Abstract
Initiation of T-lymphocyte-mediated immune responses involves two cellular processes: entry into the cell cycle (G0→G1) for clonal proliferation and coordinated changes in surface and secreted molecules that mediate effector functions. However, a point during G0→G1 beyond which T cells are committed to enter the cell cycle has not been defined. We define here a G0→G1 commitment point that occurs 3 to 5 h after CD3 and CD28 stimulation of human CD4 or CD8 T cells. Transition through this point requires cdk6/4-cyclin D, since inhibition with TAT-p16INK4A during the first 3 to 5 h prevents cell cycle entry and maintains both naive and memory T cells in G0. Transition through the G0→G1 commitment point is also necessary for T cells to increase in size, i.e., to enter the cellular growth cycle. However, transition through this point is not required for the induction of effector functions. These can be initiated while cells are maintained in G0 with TAT-p16INK4A. We have termed this quiescent, activated state G0(A). Our data provide proof of the principle that entry of T cells into the cell cycle and cellular growth cycles are coupled at the G0→G1 commitment point but that these processes can be uncoupled from the early expression of molecules of effector functions.
Many different cell types in the human body are in a quiescent (G0) state. In order to proliferate, such cells must first enter G1 before progressing through the cell cycle and undergoing division to produce two daughter cells. Progression through the cell cycle is dependent on the integration of mitogenic signals, assessment of cell size, and DNA integrity. There is a point late in G1 beyond which cells no longer need mitogenic stimulation to enter S phase and proliferate, and this is known as the restriction point (42). Commitment to enter the cell cycle from G0 is similarly important, but a point that controls the G0→G1 transition and commitment to enter the cell cycle has not been defined.
T lymphocytes are one of the few available primary cell models commonly used for studying cell cycle entry and progression. The majority of primary peripheral blood T lymphocytes rest in a true G0 state, and these can be activated through their T-cell receptor and CD28 costimulation to undergo both cell cycle progression and functional activation. T-lymphocyte-mediated immune responses involve two simultaneous processes. The first is entry into the cell cycle to expand naive T-lymphocyte clones. As they progress through the cell cycle, cells must increase in size in order that their cellular contents are maintained with each round of cell division. This process has been termed the cellular growth cycle (62). The second process is a change in surface and secreted molecules that mediate effector functions (19), giving rise to differentiation of T lymphocytes into effector or memory phenotype. Activation and clonal expansion of T cells occur normally upon interaction with an antigen-presenting cell. A defined program is thus initiated (55, 56) that leads to T-cell proliferation and the induction of effector molecules, which include cytokines and cell surface receptors (24, 38).
Peripheral blood T cells are maintained in a quiescent state by hypophosphorylated forms of pRb and p130, which are members of the retinoblastoma protein family (reviewed in reference 52). This occurs in part by repressing E2F-regulated genes. Mitogenic stimulation causes activation of a cascade of cyclin-dependent kinase (cdk)-cyclin complexes that phosphorylate and inactivate both pRb and p130. Phosphorylation of pRb is initiated by cdk6/4-cyclin D, and certain sites are known to be phosphorylated preferentially by different cdk's, e.g., S780 and S807/811 by cdk6/4-cyclin D and T821 by cdk2-cyclin E. Once pRb and p130 are hyperphosphorylated, E2F transcription factors are released from inhibition. This allows the transcription of a number of genes, such as cyclin D3, cdc2, PCNA, Cdc6, and Mcm2 to Mcm6, which are required for regulating the cell cycle and for initiating DNA synthesis.
In the study presented here we sought to analyze the molecular mechanisms involved in controlling the transition from G0 to early G1 (G1A) and to determine whether the proliferation and differentiation of human T cells are separable. Therefore, we needed to express recombinant proteins in quiescent T cells without genetic modification or prior stimulation that might affect the cells. To do this, we chose to use TAT-fusion protein transduction. This method is based on the fact that the human immunodeficiency virus TAT protein can cross cell membranes and that fusing a short sequence from TAT (YGRKKRRQRRR) to another protein is sufficient to take it into a cell. This has been shown to occur in both quiescent and proliferating cells (47, 59). Different lineages of primary hematopoietic cells are transduced rapidly and close to 100% of T lymphocytes are maximally transduced within 15 s (N. C. Lea et al., unpublished data). Furthermore, this method works for a large number of different proteins of up to 125 kDa (β-galactosidase), including proteins involved in cell proliferation, such as pRb and the cdk inhibitors p16INK4A and p27Kip1 (2, 15, 39, 41).
We show here that there is a commitment point in human peripheral blood T cells 3 to 5 h after CD3/CD28 stimulation that regulates the transition from G0 into G1. Entry into G1 from G0 requires cdk6/4-cyclin D activity since inhibition with the cdk6/4-cyclin D inhibitor protein, p16INK4A, coupled to TAT (15, 18) during the first 3 to 5 h of mitogenic stimulation stopped T cells from entering the cell cycle. Cells transduced with TAT-p16INK4A >5 h after stimulation progressed normally into G1. Transduction of quiescent (G0), nonactivated T cells with TAT-p16INK4A maintains these cells in G0, even when these cells are stimulated with CD3/CD28 or phorbol myristate acetate (PMA)-ionomycin, and prevents the increase in cell size that normally accompanies transition through G1. In spite of this, a number of effector molecules, including cytokines and cell surface proteins, are expressed normally in these quiescent cells. Furthermore, we show that CD4+ naive T cells become activated even when they are maintained in G0 by TAT-p16INK4A. Thus, our data prove the principle that there is a G0→G1 commitment point that controls entry into the cell cycle and cellular growth cycle and that early induction of effector functions does not require transition through this point.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells were obtained from normal donors by density-gradient separation. Nonactivated T lymphocytes were purified by negative selection with immunomagnetic beads conjugated to anti-CD14, anti-CD16 (a and b), anti-CD56, and anti-HLA class II (DR and DP) (all from Dynal, Oslo, Norway) according to manufacturer's instructions. CD4+ and CD8+ T cells were then isolated by selection with anti-CD8 immunomagnetic beads (Detachabead; Dynal). The unbound cells were CD4 positive, and the CD8 cells were released from the beads prior to use (typically >97% CD4+ or CD8+; n = 3). Naive CD4+ CD45RA+ T cells were isolated from nonactivated T-lymphocyte preparations by depleting the CD8+ and CD45RO+ T cells (typically >98% CD8− CD45RO−; n = 3).
T-cell stimulation.
T cells isolated as described above were seeded at 1 × 106/ml or 4 × 106/ml in RPMI 1640-10% (vol/vol) fetal calf serum. They were stimulated with PMA (10 ng/ml) and ionomycin (1 μg/ml) or by CD3 and CD28 (CD3/CD28) costimulation (30, 58) by adding anti-CD3/CD28 magnetic beads (0.5 bead/cell; Dynal). Transient CD3/CD28 stimulation was carried out by adding the CD3/CD28 beads for the times indicated, and the beads were then dissociated from the cells by pipetting them with a micropipette (p1000; Gilson). The dissociated beads were removed twice with a magnet (Dynal), and the cells were then cultured in fresh medium for the times indicated.
Flow cytometric cell cycle analysis of intracellular cytokine and cell surface antigen expression.
T lymphocytes at 1 × 106 or 4 × 106/ml in RPMI 1640-10% fetal calf serum were stimulated with PMA (10 ng/ml) and ionomycin (1 μg/ml) for 24 h, and cell surface CD69, CD44, and CD62L were detected with fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated antibodies (Becton Dickinson, Heidelberg, Germany). For the analysis of cytokine production, sodium monensin (1.4 mg/ml; Sigma, Dorset, United Kingdom) was added for the last 4 h, and labeling with conjugated antibodies to interleukin-2 (IL-2)-PE or gamma interferon (IFN-γ)-PE (Becton Dickinson) was performed by using a commercially available permeabilization kit (Fix and Perm; Caltag Laboratories, Burlingame, Calif.). Analysis of the DNA and total cell protein content was carried out as described previously (11, 51). Few apoptotic cells with <2n DNA content were observed in any of the experiments during the time periods shown (<11% up to 48 h). Cell viability was also confirmed by trypan blue exclusion.
CFSE staining.
Human T cells were loaded with 5-(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) as described previously (57), except that CFSE was used at a final concentration of 1 μM and the CFSE-loaded T cells were left overnight before use. We note that 1 μM CFSE is not toxic to T cells kept in culture for 4 days and that data obtained with CFSE-loaded T cells that were used immediately were the same as with T cells kept overnight.
Protein lysates and Western blotting.
