Abstract
Human membrane cofactor protein (MCP, CD46) is a ubiquitously expressed protein known to protect cells from complement attack. Interestingly, when we examined the expression of mouse CD46, which we recently cloned, the message was found only in testis and the protein was found on the inner acrosomal membrane of sperm. In order to elucidate the function of CD46, we produced mice carrying a null mutation in the CD46 gene by using homologous recombination. Despite the absence of CD46, the mice were healthy and both sexes were fertile. However, to our surprise, the fertilizing ability of males appeared to be facilitated by disruption of the CD46 gene, as the average number of pups born from CD46−/− males was significantly greater than that of wild-type males. It was also revealed that the incidence of the spontaneous acrosome reaction doubled in CD46−/− sperm compared to that in wild-type sperm. It was assumed that this increase caused the heightened fertilizing ability found in CD46−/− sperm. These data suggest that CD46 may have some role in regulating sperm acrosome reaction.
CD46 (membrane cofactor protein [MCP]) is known as a complement receptor that protects cells from the deleterious effects of the autologous complement system by serving as a cofactor for the cleavage of C3b and C4b by the serine protease factor I. CD46 homologues have been cloned from humans, simians, swine, guinea pigs (12), rats (19), and mice (42). All of these homologues consist of four short consensus repeats (SCR), a serine- and threonine-rich domain, a transmembrane domain, and a cytoplasmic tail with a homology of 45.3 to 51.2% to human CD46. Interestingly, in the rodent animals studied, the expression of CD46 was limited only in testis, indicating that the major function of CD46 among different species lies in fertilization. Moreover, in rodent animals, distribution of complement regulators such as transmembrane type CD55 (decay accelerating factor [DAF]), CD59b, and C4b-binding protein were preferentially expressed in reproductive organs (4, 25, 30, 37, 42). In humans, CD46 is expressed on the membrane of all kinds of nucleated cells. However, in sperm, CD46 is found on the inner acrosomal membrane but is not found on the plasma membrane located directly above. Therefore, sperm are unable to use CD46 to neutralize the possibly harmful complement which exists abundantly in the female reproductive tract during the journey to the eggs. Instead, sperm express other complement receptors such as CD59 and CD55 on their plasma membranes in humans (36).
The process of fertilization can be divided into several steps, such as the sperm's ascension to the uterus and oviduct, the penetration of cumulus layers of eggs, the attachment to the zona pellucida (ZP), the penetration of the ZP, the attachment to the egg plasma membrane, and the fusion with the eggs (45). The female reproductive tract is abundant in complement, partly because it requires a periodic removal mechanism for millions of allogeneic spermatozoa. One reported detrimental effect of complement on sperm in the female reproductive tract is that females who produce antisperm antibody become infertile when the antibody inactivates sperm with complement (8). It is also reported that the estrogen stimulates the synthesis of C3 in rat uterus (38).
During most of the fertilization steps, sperm are protected by complement receptors such as CD55 and CD59 that localize on the plasma membrane. However, at some point near the ZP penetration step, sperm need to shed part of their plasma membrane in the head area in order to release enzymes that are kept in an organelle called the acrosome. This process is called the acrosome reaction and is a prerequisite for sperm to fertilize eggs. According to our and other investigators' reports, the inner acrosomal membrane is the area where CD46 is initially localized (28, 39). Based on these results together, it is considered that CD46 on sperm may play a role in the protection of the acrosome-reacted sperm by modulating complement in the female genital tract (6, 32).
An additional role is proposed for CD46 on the inner acrosomal membrane. MH61 is one of the monoclonal antibodies (Abs) which we obtained against acrosome-reacted human sperm, and we discovered that MH61 inhibits the fusion of human sperm to hamster oocytes (28). We later found that the antigen recognized by MH61 was CD46 (29). The possibility of the involvement of CD46 in the fertilization process was indicated by results from other laboratories using different anti-CD46 Abs (1, 10). Anderson et al. (1) demonstrated that CD46 specifically binds dimeric C3b and that human sperm acrosomal proteases released during the acrosome reaction directly cleave C3, thereby facilitating its binding to CD46. In turn, these authors indicated that human eggs express complement receptors 1 and 3 (CR1 and CR3) on their surface and maintain their C3b dimer binding ability. From these findings, they proposed a sperm-egg membrane apposition model mediated by CD46, C3b/bi, and CR. The complement system has been regarded as a self-nonself recognition system as initially proposed by Scofield et al. (31) and later by Farries and Atkinson (9).
However, more direct evidence (such as that derived from gene-manipulated animals) is needed to consolidate these hypotheses. In order to elucidate the function of CD46 in mice, we disrupted the CD46 gene by homologous recombination and produced CD46-deficient mice. In spite of the fact that the CD46 is preserved over species and that testis seemed to be the only common organ that expresses CD46, the CD46−/− mice had normal testes and fertile sperm. However, in contrast with the wild-type sperm, it was found that the CD46−/− sperm showed an accelerated spontaneous acrosome reaction.
MATERIALS AND METHODS
Antibody, cells, and serum.
Fresh mouse serum was obtained from the heart as previously reported (13). All the sera were immediately stored at −80°C until use. RK13 cells (derived from rabbit kidney) were obtained from the RIKEN cell bank (Wako, Saitama, Japan). Anti-mouse CD55 monoclonal antibody (RIKO-3) and anti-mouse acrosomal matrix protein antibody (anti-MC101) were kind gifts from N. Okada (Nagoya City University, Nagoya, Japan) (27) and K. Toshimori (Miyazaki Medical College, Miyazaki, Japan) (40), respectively.
