes in complex composition.
An unusual situation arises from the fact that in solanaceous plants, Suc transporters colocalize in an enucleate cell, so that direct signaling involving the nucleus is not possible. Rather, intercellular signaling is required to control transcription. A more rapid means of regulation of phloem loading could be the direct regulation of the turnover of Suc transporters or of cooperative activity changes in the protein complexes. The regulation of protein turnover has been studied extensively for maltose permeases (Medintz et al., 2000) and amino acid transporters in yeast (Bernard and Andre, 2001) and may serve as a model. The high turnover rate of SUT1 may be taken as an indication of the regulated turnover of Suc transporters (Kühn et al., 1997). However, the possibility of the regulation of phloem transport through the formation and dynamics of higher order protein complexes has not been addressed.
Plant Suc transporters differ with respect to substrate affinity. StSUT1 and its orthologs have a high affinity for Suc. By contrast, SUT4 is a low-affinity transporter (Weise et al., 2000). When expressed in yeast, the putative Suc sensor SUT2 (Barker et al., 2000) has a low affinity and low transport rates (Schulze et al., 2000). Thus, regulation of the composition of the Suc transporter complexes would be a fast means to regulate the phloem-loading properties of the enucleate sieve element plasma membrane and adjust the loading capacity according to supply and demand. As a consequence, regulation would not require signaling to the nucleus.
One may speculate that the regulation of Suc transport occurs in the heteromeric complexes of Suc transporters that differ with respect to affinity, capacity, and potentially regulatory function, if SUT2 can function as a Suc sensor. Dominant mutations and transgenic plants, in which individual transporters have been downregulated, may help unravel the role of the interactions.
Because both the yeast and animal genomes contain 10 to 20 members of the related hexose transporter families, it is conceivable that heterooligomers are formed in these systems as well. The finding of a nonfunctional chimera of two yeast hexose transporters with a dominant-negative effect on transport mediated by the other members of the family may be a consequence of the existence of such hexose transporter complexes in the yeast plasma membrane (Sherwood et al., 2000).
Split-Ubiquitin System
Control experiments using other prey, such as the soluble ubiquitin domain NubG or a potassium channel, suggest that the interactions found in the yeast system are specific. The potential of the split-ubiquitin system to detect functional interactions was further substantiated by analysis of the functional reconstitution of two separately expressed Suc transporter halves (Reinders et al., 2002). The weak interaction of SUT2 with the hexose transporter STP1 indicates that structurally related members of the superfamily are able to interact in this system, but to a significantly weaker extent. Therefore, it is possible that these weak interactions are caused by the coexistence of the proteins in the same compartment, as shown here by the demonstration that the NubG fusion of STP1 is present at the plasma membrane because it is able to complement a yeast mutant deficient in Glc uptake.
The collision of membrane proteins that are not members of a complex localizing to the same compartment had been observed previously in the case of yeast endoplasmic reticulum membrane proteins (Wittke et al., 1999). Alternatively, the membrane polypeptides may exist in larger complexes such as rafts (Simons and Toomre, 2000). More detailed analyses, including biochemical studies, will be necessary to verify the significance of these weak interactions.
Conclusions and Outlook
The modified split-ubiquitin system was shown here to be a valuable tool to monitor and dissect membrane protein interactions. Our results indicate that the three Suc transporters with different biochemical properties can build a complex, potentially with regulatory consequences, e.g., as requirement for proton coupled transport. Another key finding is that, in analogy to other metabolite transporters, Suc transporters also may function in higher order complexes (Veenhoff et al., 2002). The major difference described here is the heterooligomerization, which may allow additional dynamics and flexibility for the regulation of Suc transport and thus for the regulation of phloem loading. The concept of heteromeric complexes involved in phloem loading and long-distance transport in plants will have to be verified in planta using transgenic plants in combination with biochemical and immunological techniques.
METHODS
Strains, Media, and Recombinant DNA Methods
Yeast strain L40 (MATa ade3 trp1 leu2 his3 LYS2::lexA-HIS3 URA3::lexA-lacZ) (Vojtek et al., 1993) was used as the host for split-ubiquitin analysis. Suc transport activity was assessed using strain SUSY7/ura3 (Riesmeier et al., 1992; Barker et al., 2000). Glc transport activity was assessed using yeast strain 18gas (Wieczorke et al., 1999), which is deficient in 18 hexose transporters. Standard procedures of yeast genetics and molecular biology were used (Guthrie and Fink, 1991). Yeast transformations were performed with a modification of the Li+ ion method (Gietz et al., 1992).