T lymphocytes were stimulated with PMA-ionomycin or CD3/CD28 beads for the times shown, and Western blotting was carried out with 4 to 12% Bis-Tris or 6% Tris glycine-sodium dodecyl sulfate (SDS) gels (Invitrogen-Novex). Chromatin-bound and soluble protein preparations were prepared as previously described (28). The antibodies used here were Mcm2 and Mcm5 (49, 50); Mcm2 (BM28; Transduction Laboratories, Becton Dickinson); Mcm3 (N19), cdc2 (17), Cdc6 (C19), cdk4 (H22), cdk6 (C21), c-myc (9E10), p130 (C20), p107 (C18), cyclin D2 (C17), and cyclin D3 (C16) (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.); cyclin D2 (Ab4; DCS3.1 and DCS5.2; Neomarker, Fremont, Calif.); phospho-pRb (S780 and S807/811; New England BioLabs, Hertfordshire, United Kingdom) (T821; Biosource, Camarillo, Calif.); or pRb (PMG3245; Becton Dickinson).
In vitro kinase assays.
cdk activity assays were performed essentially as described previously (8), with some modifications. A total of 5 × 106 T cells were centrifuged, resuspended in HB lysis buffer (50 mM HEPES, pH 7.4; 150 mM NaCl; 20 mM EDTA; 1 mM dithiothreitol; 1× Complete protease inhibitor cocktail [Roche Diagnostics, Ltd., East Sussex, United Kingdom]; 10 mM NaF; 1× phosphatase inhibitor cocktail I [Sigma-Aldrich, Ltd., Dorset, United Kingdom]; 0.5% [vol/vol] Trition X-100), and mixed end over end for 20 min at 4°C. Cell debris was removed by centrifugation, and the lysate was precleared with protein A-agarose and goat serum. cdk immunoprecipitates were prepared with agarose-conjugated anti-cdk2 (M2), anti-cdk4 (H22), and anti-cdk6 (C21) (all from Santa Cruz Biotechnology). After extensive washes with HB, immunoprecipitates were equilibrated in kinase buffer (50 mM HEPES, pH 7.4; 1 mM MnCl2; 10 mM MgCl2; 1 mM dithiothreitol; 1× Complete EDTA-free protease inhibitor cocktail; 10 mM NaF; 1× phosphatase inhibitor cocktail I). Each immunoprecipitated cdk was then incubated for 20 min at 30°C in 40 μl of 1× kinase buffer containing 0.5 μg of pRB substrate (maltose-binding protein-pRB; Santa Cruz Biotechnology), 10 μM ATP, and 0.2 μCi of [γ-32P]ATP/μl. Some assays with cdk2 also contained 0.5 μg of histone H1 as the substrate (Roche Diagnostics). Reactions were stopped by the addition of 6× SDS-sample loading buffer and separated on 4 to 12% polyacrylamide Bis-Tris gels. Proteins were transferred to nitrocellulose membranes (Hybond C-Extra; AP Biotech), which were then exposed to X-ray film. The 32P-labeled pRb signal was quantified by scanning densitometry. The presence of cdk and cyclins in each immunoprecipitate was determined on the same membranes by Western detection (ECL Plus) with the antibodies described above.
Preparation of TAT fusion proteins.
Clones encoding TAT-p16INK4A, TAT-p16INK4A(MUT), TAT-green fluorescent protein (GFP), and TAT-β-galactosidase (from S. Dowdy, St. Louis, Mo.) were expressed, and the proteins were produced as described previously (15, 18).
Transduction with TAT fusion proteins.
T lymphocytes were transduced by adding the appropriate TAT protein to the medium to a final concentration of 100 to 2,000 nM before the addition of PMA and ionomycin. For each preparation of TAT-p16INK4A, the minimum concentration that inhibited pRb phosphorylation at S807/811 was determined (typically 200 to 300 nM), and twice that concentration was used in subsequent experiments. Experiments with TAT-GFP showed that TAT-conjugated proteins enter almost all primary T cells in the culture within 15 s (>98% of cells; Lea et al., unpublished). Cells transduced with TAT-GFP were detected by flow cytometry and confocal microscopy of FITC-TAT-β-galactosidase showed that the protein entered into cells and was not simply bound to the surface (data not shown).
RESULTS
Defining the G0-to-G1 transition in human primary T lymphocytes.
Nonactivated human T cells were isolated from peripheral blood. These HLA class II (DP and DR) negative cells are small and have 2n DNA and a low protein content. Stimulation with anti-CD3/CD28 beads caused an increase in the cellular protein content (i.e., cell size) between 8 and 16 h (FITC staining; Fig. 1A, y axis) without an increase in DNA content (propidium iodide [PI] staining; Fig. 1A, x axis), a finding consistent with entry into the growth cycle (34, 44, 62). The cells then entered S phase by between 30 and 48 h (Fig. 1A). Staining with CFSE (43) showed that <1% of the cells had divided by 24 h and that 16.4% had divided by 48 h. At 72 h, 36% had divided once and <1% had divided twice (data not shown). These kinetics of cell cycle entry and proliferation are slower than those reported for murine T cells, which can undergo multiple rounds of cell division between 24 and 48 h after stimulation, with a cell cycle time as short as 5 to 6 h (57).
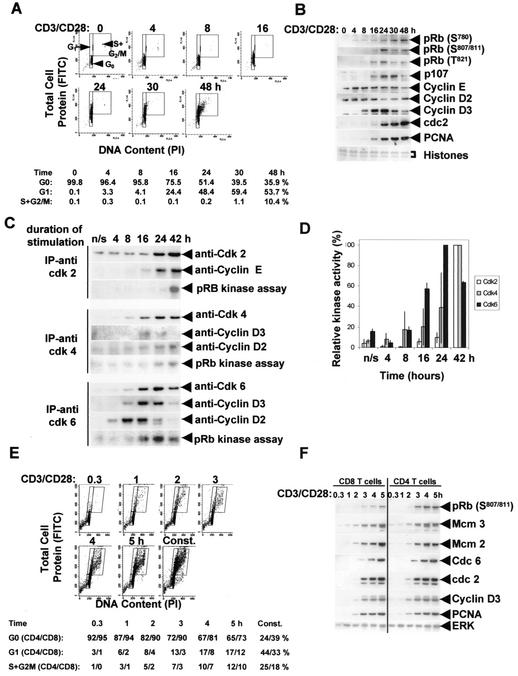
FIG. 1.
Identifying a G0→G1 commitment point in nonactivated human T cells stimulated with CD3/CD28. (A) Samples were taken at the times shown after CD3/CD28 stimulation, and the cellular DNA (PI) and protein (FITC) content were determined by flow cytometry. Cell size and protein content increase as cells enter and traverse G1, and the percentages in the G0, G1, and S+G2/M phases are shown. (B) The kinetics of pRb phosphorylation and the induction of proteins encoded by several E2F-regulated genes during cell cycle entry were determined by Western blotting, with samples from the same experiment as shown in panel A. Antibodies used recognize phosphorylation at the cdk6/4-cyclin D-specific sites S780 and S807/811, as well as a site phosphorylated by cdk2-cyclin E, T821. Proteins of <30 kDa (mainly histones) were stained with Coomassie blue as a loading control. (C and D) In vitro kinase assays. cdk6, cdk4, and cdk2 were immunoprecipitated at the times shown after CD3/CD28 stimulation, and their activities were assayed with recombinant pRb as the substrate. The 32P-labeled pRb was separated by gel electrophoresis, transferred to a nitrocellulose membrane, and detected by exposure to film. The assays were carried out with three independent T-cell preparations, and each assay was consistent with the data shown in panel C. Complete time courses were available for two of the experiments, and these are quantified in panel D (mean ± range). After autoradiography, the membranes were subjected to Western blotting for cyclin D2, D3, and E in order to detect the association of these cyclins with the relevant cdk at each time point (C). (E and F) The CD4+ and CD8+ T-cell subsets were stimulated transiently with CD3/CD28 for the times shown. Samples were obtained at 48 h, and the cellular DNA and protein content (E), as well as the pRb phosphorylation and E2F-dependent protein expression (F), were evaluated. In panel F, blots were probed for ERK as a loading control. In panel E, data for CD8 cells are shown with the percentages in the G0, G1, and S+G2/M phases for both the CD4 and the CD8 subsets. The data are representative of three separate experiments.