Preparation of rabbit anti-mouse CD46 polyclonal antibody.
Rabbit anti-mouse CD46 polyclonal antibody (anti-mCD46 Ab) was produced by a method established previously (13). Briefly, RK13 cells (107) were transiently transfected with pME18s-full-length CD46-His6 tag by using LipofectAMINE PLUS reagent (Invitrogen, San Diego, Calif.). After 2 days, transfected RK13 cells were collected with 10 mM EDTA-phosphate-buffered saline (PBS) and suspended in 0.5 ml of PBS after three cycles of washing with PBS. RK13 cell suspensions were then mixed and emulsified with 0.6 ml of Freund's complete adjuvant (Difco, Detroit, Mich.) and used for immunization of rabbits. Immunization was performed four times at 7-day intervals, and booster immunizations were performed before blood was drawn. Immunoglobulin G (IgG) was purified by 33% ammonium sulfate precipitation and kept frozen at −80°C until it was used following dialysis against PBS.
Tissue blot analysis.
Various mouse tissues were solubilized with lysis buffer containing 1% (vol/vol) NP-40, 0.14 M NaCl, 0.01 M EDTA, 20 mM Tris-HCl (pH 7.4), 1 mg of iodoacetamide/ml, and 1 mM phenylmethylsulfonyl fluoride by using a Potter-type homogenizer. After incubation at 4°C for 30 min, each lysate was centrifuged at 15,000 × g at 4°C for 30 min. The supernatant was collected, and the protein concentration was measured with a protein assay kit (Bio-Rad Laboratories, Hercules, Calif.). Equal amounts of total cellular protein were electrophoresed on sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride membranes. CD46 was visualized by using an ECL detection system (Amersham Bioscience, Piscataway, N.Y.) after incubation with rabbit anti-mCD46 Ab and a horseradish peroxidase-linked goat anti-rabbit secondary Ab (Biosource International, Camarillo, Calif.).
Construction of the CD46 gene disruption vector.
The targeting construct was generated from the 129/SVJ genomic library as previously described (24). The 5′ homology arm of the construct was derived from a 1.2-kb NotI/XhoI genomic fragment containing a noncoding sequence upstream of the ATG initiation codon. This fragment was obtained by PCR using genomic DNA in D3 embryonic stem (ES) cells as a template. The PCR primers used were as follows: 5′-TTGCGGCCGCTAATAGTCCCACTCCCTATG-3′ and 5′-CCCTCGAGAGCCAGAGAGCAAGTTTTAA-3′. The 3′ homology arm of the targeting construct was derived from a 7.5-kb EcoRI/SalI genomic fragment located between exons 3 and 7 of the CD46 gene. This fragment was subcloned into EcoRI/SalI-restricted pBluescript SK(+) and then digested with BamHI and KpnI. Between these two homology arms was placed a phosphoglycerine kinase (PGK)-neomycin selectable marker cassette (43). Successful homologous recombination of the targeting construct and the endogenous CD46 allele resulted in the deletion of exons 1 to 3, thus resulting in a disruption of the CD46 protein. A negative selectable marker, a herpes simplex virus thymidine kinase, was located adjacent to the 3′ homology region. The targeting construct was linearized with NotI and electroporated into D3 ES cells, and the colonies were screened.
Generation of CD46-deficient mice.
G418-resistant colonies were amplified, and genomic DNA was prepared from them and screened by Southern blot analysis. Probes derived from genomic CD46 sequences upstream and downstream of the original targeting construct were used to screen for correctly targeted clones (data not shown). Several of the recombinant ES cell lines carrying the disrupted CD46 allele were identified and subsequently used to generate chimeras by injection into C57BL/6 Cr blastocysts (at embryonic day 3.5) purchased from Japan SLC (Shizuoka, Japan). The blastocysts were transferred to ICR pseudopregnant foster mothers, resulting in the birth of two lines of male chimeric mice. Two male chimeric offspring, identified by their agouti coat color, were mated with C57BL/6 females. The F1 agouti offspring were then analyzed for CD46 disruption by Southern blotting and PCR analysis. The CD46-deficient mice were generated by the intercrossing of F1 offspring mice or by mating with C57BL/6 mice after backcrossing to C57BL/6 mice for more than five generations.
All experiments were performed with the consent of the Animal Care and Use Committee of Osaka University.
Southern blot hybridization and PCR analysis.
For Southern blot analysis, genomic DNAs from ES cells and tail biopsy specimens were prepared as described by Laird et al. (15). BamHI-digested genomic DNA (approximately 20 μg) was electrophoresed though an 0.8% agarose gel, transferred to a Hybond N+ membrane (Amersham Bioscience), and hybridized with the 32P-labeled 93-bp sequence of the exon 7 probe depicted below (see Fig. 2A). Genomic DNA derived from tail biopsy specimens of CD46+/+, CD46+/−, and CD46−/− mice was used as the template for PCR analysis. The primers used were derived from sequences contained in the 5′ flanking region (forward, 5′-TGTGAGGAAATACCATGACCAACACAACTC-3′) and intron 1 (reverse, 5′-GGATGCCCACCCCTTAACTACTGCGT-3′) of the CD46 gene and upstream of the PGK promoter (reverse, 5′CCGGTGGATGTGGAATGTGTGCGAGGCC-3′) of the neomycin resistance gene.
