Abstract
The adapter SLP-76 plays an essential role in FcɛRI signaling, since SLP-76−/− bone marrow-derived mast cells (BMMC) fail to degranulate and release interleukin-6 (IL-6) following FcɛRI ligation. To define the role of SLP-76 domains and motifs in FcɛRI signaling, SLP-76−/− BMMC were retrovirally transduced with SLP-76 and SLP-76 mutants. The SLP-76 N-terminal and Gads binding domains, but not the SH2 domain, were critical for FcɛRI-mediated degranulation and IL-6 secretion, whereas all three domains are essential for T-cell proliferation following T-cell receptor (TCR) ligation. Unexpectedly, the three tyrosine residues in SLP-76 critical for TCR signaling, Y112, Y128, and Y145, were not essential for IL-6 secretion, but were required for degranulation and mitogen-activated protein kinase activation. Furthermore, a Y112/128F SLP-76 mutant, but not a Y145F mutant, strongly reconstituted mast cell degranulation, suggesting a critical role for Y145 in FcɛRI-mediated exocytosis. These results point to important differences in the function of SLP-76 between T cells and mast cells.
The high-affinity receptor for immunoglobulin E (IgE) (FcɛRI) is a multimolecular complex of the IgE-binding α subunit, two signal-transducing γ subunits, and a β subunit that promotes assembly of the receptor and amplifies signal transduction (3, 32). Both γ and β chains contain immunoreceptor tyrosine-based activation motifs (ITAMs) within their intracellular domains. Upon FcɛRI cross-linking, the ITAMs of the γ and β subunits become phosphorylated by the Src family tyrosine kinase lyn and recruit the protein tyrosine kinase Syk, which in turn phosphorylates intracellular proteins such as LAT, phospholipase C-γ (PLC-γ), Vav, and the adapter protein SLP-76 (9, 21, 28, 35).
SLP-76 is predominantly expressed in hematopoietic cells and has three major protein-interacting domains (7, 25, 38, 46). Three tyrosine residues (Y113, Y128, and Y145) in the N-terminal domain become phosphorylated by Syk family protein tyrosine kinases following T-cell receptor (TCR) engagement and provide binding sites for the SH2 domains of Vav, Nck, and Itk. The binding of Vav and Nck to phosphotyrosine residues Y113 and Y128 may link SLP-76 to the JNK (Jun amino-terminal kinase) pathway and to the actin cytoskeleton (5, 10, 54-56). Y145 has been implicated in the binding of SLP-76 to Itk (6, 53). Direct interaction of PLC-γ with SLP-76 as well as formation of a complex involving LAT and Itk, which, respectively, bind and phosphorylate PLC-γ, may be required for PLC-γ activation (49, 57, 59). SLP-76 associates constitutively via its central proline-rich domain with the SH3 domain of Gads, which recruits it to LAT following TCR stimulation (1, 31, 33). This allows the translocation of SLP-76 to glycolipid-enriched microdomains (GEMs) (24) and may also link it via Sos to the Ras/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated protein kinase (ERK) pathway (29, 36). Proteins that directly interact with the SLP-76 SH2 domain include ADAP (formerly known as SLAP-130/FYB), the Ser/Thr kinase HPK1, and a 62-kDa phosphoprotein (11, 36, 37, 48).
SLP-76−/− mice lack T cells, indicating that signals integrated by SLP-76 are critical for T-cell development (8, 43). SLP-76 also plays an important role in TCR signal transduction and T-cell activation. SLP-76-deficient Jurkat cells exhibit severely impaired signaling after stimulation through the TCR-CD3 complex. PLC-γ1 activation, calcium mobilization, ERK1/2 phosphorylation, and interleukin-2 (IL-2) production are all severely compromised (59).
SLP-76-deficient mice have normal numbers of mast cells in their skin and bronchi, and their bone marrow cells differentiate normally in vitro into mast cells upon culture in IL-3-containing medium (44). However, SLP-76−/− bone marrow-derived mast cells (BMMC) fail to release the granular enzyme β-hexosaminidase and to secrete IL-6 after FcɛRI cross-linking. These findings indicate that SLP-76 plays an essential role in FcɛRI signaling. We took advantage of the availability of SLP-76−/− BMMC and transduced them retrovirally with SLP-76 mutants to address the role of SLP-76 domains and residues for its adapter function in signaling via FcɛRI.
MATERIALS AND METHODS
Cells and cell culture.
Bone marrow cells were cultured in WEHI-3-conditioned medium (WCM) as a source of IL-3 (44). After 3 to 5 weeks of culture, 90% or more of the cells derived from wild-type (WT) and SLP-76−/− bone marrow are mast cells, as evidenced by fluorescence-activated cell sorting (FACS) analysis for FcɛRI expression. To assess FcɛRI expression, the cells were successively incubated with mouse IgE, biotinylated rat anti-mouse IgE, and streptavidin-CyChrome (all from PharMingen). Cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems).
cDNA constructs and viral constructs.
SLP-76 mutants were generated from mouse SLP-76 cDNA by PCR and then cloned into the Moloney murine leukemia virus (MLV)-based retroviral pMMP vector. SLP-76 cDNA was cloned upstream of an internal ribosomal entry site that precedes the gene encoding green fluorescent protein (GFP). This vector construct once integrated into the host genome directs expression of a bicistronic mRNA encoding both SLP-76 and GFP.
Virus production and viral transduction of BMMC.
High-titer vesicular stomatitis virus G protein (VSV-G)-pseudotyped retrovirus-containing supernatants were obtained after calcium phosphate transfection of the 293T packaging cell line (50) with individual retroviral vectors as well as expression plasmids for MLV Gag-Pol and VSV-G (41). Viral particle-containing supernatant was added to SLP-76-deficient BMMC cultures prestimulated with stem cell factor (Biosource International) and 8 μg of Polybrene per ml (Sigma, St. Louis, Mo.) in fibronectin (Sigma)-coated plates. After 24 h, the medium was changed to WCM. GFP expression was assessed after 2 to 3 days of culture, and GFP-expressing cells were sorted (FACSVantage SE flow cytometer; Becton Dickinson).
β-Hexosaminidase release assay.
BMMC (106) were incubated in WCM containing 2.5 μg of rat IgE per ml for 1 h on ice. After washing, pellets were resuspended on ice in WCM containing 0.1, 1, and 10 μg of F(ab′)2 fragments per ml of mouse anti-rat immunoglobulins (Igs) (Jackson ImmunoResearch) and incubated at 37°C for 20 min. The reaction was stopped by centrifugation. The pellets were resuspended in their original volume and lysed with WCM containing 0.5% Triton X-100. Aliquots of supernatants and cell lysates were incubated in duplicates with substrate solution (1.3 mg of p-nitrophenyl-β-d-2-acetamido-2-deoxyglucopyranozide per ml in 0.1 M citrate [pH 4.5]) as described previously (44). The percent release values for each experimental condition were calculated by the formula [S/(S + P)] × 100, where S and P, respectively, are the β-hexosaminidase contents of the supernatant and pellet from each sample. The net release values were obtained by subtracting the optical density for medium alone from those for the supernatant and pellet.