Analysis of the kinetics of activation of cell cycle proteins in human T cells showed that pRb was phosphorylated at the cdk6/4-cyclin D-specific site S780 by 4 to 8 h and at the S807/811 site by 8 to 16 h after stimulation. Cyclin D2 is present in quiescent T cells, but they do not express cyclin D3, which is induced by 8 h after stimulation (Fig. 1B). In contrast, cyclin D1 is not detectable even after stimulation (not shown). Therefore, phosphorylation of pRb is probably initiated by cyclin D2-cdk6/4. The phosphorylation of pRb at a site phosphorylated by cdk2-cyclin E, i.e., T821, was then detectable by 16 h, and proteins encoded by E2F-regulated genes, such as PCNA, cdc2, and p107 were detected by 16 to 24 h (Fig. 1B). Similar data were obtained when cells were stimulated with PMA-ionomycin (not shown).
Next we performed in vitro kinase assays to gauge the kinetics of cdk6, cdk4, and cdk2 activation after T-cell stimulation. We also probed the immunoprecipitates for the association of each cdk with the appropriate cyclin. These results are shown in Fig. 1C and D. cdk6 coimmunoprecipitates with cyclin D2 beginning at 4 h, peaking at 8 to 16 h and decreasing thereafter. The cyclin D3-cdk6 complex formed later; it was detectable by 8 h, peaked at 16 to 24 h, and then declined. cdk6 is the first of the cdk's to be activated, and its activity was detectable 8 to 16 h after stimulation, peaked at 24 h, and then declined (Fig. 1C and D). We were not able to reliably detect cdk6 activation before this time, possibly due to the inefficiency of this in vitro assay. However, it is clear that cdk6 was activated at least 24 h before cdk2. cdk2 associated with cyclin E by 24 h, and kinase activity was detectable by 42 h. Similar data on the kinetics of cdk2 activation were obtained by using histone H1 as the substrate (data not shown). cdk4 activation occurred after cdk6 and was detectable 24 to 42 h after stimulation.
The data described above are consistent with cells engaging the cell cycle, i.e., entering G1A, as early as 4 h after CD3/CD28 stimulation. If this is the case, these early changes in cell cycle proteins might define a G0→G1 commitment point. For this to be the case, transient stimulation with CD3/CD28 past this point must be sufficient for cells to enter G1. To investigate whether this occurs, we isolated human CD4+ and CD8+ T cells from peripheral blood and stimulated each with CD3/CD28-conjugated beads for 0.3, 1, 2, 3, 4, or 5 h. The beads were then removed (see Materials and Methods), and the cells were cultured in fresh medium with no CD3/CD28. Analyses were carried out at 48 h, by which time the majority of cells that had entered the cell cycle would be expected to be in late G1 or early S phase and pRb phosphorylation and E2F-regulated proteins would be detectable. Cell cycle analyses of DNA and protein content showed that few cells responded to CD3/CD28 stimulated for 0.3 or 1 h, but a proportion of the cells did after 2 to 5 h of stimulation (Fig. 1E). As reported by others, not all T cells respond to CD3/CD28 stimulation, and our data pertain to those that enter the cell cycle most rapidly. Phosphorylation of pRb (assayed at the cdk4/6-cyclin D sites [S807/811]) and induction of E2F-regulated genes encoding cdc2, cyclin D3, and PCNA also required that the cells were stimulated for 2 to 5 h (Fig. 1F). This was also necessary for induction of Cdc6 and minichromosome maintenance (MCM) proteins that are necessary for the formation of DNA replication complexes that “license” cells in G1 to enter S phase (Fig. 1F). Thus, our data show that there is a commitment point 2 to 5 h after CD3/CD28 stimulation that regulates the transition from G0 to G1 and that progression through this transition coincides with phosphorylation of pRb at cdk6/4-cyclin D-dependent sites and allows the induction of E2F-regulated genes. The timing of cyclin D2 association with cdk6, as judged by coimmunoprecipitation experiments (Fig. 1C), coincides with the timing of the commitment point (Fig. 1E and F). Association of cdk6 with cyclin D3, which occurs ∼8 h later (Fig. 1C), coincides with the induction of cyclin D3 (Fig. 1B) and an increase in cell size (Fig. 1A) as cells enter the growth cycle.
TAT-p16INK4A transduction maintains T cells in G0.
The data shown above indicated that activation of cdk6/4-cyclin D2 coincides with progression through the G0→G1 commitment point. If this were the case, inhibiting cyclin D2-cdk6/4 activation would be sufficient to maintain these cells in G0. The p16INK4A protein is a selective cellular cdk inhibitor of cdk4/6 (48), and we used recombinant TAT-p16INK4A as a reagent to inhibit endogenous T-cell cdk4/6-cyclin D. We used, as a control, a TAT-p16MUT that does not bind cdk6 or cdk4 (15). To test whether activation of cdk6/4-cyclin D2 is necessary for G0→G1 commitment, we isolated nonactivated, quiescent, human peripheral blood T lymphocytes; transduced these cells with the cdk6/4-cyclin D inhibitor protein p16INK4A (TAT-p16INK4A) or the inactive TAT-p16MUT control; and stimulated the cells to enter the cell cycle with anti-CD3/CD28 beads. Our results showed that TAT-p16INK4A prevented the phosphorylation of endogenous pRb by cdk6/4-cyclin D, as assayed by the phosphorylation at S807/811. The induction of the E2F-regulated genes encoding cyclin D3, Cdc6, and cdc2 was also inhibited (Fig. 2A). In case such inhibition was specific to receptor-mediated activation, we also determined whether cell cycle entry caused by direct stimulation via PKC and Ca2+ mobilization was also inhibited by TAT-p16INK4A. Again, the phosphorylation of pRb at S807/811 and hyperphosphorylation of p130, which is also cdk dependent, were inhibited and cyclin D3, PCNA, cdc2, and p107 were not induced (Fig. 2B and C). Therefore, regardless of the stimulus, TAT-p16INK4A inhibited cdk6/4-cyclin D activation, pRb and p130 phosphorylation, and E2F-dependent gene activation.
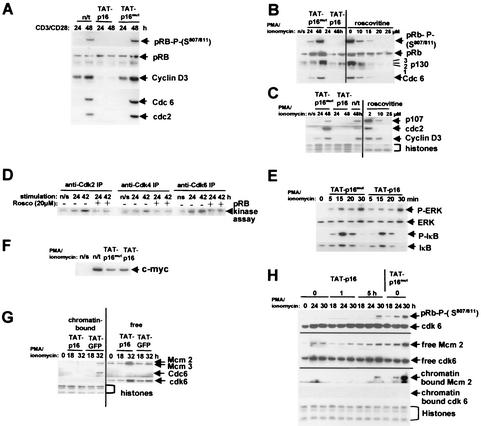
FIG. 2.
pTAT-p16INK4A and roscovitine inhibit the pRb/p130 pathways and prevent transition through the G0→G1 commitment point. (A to C) Nonactivated T cells were transduced with TAT-p16INK4A or TAT-p16MUT (inactive mutant of p16INK4A), or roscovitine was added at the concentrations shown. The cells were stimulated with CD3/CD28 (A) or PMA-ionomycin (B and C), and samples were taken at 24 and 48 h. The pRb phosphorylated on S807/811, the total pRb and p130 proteins, and the E2F-regulated Cdc6, cdc2 p107, and cyclin D3 proteins were analyzed by Western blotting. pRb, total pRb protein; 3, hyperphosphorylated p130; 1,2, hypophosphorylated p130. n/s, not stimulated with CD3/CD28 or PMA-ionomycin. n/t, not transduced with TAT-p16INK4A or TAT-p16MUT. Histones stained with Coomassie blue were a loading control for panel C. (D) Inhibition of cdk activity by roscovitine. T cells were stimulated with CD3/CD28 in the presence (+) and absence (−) of 20 μM roscovitine, and the activity of each cdk was determined by in vitro kinase assays. (E) Western blot of phospho-IκB (S32) and ERK1/2 phosphorylated at T202/Y204. Blots of total cell IκB and ERK1/2 proteins are also shown. (F) Western blot of c-myc 5 h after stimulation. n/s, not stimulated; n/t, stimulated but not transduced with TAT protein. (G) Inhibition of cdk activation prevents the formation of DNA origin recognition complexes. T cells were transduced with TAT-p16INK4A or TAT-GFP (as a control), and extracts of chromatin-bound and free proteins were prepared at the times shown. The presence of Mcm2, Mcm3, and Cdc6 in each sample was determined by Western blotting. Histones and cdk6 are loading controls for chromatin-bound and free proteins, respectively. (H) The T cells were stimulated with PMA-ionomycin and transduced with TAT-p16INK4A or TAT-p16MUT at 0, 1, or 5 h after stimulation. Samples were obtained at 18, 24, and 30 h for the analysis of pRb phosphorylation and chromatin binding of Mcm2. cdk6 was used as a loading control for total cell lysates and for extracts of non-chromatin-bound (free) protein and histones stained with Coomassie blue for chromatin. The data shown in panels B, C, and G are each representative of six separate experiments; the data shown in panels A, E, F, and H are each representative of three experiments, and the data shown in panel D are representative of two experiments.