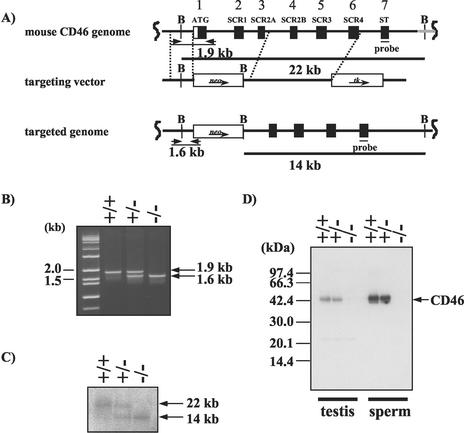
FIG. 2.
Generation of mice lacking CD46. (A) The top diagram is a schematic representation of the normal CD46 allele; the middle diagram shows the targeting construct with PGK-neomycin resistance and PGK-thymidine kinase genes as positive and negative selectable markers, respectively; this construct was designed to delete the 7.4 kb of CD46 that included the putative translational start site and the first three exons. Homologous regions included 34 bp upstream of the ATG initiation codon to 495 bp downstream of exon 3 that encompassed exons 4 to 6. The bottom diagram shows the CD46 allele mutated by homologous recombination. The exons are represented by black boxes, thick horizontal lines indicate the extent of the homologous DNA, and the positions of the 3′ probe are indicated under the null allele. Restriction enzyme cut sites are indicated by B (BamHI). Arrows indicate the directions of transcription of the two selectable markers. (B) Genotyping of mouse tail DNA by PCR analysis with primers indicated in the figure (short arrow [A]). Normal and mutant alleles showed 1.9- and 1.6-kb fragments, respectively. (C) Genotyping by Southern blot analysis of DNA purified from the tails of F2 mice generated from crossing with CD46+/− F1 pups. After restriction digestion with BamHI, the DNA was hybridized with the 32P-labeled 3′ probe. Heterozygous (+/−), homozygous (−/−), and normal (+/+) genotypes were present in the expected Mendelian ratios for a single-copy mutant gene. (D) Western blot analysis was performed with solubilized proteins (100 μg) from wild-type (+/+), heterozygous (+/−), and knockout (−/−) mouse testis and sperm. The extracts were separated by SDS-12.5%PAGE under nonreducing conditions and transblotted onto membrane. The CD46 was then visualized with anti-mCD46 Ab as described in Materials and Methods.
Flow cytometry.
A total of 107 sperm were incubated for 60 min at 4°C with a 50-μg/ml concentration of anti-mCD46 Ab or a 25-μg/ml concentration of RIKO 3, which recognizes both transmembrane- and glycosylphosphatidylinositol-anchored types of CD55 in 50 μl of staining buffer (0.1% NaN3-0.1% BSA in PBS). The sperm were then washed three times in the same buffer and incubated for 30 min with fluorescein isothiocyanate (FITC)-conjugated secondary Ab under the same conditions. Both incubations were performed in the presence of 10% heat-inactivated normal goat serum to avoid nonspecific binding of Abs. After being washed with staining buffer, the stained sperm were analyzed with a FACSCalibur flow cytometer (Becton-Dickinson, Franklin Lakes, N.J.). For the measurement of the total amount of protein, sperm were fixed with 1% paraformaldehyde in 37.5 mM phosphate buffer (pH 7.4) containing 75 mM lysine and 10 mM sodium periodate following permeabilization with 0.4 mg of saponin per ml.
Immunofluorescence analysis of CD46 on sperm.
The spermatozoa prepared from cauda epididymis were washed twice with PBS, spotted onto slides, air dried, and rinsed briefly in PBS. Nonspecific protein binding sites were blocked with 1% bovine serum albumin (BSA)-PBS at room temperature for 30 min. The slides were then incubated with 20 μg of anti-mCD46 Ab per ml at 4°C overnight. After being washed with 1% BSA-PBS, the slides were incubated in the dark with FITC-conjugated anti-rabbit IgG (American Quiaex International, San Clemente, Calif.) at 37°C for 30 min in PBS containing 10% (wt/vol) Block Ace (Yukijirushi Co., Tokyo, Japan). After being washed, the slides were mounted in PBS containing 2.3% 1,4-diazabiocyclo-2-octane and 50% glycerol. The stained cells were observed under a confocal microscope (FLUOVIEW; Olympus Co., Tokyo, Japan).
C3 deposition assay.
The C3 deposition on CD46−/− and CD46+/+ sperm was determined with a flow cytometer. Sperm dispersed from cauda epididymis were preincubated in a 0.4-ml droplet (107 sperm/ml) of TYH medium (41) at 37°C for 2 h. Sperm were then incubated with 10 μM A23187 (Ca2+ ionophore) for 20 min to induce the acrosome reaction. After several washes, sperm were incubated with a 1:10,000 dilution of anti-acrosome OBF13 monoclonal Ab (IgM) and with 10% mouse serum in Ca2+- and Mg2+-containing medium (gelatin Veronal buffer) for 20 min at 37°C to allow complement activation. Sperm were washed three times with PBS containing 0.1% BSA and 0.1% sodium azide and were treated with a 1:50 dilution of goat anti-mouse C3 F(ab)2 polyclonal Ab (ICN Pharmaceuticals, Inc., Costa Mesa, Calif.) followed by staining with FITC-conjugated secondary Ab.
Assessment of the fertilizing ability of CD46-disrupted mice.