Measurement of intracellular calcium.
BMMC (10 × 106/ml) were preloaded for 1 h on ice with 5 μg of rat IgE per ml and the calcium-sensitive dye indo 1-AM (5 μM) in calcium buffer (phosphate-buffered saline [pH 7.4], supplemented with 5 mM glucose, 1 mM CaCl2, 0.5 mM MgCl2, 10 mM HEPES, and 1% bovine serum albumin). Cells were washed twice with calcium buffer and resuspended to the original concentration. Three hundred microliters of cells was analyzed for calcium mobilization in a spectrophotometer (LS50; Perkin-Elmer Cetus Instruments). Excitation and emission wavelengths were 331 and 410 nm, respectively. Receptor-mediated calcium release was monitored after addition of F(ab′)2 anti-rat Ig (25 μg/ml), and maximal release was determined after addition of ionomycin (10 μM final concentration). Values were plotted as a percentage of calcium release triggered by ionomycin.
Measurement of IL-6 production.
BMMC (106) preloaded for 1 h on ice with 2.5 μg of rat IgE per ml were incubated with 1 μg of F(ab′)2 anti-rat Ig per ml for 24 h in WCM at 37°C. Supernatants were assayed for IL-6 with an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems).
Western blotting and immunoprecipitation.
SLP-76 proteins were detected after lysis in Laemmli buffer and Western blotting with a rabbit anti-SLP-76 antiserum (30). For immunoprecipitation experiments, BMMC were preloaded for 1 h on ice with 2.5 μg of rat IgE per ml and then stimulated for 2 min (SLP-76 immunoprecipitation) or 7 min (PLC-γ immunoprecipitation) at 37°C with 10 μg of F(ab′)2 anti-rat Ig per ml in WCM. After centrifugation, cells were lysed for 15 min on ice in lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40) supplemented with the protease inhibitors diisopropyl fluorophosphate (3 mM; Sigma), phenylmethylsulfonyl fluoride (PMSF; 1 mM; Sigma), antipain (50 μg/ml), (4-amidinophenyl)-methane-sulfonyl fluoride (APMSF; 20 μg/ml), and complete protease inhibitor cocktail (all from Roche Molecular Biochemicals), as well as phosphatase inhibitors (0.5 mM Na3VO4, 5 mM NaF). Clarified cell lysates were subjected to immunoprecipitation with antibodies to SLP-76 (30), PLC-γ1 (Upstate Biotechnology), or PLC-γ2 (Santa Cruz Biotechnology). Immunoblotting was performed with antiphosphotyrosine monoclonal antibody 4G10 (Upstate Biotechnology), anti-pTyr (Sigma), anti-btk (BD Biosciences), and anti-Vav (Upstate Biotechnology).
Activated MAPKs were detected by immunoblotting cell lysates with phosphoprotein-specific antibodies (phospho-ERK1/2; Santa Cruz Biotechnology), phospho-SAPK/JNK and phospho-p38 (both from NewEngland Biolabs). Immunoreactive bands were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech).
Statistical analysis and densitometry.
Statistical analyses of the data were performed with the Prism software (version 3.0a). Blots were scanned and analyzed with NIH Image software (version 1.62). Results were expressed as fold induction over baseline and were normalized to signal for loading.
RESULTS
Functional reconstitution of SLP-76−/− BMMC by retroviral transfer of WT SLP-76.
BMMC from SLP76−/− mice were reconstituted by retroviral transfer of a bicistronic construct encoding WT SLP-76 and GFP or of a control vector encoding only GFP. GFP-expressing cells were sorted and analyzed. All cultures contained >90% GFP-positive cells at the time of analysis. The levels of FcɛRI expression were equivalent in BMMC from WT mice and SLP-76−/− mice, as well as virally transduced BMMC from SLP76−/− mice (Fig. 1A). Expression of WT SLP-76 protein was confirmed by Western blotting (Fig. 1B).
FIG. 1.
Retroviral transduction with SLP-76 reconstitutes FcɛRI-mediated signaling in SLP-76−/− mast cells. (A) IgE receptor expression on BMMC from WT, SLP-76−/− (KO) mice, and SLP-76−/− BMMC reconstituted (reconst.) with control vector or WT SLP-76. Cells were treated with mouse IgE and then incubated with biotinylated anti-IgE and streptavidin-CyChrome (solid line). Control staining was with biotinylated anti-IgE and streptavidin-CyChrome alone (dashed line). (B) SLP-76 protein expression was assessed by immunoblotting after separation of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (C) Calcium mobilization was detected in BMMC loaded with indo 1-AM. BMMC (10 × 106/ml) were sensitized with IgE (5 μg/ml) for 1 h at room temperature and then stimulated with 25 μg of F(ab′)2 anti-rat Ig per ml followed later by ionomycin (10 μM) at the indicated time points. Results are expressed as percentage of ionomycin-induced calcium mobilization. (D) Release of β-hexosaminidase. BMMC (106) were incubated with 2.5 μg of IgE per ml for 1 h on ice and then stimulated with F(ab′)2 anti-rat Ig at the indicated concentrations. The results represent the mean ± standard deviation of three experiments, each performed in duplicate. **, P < 0.01; *, P < 0.05. ns, not significant as determined by Student's t test compared to WT. (E) IL-6 release by BMMC. BMMC (106) preloaded for 1 h on ice with 2.5 μg of rat IgE per ml were incubated with 1 μg of F(ab′)2 anti-rat Ig per ml for 24 h. IL-6 release was determined by ELISA. The results represent the mean ± standard deviation of four experiments. **, P < 0.01. ns, not significant compared to WT, as determined by Student's t test.
SLP-76−/− BMMC are severely deficient in their ability to mobilize calcium, degranulate, and secrete IL-6 following FcɛRI ligation (44). To validate the retroviral transduction system as a tool to examine the ability of SLP-76 mutants to restore SLP-76 function, BMMC transduced with WT SLP-76 were loaded with rat IgE and challenged with F(ab′)2 anti-rat Ig. Retroviral transduction of SLP-76−/− BMMC with WT SLP-76-encoding vector, but not control vector, restored to normal their capacity to mobilize calcium (Fig. 1C) and to release the granule enzyme β-hexosaminidase at all three concentrations of cross-linking reagent tested (Fig. 1D). Retroviral transduction of WT SLP-76 also restored to normal the capacity of SLP-76−/− BMMC to secrete IL-6 following FcɛRI ligation (Fig. 1E).
Reconstitution of SLP-76−/− BMMC with SLP-76 mutants.