Next, we determined whether the action of TAT-p16INK4A was specific to cdk's. To this end, we assayed the phosphorylation of signal transduction proteins involved in T-cell activation that are known to be due to other kinases. Phosphorylation of ERK1/2 and IκB occurred with normal kinetics, and IκB was then degraded normally (Fig. 2E). Further, induction of c-myc occurred normally (Fig. 2F). Thus, TAT-p16INK4A inhibited endogenous cdk6/4-cyclin D activity but did not prevent the activation of other kinases involved in T-cell activation or the induction of c-myc.
Because IκB and ERK1/2 were phosphorylated and c-myc was induced normally in TAT-p16INK4A-transduced T cells, we investigated whether such cells enter G1 and S phase in spite of the fact that cdk-dependent pRb and p130 phosphorylation did not occur. This was investigated because transfection of c-myc can induce cell growth and proliferation in the absence of mitogenic stimulation (3) and so might bypass inhibition by pRb and p130. Cells can only enter S phase if the appropriate complex containing ORC, Cdc6, and MCM proteins binds at origins to “license” DNA replication (54). Several MCM proteins (Mcm2, Mcm3, and Mcm5) are expressed at a basal level even in quiescent T cells (Fig. 2G). However, although present, the MCM proteins in T cells only bind chromatin when the cells are in G1, some 24 h after stimulation, and their abundance increases further in late G1 (32 to 48 h; S. J. Orr et al., unpublished data). In contrast, Cdc6 is not present in quiescent T lymphocytes but it is induced upon stimulation and binds chromatin at between 24 and 32 h. This is consistent with the acquisition of replication competence and pre-replication complex formation in the middle to late G1 phase that we have observed in fibroblasts after reentry into the cell cycle (49). We determined that induction and DNA binding of MCM and Cdc6 proteins did not occur in primary T lymphocytes transduced with TAT-p16INK4A (Fig. 2G). Thus, the transduced T cells do not bypass pRb and p130 controls to enter the cell cycle and form DNA replication complexes.
The protein and RNA content of T cells increase as they exit G0 and traverse G1 (34), and cells must attain a critical size before entering S phase and then dividing (44). Inhibition of T cells with TAT-p16INK4A prevented the size increase that normally accompanies the G0-to-G1 transition. This was determined in two ways. The first was staining for the total cell protein content (with FITC) and for DNA content (with PI) (Fig. 3A, y and x axes, respectively). These data showed that T cells remained small with a low total protein content and 2n DNA content in the presence of TAT-p16INK4A. Second, cell size was analyzed indirectly by measuring forward scatter, which showed that TAT-p16INK4A prevented the increase in forward scatter that normally accompanies entry into G1 (Fig. 3B).
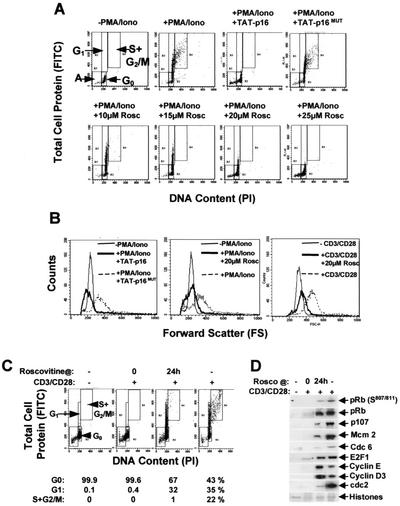
FIG. 3.
TAT-p16INK4A prevents T lymphocytes from entering the cell cycle and increasing in size. Nonactivated T cells were transduced with TAT-p16INK4A or TAT-p16MUT and stimulated for 48 h with PMA-ionomycin (PMA/Iono). Similarly, roscovitine at the concentrations shown was added, and the cells were stimulated with PMA-ionomycin or CD3/CD28. (A) Total cell protein (stained with FITC) and DNA (stained with PI) were quantified by flow cytometry. (B) Forward scatter was used as a measure of cell size. (C and D) Inhibition of cell cycle progression by roscovitine. T cells were stimulated with CD3/CD28, and 20 μM roscovitine was added at 0 or 24 h. Samples were taken at 48 h and assayed for cell cycle progression and the percentages of the cells in G0, G1, and S+G2/M are indicated in panel C. pRb phosphorylation and induction of proteins associated with cell cycle entry were also analyzed in samples from the same experiment (shown in panel D). The data are representative of six (A and B) and two (C and D) separate experiments, respectively.
In order to verify our data with TAT-p16INK4A, we inhibited cdk activation with roscovitine, a compound that also inhibits cdk activity (20, 36). There was a dose-dependent inhibition of pRb and p130 phosphorylation and E2F-dependent gene activation, and complete inhibition occurred at 20 μM in our experimental system (Fig. 2B and C). In vitro kinase assays showed that this concentration of roscovitine significantly inhibited cdk6, cdk4, and cdk2 activities in primary T cells (Fig. 2D). This concentration also inhibited Cdc6 induction (Fig. 2B) and the binding of MCM proteins to chromatin (not shown). In addition, 20 μM roscovitine prevented an increase in cell size, i.e., entry into the growth cycle (Fig. 3A to C), a finding consistent with maintaining the T cells in G0. The cdk inhibitor butyrolactone I (25 μM) also inhibited cdk activation, cell cycle entry, and the increase in cell size, as judged by the same criteria (not shown). The addition of roscovitine 24 h after CD3/CD28 stimulation just before cdk2 becomes active prevented cells from entering S phase (Fig. 3C). This occurred in cells containing cdk6/4-cyclin D-phosphorylated pRb (S807/811), as well as proteins such as Cdc6 and Mcm2 that license cells to enter S phase (Fig. 3D). We have demonstrated therefore that inhibiting cdk6/4-cyclin D activation by different means prevented T cells from engaging the cell cycle, as judged by the inhibition of pRb and p130 phosphorylation, the induction of E2F-regulated genes, and the formation of DNA origin recognition complexes. This also inhibited entry into the cellular growth cycle that controls cell size (62). Inhibition occurs regardless of whether the cells are stimulated with CD3/CD28 or PMA-ionomycin. Furthermore, primary T lymphocytes do not contain cyclin D1 and, since cyclin D3 is not detectable in cells inhibited by TAT-p16INK4A or 20 μM roscovitine (Fig. 2A and C), this implicates activation of cyclin D2-cdk6/4 as rate limiting for the transition of T cells from G0 into G1.
TAT-p16INK4A inhibits G0→G1 commitment.
In order to determine whether cyclin D2-cdk6/4 could be involved in regulating transition through the G0→G1 commitment point, we made use of the fact that TAT proteins enter cells very rapidly. Maximal transduction occurs within 15 s for TAT-GFP (N. C. Lea et al., unpublished), and this allowed us to introduce TAT-p16INK4A into T cells at precise times after stimulation. Nonactivated T cells were stimulated with PMA-ionomycin and transduced with TAT-p16INK4A simultaneously (0 h) or 1 or 5 h later. The cells were then cultured, and samples were taken for Western blot analysis of pRb phosphorylation at 18, 24, and 30 h. The addition of TAT-p16INK4A at 0 or 1 h prevented pRb (S807/811) from being phosphorylated, but addition at 5 h did not (Fig. 2H). We also determined whether the cells entered the cell cycle and formed DNA replication complexes containing chromatin-bound Mcm2. Addition of TAT-p16INK4A at 5 h did not prevent Mcm2 binding to chromatin, whereas addition at 0 or 1 h did (Fig. 2H). Thus, TAT-p16INK4A prevents T cells from progressing from G0 to G1 but only if added less than 5 h after stimulation. The timing coincides with the G0→G1 commitment point and is consistent with a role for cyclin D2-cdk6/4 in regulating the transition from G0 into G1. However, the cells maintained in G0 by TAT-p16INK4A differ from cells that have not been stimulated. For example, c-myc is induced, and ERK1/2 and IκB are phosphorylated. Because of this we redefined this quiescent, activated state as G0(A) for activated cells in G0.
Effector functions are induced in T cells maintained in G0(A).