Various combinations of sexually mature male and female (≥12 and ≥8 weeks of age, respectively) mice of CD46+/+ and CD46−/− genotypes were caged together for mating, and the number of pups in each cage was counted within a week of birth. For the mating with wild-type C57BL/6 and DBA/2N strain mice, more than five males backcrossed with C57BL/6 mice for at least five generations were used. The same males were also used for the assessment of fertilizing ability against superovulated eggs. Female mice of the (C57BL/6 × DBA2) F1 (BDF1) strain were superovulated by intraperitoneal injections of 5 IU of pregnant mare serum gonadotropin and 5 IU of human chorionic gonadotropin (hCG) at a 48-h interval and caged with CD46+/+ or CD46−/− males. The eggs were recovered at 24 h after hCG injection, and the formation of the pronucleus was examined under a fluorescent microscope equipped with a micromanipulator following nucleus staining with Hoechst stain 33342. The eggs with one female and one male pronucleus were counted as fertilized, while those with the spindle that is characteristic of metaphase II were counted as unfertilized. Polyspermy eggs and degenerated eggs were not counted.
Analysis of acrosome reaction.
To investigate the influence of CD46 disruption on sperm acrosome reaction, the females from a transgenic mouse line carrying acrosin-enhanced green fluorescent protein (EGFP) were crossed with CD46−/− males. CD46+/− and acrosin-EGFP+/− double-transgenic F1 pups were mated to generate F2 CD46−/− and acrosin-EGFP+/− double-transgenic mice. The cauda epididymal sperm from the double-transgenic mice and wild-type mice were incubated in TYH medium (41) to induce a spontaneous acrosome reaction and subjected to flow cytometric analysis at 15-min intervals for up to 3 h. The viable sperm were selected by staining with propidium iodide (final concentration, 10 μg/ml), and their acrosomal integrity was examined as indicated in our previous paper (21).
RESULTS
Expression of CD46 in testis and glycosylation of CD46 in sperm.
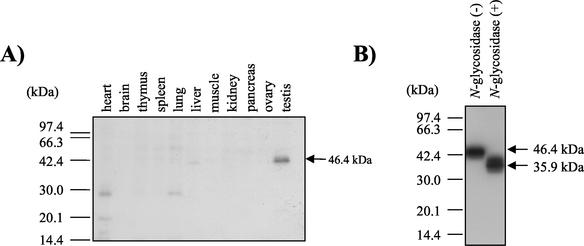
In humans, various splice isoforms of CD46 are known to exist within an organ and also in an organ-specific manner (35). In mice, a 1.5-kb message is expressed predominantly in testis, as determined by Northern blot analysis (42). However, the distribution of mouse CD46 protein has remained unknown. We therefore produced rabbit polyclonal Ab against whole mouse CD46 protein and performed Western blot analysis using various tissues. Mouse CD46 protein was shown to be absent in all tested organs except testis (Fig. 1A). Although several alternative splicing forms of mRNA were detected, only a 46.4-kDa band was seen in Western blotting results. When sperm were treated with N-glycosidase, the molecular mass of CD46 decreased to 36 kDa (Fig. 1B). Since the 36-kDa size corresponds to a molecular mass deduced from the CD46 amino acid sequence, CD46 was suggested to be N glycosylated but not O glycosylated. A similar result is reported for CD46 in human sperm (11).
FIG. 1.
Tissue blots of mouse CD46 protein in testis and sperm. (A) Immunoblotting analysis of various mouse tissues. Solubilized proteins (100 μg) were extracted from various mouse tissues, fractionated through an SDS-12.5% PAGE gel, and transferred to a nitrocellulose membrane. Immunodetection with Abs against mouse CD46 was performed. A 46.4-kDa band was observed only with testis extract. Two faint 29- and 46-kDa bands were found in heart and lung tissues; the 46-kDa bands in liver extracts were found to be nonspecific bands by comparing them with the extracts from CD46-deficient mice. (B) Deglycosylation analysis of sperm CD46. The sperm-derived solubilized protein (50 μg) was treated with N-glycosidase and subjected to SDS-PAGE followed by immunoblotting with anti-mCD46 Ab.
Establishment of CD46-deficient mice.
The mouse CD46 gene consists of 11 exons and is mapped to chromosome 1 (24). To disrupt the CD46 gene, the targeting vector was designed to remove the first three exons of CD46, which included the translational start site and two of the first SCR domains (Fig. 2A). After linearization, the targeting plasmid was electroporated into D3 ES cells and potentially targeted cells were identified by positive-negative selection with G418 and ganciclovir. The selected clones were then checked by PCR for homologous recombination on the 5′ end (Fig. 2B), and the positive clones were assayed by Southern blotting for their recombination on the 5′ and 3′ ends by using several CD46 fragments as probes (data not shown). The targeted ES cell lines were injected into C57BL/6 Cr host blastocysts, and chimeric male mice were obtained. They were bred to C57BL/6 females, and the F1 mice that possessed the CD46−/− mutation were bred to obtain CD46+/+, CD46+/−, and CD46−/− mice. By Southern blotting with a 93-bp sequence corresponding to exon 7 as a probe, the normal (Fig. 2C, lane 1) and targeted (Fig. 2C, lanes 2 and 3) CD46 alleles were detected as single 22- or 14-kbp bands, respectively, after digestion with BamHI.