Six SLP-76 mutants were generated in order to delineate the role of individual SLP-76 domains and tyrosine residues in signaling through FcɛRI. Three deletion mutants included an N-terminal deletion mutant (amino acids [aa] 2 to 156; SLP-76 Δ2-156), a Gads binding site deletion mutant (aa 224 to 244; SLP-76 Δ224-244) and an SH2-domain deletion mutant (aa 422 to 533; SLP-76 ΔSH2). In three other mutants, tyrosine (Y) residues Y112, Y128, and Y145 in the N-terminal domain of murine SLP-76, which correspond to Y113, Y128, and Y145 in human SLP-76, were substituted for with phenylalanine (F): Y112/128/145F in SLP-76 FFF, Y112/128F, in SLP-76 FFY, and Y145F in SLP-76 YYF (Fig. 2A). The FFY mutant was specifically chosen because it might be uncoupled from Vav (14, 45) yet retain the ability to bind Itk via Y145 (6). Surface FcɛRI expression in BMMC reconstituted with each of the constructs and sorted for GFP expression was comparable to that of WT BMMC (Fig. 2B). The level of expression of SLP-76 mutant proteins was verified by Western blotting and was similar to or higher than SLP-76 expression in WT BMMC (Fig. 2C).
FIG. 2.
Reconstitution of SLP-76−/− BMMC with SLP-76 and SLP-76 mutants. (A) Schematic representation of SLP-76 mutants. (B) IgE receptor expression on BMMC reconstituted with SLP-76 constructs. Cells were labeled as described in the legend to Fig. 1. Levels of FcɛRI expression on WT, KO, and control vector- and WT SLP-76-transduced cells, are shown in Fig. 1A. (C) SLP-76 protein expression in WT, KO, and transduced BMMC as assessed by immunoblotting (Western blotting [WB]) with rabbit anti-SLP-76 antiserum. The membrane was reprobed with anti-PLC-γ2 to control for loading.
FcɛRI-mediated β-hexosaminidase release.
Reconstitution with either SLP-76 Δ2-156 or SLP-76 Δ224-244, completely failed to restore the capacity of SLP-76−/− BMMC to release β-hexosaminidase after FcɛRI ligation (Fig. 3A). In contrast, β-hexosaminidase release was substantially restored by SLP-76 ΔSH2 (to 77% of WT reconstituted BMMC at 10 μg of anti-IgE per ml; n = 3). These results suggest that both the N-terminal domain and the Gads binding site of SLP-76, but not its SH2 domain, are essential for FcɛRI-mediated mast cell granule exocytosis.
FIG. 3.
IgE-mediated in vitro degranulation and IL-6 release of BMMC reconstituted (reconst.) with SLP-76 constructs. (A) β-Hexosaminidase release of BMMC with mutant construct is shown in comparison with BMMC transduced with WT SLP-76. The results represent the mean ± standard deviation of three experiments. Cells were sensitized and stimulated as described in the legend to Fig. 1C. *, P < 0.05; **, P < 0.01; ***, P < 0.001, as determined by analysis of variance. (B) BMMC were sensitized and stimulated as described in the legend to Fig. 1E, and secreted IL-6 was quantitated 24 h later. The results shown represent the mean ± standard deviation of four experiments. **, P < 0.01; ***, P < 0.001. ns, not significant as determined by Student's t test compared to SLP-76.
SLP-76 Δ2-156 lacks the tyrosine residues that have been shown to play an important role in signaling via the TCR. We assessed the role of these residues in FcɛRI signaling by examining the capacity of SLP-76 FFF, SLP-76 FFY, and SLP-76 YYF mutants to restore degranulation in SLP-76-deficient BMMC. SLP-76 FFF and SLP-76 YYF only partially restored the capacity of SLP-76−/− BMMC to release β-hexosaminidase following FcɛRI ligation (to 43 and 32% of WT reconstituted BMMC, respectively, at 10 μg of anti-IgE per ml; n = 3). In contrast, SLP-76 FFY substantially restored β-hexosaminidase release (to 78% of WT reconstituted BMMC, at 10 μg of anti-IgE per ml; n = 3) (Fig. 3A). These results suggest that Y145 plays an important role in FcɛRI-mediated granule exocytosis.
IL-6 production.
IL-6 secretion remained severely impaired following reconstitution with SLP-76 Δ2-156 and SLP-76 Δ224-244, but was partially restored following reconstitution with SLP-76 ΔSH2 (to 42% of WT reconstituted BMMC; n = 4) (Fig. 3B). These results suggest that both the N-terminal domain and the Gads binding site of SLP-76 are essential for FcɛRI-mediated IL-6 secretion by mast cells. However, the SH2 domain is required for optimal IL-6 secretion.
IL-6 secretion was restored to a large extent by all three Y-to-F mutants: SLP-76 FFF, SLP-76 FFY, and SLP-76 YYF (to 65, 72, and 83%, respectively, of IL-6 secretion by BMMC reconstituted with WT SLP-76; n = 4) (Fig. 3B). This suggests that phosphorylation of the SLP-76 N-terminal tyrosine residues is not critical for FcɛRI-mediated IL-6 secretion.
Calcium mobilization.
The rapid rise in intracellular calcium concentration that follows FcɛRI engagement is impaired in SLP-76−/− BMMC (44). Calcium mobilization in SLP-76 Δ2-156- and in SLP-76 Δ224-244 BMMC remained indistinguishable from that of SLP-76−/−-transduced BMMC (Fig. 4). In contrast, BMMC transduced with SLP-76 ΔSH2 responded similarly to WT BMMC (Fig. 4). These results suggest that the N-terminal domain and the Gads binding site of SLP-76, but not its SH2 domain, are essential for FcɛRI-mediated calcium mobilization in mast cells.
FIG. 4.
Calcium mobilization in reconstituted BMMC in response to FcɛRI ligation. Change of fluorescence of the calcium-sensitive dye indo 1-AM was monitored for the indicated time. IgE-sensitized cells were stimulated with F(ab′)2 anti-rat Ig and ionomycin at the indicated time points (▴). BMMC reconstituted (reconst.) with a mutant construct are shown in comparison with SLP-76−/− (KO) or WT BMMC analyzed in parallel. Results are expressed as percentage of ionomycin-induced calcium mobilization. Similar results were obtained in at least five experiments for each of the mutants.
BMMC reconstituted with SLP76 FFF exhibited decreased calcium flux following FcɛRI ligation. The calcium response was restored by SLP-76 YYF and SLP-76 FFY. These results suggest that tyrosine phosphorylation of tyrosine residues in the N-terminal domain of SLP-76 is required for FcɛRI-mediated calcium fluxes, but that the function of Y112 and Y128 is redundant with that of Y145.
Tyrosine phosphorylation of SLP-76 and coimmunoprecipitation with btk and Vav.
FcɛRI ligation induces the rapid tyrosine phosphorylation SLP-76 (44). Figure 5A shows that FcɛRI ligation, resulted in tyrosine phosphorylation of WT SLP-76 and, to a lesser extent, the three SLP-76 Y-to-F mutants (WT>YYF>FFY>FFF), indicating that Y112, Y128, and Y145 are the major sites of tyrosine phosphorylation following FcɛRI ligation.
FIG. 5.