We have defined a G0→G1 cell cycle commitment point that also determines whether T cells increase in size, i.e., controlling entry into the cell cycle and cellular growth cycle. However, in response to CD3/CD28 or PMA-ionomycin T cells also become functionally activated by the induction of cell surface and intracellular effector molecules. In order to determine whether the G0→G1 commitment point purely couples entry into the cell cycle and growth cycle, we determined whether transition from G0 to G1 was necessary for the induction of proteins associated with T-cell effector functions. Thus, T lymphocytes were transduced with TAT-p16INK4A and stimulated with PMA and ionomycin, and the induction of IFN-γ, IL-2, CD69, and CD44 and the downregulation of CD62L were assayed by flow cytometry. The data show that each is regulated normally whether or not the cells pass through the G0→G1 commitment point (Fig. 4A, compare panels labeled “+PMA/Iono+TAT-p16” to those labeled “+PMA/Iono+TAT-p16MUT”). Note that TAT-p16INK4A prevents T cells from increasing in size (forward scatter) in response to PMA-ionomycin or CD3/CD28 (Fig. 3B). Therefore, induction and downregulation of these effector molecules occur in cells that have the same forward scatter as unstimulated cells (Fig. 4A, compare panels labeled “−PMA/Iono” to those labeled “+PMA/Iono+TAT-p16”). We verified these data by repeating the experiment with T cells maintained in G0(A) with roscovitine (Fig. 4A) or butyrolactone I (data not shown). IL-2, CD69, and CD44 were induced and CD62L was downregulated upon stimulation with CD3/CD28 or PMA-ionomycin, respectively. Roscovitine inhibits ERK activation in vitro at 14 μM (36). However, inhibition of ERK activation in T cells with the MEK1 inhibitor PD98059 prevents the production of IL-2 and IFN-γ (13). Both IL-2 and IFN-γ were produced in T cells in the presence of 20 μM roscovitine (Fig. 4A). Therefore, inhibition of the G0→G1 transition in primary T cells by roscovitine (Fig. 2B and C and 3) was not due to preventing ERK activation. Thus, activation and effector molecules are induced in cells maintained in G0(A) by TAT-p16INK4A or with drugs that inhibit cdk activity and do not require cells to progress through the G0→G1 commitment point.
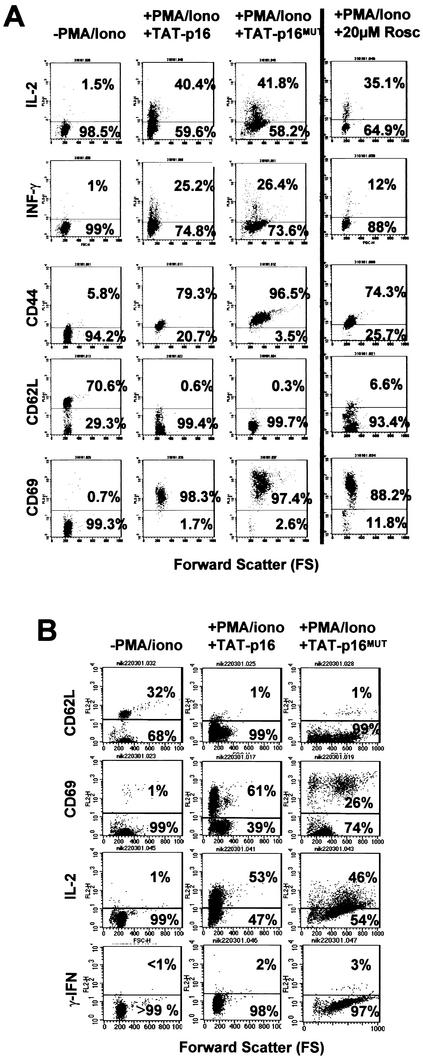
FIG. 4.
Effector molecules are regulated normally in memory and naive T cells in G0(A). (A) T lymphocytes maintained in G0 by cdk inhibition by TAT-p16INK4A or roscovitine (20 μM) produce IL-2 and IFN-γ, upregulate CD69 and CD44, and downregulate CD62L when stimulated for 24 h with PMA and ionomycin. (B) CD4+ naive T cells (95% CD45RO+ and CD8+) also produce IL-2, upregulate CD69, and downregulate CD62L in response to PMA and ionomycin when maintained in G0 by TAT-p16INK4A. The level of IFN-γ produced was below the limits of detection. The data shown are each representative of three separate experiments.
Naive CD4+ T cells become activated and produce IL-2 in G0(A).
The cells that respond in the experiments shown in Fig. 4A will predominantly be memory T cells. The finding that cells that were CD62L+ before stimulation were activated even when cell cycle entry was blocked suggested that cell cycle entry and effector molecule expression might be dissociable also in naive T cells. The results (Fig. 4B) show that purified, naive (CD45RO− CD4+) T cells can also be activated without traversing from G0 to G1, as evidenced by the upregulation of CD69, the downregulation of CD62L, and the induction of IL-2. As expected, we did not detect IFN-γ production within the experimental time period even in the absence of TAT-p16INK4A. This was also true when the cells were stimulated via CD3/CD28 and the production of IFN-γ was assayed by flow cytometry for intracellular cytokines, reverse transcription-PCR, or mRNA enzyme-linked immunosorbent assay (<18 molecules/cell [data not shown]). These data, and those shown earlier in this study, provide proof that a number of activation and effector molecules can be induced in either memory or naive T cells that are in a quiescent, i.e., G0(A), state and that cell cycle entry is not required for their induction.
DISCUSSION
We have shown here that there is a commitment point in human T cells that controls the transition from G0 into the cell cycle and also the cellular growth cycle. Our data suggest that activation of cdk6/4-cyclin D2 is rate limiting for progression through this point and that quiescent cells transduced with TAT-p16INK4A do not enter G1 in response to costimulation via CD3 and CD28 or with PMA-ionomycin. Although entries into the cell and growth cycles appear to be coupled at the commitment point, this does not tie the activation of effector functions to the initiation of T-cell proliferation since transition through G0→G1 is not necessary for the induction of effector functions to be initiated in memory or naive T cells.
We believe that the nonactivated primary T cells used in the present study represent a population of cells that are in G0. They are nondividing cells with a 2n DNA content that are able to enter the cell cycle and divide given the appropriate mitogenic stimulus. As classically defined, G0 cells take longer to enter the S phase for the first time from quiescence than in subsequent cell divisions. This is true for nonactivated T cells stimulated with CD3/CD28. The data in the present study show that, on a population basis, they take 30 to 48 h to enter S phase. Consistent with this timing of G0→G1→S phase, CFSE cell division data indicate that only 16% of cells had divided once by 48 h and 36% had divided once by 72 h. Once proliferating, we have determined by manual cell counting that the fastest population doubling time over a 3- to 4-week period in optimal IL-2 is 1.07 days (25.6 h; doublings three and four; data not shown). Thus, the time taken for one complete cell cycle is far less than the time taken initially to progress from G0 through G1 and into the first S phase. Since there is not a universally accepted criterion that defines that a cell is in G0, we have used a number of assays to define the state of our cells, which are discussed below.
In order to investigate the transition of quiescent T cells into the cell cycle, we first investigated whether there was a CD3/CD28-dependent commitment point that regulated the transition from G0 to G1. In order to do this, we developed a method for transiently costimulating T cells with CD3- and CD28-conjugated magnetic beads so that the stimulus could be applied for a defined time before being removed. By using this method we showed that CD3/CD28 costimulation was required for 3 to 5 h in order for quiescent T cells to enter G1 from G0. Costimulation for this period induced a number of molecular mechanisms involved in cell cycle entry. Both pRb and p130 were phosphorylated, and several E2F-regulated genes, such as those encoding cyclin D3 (60), Cdc6 (22, 40, 61), and PCNA (23), were induced. Such stimulation was also sufficient to induce formation of DNA origin recognition complexes containing Cdc6 and MCM proteins. It should be noted that quiescent T cells do not contain Cdc6 but do contain low levels of MCM proteins. This is different from nonproliferating cells in organs such as the liver, colon, prostate, or brain in which nondividing cells do not express MCM proteins but is similar to mammary luminal epithelial acinar cells of the premenopausal breast (50). As we have suggested previously for these cells, MCM expression in T lymphocytes may be an adaptation that allows rapid periodic cell expansion. Consistent with this, we note that chromatin-bound MCM proteins are detected before they are induced to the levels observed in proliferating cells. However, Cdc6 is necessary for chromatin association of MCM proteins (10, 33, 49, 50), and binding of MCM proteins to DNA in T cells only occurs once Cdc6 is induced. Therefore, T cells that do not receive sufficient CD3/CD28 activation to pass the G0→G1 commitment point do not contain a number of proteins that would make them competent to enter S phase. These include Cdc6, DNA-bound MCM proteins, and PCNA. Similarly, they do not contain regulatory proteins such as cdc2. Each of these proteins is present in proliferating cells that are in G1 phase.