The targeted disruption of the CD46 gene resulted in the absence of expression of the CD46 protein. Western blot analysis with polyclonal anti-CD46 Ab identified the 46.4-kDa CD46 protein in testicular and sperm extracts from wild-type and heterozygous mice, but the protein was not identified in extracts from CD46−/− homozygous mice (Fig. 2D).
Localization of CD46 on sperm.
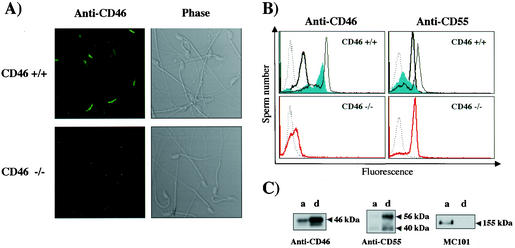
The CD46 in sperm was localized in the acrosomal cap area when detected by immunostaining (Fig. 3A). The subcellular localization of CD46 was then analyzed by flow cytometry for (i) fresh, (ii) acrosome-reacted, and (iii) permeabilized sperm. Fresh sperm showed only a slight reactivity to the CD46 Ab, while the ionophore-treated (acrosome-reacted) sperm showed much intense reactivity. This indicates that the majority of the CD46 protein is not expressed on the plasma membrane but on the inner acrosomal membrane. In CD46−/− animals, sperm showed no reactivity to the Ab even after the permeabilization. The localization on the inner acrosomal membrane was characteristic for CD46 because the other complement receptor, CD55, was found on fresh sperm and decreased after the acrosome reaction. The disappearance of CD46 from sperm did not alter the presence of CD55 on sperm (Fig. 3B). Moreover, to verify that CD46 is an inner acrosomal-membrane protein, the protein product was extracted with Triton X-114 for phase separation experiments. As shown in Fig. 3C, the majority of the CD55 and MC101 proteins were extracted into the detergent and aqueous phases, respectively. In contrast, the CD46 protein was slightly extracted into the aqueous phase (including the acrosomal matrix component), but CD46 was mainly extracted into the detergent phase (including the membrane component). Considering that CD46 exists only in sperm and that the protein does not appear on the sperm surface until the acrosome reaction takes place, we expected CD46 to play a significant role in fertilization.
FIG. 3.
Subcellular localization of CD46 protein in sperm. (A) Immunostaining of mouse sperm. Sperm from wild-type (top) and from CD46−/− (bottom) mice were stained and were observed by fluorescent microscopy (left) or by phase-contrast microscopy (right). (B) Flow cytometric analysis after permeabilization. (Upper left) Fresh wild-type sperm were not strongly stained with anti-mCD46 Ab (thick line), but after the acrosome reaction, the fluorescent peak increased significantly (filled area). The reactivity became even higher when sperm were treated for permeabilization before staining (thin line). (Lower left) There was no reactivity to CD46 against CD46−/− sperm even after permeabilization (red line). The dotted line indicates the control IgG. (Upper right) Sperm were stained with anti-CD55 Ab (RIKO-3). Various lines indicate fresh (thick), acrosome-reacted (filled area) and permeabilized (thin line) sperm, respectively. (Lower right) CD55 expression in fresh sperm (red line). The dotted line indicates the control IgG. (C) Western blotting of CD46, CD55, and MC101 antigens after Triton X-114 extraction. Sperm were incubated for 5 min at 37°C with 1.0% Triton X-114 and then layered on top of a 6% sucrose cushion and centrifuged at 500 × g for 15 min. The aqueous (a) and the detergent (d) phases were analyzed by immunoblotting analysis after SDS-PAGE with anti-mCD46 Ab (left panel), anti-DAF (RIKO-3) (middle panel), and anti-MC101 (right panel).
C3 deposition on CD46−/− mouse sperm.
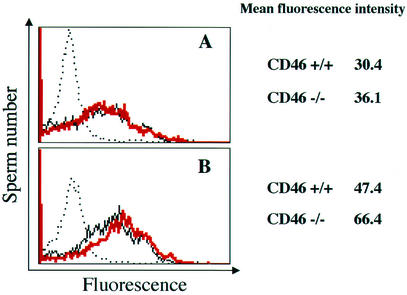
We first analyzed the complement regulation ability of sperm CD46 by performing the C3 deposition assay with CD46-deficient mice and found no difference in the quantity of C3 deposited between the CD46−/− and CD46+/+ sperm before the acrosome reaction. This was in accordance with the fact that only small amounts of CD46 were expressed on the intact sperm membrane (Fig. 4A). On the other hand, in CD46−/− mice, a slightly increased deposition of C3 on the sperm surface was found after the acrosome reaction (mean fluorescence intensity shifted from 47.4 to 66.4) (Fig. 4B). However, the difference may not be enough for CD46 to act as a major complement regulator in sperm.
FIG. 4.
C3 deposition assay. The deposition was measured by flow cytometry by using anti-mouse C3 Ab as described in Materials and Methods. The solid black line indicates C3 deposition on sperm carrying the wild-type allele, and the red line indicates C3 deposition for CD46−/− sperm. The deposition of C3 on sperm with control IgG to CD46−/− sperm is indicated by the dotted line. The top panel (A) indicates the result when fresh sperm were used; the bottom panel (B) shows results when acrosome-reacted (CD46-exposed) sperm were used.
Fertilizing ability of CD46−/− mice.