Tyrosine phosphorylation of PLC-γ in response to FcɛRI ligation. (A) Tyrosine phosphorylation of SLP-76 and coimmunoprecipitation with btk and Vav. BMMC were sensitized with rat IgE (2.5 μg of rat IgE per ml) followed by cross-linking with F(ab′)2 anti-rat Ig (10 μg/ml) and incubated for 2 min at 37°C. Cell lysates were immunoprecipitated (IP) with rabbit anti-SLP-76 antiserum. Membrane was successively probed with anti-pTyr, anti-btk, anti-SLP-76, and anti-Vav antibodies. The degree of association of SLP-76 with btk and Vav after stimulation was normalized to the signal for SLP-76 as determined by densitometry. Similar results were obtained in two experiments. (B) Tyrosine phosphorylation of PLC-γ1 and PLC-γ2 in WT and SLP-76−/− BMMC. BMMC were sensitized with IgE and stimulated for 7 min as described above. Cell lysates were immunoprecipitated with anti-PLC-γ1 and anti-PLC-γ2. PLC-γ1/2 tyrosine phosphorylation was analyzed by immunoblotting with antiphosphotyrosine antibody (4G10). The membranes were reprobed with anti-PLC-γ1 and anti-PLC-γ2 to control for loading. (C) Tyrosine phosphorylation of PLC-γ2 in SLP-76-reconstituted BMMC. The top two sets of lanes represent a single experiment with cells stimulated simultaneously and then processed in parallel. The bottom set of lanes represents a separate experiment for SLP-76 YYF. Membranes were reprobed with anti-PLC-γ2 to control for loading. Similar results were found in three different experiments. Fold induction normalized to signal for loading was determined by densitometry.
Y112 and Y128 have been reported to be important for the association of Vav with SLP-76, and Y145 has been shown to be implicated in the association of the Tec family kinase Itk with SLP-76 following TCR ligation (6, 40, 53, 55). There was a baseline association of Vav and btk with WT SLP-76 in mast cells, as previously described for Vav and Itk in Jurkat T cells (53, 55). FcɛRI ligation resulted in increased association of Vav with WT SLP-76 and SLP-76 YYF, but not with SLP-76 FFY or SLP-76 FFF. FcɛRI ligation resulted in a modest increase in the association of the Tec family kinase btk with WT SLP-76 and the SLP-76 FFY mutant and a weaker increase in the association of btk with SLP-76 YYF and SLP-76 FFF. These results suggest that Y112 and Y128 are important for the increased association of Vav with SLP-76 and that Y145 is important for the increased association of btk with SLP-76 after FcɛRI ligation in mast cells. However, contributions from additional residues or domains of SLP-76 to its association with Vav and btk cannot be ruled out.
Tyrosine phosphorylation of PLC-γ2.
Both PLC-γ1 and PLC-γ2 become rapidly tyrosine phosphorylated after ligation of FcɛRI in mast cells (61). Phosphorylation of PLC-γ1 was reduced and phosphorylation of PLC-γ2 was virtually absent in SLP-76−/− BMMC after FcɛRI ligation (Fig. 5B). This decrease in PLC-γ phosphorylation did not simply reflect a global decrease in protein tyrosine phosphorylation as total protein tyrosine phosphorylation following FcɛRI ligation in SLP-76−/− mast cells is indistinguishable from that in WT mast cells (44).
We specifically focused on the capacity of SLP-76 mutants to restore PLC-γ2 phosphorylation. SLP-76 Δ2-156 and SLP-76 Δ224-244 failed to restore PLC-γ2 phosphorylation, whereas a modest increase in PLC-γ2 phosphorylation was detected in several independent experiments in BMMC reconstituted with SLP-76 ΔSH2 (Fig. 5C). These results suggest that the N-terminal domain and the Gads binding site of SLP-76 are essential for FcɛRI-mediated PLC-γ2 activation.
Reconstitution with SLP76 FFF, SLP-76 FFY, and SLP-76 YYF resulted in normal phosphorylation of PLC-γ2 following FcɛRI cross-linking (Fig. 5C) suggesting that phosphorylation of Y112, Y128, and Y145 may not be critical for FcɛRI-mediated PLC-γ2 activation in mast cells.
MAPK activation.
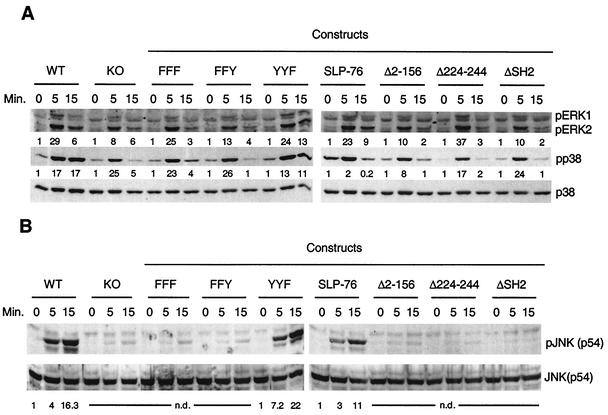
Phosphorylation of ERK1/2 and p38 after FcɛRI cross-linking was modestly reduced and less sustained in SLP-76−/− BMMC than in WT BMMC (Fig. 6A). In contrast, phosphorylation of p54 JNK after FcɛRI cross-linking was severely reduced in SLP-76−/− BMMC (Fig. 6B). This suggests that SLP-76 is essential for JNK activation, but not for ERK1/2 and p38 activation.
FIG. 6.
Activation of MAPKs in response to FcɛRI ligation in BMMC. BMMC were stimulated for the indicated times and lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Aliquots of the lysates were analyzed in parallel for phosphorylation of ERK1/2 and p38 (A) and SAPK/JNK (B) by Western blotting with the corresponding phosphospecific antibodies. The two sets of lanes in panels A and B represent the same experiment with cells stimulated simultaneously and then processed in parallel. Membranes were reprobed with kinase-specific antibodies to control for loading. Fold induction normalized to signal for loading was determined by densitometry. n.d., not determined.
Reconstitution with SLP-76 Δ2-156, SLP-76 Δ224-244, and SLP-76 ΔSH2 failed to rescue the defect in MAPK phosphorylation after FcɛRI ligation. However, SLP-76 YYF, but not SLP-76 FFF or SLP-76 FFY, restored the phosphorylation of ERK, p38, and JNK to normal. This suggests that phosphorylation of the tyrosine residues Y112 and Y128 in the N-terminal domain of SLP-76 is critical for MAPK activation in mast cells.
DISCUSSION
In this study, we used SLP-76−/− BMMC retrovirally transduced with SLP-76 mutants to address the structural requirements for SLP-76 in signaling via FcɛRI. The results obtained are summarized in Table 1. They demonstrate a differential role for SLP-76 domains in mast cell function and a critical role of residue Y145 in IgE-mediated degranulation.
TABLE 1.