Immunocytochemistry
The generation and purification of specific peptide antisera have been described: SUT1, 5′-EIDEKLAGAGKSK-3′ (Kühn et al., 1997); SUT2, 5′-MDAVSIRVPYKNLKC-3′ (Barker et al., 2000); SUT4, 5′-GSSHTGEEIDESSHGQEEAFLW-3′ (Weise et al., 2000). Fluorescent immunodetection of SUTs was performed with modifications according to Kühn et al. (1997) using fluorescein isothiocyanate– or cy3-coupled anti-rabbit IgG (Sigma). Photographs were taken with a fluorescence microscope (Axiophot; Zeiss, Jena, Germany) using fluorescein isothiocyanate filters, broad-spectrum cy3 filters, or specific cy3 filters. Electron microscopy and immunogold labeling were performed as described (Kühn et al., 1997; Barker et al., 2000).
Split-Ubiquitin Analysis
The interaction of Suc transporters was tested by split-ubiquitin analysis using a modified version with a weak WBP promoter for the NubG construct as described in Reinders et al. (2002). Full-length or partial cDNAs of interest were cloned in frame with either the C-terminal (Cub) or the N-terminal (NubG; wild-type I-13 replaced by G) (Stagljar et al., 1998) subdomain of ubiquitin. Interaction was tested by analysis of reporters (i.e., His auxotrophy and β-galactosidase activity).
Full-length coding regions of LeSUT1, LeSUT2, and LeSUT4 were amplified by PCR using Deep Vent DNA polymerase (New England Biolabs, Beverly, MA). Appropriate restriction sites were introduced with the primers. After restriction, PCR fragments were cloned into NdelI-PstI-cleaved pY-CubPLV (LeSUT1) on NdeI-SpeI–cleaved pY-CubPLV (LeSUT2) or SalI-PstI–cleaved (LeSUT1 and LeSUT4) and SalI-SalI–cleaved (LeSUT2) pNubG-X to yield plasmids pLeSUT2-CubPLV, pLeSUT1-CubPLV, pNubG-LeSUT1, pNubG-LeSUT4, and pNubG-LeSUT2, respectively. pNubG-AtKAT1 and pNubG-AtSTP1 have been described (Reinders et al., 2002). pLeSUT2-CubPLV was integrated into the LEU2 locus of strain L40 after linearization with ClaI. Integration of the expression cassette was confirmed by PCR using primers for the WBP1 promoter and SUT.
β-Galactosidase Assays
β-Galactosidase activity was determined using filter assays (Ramer et al., 1992). Cells were streaked on nitrocellulose (BA-S83; Schleicher & Schüll), placed on synthetic minimal medium-agar plates containing appropriate supplements, and grown for 2 days at 27°C. Filters were removed, frozen briefly in liquid nitrogen, defrosted, and placed in Petri dishes onto filter paper saturated with Z buffer (60 mM Na2PO4, 40 mM NaH2PO4, 10 mM KCl, and 1 mM MgSO4) containing 35 mM β-mercaptoethanol (v/v) and 1.5 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-galactoside (Sigma). For development, filters were incubated at 37°C overnight.
Quantitative β-galactosidase assays were performed by harvesting 30-mL cultures at an OD600 of ∼0.8, washing with 10 mL of water, and resuspending cells in 0.5 mL of Z buffer. Cells were broken by vortexing with glass beads. After the addition of 0.2 mL of Z buffer, debris was pelleted at 14,000 rpm for 2 min. Supernatant was used for assays. In microtiter plates, 200-μL protein aliquots were incubated with 40 μL of o-nitrophenylglucoside (4 mg/mL). The kinetics of change in absorbance was measured at 415 nm (Biotek, Neufahrn, Germany).
Suc Uptake in Yeast
Yeast strain SUSY7/ura3 (Riesmeier et al., 1992; Barker et al., 2000) was used to measure 14C-Suc uptake. Plasmids with different selectable markers, bearing StSUT1 or LeSUT2 under the ADH1 promoter (vector 112A1NE; Riesmeier et al., 1992) or the PMA1 promoter fragment (plasmid pDR196; Rentsch et al., 1995), were used to coexpress StSUT1 and LeSUT2. 14C-Suc uptake was measured as described (Weise et al., 2000).
Expression of Green Fluorescent Protein Fusion Proteins in Yeast
LeSUT1-cDNA was amplified by PCR (Pfu) using T3 primer and 5′-CTTTTGTAATGCGGCCGCATGGAAACCCATGGCG-3′. The PCR product was treated with PstI and NotI and ligated with a NotI-EcoRI green fluorescent protein fragment (Clontech, Palo Alto, CA) into the yeast expression vector 112A1NE (Riesmeier et al., 1992) after linearization with PstI-EcoRI. The fusion construct was still functional and able to complement Suc uptake–deficient yeast mutants. Coexpression was analyzed using yeast strain SUSY7/ura− transformed by the frozen-cell method (Dohmen et al., 1991). Green fluorescent protein fluorescence was detected with Leica confocal microscope TCS SP (Wetzlar, Germany).