The F1 offspring carrying the heterozygously CD46-disrupted allele were crossed, and 27 wild-type, 58 heterozygous, and 28 homozygous mice were obtained, respectively, in accordance with the principles of Mendelian inheritance. Mice of various genotype combinations showed normal fertility and produced litters. The average litter size of each combination is shown in Table 1 (experiment 1). The litter size appeared larger when females were mated with CD46−/− males. However, litter sizes were statistically indistinguishable when analyzed by Student's unpaired t test. In the next experiment, we prepared females of the inbred C57BL/6 and DBA/2N strains. DBA/2N is a strain known for its small litter size. We then mated the CD46−/− males which had been backcrossed with the C57BL/6 strain for more than five generations with the C57BL/6 and DBA/2N females, and the litter sizes were compared with those of females mated with CD46+/+ males. As shown in Table 1 (experiment 2), the average litter size was found to be significantly high (P < 0.02) in CD46−/− males when they were mated with the DBA/2N strain. In order to discover whether the difference was derived from the implantation or from the fertilization step, females from the genetic background of (C57BL/6 × DBA/2N)F1 (B6D2) were induced to superovulate and were caged with males. The B6D2 females were used as the oocyte donors because the females produce many oocytes during ovulation and mate with males with a high probability of success. The eggs were recovered from the oviducts 24 h after the hCG injection, the pronuclear formation was observed after staining with Hoechst stain 33342, and the efficiencies of fertilization were compared. As summarized in Table 1 (experiment 3), the CD46−/− sperm showed a significantly higher fertilizing ability (P < 0.02) against superovulated eggs than sperm from the CD46+/+ mice.
TABLE 1.
Assessment of fertilizing ability of CD46-disrupted micea
| Male genotype | Avg litter size (no. of births) of female of indicated genotype
|
Fertilization (%) of oviductal eggs (total no. of eggs/no. of females) | |||
|---|---|---|---|---|---|
| Expt 1
|
Expt 2
|
||||
| CD46−/− | CD46+/+ | C57BL/6 | DBA/2N | B6D2F1 | |
| CD46−/− | 8.2 ± 2.1 (20) | 9.0 ± 2.4 (21) | 8.9 ± 1.9 (39) | 7.5 ± 2.2 (67)∗ | 92.1 ± 7.2 (302/10)∗ |
| CD46+/+b | 7.4 ± 1.3 (10) | 7.9 ± 1.8 (16) | 9.0 ± 2.1 (40) | 6.5 ± 2.6 (57) | 83.3 ± 8.1 (315/10) |
The results are expressed as means ± standard deviations. The number of females used is indicated in parentheses. At least five different males were used in each experiment. All comparisons were made using the males from the same litter. In experiment 3, the eggs were recovered from the oviduct and were assessed for fertilization. ∗, P < 0.02 compared to results for CD46+/+ males.
CD46+/+ and CD46−/− mice used were littermates.
Increased spontaneous acrosome reaction in CD46−/− sperm.
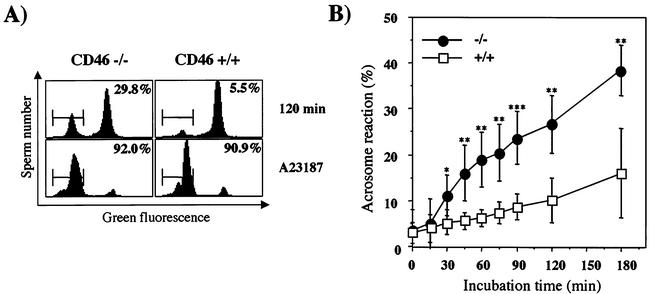
The acrosome reaction is a prerequisite for sperm to fertilize eggs and is reported to take place during the sperm penetration through the ZP. The acrosome reaction may also take place spontaneously without the aid of the ZP, and spontaneously acrosome-reacted sperm are also known to fertilize ZP-free eggs without difficulty. We therefore tried to examine the efficiency of inducing spontaneous acrosome reaction during the incubation of CD46−/− sperm in a fertilization medium. The acrosome of mouse sperm is small, and it is difficult to ascertain the acrosomal status under normal microscopic observation. In order to circumvent the problem and observe the acrosome reaction noninvasively, we used a “green sperm” transgenic mouse line. Sperm from this line have normal fertilizing ability but accumulate green fluorescent protein (GFP) in their acrosomes, allowing analysis of acrosomal status without any pretreatment and on a real-time basis (21, 22). In the present experiment, a green sperm transgenic mouse line was bred with a CD46−/− mouse line. At their F2 generation, CD46−/− sperm tagged with GFP in their acrosomes were produced and used to observe spontaneous acrosome reaction in vitro with a flow cytometer. As a result, the induction of spontaneous acrosome reaction was observed in a significantly larger population of the CD46−/− sperm than in the wild-type sperm during all stages of the incubation period examined. However, when the sperm were challenged with A23187, all of the wild-type and CD46−/− sperm responded equally to the ionophore and had induced acrosome reactions (Fig. 5). The difference was not due to the difference in the viabilities of the sperm. In both genetic backgrounds of mice, the viabilities of the sperm were not different when observed by flow cytometry after propidium iodide staining.
FIG. 5.