Summary of IgE-mediated mast cell activation events in SLP-76−/− BMMC reconstituted with SLP-76 mutants
| SLP-76 | Degranulation | IL-6 production | Ca2+ mobilization | Phosphorylation
|
|
|---|---|---|---|---|---|
| PLC-γ2 | JNK | ||||
| WT | ++++ | ++++ | ++++ | ++++ | ++++ |
| KO | − | − | + | − | − |
| Δ2-156 | − | − | + | − | − |
| Δ224-244 | − | − | + | − | − |
| ΔSH2 | +++ | ++ | ++++ | ++ | − |
| FFF | + | +++ | ++ | ++++ | − |
| FFY | +++ | +++ | ++++ | ++++ | − |
| YYF | + | +++ | ++++ | ++++ | ++++ |
SLP-76−/− mast cells transduced with a SLP-76 deletion mutant lacking the N-terminal domain (SLP-76 Δ2-156) were indistinguishable from SLP-76−/− mast cells in all FcɛRI-mediated activation events we tested. The critical role of the N-terminal SLP-76 domain in mast cell activation is consistent with its requirement for T-cell development and activation. Transgenic expression of SLP-76 Δ2-156 in SLP-76−/− mice failed to rescue the block in thymocyte development from the DP to DN stage (30). Expression of the same mutant in SLP-76-deficient Jurkat J14 cells did not reconstitute NFAT activation following stimulation with soluble anti-CD3 monoclonal antibody (57).
A major contribution to the function of the SLP-76 N-terminal domain in T cells is made by tyrosine residues Y112, Y128, and Y145, which become phosphorylated after TCR stimulation (15). FcɛRI ligation resulted in tyrosine phosphorylation of WT SLP-76 and, to a lesser extent, the three SLP-76 Y-to-F mutants (WT>YYF>FFY>FFF), indicating that Y112, Y128, and Y145 are the major sites of tyrosine phosphorylation of SLP-76 following FcɛRI ligation in mast cells. The SLP-76 FFF mutant in which all three tyrosine residues are substituted for with phenylalanine partially reconstituted β-hexosaminidase release and calcium fluxes and substantially reconstituted IL-6 release, whereas JNK kinase phosphorylation remained impaired. The fact that calcium fluxes were reduced in the face of normal PLC-γ2 phosphorylation is not entirely surprising, because the degree of global phosphorylation of PLC-γ may not always correlate with its activity (16). Taken together with the complete failure of SLP-76 Δ2-156 to reconstitute FcɛRI signaling in SLP-76−/− BMMC, the findings with SLP-76 FFF suggest that residues or motifs in addition to tyrosines in the N-terminal domain of SLP-76 may contribute to its adapter function in mast cells. This is supported by recent reports that SLP-76 FFF partially restores NF-κB and NFAT signaling in SLP-76-deficient Jurkat T cells (22, 57). Furthermore, reconstitution of SLP-76−/− mice with SLP-76 FFF mutant partially rescues T-cell development (26, 39); however, the function of the T cells in these mice is severely impaired. In contrast to our findings in mast cells, reconstitution with SLP-76 FFF completely fails to correct the defect in calcium mobilization and PLC-γ phosphorylation in response to TCR ligation. Furthermore, T cells from SLP-76−/− mice reconstituted with SLP-76 FFF fail to cluster their TCR and proliferate in response to anti-CD3 (39).
In an attempt to further define the roles of individual N-terminal domain tyrosine residues in FcɛRI-mediated activation of mast cells, we reconstituted SLP-76−/− BMMC with two mutants, SLP-76 FFY and SLP-76 YYF. These two mutants were chosen on the basis of the observations in T cells that Y112 and Y128 (both within pYESP motifs), which are mutated in SLP-76 FFY, upon phosphorylation mediate the binding of SLP-76 to Vav and Nck (5, 10, 54-56), whereas Y145 (pYEPP motif), which is mutated in SLP-76 YYF, is implicated in the association of SLP-76 and the Tec family kinase Itk (6, 53). Our results suggest that Y112 and Y128 are important for the increased association of Vav with SLP-76, and that Y145 is important for the increased association of btk with SLP-76 after FcɛRI ligation in mast cells. Granule exocytosis was restored by the SLP-76 FFY mutant, but not by the SLP-76 YYF mutant. This suggests that Y145 is critical for degranulation, possibly by recruiting btk or other Tec family kinases to the SLP-76-Gads-LAT-PLC-γ complex. This hypothesis is supported by the finding that degranulation is impaired in mast cells from btk-deficient mice (20). Vav, which has been shown to associate with SLP-76 YYF (14, 45), but not with SLP-76 FFY, is important for IgE-mediated exocytosis. Our results suggest that direct association of Vav with SLP-76 may not be required, since SLP-76 FFY restored exocytosis (34). It should be noted that Vav phosphorylation following FcɛRI cross-linking is normal in SLP-76−/− mast cells and that Vav can associate with FcɛRI (44, 51).
Calcium mobilization and PLC-γ2 phosphorylation following FcɛRI ligation were restored by both SLP-76 FFY and SLP-76 YYF. This suggests that Y112 and/or Y128 plays a role in calcium mobilization that is redundant with that of Y145. Y145-mediated recruitment of Tec kinases to the SLP-76-Gads-LAT-PLC-γ complex may explain the capacity of SLP-76 FFY to restore calcium mobilization in SLP-76−/− mast cells. The finding that SLP-76 YYF restores calcium mobilization suggests that interaction of Vav and SLP-76 may be important for this process. The observation that calcium fluxes, but not exocytosis, was restored by SLP-76 YYF suggests that while calcium mobilization is essential for degranulation, other signaling pathways, which are dependent on phosphorylation of the SLP-76 residue Y145, are also involved.
Phosphorylation of JNK was severely reduced in SLP-76−/− BMMC. Expression of SLP-76 YYF, but not SLP-76 FFY, restored JNK phosphorylation. Vav1-deficient BMMC fail to activate JNK after FcɛRI stimulation (34, 52). Restoration of JNK activation by SLP-76 YYF supports the notion that a functional association between SLP-76 and Vav is required for optimal activation of the JNK kinase pathway. In addition, the adaptor protein Nck, which, like Vav, binds to phosphorylated Y113 and Y128 may contribute to JNK activation by recruiting PAK1 to the Vav-SLP-76-containing complex (5, 56, 58). The finding that SLP-76 FFF and SLP-76 FFY restored IL-6 secretion, but not JNK phosphorylation, may reflect the presence of JNK-independent pathways for the induction of FcɛRI-mediated IL-6 production. The activation of such pathways would be dependent on motifs in the N-terminal domain of SLP-76 other than tyrosine residues, since IL-6 secretion is abolished in mast cells reconstituted with SLP-76 Δ2-156.
SLP-76 YYF, but not SLP-76 FFY, restored ERK1/2 and p38 phosphorylation to WT levels, suggesting that optimal activation of the ERK1/2 and p38 pathways may require the association of SLP-76 with Vav and/or Nck. It should be noted that FcɛRI-mediated ERK1/2 and p38 phosphorylation was nevertheless substantial in the absence of SLP-76. In contrast, ERK1/2 activation is undetectable in SLP-76-deficient Jurkat T cells following TCR ligation (59). These differences suggest that FcɛRI, but not the TCR, may be coupled to ERK1/2 activation independently of SLP-76. This could possibly be via LAT/Grb2/Sos (29, 36, 47) and/or Vav, which may be linked to ERK via a Rac-PAK1-MEK cascade (2, 17). However, since LAT and Vav are both phosphorylated normally in SLP-76-deficient T cells and in SLP-76−/− BMMC (44, 59), it is unlikely that these two pathways explain the differential phosphorylation of ERK1/2 in these two cell types. RasGRP family members, which are expressed in both mast cells and T cells, can activate the Ras/MAPK pathway through membrane recruitment by the PLC-γ product diacylglycerol (DAG) (12, 13, 60). PLC-γ1 phosphorylation is observable after FcɛRI ligation in SLP76−/− BMMC, but is undetectable in SLP-76-deficient Jurkat T cells following TCR ligation (59). This suggests that activation of ERK1/2 in SLP76−/− BMMC may be mediated by RasGRP.