Quiescent, primary T lymphocytes contained cyclin D2 but not cyclin D1 and since cyclin D3 was not detectable, this implicates activation of cyclin D2-cdk6/4 as rate limiting for G0→G1 commitment. Consistent with this, cyclin D2 coimmunoprecipitated with cdk6, was detectable by 4 h after CD3/CD28 stimulation, and was maximal by 8 h. Analysis of cdk activation by in vitro kinase assays showed that cdk6 was the first cdk to become activated and preceded activation of cdk2 by at least 24 h. These data are consistent with our previous work which showed that B cells from cyclin D2−/− animals took longer to enter the cell cycle in response to BCR/CD40 stimulation than cells from normal littermates and coincided with a compensatory induction of cyclin D3 (29). The induction of cyclin D3 in T cells during G1 occurs in response to IL-2 when they are stimulated to proliferate, deprived of IL-2, and then restimulated (7). The same may occur in T cells entering G1 from G0 by autocrine production of IL-2, and our data do not preclude a role for cyclin D3-cdk6/4 activation during the transition through G1. Indeed, cyclin D3 is detected in cdk6 complexes by coimmunoprecipitation as cells progress into and through G1.
We investigated the molecular mechanisms that control transition from G0 to G1 by using TAT protein transduction. This enabled us to introduce active recombinant proteins rapidly and efficiently into quiescent human T cells. We used TAT-p16INK4A as a reagent that inhibits cdk4/6-cyclin D activity. Because TAT proteins enter cells rapidly, we were able to add the TAT-p16INK4A to inhibit cdk4/6-cyclin D2 at defined time points after stimulation with CD3/CD28 or PMA-ionomycin. We showed that cdk4/6-cyclin D2 was only required for the first 5 h, and thereafter inhibition with TAT-p16INK4A did not prevent quiescent T cells from entering G1. p16INK4A inhibits cdk6 directly, but it can also cause inhibition of cdk2. It does so by displacing p27Kip1 or p21Cip1 from cdk6 complexes that then bind and inhibit cdk2 (35). However, inhibition of cdk2 by p27Kip1/p21Cip1 displacement cannot be the mechanism by which TAT-p16INK4A prevents transition through the commitment point as in vitro kinase assays show that cdk2 is activated at least 24 h after cdk6, which correlates with the G1-to-S-phase transition rather than G0 to G1. Cyclin D3 was not detected in cells that were stimulated but held in G0(A) by TAT-p16INK4A. These data are again consistent with a role for cdk4/6-cyclin D2 in regulating progression through the G0→G1 commitment point. Our data are also consistent with a role for pRb phosphorylation during the transition from G0 to early G1 reported by others (15). It should be noted that our work does not imply that endogenous cellular p16INK4A regulates the G0→G1 transition in T cells. Another member of the INK4 family, p18INK4C, is important for regulating T-cell proliferation in mice by inhibiting the activity of cdk6 (27), and further studies are required to determine whether p18INK4C controls the transition of human T cells through the G0→G1 commitment point defined here.
We showed that inhibition with TAT-p16INK4A also prevented T cells from increasing in size. Upon progressing from G0 and entering G1A, cells normally carry out a program of commitment, which requires the synthesis of new proteins. During the progression from G1A to late G1 (G1B), cell size, protein, and RNA content increase markedly and cells only enter S phase when they have reached a certain size. Cell size continues to increase during the remainder of the cell cycle. This is necessary since cells must ultimately divide into two cells while maintaining a characteristic size. In this case the cell cycle and the cellular growth cycle are coordinated and controlling this size homeostasis has been termed the cellular growth cycle (62). The mechanisms that regulate cell size in human T cells have yet to be elucidated. However, cdk4-cyclin D regulates cell size in Drosophila melanogaster (12, 37), and mice lacking cdk4, cyclin D1, or cyclin D2 genes are small and display hypoplasia of certain tissues (45, 53). Thus, our data suggest that a similar cdk4/6-dependent mechanism regulates cell size in human T lymphocytes. This finding is at odds with a study of mouse and human fibroblasts (16). That study showed that proliferating cells continued to increase in size when the cell cycle was inhibited in G1 by the expression of p16INK4A. We note that our study differs not only because different cell types were used (immortalized fibroblasts [rat1a, NIH 3T3, and WI38] versus primary T lymphocytes) but also because we analyzed the progression from G0 to G1 rather than the M→G1→S-phase transition. As noted above, the formation of cyclin D2-cdk6 complexes coincides with the commitment point, followed 4 to 8 h later by the formation of cyclin D3-cdk6. Further studies are required to determine whether one of these kinases is involved in initiating entry into the growth cycle. We note also that inhibition of cell size by TAT-p16INK4A occurred even though c-myc was induced. In other cell types, overexpression of c-myc can cause an increase in cell growth and entry into the cell cycle (3). However, in primary T cells normal expression of endogenous c-myc is not sufficient to cause primary T cells to enter the cell and growth cycles when pRb and p130 are still active.
Although transduction with TAT-p16INK4A inhibited pRb and p130 phosphorylation by cdk6/4-cyclin D2, phosphorylation of ERK and IκB and IκB degradation occurred with normal kinetics. Therefore, the TAT-p16INK4A did not inhibit phosphorylation events carried out by kinases other than cdk’s. Certain signaling pathways are known to be involved in induction of effector molecules such as IFN-γ and CD69, as well as cell proliferation. These include the mitogen-activated protein kinase (6), phosphatidylinositol 3-kinase/Akt (1, 7, 25), and NF-κB pathways (see reference 9 and references therein). However, although inhibiting the cdk/pRb/E2F pathway prevents the G0→G1 transition, this does not seem to affect activation of other pathways that have been shown to be necessary for the induction of effector molecules.
The c-myc protein was also induced normally in cells maintained in G0(A), but in spite of this the cells did not enter the cell cycle, and significant apoptosis did not occur during the period of study. This result was in spite of the fact that the presence of c-myc might be expected to cause apoptosis in the absence of cell proliferation (14). However, there is considerable cell death when T cells are stimulated for prolonged periods (48 to 72 h) in the presence of either TAT-p16INK4A or roscovitine, and the mechanisms involved merit further investigation.
We then investigated whether we were dealing with a cell and growth cycle control point that also coordinated the induction of effector functions such as IL-2, IFN-γ, CD69, and CD44 and the downregulation of CD62L. We showed that the transition of T cells from G0 to G1 is not necessary for memory or naive T cells to become activated and express molecules of effector function. Our findings would be consistent with a previously suggested stochastic model of cytokine gene activation in the T-cell lineage (26). Several studies on murine T cells in vitro and in vivo have addressed whether effector functions, such as the production of IL-2, IFN-γ, and IL-4, as well as the induction of cell surface proteins such as CD69 and CD44, and the downregulation of CD62L, are dependent on cell proliferation. It has been suggested that cell division is necessary for murine T cells to be able to transcribe the IFN-γ and IL-4 genes (5, 17) and, in another study, entry into S phase was required for the expression of IL-4 and IL-10 (46). However, a recent study has shown that, although cell division affects the frequency of cells that can produce IFN-γ and IL-4, both can be produced by T cells, including naive peripheral CD4+ cells, that have been stimulated but have not entered S phase (4). Demethylation and chromatin remodeling have been suggested to play a role in regulating cytokine expression, and the role of methylation in limiting the expression of IFN-γ, IL-2, IL-3, and IL-4 by naive CD4 T cells was demonstrated recently by the analysis of cells from Dnmt1−/− mice (32). In mice in vivo, although CD4+ T cells that proliferated in response to antigenic stimulus expressed effector functions, there was also a cohort of T cells that were arrested in G1, as judged by the fact that they expressed cyclin D3 but still produced IFN-γ (31). CD69 and CD44 were also induced on such cells and, in addition, induction of IL-2 and CD25 and downregulation of CD62L did not require entry into S phase (46). Others reported that induction of CD44 was dependent on cell cycle progression (21). In all of these studies the cell cycle was engaged but either the T cells were arrested in vivo in G1 rather than being maintained in G0 (46) or the cell cycle was inhibited in late G1 in vitro with drugs (21). Although there are conflicting data, these studies established that at least some effector functions can be regulated in cells that have entered the cell cycle but have not yet progressed into S phase and divided. However, none of the studies to date have shown whether effector functions can be induced before cell cycle entry, while T cells are in a quiescent [G0(A)] state, i.e., before transition through the G0→G1 commitment point described here. This is important for understanding the very first events of initiation and modulation of immune responses.