Competency of acrosome reaction in CD46−/− mice. (A) When the mice with the CD46−/− allele were bred with a green sperm transgenic mouse line and a double-transgenic mouse line, CD46−/− mice with green sperm were obtained. Sperm from cauda epididymis from this double-transgenic mouse line were incubated in TYH medium and analyzed for their acrosomal integrity by flow cytometry. The figure shows a sample histogram view of the measurement of a spontaneous acrosome reaction after 120 min of incubation. When sperm were treated with A23187 (Ca2+ ionophore), an acrosome reaction was induced in almost all the population of sperm in both the CD46+/+ and CD46−/− mouse lines. The GFP-positive peak indicates the acrosome-intact population, and the GFP-negative peak indicates the acrosome-reacted population. (B) Periodical observation of the percentage of spontaneous acrosome-reacted sperm during incubation in TYH medium. Sperm from CD46−/− (•) and wild-type (□) littermate mice were compared in six independent experiments. The values represent means ± standard deviations (two-tailed P values calculated were <0.03 [*], <0.01 [**], and <0.001 [***]).
DISCUSSION
It is clear that complement may have some effects on the implantation stage of reproduction. When complement regulatory protein Crry is disrupted, the Crry−/− embryos are developmentally arrested by complement up to day 9.5 of their embryogenesis. However, those Crry−/− embryos can be delivered from complement-deficient C3−/− mothers (44). Likewise, in earlier stages of reproduction, it is assumed that there is a mechanism to protect sperm from complement attack in the female reproductive tract. For this purpose, complement regulators such as CD55 and CD59 are reported to protect sperm from complement attack while sperm ascend the uterus and oviducts, where complement is abundant (5). Sperm need to undergo a morphological change called acrosome reaction before fertilizing eggs, and this change includes the alteration of the sperm plasma membrane. During the acrosome reaction, sperm lose their plasma membranes in the acrosome region of their heads, with concomitant loss of the plasma membrane-associated complement-regulating factors. The inner acrosomal membrane becomes the outermost membrane after the acrosome reaction in this area. CD46 is expressed exactly in this area where other complement regulators become absent. Naturally, it was assumed that CD46 must be necessary to protect acrosome-reacted sperm from complement attack. However, males deficient in the CD46 protein did not exhibit a detectable abnormality in spermatogenesis (data not shown) and, as demonstrated in the present experiments, the CD46-disrupted males showed no decrease in their fecundity at maturity. When we examined the complement deposition on acrosome-reacted sperm, the difference observed was negligible (Fig. 4). Combining these facts, we concluded that the primary role of CD46 on acrosome-reacted sperm is not likely the protection of the sperm from complement attack.
Another role of CD46 is also hypothesized for human sperm. Human sperm acrosomal proteases are released during the acrosome reaction, and the proteases cleave C3. Since CD46 on sperm can specifically bind to dimeric C3b, and human and hamster oocytes can activate the alternative pathway of complement and bind to human C3 fragments via complement receptors, the sperm CD46 protein functions as an egg-binding molecule through dimeric C3b (1). However, as shown in the present paper, CD46-deficient sperm retained their fertilizing ability in mice. Therefore, the hypothesis that CD46 is an egg-binding molecule also seemed improbable—at least in mice.
It is not surprising that the hypothesis based on the Ab- and/or ligand-added experiments became inapplicable when examined in gene disruption experiments. For example, acrosin (3), galactosyltransferase (18), and hyaluronidase (2) are all clearly shown to be significantly involved in sperm-egg interaction. However, none of these gene disruption mice became sterile. If the primary role of CD46 is not protection of sperm from complement or assistance with sperm-egg interaction, what in fact is it?
As shown in the present experiment, the CD46−/− sperm showed apparently increased spontaneous acrosome reaction by producing double-transgenic mouse lines that have a CD46−/− gene with an additional green sperm transgene (21). It is well known that spontaneously acrosome-reacted mouse sperm are capable of fertilizing ZP-free eggs and producing normal, fertile offspring (20). Moreover, the analysis of the spontaneous acrosome reaction in human sperm is reported to be useful for estimating the fertilizing ability of human sperm in the clinical field (26). The increased competency of spontaneous acrosome reaction demonstrated in the present paper could have been the cause of the increased fertilizing ability of CD46−/− sperm demonstrated by the increased litter size and the increased fertilization ratio in superovulated oviducts (Table 1). The mechanism of acceleration of the acrosome reaction remains unclear. The acrosome reaction is the phenomenon induced by the fusion between the plasma membrane and the outer acrosomal membrane. Topologically, this is not the area where CD46 can directly participate for the event. Therefore, it was presumed that the disappearance of CD46 from the acrosomal membrane may affect the distribution of membrane proteins which may associate with CD46. For example, all types of β1 integrins and tetraspanin proteins are demonstrated to bind with CD46 (16). In any case, it might be reasonable to assume that the disruption of CD46 could possibly change ion channel behavior so that ionic changes occur, thereby leading to somewhat elevated levels of cytoplasmic calcium that trigger the spontaneous acrosome reaction.
However, not only fertilin beta but also fertilin alpha and cyritestin disappear from sperm (7, 23, 34) in the case of fertilin beta disruption. Likewise, the cyritestin disruption caused the concomitant loss of fertilin beta from sperm. Therefore, it may not be proper to link the phenotype and the disrupted gene directly unless the phenotype can be explained with the gene on a molecular basis.
There is a notion that the spontaneous acrosome reaction is a nonphysiological event that occurs only in damaged sperm. However, the spontaneous acrosome reaction might be occurring naturally as a consequence of capacitation. According to a new paradigm of acrosome reaction postulated by Kim et al. (14), the spontaneous acrosome reaction might be a physiological event that occurs naturally as a consequence of capacitation. In this model, the ZP does not initiate the acrosome reaction but rather accelerates the rate of acrosomal exocytosis in sperm that have already initiated exocytosis. In fact, we previously demonstrated a swift release of GFP at the initial stages of the acrosome reaction followed by a delayed release of some other acrosomal antigens (22). Therefore, one can assume that in the CD46-null mice, larger numbers of competent sperm (i.e., sperm that have begun acrosomal exocytosis) reach the oocytes and are more proficient at fertilizing them, thus yielding a better fertilization rate.