SLP-76 Δ224-244, which lacks the Gads binding site, failed to reconstitute all aspects of FcɛRI signaling examined. This suggests an absolute requirement for Gads binding in targeting SLP-76 to signaling complexes in mast cells. However, the same mutant was able to partially restore T-cell development when introduced in the SLP-76−/− background and to partially restore calcium fluxes in the T cells from these mice (30, 39) as well as in SLP-76-deficient Jurkat T cells (4, 38, 57). Thus, while SLP-76 binding to Gads is obligatory for FcɛRI signaling in mast cells, it is in part dispensable for TCR signaling in T cells. Residual signaling in T cells reconstituted with SLP-76 Δ224-244 might result from a Gads-independent recruitment of SLP-76 to LAT and/or GEMs in T cells, as suggested by the partially restored TCR signaling in LAT-deficient T cells expressing a LAT mutant that does not recruit Gads (62).
The SH2 domain of SLP-76 mediates its association with ADAP/FYB/SLAP130 and with HPK1 (11, 37, 48). Our results demonstrate that the SH2 domain of SLP-76 plays a minimal role in FcɛRI-mediated calcium fluxes and degranulation, although one of its ligands, ADAP, has been reported to promote mediator release upon overexpression in RBL-2H3 cell line (18). On the other hand, the SH2 domain of SLP-76 is required for optimal IL-6 release and for JNK activation after FcɛRI ligation, possibly because it may be linked via HPK1 to MAPK pathways (23, 27). In light of the results from the other mutants examined, JNK activation following FcɛRI ligation may require contributions from Y112, Y128, the Gads binding domain and the SH2 domain of SLP-76.
The restricted role of the SH2 domain in FcɛRI-mediated activation is consistent with the observations that deletion or mutation of the SH2 domain has a modest effect on the capacity of SLP-76 to reconstitute TCR signaling and suggest that the SH2 domain functions similarly in mast cells and T cells. SLP-76 ΔSH2 and SLP-76 SH2 domain mutant (R448K) reconstitute T-cell development in SLP-76−/− mice and the T cells of these mice mobilize calcium normally following TCR ligation, although they fail to proliferate (39). Furthermore, the SLP-76 SH2 domain mutant reconstitutes calcium fluxes and NFAT activation in Jurkat T cells (4, 38, 57). It is interesting to note that the phenotype of ADAP-deficient mice resembles that of SLP-76−/− mice reconstituted with SLP-76 ΔSH2, as proximal TCR signaling pathways are normal in T cells from these mice, but proliferation and IL-2 secretion are impaired (19, 22). The role of ADAP and HPK1 in FcɛRI-mediated signaling has not been determined.
The SLP-76 family of adaptor includes SLP-76, Blnk, and Clnk. Clnk is expressed in mast cells and in cytokine-stimulated T cells, but not in resting T cells (18a). Clnk is tyrosine phosphorylated upon FcɛRI cross-linking and associates with PLC-γ, Vav, Grb2, and LAT. More importantly, overexpression of a mutant form of Clnk inhibits FcɛRI-mediated degranulation, calcium mobilization, NFAT activation, and phosphorylation of LAT. Signaling via Clnk may underlie the residual calcium mobilization and PLC-γ1 phosphorylation observed in SLP-76−/− mast cells and may account for some of the differences between the capacity of SLP-76 mutants to reconstitute FcɛRI signaling in mast cells and T-cell function in SLP-76−/− transgenic mice.
IgE-mediated mast cell degranulation plays a very important role in allergic diseases. The critical role of the SLP-76 residue Y145 in mast cell degranulation demonstrated in this study suggests that it may provide a good target for therapeutic interventions aimed at inhibiting the allergic response.
Acknowledgments
We thank A. Flint for sorting GFP-expressing cells. Furthermore, we thank H. Oettgen and M. de la Fuente for critical evaluation of the manuscript.
A.K. received a research fellowship from the Swiss National Science Foundation and an AAAAI Zeneca Research Award. This work was supported by USPHS grants AI-35714, in part by research grant no. 1-FY00-673 from The March of Dimes Birth Defects Foundation, and by research grants from the Hood Foundation and the Baxter, Aventis, and Gentiva Corporations.
REFERENCES
- 1.Asada, H., N. Ishii, Y. Sasaki, K. Endo, H. Kasai, N. Tanaka, T. Takeshita, S. Tsuchiya, T. Konno, and K. Sugamura. 1999. Grf40, a novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J. Exp Med. 189:1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrodia, S., B. Derijard, R. J. Davis, and R. A. Cerione. 1995. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270:27995-27998. [DOI] [PubMed] [Google Scholar]
- 3.Blank, U., C. Ra, L. Miller, K. White, H. Metzger, and J.-P. Kinet. 1989. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature 337:187-189. [DOI] [PubMed] [Google Scholar]
- 4.Boerth, N. J., J. J. Sadler, D. E. Bauer, J. L. Clements, S. M. Gheith, and G. A. Koretzky. 2000. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J. Exp. Med. 192:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bubeck Wardenburg, J., R. Pappu, J. Y. Bu, B. Mayer, J. Chernoff, D. Straus, and A. C. Chan. 1998. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity 9:607-616. [DOI] [PubMed] [Google Scholar]
- 6.Bunnell, S. C., M. Diehn, M. B. Yaffe, P. R. Findell, L. C. Cantley, and L. J. Berg. 2000. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J. Biol. Chem. 275:2219-2230. [DOI] [PubMed] [Google Scholar]
- 7.Clements, J. L., S. E. Ross-Barta, L. T. Tygrett, T. J. Waldschmidt, and G. A. Koretzky. 1998. SLP-76 expression is restricted to hemopoietic cells of monocyte, granulocyte, and T lymphocyte lineage and is regulated during T cell maturation and activation. J. Immunol. 161:3880-3889. [PubMed] [Google Scholar]
- 8.Clements, J. L., B. Yang, S. E. Ross-Barta, S. L. Eliason, R. F. Hrstka, R. A. Williamson, and G. A. Koretzky. 1998. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science 281:416-419. [DOI] [PubMed] [Google Scholar]
- 9.Costello, P. S., M. Turner, A. E. Walters, C. N. Cunningham, P. H. Bauer, J. Downward, and V. L. Tybulewicz. 1996. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene 13:2595-2605. [PubMed] [Google Scholar]