From the data presented here it is clear that certain cellular programs that mediate at least some human T-lymphocyte effector functions are separable from those which engage the cell cycle and the cellular growth cycle. Thus, functional activation of T lymphocytes can be initiated in G0(A) without the cells first entering the cell cycle. These data, along with those shown previously, provide proof that a number of activation and effector molecules can be induced in either memory or naive T cells that are in a quiescent [G0(A)] state and that cell cycle entry is not required for their induction. This may be important for rapid T-cell cytokine responses at the site of infection in the case of memory T cells. In naive CD4+ T cells, rapid release of IL-2 before proliferation would initiate the immune response through its known autocrine and paracrine effects. Therefore, such T-cell activation in G0(A) is important since activation of neighboring cells could then occur before the T-cell numbers increase. Studies of T cells activated in vivo show that T-cell activation can occur without proliferation. For example, cytokine release or cell killing by murine T cells can take place without prior proliferation (reference 31 and references therein). However, as described above, such activation occurs in nonproliferating cells that are in G1 rather than in G0.
The experimental approaches used in our study provide a valuable tool to help elucidate the cellular mechanisms of T-cell memory and homeostasis, as well as the potential for induction of differentiation in lymphoid malignancies. Inhibition of T-cell proliferation is also important for the immunosuppression observed in patients with a number of different cancers and is a mechanism adopted by tumors to evade immune rejection (see reference 9 and references therein). This is true of acute myeloid leukemia (AML), and we showed recently that cells from patients with AML prevent quiescent T cells from entering G1. The immune suppression in AML occurs by a combination of mechanisms since AML cells not only prevent T cells passing through the G0→G1 commitment point but also inhibit the induction of c-myc and the production of IL-2 and IFN-γ (9). Description of the G0→G1 commitment point and the molecular mechanisms involved is therefore important not only for our understanding of immunobiology but also for targets for reversing the immune suppression seen in a number of different diseases such as AML.
Acknowledgments
We are indebted to Steve Dowdy for vectors encoding TAT proteins and methods for purification and to Sibylle Mittnacht for in vitro kinase methods. We thank our colleagues in Leukaemia Sciences and Adrian Hayday and Farzin Farzaneh for insightful comments on the manuscript.
N.C.L. and S.J.O. contributed equally to this study.
This research was funded by the Charles Wolfson Charitable Trust (N.C.L., S.J.O., and N.S.B.T.), the Leukemia Research Fund (N.S.B.T. and E.W.-F.L.), and Cancer Research UK (K.S., G.H.W., and E.W.-F.L.).
REFERENCES
- 1.Appleman, L. J., A. A. van Puijenbroek, K. M. Shu, L. M. Nadler, and V. A. Boussiotis. 2002. CD28 costimulation mediates downregulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 168:2729-2736. [DOI] [PubMed] [Google Scholar]
- 2.Banerji, L., J. Glassford, N. C. Lea, N. S. B. Thomas, G. G. Klaus, and E. W.-F. Lam. 2001. BCR signals target p27Kip1 and cyclin D2 via the PI3-K signalling pathway to mediate cell cycle arrest and apoptosis of WEHI 231 B cells. Oncogene 20:7352-7367. [DOI] [PubMed] [Google Scholar]
- 3.Beier, R., A. Burgin, A. Kiermaier, M. Fero, H. Karsunky, R. Saffrich, T. Moroy, W. Ansorge, J. Roberts, and M. Eilers. 2000. Induction of cyclin E-cdk2 kinase activity, E2F-dependent transcription, and cell growth by Myc are genetically separable events. EMBO J. 19:5813-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Sasson, S. Z., R. Gerstel, J. Hu-Li, and W. E. Paul. 2001. Cell division is not a “clock” measuring acquisition of competence to produce IFN-γ or IL-4. J. Immunol. 166:112-120. [DOI] [PubMed] [Google Scholar]
- 5.Bird, J. J., D. R. Brown, A. C. Mullen, N. H. Moskowitz, M. A. Mahowald, J. R. Sider, T. F. Gajewski, C. R. Wang, and S. L. Reiner. 1998. Helper T-cell differentiation is controlled by the cell cycle. Immunity 9:229-237. [DOI] [PubMed] [Google Scholar]
- 6.Borovsky, Z., G. Mishan-Eisenberg, E. Yaniv, and J. Rachmilewitz. 2002. Serial triggering of T-cell receptors results in incremental accumulation of signaling intermediates. J. Biol. Chem. 277:21529-21536. [DOI] [PubMed] [Google Scholar]
- 7.Brennan, P., J. W. Babbage, B. M. Burgering, B. Groner, K. Reif, and D. A. Cantrell. 1997. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity 7:679-689. [DOI] [PubMed] [Google Scholar]
- 8.Broceno, C., S. Wilkie, and S. Mittnacht. 2002. RB activation defect in tumor cell lines. Proc. Natl. Acad. Sci. USA 99:14200-14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buggins, A. G., D. Milojkovic, M. J. Arno, N. C. Lea, G. J. Mufti, N. S. B. Thomas, and W. J. Hirst. 2001. Microenvironment produced by acute myeloid leukemia cells prevents T-cell activation and proliferation by inhibition of NF-κB, c-Myc, and pRb pathways. J. Immunol. 167:6021-6030. [DOI] [PubMed] [Google Scholar]
- 10.Cook, J. G., C. H. Park, T. W. Burke, G. Leone, J. DeGregori, A. Engel, and J. R. Nevins. 2002. Analysis of Cdc6 function in the assembly of mammalian prereplication complexes. Proc. Natl. Acad. Sci. USA 99:1347-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darzynkiewicz, Z., F. Traganos, and M. R. Melamed. 1980. New cell cycle compartments identified by multiparameter flow cytometry. Cytometry 1:98-108. [DOI] [PubMed] [Google Scholar]
- 12.Datar, S. A., H. W. Jacobs, A. F. de la Cruz, C. F. Lehner, and B. A. Edgar. 2000. The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J. 19:4543-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont, F. J., M. J. Staruch, P. Fischer, C. DaSilva, and R. Camacho. 1998. Inhibition of T-cell activation by pharmacologic disruption of the MEK1/ERK MAP kinase or calcineurin signaling pathways results in differential modulation of cytokine production. J. Immunol. 160:2579-2589. [PubMed] [Google Scholar]
- 14.Evan, G. I., A. H. Wyllie, C. S. Gilbert, T. D. Littlewood, H. Land, M. Brooks, C. M. Waters, L. Z. Penn, and D. C. Hancock. 1992. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119-128. [DOI] [PubMed] [Google Scholar]
- 15.Ezhevsky, S. A., A. Ho, M. Becker-Hapak, P. K. Davis, and S. F. Dowdy. 2001. Differential regulation of retinoblastoma tumor suppressor protein by G1 cyclin-dependent kinase complexes in vivo. Mol. Cell. Biol. 21:4773-4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingar, D. C., S. Salama, C. Tsou, E. Harlow, and J. Blenis. 2002. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16:1472-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gett, A. V., and P. D. Hodgkin. 1998. Cell division regulates the T-cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc. Natl. Acad. Sci. USA 95:9488-9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gius, D. R., S. A. Ezhevsky, M. Becker-Hapak, H. Nagahara, M. C. Wei, and S. F. Dowdy. 1999. Transduced p16INK4a peptides inhibit hypophosphorylation of the retinoblastoma protein and cell cycle progression prior to activation of Cdk2 complexes in late G1. Cancer Res. 59:2577-2580. [PubMed] [Google Scholar]
- 19.Goldsby, R. A. 2002. T-cell maturation, activation, and differentiation, p. 221-246. In R. A. Goldsby, T. J. Kindt, B. A. Osborne, and J. Kuby (ed.), Immunology. W. H. Freeman & Co., New York, N.Y.