Acknowledgments
We thank Elena Wiederhold for help with immunochemistry. This work was supported by the Deutsche Forschungsgemeinschaft by a fellowship to A.R. (Grant RE 1454/1) and a grant to W.B.F. (Grant SFB446).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.002428.
References
- Barker, L., Kühn, C., Weise, A., Schulz, A., Gebhardt, C., Hirner, B., Hellmann, H., Schulze, W., Ward, J.M., and Frommer, W.B. (2000). SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12, 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, F., and Andre, B. (2001). Ubiquitin and the SCF(Grr1) ubiquitin ligase complex are involved in the signalling pathway activated by external amino acids in Saccharomyces cerevisiae. FEBS Lett. 496, 81–85. [DOI] [PubMed] [Google Scholar]
- Boorer, K.J., Loo, D.D.F., Frommer, W.B., and Wright, E.M. (1996). Transport mechanism of the cloned potato H+/sucrose cotransporter StSUT1. J. Biol. Chem. 271, 25139–25144. [DOI] [PubMed] [Google Scholar]
- Bürkle, L., Hibberd, J.M., Quick, W.P., Kühn, C., Hirner, B., and Frommer, W.B. (1998). The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol. 118, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot, S., and Bonnemain, J.L. (1981). Involvement of protons as a substrate for the sucrose carrier during phloem loading in Vicia faba leaves. Plant Physiol. 67, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen, R.J., Strasser, A.W., Honer, C.B., and Hollenberg, C.P. (1991). An excellent transformation procedure enabling long-term storage of competent cells of various yeast genera. Yeast 7, 691–692. [DOI] [PubMed] [Google Scholar]
- Gietz, D., St. Jean, A., Woods, R.A., and Schiestl, R.H. (1992). Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20, 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald, J.R., Krysan, P.J., Young, J.C., Evert, R.F., and Sussman, M.R. (2000). Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl. Acad. Sci. USA 97, 13979–13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G.R. (1991). Guide to Yeast Genetics and Molecular Biology. (San Diego: Academic Press), p. 194.
- Hamill, S., Cloherty, E.K., and Carruthers, A. (1999). The human erythrocyte sugar transporter presents two sugar import sites. Biochemistry 38, 16974–16983. [DOI] [PubMed] [Google Scholar]
- Hebert, D., and Carruthers, A. (1992). Glucose transporter oligomeric structure determines transporter function: Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. J. Biol. Chem. 267, 23829–23838. [PubMed] [Google Scholar]
- Heuberger, E.H., Veenhoff, L.M., Duurkens, R.H., Friesen, R.H., and Poolman, B. (2002). Oligomeric state of membrane transport proteins analyzed with blue native electrophoresis and analytical ultracentrifugation. J. Mol. Biol. 317, 591–600. [DOI] [PubMed] [Google Scholar]
- Ito, T., Chiba, T., Ozawa, R., Yoshida, M., Hattori, M., and Sakaki, Y. (2001). A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98, 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., Tashiro, K., Muta, S., Ozawa, R., Chiba, T., Nishizawa, M., Yamamoto, K., Kuhara, S., and Sakaki, Y. (2000). Toward a protein-protein interaction map of the budding yeast: A comprehensive system to examine two-hybrid interactions in all possible combinations between yeast proteins. Proc. Natl. Acad. Sci. USA 97, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson, N., and Varshavsky, A. (1994). Split-ubiquitin as a sensor of protein interactions in vivo. Proc. Natl. Acad. Sci. USA 91, 10340–10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn, C., Franceschi, V.R., Schulz, A., Lemoine, R., and Frommer, W.B. (1997). Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275, 1298–1300. [DOI] [PubMed] [Google Scholar]
- Kühn, C., Quick, W.P., Schulz, A., Riesmeier, J.W., Sonnewald, U., and Frommer, W.B. (1996). Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ. 19, 1115–1123. [Google Scholar]
- Lalonde, S., Boles, E., Hellmann, H., Barker, L., Patrick, J.W., Frommer, W.B., and Ward, J.M. (1999). The dual function of sugar carriers: Transport and sugar sensing. Plant Cell 11, 707–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine, R., Kühn, C., Thiele, N., Delrot, S., and Frommer, W.B. (1996). Antisense inhibition of the sucrose transporter in potato: Effects on amount and activity. Plant Cell Environ. 19, 1124–1131. [Google Scholar]
- Li, Z.-S., Gallet, O., Gaillard, C., Lemoine, R., and Delrot, S. (1991). Reconstitution of active sucrose transport in plant proteoliposomes. FEBS Lett. 286, 117–120. [DOI] [PubMed] [Google Scholar]