- 10.Crespo, P., K. E. Schuebel, A. A. Ostrom, J. S. Gutkind, and X. R. Bustelo. 1997. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385:169-172. [DOI] [PubMed] [Google Scholar]
- 11.da Silva, A. J., Z. Li, C. de Vera, E. Canto, P. Findell, and C. E. Rudd. 1997. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc. Natl. Acad. Sci. USA 94:7493-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dower, N. A., S. L. Stang, D. A. Bottorff, J. O. Ebinu, P. Dickie, H. L. Ostergaard, and J. C. Stone. 2000. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1:317-321. [DOI] [PubMed] [Google Scholar]
- 13.Ebinu, J. O., S. L. Stang, C. Teixeira, D. A. Bottorff, J. Hooton, P. M. Blumberg, M. Barry, R. C. Bleakley, H. L. Ostergaard, and J. C. Stone. 2000. RasGRP links T-cell receptor signaling to Ras. Blood 95:3199-3203. [PubMed] [Google Scholar]
- 14.Fang, N., and G. A. Koretzky. 1999. SLP-76 and Vav function in separate, but overlapping pathways to augment interleukin-2 promoter activity. J. Biol. Chem. 274:16206-16212. [DOI] [PubMed] [Google Scholar]
- 15.Fang, N., D. G. Motto, S. E. Ross, and G. A. Koretzky. 1996. Tyrosines 113, 128, and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J. Immunol. 157:3769-3773. [PubMed] [Google Scholar]
- 16.Fluckiger, A. C., Z. Li, R. M. Kato, M. I. Wahl, H. D. Ochs, R. Longnecker, J. P. Kinet, O. N. Witte, A. M. Scharenberg, and D. J. Rawlings. 1998. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 17:1973-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frost, J. A., H. Steen, P. Shapiro, T. Lewis, N. Ahn, P. E. Shaw, and M. H. Cobb. 1997. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 16:6426-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng, L., S. Pfister, S. K. Kraeft, and C. E. Rudd. 2001. Adaptor FYB (Fyn-binding protein) regulates integrin-mediated adhesion and mediator release: differential involvement of the FYB SH3 domain. Proc. Natl. Acad. Sci. USA 98:11527-11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Goitsuka, R., H.Kanazashi, H. Sasanuma, Y. Fujimura, Y. Hidaka, A. Tatsuno, C. Ra, K. Hayashi, and D. Kitamura. 2000. A BASH/SLP-76-related adaptor protein MIST/Clnk involved in IgE receptor-mediated mast cell degranulation. Int. Immunol. 12:573-580. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths, E. K., C. Krawczyk, Y. Y. Kong, M. Raab, S. J. Hyduk, D. Bouchard, V. S. Chan, I. Kozieradzki, A. J. Oliveira-Dos-Santos, A. Wakeham, P. S. Ohashi, M. I. Cybulsky, C. E. Rudd, and J. M. Penninger. 2001. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science 293:2260-2263. [DOI] [PubMed] [Google Scholar]
- 20.Hata, D., Y. Kawakami, N. Inagaki, C. S. Lantz, T. Kitamura, W. N. Khan, M. Maeda-Yamamoto, T. Miura, W. Han, S. E. Hartman, L. Yao, H. Nagai, A. E. Goldfeld, F. W. Alt, S. J. Galli, O. N. Witte, and T. Kawakami. 1998. Involvement of Bruton's tyrosine kinase in FcepsilonRI-dependent mast cell degranulation and cytokine production. J. Exp. Med. 187:1235-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendricks-Taylor, L. R., D. G. Motto, J. Zhang, R. P. Siraganian, and G. A. Koretzky. 1997. SLP-76 is a substrate of the high affinity IgE receptor-stimulated protein tyrosine kinases in rat basophilic leukemia cells. J. Biol. Chem. 272:1363-1367. [DOI] [PubMed] [Google Scholar]
- 22.Herndon, T. M., X. C. Shan, G. C. Tsokos, and R. L. Wange. 2001. Zap-70 and slp-76 regulate protein kinase C-theta and NF-kappab activation in response to engagement of CD3 and CD28. J. Immunol. 166:5654-5664. [DOI] [PubMed] [Google Scholar]
- 23.Hu, M. C., W. R. Qiu, X. Wang, C. F. Meyer, and T. H. Tan. 1996. Human HPK1, a novel human hematopoietic progenitor kinase that activates the JNK/SAPK kinase cascade. Genes Dev. 10:2251-2264. [DOI] [PubMed] [Google Scholar]
- 24.Ishiai, M., M. Kurosaki, K. Inabe, A. C. Chan, K. Sugamura, and T. Kurosaki. 2000. Involvement of LAT, gads, and grb2 in compartmentation of SLP-76 to the plasma membrane. J. Exp. Med. 192:847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackman, J. K., D. G. Motto, Q. Sun, M. Tanemoto, C. W. Turck, G. A. Peltz, G. A. Koretzky, and P. R. Findell. 1995. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J. Biol. Chem. 270:7029-7032. [DOI] [PubMed] [Google Scholar]
- 26.Judd, B. A., P. S. Myung, A. Obergfell, E. E. Myers, A. M. Cheng, S. P. Watson, W. S. Pear, D. Allman, S. J. Shattil, and G. A. Koretzky. 2002. Differential requirement for LAT and SLP-76 in GPVI versus T cell receptor signaling. J. Exp. Med. 195:705-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiefer, F., L. A. Tibbles, M. Anafi, A. Janssen, B. W. Zanke, N. Lassam, T. Pawson, J. R. Woodgett, and N. N. Iscove. 1996. HPK1, a hematopoietic protein kinase activating the SAPK/JNK pathway. EMBO J. 15:7013-7025. [PMC free article] [PubMed] [Google Scholar]
- 28.Kinet, J. P., M. H. Jouvin, R. Paolini, R. Numerof, and A. Scharenberg. 1996. IgE receptor (Fc epsilon RI) and signal transduction. Eur. Respir. J. Suppl. 22:116s-118s. [PubMed]
- 29.Koretzky, G. 1997. The role of Grb2-associated proteins in T-cell activation. Immunol. Today 18:401-406. [DOI] [PubMed] [Google Scholar]
- 30.Kumar, L., V. Pivniouk, M. A. de la Fuente, D. Laouini, and R. S. Geha. 2002. Differential role of SLP-76 domains in T cell development and function. Proc. Natl. Acad. Sci. USA 99:884-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law, C. L., M. K. Ewings, P. M. Chaudhary, S. A. Solow, T. J. Yun, A. J. Marshall, L. Hood, and E. A. Clark. 1999. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J. Exp. Med. 189:1243-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, S., C. Cicala, A. M. Scharenberg, and J. Kinet. 1996. The FcɛRIβ subunit functions as an amplifier of FcɛRIγ-mediated cell activation signals. Cell 85:985-995. [DOI] [PubMed] [Google Scholar]
- 33.Liu, S. K., N. Fang, G. A. Koretzky, and C. J. McGlade. 1999. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 9:67-75. [DOI] [PubMed] [Google Scholar]
- 34.Manetz, T. S., C. Gonzalez-Espinosa, R. Arudchandran, S. Xirasagar, V. Tybulewicz, and J. Rivera. 2001. Vav1 regulates phospholipase Cγ activation and calcium responses in mast cells. Mol. Cell. Biol. 21:3763-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolis, B., P. Hu, S. Katzav, W. Li, J. M. Oliver, A. Ullrich, A. Weiss, and J. Schlessinger. 1992. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature 356:71-74. [DOI] [PubMed] [Google Scholar]