- 20.Gray, N., L. Detivaud, C. Doerig, and L. Meijer. 1999. ATP-site directed inhibitors of cyclin-dependent kinases. Curr. Med. Chem. 6:859-875. [PubMed] [Google Scholar]
- 21.Gudmundsdottir, H., A. D. Wells, and L. A. Turka. 1999. Dynamics and requirements of T-cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J. Immunol. 162:5212-5223. [PubMed] [Google Scholar]
- 22.Hateboer, G., A. Wobst, B. O. Petersen, L. Le Cam, E. Vigo, C. Sardet, and K. Helin. 1998. Cell cycle-regulated expression of mammalian CDC6 is dependent on E2F. Mol. Cell. Biol. 18:6679-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, D. Y., and M. B. Prystowsky. 1996. Identification of an essential cis-element near the transcription start site for transcriptional activation of the proliferating cell nuclear antigen gene. J. Biol. Chem. 271:1218-1225. [DOI] [PubMed] [Google Scholar]
- 24.Jain, J., C. Loh, and A. Rao. 1995. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 7:333-342. [DOI] [PubMed] [Google Scholar]
- 25.Kane, L. P., P. G. Andres, K. C. Howland, A. K. Abbas, and A. Weiss. 2001. Akt provides the CD28 costimulatory signal for upregulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 2:37-44. [DOI] [PubMed] [Google Scholar]
- 26.Kelso, A., P. Groves, A. B. Troutt, and K. Francis. 1995. Evidence for the stochastic acquisition of cytokine profile by CD4+ T cells activated in a T helper type 2-like response in vivo. Eur. J. Immunol. 25:1168-1175. [DOI] [PubMed] [Google Scholar]
- 27.Kovalev, G. I., D. S. Franklin, V. M. Coffield, Y. Xiong, and L. Su. 2001. An important role of CDK inhibitor p18INK4c in modulating antigen receptor-mediated T-cell proliferation. J. Immunol. 167:3285-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krude, T., C. Musahl, R. A. Laskey, and R. Knippers. 1996. Human replication proteins hCdc21, hCdc46 and P1Mcm3 bind chromatin uniformly before S-phase and are displaced locally during DNA replication. J. Cell Sci. 109(Pt. 2):309-318. [DOI] [PubMed] [Google Scholar]
- 29.Lam, E. W.-F., J. Glassford, L. Banerji, N. S. Thomas, P. Sicinski, and G. G. Klaus. 2000. Cyclin D3 compensates for loss of cyclin D2 in mouse B-lymphocytes activated via the antigen receptor and CD40. J. Biol. Chem. 275:3479-3484. [DOI] [PubMed] [Google Scholar]
- 30.Lanzavecchia, A., and F. Sallusto. 2000. From synapses to immunological memory: the role of sustained T-cell stimulation. Curr. Opin. Immunol. 12:92-98. [DOI] [PubMed] [Google Scholar]
- 31.Laouar, Y., and I. N. Crispe. 2000. Functional flexibility in T cells: independent regulation of CD4+ T-cell proliferation and effector function in vivo. Immunity 13:291-301. [DOI] [PubMed] [Google Scholar]
- 32.Lee, P. P., D. R. Fitzpatrick, C. Beard, H. K. Jessup, S. Lehar, K. W. Makar, M. Perez-Melgosa, M. T. Sweetser, M. S. Schlissel, S. Nguyen, S. R. Cherry, J. H. Tsai, S. M. Tucker, W. M. Weaver, A. Kelso, R. Jaenisch, and C. B. Wilson. 2001. A critical role for Dnmt1 and DNA methylation in T-cell development, function, and survival. Immunity 15:763-774. [DOI] [PubMed] [Google Scholar]
- 33.Madine, M. A., M. Swietlik, C. Pelizon, P. Romanowski, A. D. Mills, and R. A. Laskey. 2000. The roles of the MCM, ORC, and Cdc6 proteins in determining the replication competence of chromatin in quiescent cells. J. Struct. Biol. 129:198-210. [DOI] [PubMed] [Google Scholar]
- 34.Mao, X., J. M. Green, B. Safer, T. Lindsten, R. M. Frederickson, S. Miyamoto, N. Sonenberg, and C. B. Thompson. 1992. Regulation of translation initiation factor gene expression during human T-cell activation. J. Biol. Chem. 267:20444-20450. [PubMed] [Google Scholar]
- 35.McConnell, B. B., F. J. Gregory, F. J. Stott, E. Hara, and G. Peters. 1999. Induced expression of p16INK4a inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol. Cell. Biol. 19:1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2, and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 37.Meyer, C. A., H. W. Jacobs, S. A. Datar, W. Du, B. A. Edgar, and C. F. Lehner. 2000. Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. EMBO J. 19:4533-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T-cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 39.Nagahara, H., A. M. Vocero-Akbani, E. L. Snyder, A. Ho, D. G. Latham, N. A. Lissy, M. Becker-Hapak, S. A. Ezhevsky, and S. F. Dowdy. 1998. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4:1449-1452. [DOI] [PubMed] [Google Scholar]
- 40.Ohtani, K., A. Tsujimoto, M. Ikeda, and M. Nakamura. 1998. Regulation of cell growth-dependent expression of mammalian CDC6 gene by the cell cycle transcription factor E2F. Oncogene 17:1777-1785. [DOI] [PubMed] [Google Scholar]
- 41.Parada, Y., L. Banerji, J. Glassford, N. C. Lea, M. Collado, C. Rivas, J. L. Lewis, M. Y. Gordon, N. S. Thomas, and E. W.-F. Lam. 2001. BCR-ABL and interleukin 3 promote haematopoietic cell proliferation and survival through modulation of cyclin D2 and p27Kip1 expression. J. Biol. Chem. 276:23572-23580. [DOI] [PubMed] [Google Scholar]
- 42.Pardee, A. B. 1989. G1 events and regulation of cell proliferation. Science 246:603-608. [DOI] [PubMed] [Google Scholar]
- 43.Parish, C. R. 1999. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol. Cell Biol. 77:499-508. [DOI] [PubMed] [Google Scholar]
- 44.Polymenis, M., and E. V. Schmidt. 1999. Coordination of cell growth with cell division. Curr. Opin. Genet. Dev. 9:76-80. [DOI] [PubMed] [Google Scholar]
- 45.Rane, S. G., P. Dubus, R. V. Mettus, E. J. Galbreath, G. Boden, E. P. Reddy, and M. Barbacid. 1999. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat. Genet. 22:44-52. [DOI] [PubMed] [Google Scholar]
- 46.Richter, A., M. Lohning, and A. Radbruch. 1999. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J. Exp. Med. 190:1439-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarze, S. R., A. Ho, A. Vocero-Akbani, and S. F. Dowdy. 1999. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569-1572. [DOI] [PubMed] [Google Scholar]
- 48.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 49.Stoeber, K., A. D. Mills, Y. Kubota, T. Krude, P. Romanowski, K. Marheineke, R. A. Laskey, and G. H. Williams. 1998. Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. EMBO J. 17:7219-7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoeber, K., T. D. Tlsty, L. Happerfield, G. A. Thomas, S. Romanov, L. Bobrow, E. D. Williams, and G. H. Williams. 2001. DNA replication licensing and human cell proliferation. J. Cell Sci. 114:2027-2041. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, N. S., A. R. Pizzey, S. Tiwari, C. D. Williams, and J. Yang. 1998. p130, p107, and pRb are differentially regulated in proliferating cells and during cell cycle arrest by alpha-interferon. J. Biol. Chem. 273:23659-23667. [DOI] [PubMed] [Google Scholar]
- 52.Thomas, N. S. B. 1999. Cell cycle regulation, p. 133-152. In L. Degos, D. C. Linch, and B. Lowenberg (ed.), Textbook of malignant haematology. Martin Dunitz, London, England.
- 53.Tsutsui, T., B. Hesabi, D. S. Moons, P. P. Pandolfi, K. S. Hansel, A. Koff, and H. Kiyokawa. 1999. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol. 19:7011-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tye, B. K. 1999. MCM proteins in DNA replication. Annu. Rev. Biochem. 68:649-686. [DOI] [PubMed] [Google Scholar]
- 55.van Leeuwen, J. E., and L. E. Samelson. 1999. T-cell antigen-receptor signal transduction. Curr. Opin. Immunol. 11:242-248. [DOI] [PubMed] [Google Scholar]
- 56.van Oers, N. S. 1999. T-cell receptor-mediated signs and signals governing T-cell development. Semin. Immunol. 11:227-237. [DOI] [PubMed] [Google Scholar]
- 57.van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423-429. [DOI] [PubMed] [Google Scholar]
- 58.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283:680-682. [DOI] [PubMed] [Google Scholar]
- 59.Vocero-Akbani, A., N. A. Lissy, and S. F. Dowdy. 2000. Transduction of full-length Tat fusion proteins directly into mammalian cells: analysis of T-cell receptor activation-induced cell death. Methods Enzymol. 322:508-521. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Z., P. Sicinski, R. A. Weinberg, Y. Zhang, and K. Ravid. 1996. Characterization of the mouse cyclin D3 gene: exon/intron organization and promoter activity. Genomics 35:156-163. [DOI] [PubMed] [Google Scholar]
- 61.Yan, Z., J. DeGregori, R. Shohet, G. Leone, B. Stillman, J. R. Nevins, and R. S. Williams. 1998. Cdc6 is regulated by E2F and is essential for DNA replication in mammalian cells. Proc. Natl. Acad. Sci. USA 95:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zetterberg, A. 1996. Cell growth and cell cycle progression in mammalian cells, p. 17-36. In N. S. B. Thomas (ed.), Apoptosis and cell cycle control in cancer. Bios Scientific Publishers, Ltd., Oxford, United Kingdom.