- Ludewig, U., Pusch, M., and Jentsch, T. (1996). Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature 383, 340–343. [DOI] [PubMed] [Google Scholar]
- Marini, A.-M., Springael, J.-Y., Frommer, W.B., and André, B. (2000). Cross-talk between ammonium transporters in yeast and interference by the soybean SAT1 protein. Mol. Microbiol. 35, 378–385. [DOI] [PubMed] [Google Scholar]
- Medintz, I., Wang, X., Hradek, T., and Michels, C.A. (2000). A PEST-like sequence in the N-terminal cytoplasmic domain of Saccharomyces maltose permease is required for glucose-induced proteolysis and rapid inactivation of transport activity. Biochemistry 39, 4518–4526. [DOI] [PubMed] [Google Scholar]
- Özcan, S., Dover, J., Rosenwald, A.G., Wölfel, S., and Johnston, M. (1996). Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc. Natl. Acad. Sci. USA 93, 12428–12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer, S.W., Elledge, S.J., and Davis, R.W. (1992). Detection of altered protein conformations in living cells. Proc. Natl. Acad. Sci. USA 89, 11589–11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders, A., Thaminy, S., Stagljar, I., Frommer, W.B., and Ward, J.M. (2002). Intra- and intermolecular interactions in sucrose transporters at the plasma membrane detected by the split-ubiquitin system and functional assays. Structure 10, 763–772. [DOI] [PubMed] [Google Scholar]
- Rentsch, D., Laloi, M., Rouhara, I., Schmelzer, E., Delrot, S., and Frommer, W.B. (1995). NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett. 370, 264–268. [DOI] [PubMed] [Google Scholar]
- Riesmeier, J.W., Flügge, U.I., Schulz, B., Heineke, D., Heldt, H.W., Willmitzer, L., and Frommer, W.B. (1993). Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc. Natl. Acad. Sci. USA 90, 6160–6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier, J.W., Willmitzer, L., and Frommer, W.B. (1992). Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 11, 4705–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier, J.W., Willmitzer, L., and Frommer, W.B. (1994). Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J. 13, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze, W., Weise, A., Frommer, W.B., and Ward, J.M. (2000). Function of the cytosolic N-terminus of sucrose transporter AtSUT2 in substrate affinity. FEBS Lett. 485, 189–194. [DOI] [PubMed] [Google Scholar]
- Schwikowski, B., Uetz, P., and Fields, S. (2000). A network of protein-protein interactions in yeast. Nat. Biotechnol. 18, 1257–1261. [DOI] [PubMed] [Google Scholar]
- Sherwood, P.W., Katic, I., Sanz, P., and Carlson, M. (2000). A glucose transporter chimera confers a dominant negative glucose starvation phenotype in Saccharomyces cerevisiae. Genetics 155, 989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Stagljar, I., Korostensky, C., Johnsson, N., and Te Heesen, S. (1998). A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc. Natl. Acad. Sci. USA 95, 5187–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhoff, L.M., Heuberger, E.H., and Poolman, B. (2001). The lactose transport protein is a cooperative dimer with two sugar translocation pathways. EMBO J. 20, 3056–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhoff, L.M., Heuberger, E.H., and Poolman, B. (2002). Quarternary structure and function of transport proteins. Trends Biochem. Sci. 27, 242–249. [DOI] [PubMed] [Google Scholar]
- Vojtek, A.B., Hollenberg, J.A., and Cooper, J.A. (1993). Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74, 205–214. [DOI] [PubMed] [Google Scholar]
- Weise, A., Barker, L., Kühn, C., Lalonde, S., Buschmann, H., Frommer, W.B., and Ward, J.M. (2000). A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12, 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes, P., Xu, X., Li, H., Chien, F., Doberstein, S., and Montell, C. (1999). Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nat. Neurosci. 2, 447–453. [DOI] [PubMed] [Google Scholar]
- Wieczorke, R., Krampe, S., Weierstall, T., Freidel, K., Hollenberg, C.P., and Boles, E. (1999). Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464, 123–128. [DOI] [PubMed] [Google Scholar]
- Wittke, S., Lewke, N., Müller, S., and Johnsson, N. (1999). Probing the molecular environment of membrane proteins in vivo. Mol. Biol. Cell 10, 2519–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottola, R.J., Cloherty, E.K., Coderre, P.E., Hansen, A., Hebert, D.N., and Carruthers, A. (1995). Glucose transporter function is controlled by transporter oligomeric structure: A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry 34, 9734–9747. [DOI] [PubMed] [Google Scholar]