- 36.Motto, D. G., S. E. Ross, J. Wu, L. R. Hendricks-Taylor, and G. A. Koretzky. 1996. Implication of the GRB2-associated phosphoprotein SLP-76 in T cell receptor-mediated interleukin 2 production. J. Exp. Med. 183:1937-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musci, M. A., L. R. Hendricks-Taylor, D. G. Motto, M. Paskind, J. Kamens, C. W. Turck, and G. A. Koretzky. 1997. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J. Biol. Chem. 272:11674-11677. [DOI] [PubMed] [Google Scholar]
- 38.Musci, M. A., D. G. Motto, S. E. Ross, N. Fang, and G. A. Koretzky. 1997. Three domains of SLP-76 are required for its optimal function in a T cell line. J. Immunol. 159:1639-1647. [PubMed] [Google Scholar]
- 39.Myung, P. S., G. S. Derimanov, M. S. Jordan, J. A. Punt, Q. H. Liu, B. A. Judd, E. E. Meyers, C. D. Sigmund, B. D. Freedman, and G. A. Koretzky. 2001. Differential requirement for SLP-76 domains in T cell development and function. Immunity 15:1011-1026. [DOI] [PubMed] [Google Scholar]
- 40.Onodera, H., D. G. Motto, G. A. Koretzky, and D. M. Rothstein. 1996. Differential regulation of activation-induced tyrosine phosphorylation and recruitment of SLP-76 to Vav by distinct isoforms of the CD45 protein-tyrosine phosphatase. J. Biol. Chem. 271:22225-22230. [DOI] [PubMed] [Google Scholar]
- 41.Ory, D. S., B. A. Neugeboren, and R. C. Mulligan. 1996. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 93:11400-11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson, E. J., M. L. Woods, S. A. Dmowski, G. Derimanov, M. S. Jordan, J. N. Wu, P. S. Myung, Q. H. Liu, J. T. Pribila, B. D. Freedman, Y. Shimizu, and G. A. Koretzky. 2001. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science 293:2263-2265. [DOI] [PubMed] [Google Scholar]
- 43.Pivniouk, V., E. Tsitsikov, P. Swinton, G. Rathbun, F. W. Alt, and R. S. Geha. 1998. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell 94:229-238. [DOI] [PubMed] [Google Scholar]
- 44.Pivniouk, V. I., T. R. Martin, J. M. Lu-Kuo, H. R. Katz, H. C. Oettgen, and R. S. Geha. 1999. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J. Clin. Investig. 103:1737-1743. [PMC free article] [PubMed] [Google Scholar]
- 45.Raab, M., A. J. da Silva, P. R. Findell, and C. E. Rudd. 1997. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR zeta/CD3 induction of interleukin-2. Immunity 6:155-164. [DOI] [PubMed] [Google Scholar]
- 46.Robinson, A., J. Gibbins, B. Rodriguez-Linares, P. M. Finan, L. Wilson, S. Kellie, P. Findell, and S. P. Watson. 1996. Characterization of Grb2-binding proteins in human platelets activated by Fc gamma RIIA cross-linking. Blood 88:522-530. [PubMed] [Google Scholar]
- 47.Saitoh, S., R. Arudchandran, T. S. Manetz, W. Zhang, C. L. Sommers, P. E. Love, J. Rivera, and L. E. Samelson. 2000. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity 12:525-535. [DOI] [PubMed] [Google Scholar]
- 48.Sauer, K., J. Liou, S. B. Singh, D. Yablonski, A. Weiss, and R. M. Perlmutter. 2001. Hematopoietic progenitor kinase 1 associates physically and functionally with the adaptor proteins B cell linker protein and SLP-76 in lymphocytes. J. Biol. Chem. 276:45207-45216. [DOI] [PubMed] [Google Scholar]
- 49.Schaeffer, E. M., and P. L. Schwartzberg. 2000. Tec family kinases in lymphocyte signaling and function. Curr. Opin. Immunol. 12:282-288. [DOI] [PubMed] [Google Scholar]
- 50.Soneoka, Y., P. M. Cannon, E. E. Ramsdale, J. C. Griffiths, G. Romano, S. M. Kingsman, and A. J. Kingsman. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song, J. S., J. Gomez, L. F. Stancato, and J. Rivera. 1996. Association of a p95 Vav-containing signaling complex with the FcepsilonRI gamma chain in the RBL-2H3 mast cell line. Evidence for a constitutive in vivo association of Vav with Grb2, Raf-1, and ERK2 in an active complex. J. Biol. Chem. 271:26962-26970. [DOI] [PubMed] [Google Scholar]
- 52.Song, J. S., H. Haleem-Smith, R. Arudchandran, J. Gomez, P. M. Scott, J. F. Mill, T. H. Tan, and J. Rivera. 1999. Tyrosine phosphorylation of Vav stimulates IL-6 production in mast cells by a Rac/c-Jun N-terminal kinase-dependent pathway. J. Immunol. 163:802-810. [PubMed] [Google Scholar]
- 53.Su, Y. W., Y. Zhang, J. Schweikert, G. A. Koretzky, M. Reth, and J. Wienands. 1999. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur. J. Immunol. 29:3702-3711. [DOI] [PubMed] [Google Scholar]
- 54.Tuosto, L., F. Michel, and O. Acuto. 1996. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J. Exp. Med. 184:1161-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, J., D. G. Motto, G. A. Koretzky, and A. Weiss. 1996. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity 4:593-602. [DOI] [PubMed] [Google Scholar]
- 56.Wunderlich, L., A. Farago, J. Downward, and L. Buday. 1999. Association of Nck with tyrosine-phosphorylated SLP-76 in activated T lymphocytes. Eur. J. Immunol. 29:1068-1075. [DOI] [PubMed] [Google Scholar]
- 57.Yablonski, D., T. Kadlecek, and A. Weiss. 2001. Identification of a phospholipase C-γ1 (PLC-γ1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-γ1 and NFAT. Mol. Cell. Biol. 21:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yablonski, D., L. P. Kane, D. Qian, and A. Weiss. 1998. A nck-pak1 signaling module is required for T-cell receptor-mediated activation of NFAT, but not of JNK. EMBO J. 17:5647-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yablonski, D., M. R. Kuhne, T. Kadlecek, and A. Weiss. 1998. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science 281:413-416. [DOI] [PubMed] [Google Scholar]
- 60.Yang, Y., L. Li, G. W. Wong, S. A. Krilis, M. S. Madhusudhan, A. Sali, and R. L. Stevens. 2002. RasGRP4, a new mast cell-restricted Ras guanine nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. J. Biol. Chem. 277:25756-25774. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, J., E. H. Berenstein, R. L. Evans, and R. P. Siraganian. 1996. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J. Exp. Med. 184:71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, W., B. J. Irvin, R. P. Trible, R. T. Abraham, and L. E. Samelson. 1999. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int. Immunol. 11:943-950. [DOI] [PubMed] [Google Scholar]