CD46 is the gene preserved in humans, simians, swine, mice, rats, and guinea pigs. However, in mice, rats, and guinea pigs, the testis is the only organ that expresses CD46 (33). Moreover, in both mice and humans, the inner acrosomal membrane is the site where CD46 resides in sperm. Therefore, it was surprising for us to find no detrimental effect in the reproductive system of CD46-disrupted mice. We may not be able to conclude simply that CD46 does not function in sperm-egg interaction. When we consider the role of CD46, we also need to remember the case of Tyro-3 family gene disruption. Mice lacking any single receptor, or lacking any two of the three receptors, are viable and fertile, but male mice that lack all three receptors produce no mature sperm (17). The CD46-disrupted mice might show a very drastic phenotype when combined with other gene-disrupted mouse lines. The putative role of CD46 is the stabilization of the acrosomal membrane, but further investigation is necessary before we address a definite conclusion.
Acknowledgments
We are grateful to A. Fukui, A. Tsujimura, S. B. Moss, G. L. Gerton, and K. Nunoue for helpful discussions. We also thank N. Okada (Nagoya City University, Nagoya, Japan) and K. Toshimori (Miyazaki Medical College, Miyazaki, Japan) for providing the anti-mouse CD55 monoclonal Ab (RIKO-3) and anti-MC101, respectively.
This work was supported by grants from the Ministry of Education, Science, Sports, and Culture (11234203 and 11444).
REFERENCES
- 1.Anderson, D. J., A. F. Abbott, and R. M. Jack. 1993. The role of complement component C3b and its receptors in sperm-oocyte interaction. Proc. Natl. Acad. Sci. USA 90:10051-10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, D., S. I. Kashiwabara, A. Honda, K. Yamagata, Q. Wu, M. Ikawa, M. Okabe, and T. Baba. 2002. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J. Biol. Chem. 277:30310-30314. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T., S. Azuma, S. Kashiwabara, and Y. Toyoda. 1994. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem. 269:31845-31849. [PubMed] [Google Scholar]
- 4.Bookbinder, L. H., A. Cheng, and J. D. Bleil. 1995. Tissue- and species-specific expression of sp56, a mouse sperm fertilization protein. Science 269:86-89. [DOI] [PubMed] [Google Scholar]
- 5.Bozas, S. E., L. Kirszbaum, R. L. Sparrow, and I. D. Walker. 1993. Several vascular complement inhibitors are present on human sperm. Biol. Reprod. 48:503-511. [DOI] [PubMed] [Google Scholar]
- 6.Cervoni, F., T. J. Oglesby, E. M. Adams, C. Milesifluet, M. Nickells, P. Fenichel, J. P. Atkinson, and B. L. Hsi. 1992. Identification and characterization of membrane cofactor protein of human spermatozoa. J. Immunol. 148:1431-1437. [PubMed] [Google Scholar]
- 7.Cho, C., D. O. Bunch, J. E. Faure, E. H. Goulding, E. M. Eddy, P. Primakoff, and D. G. Myles. 1998. Fertilization defects in sperm from mice lacking fertilin beta. Science 281:1857-1859. [DOI] [PubMed] [Google Scholar]
- 8.D'Cruz, O. J., C. A. Toth, and G. G. Haas, Jr. 1996. Recombinant soluble human complement receptor type 1 inhibits antisperm antibody- and neutrophil-mediated injury to human sperm. Biol. Reprod. 54:1217-1228. [DOI] [PubMed] [Google Scholar]
- 9.Farries, T. C., and J. P. Atkinson. 1991. Evolution of the complement system. Immunol. Today 12:295-300. [DOI] [PubMed] [Google Scholar]
- 10.Fenichel, P., G. Dohr, C. Grivaux, F. Cervoni, M. Donzeau, and B. L. Hsi. 1990. Localization and characterization of the acrosomal antigen recognized by GB24 on human spermatozoa. Mol. Reprod. Dev. 27:173-178. [DOI] [PubMed] [Google Scholar]
- 11.Hara, T., Y. Suzuki, T. Nakazawa, H. Nishimura, S. Nagasawa, M. Nishiguchi, M. Matsumoto, M. Hatanaka, M. Kitamura, and T. Seya. 1998. Post-translational modification and intracellular localization of a splice product of CD46 cloned from human testis: role of the intracellular domains in O-glycosylation. Immunology 93:546-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa, M., M. Nonaka, N. Okada, and H. Okada. 1996. Molecular cloning of guinea pig membrane cofactor protein: preferential expression in testis. J. Immunol. 157:4946-4952. [PubMed] [Google Scholar]
- 13.Inoue, N., A. Fukui, M. Nomura, M. Matsumoto, Y. Nishizawa, K. Toyoshima, and T. Seya. 2001. A novel chicken membrane-associated complement regulatory protein: molecular cloning and functional characterization. J. Immunol. 166:424-431. [DOI] [PubMed] [Google Scholar]
- 14.Kim, K. S., J. A. Foster, and G. L. Gerton. 2001. Differential release of guinea pig sperm acrosomal components during exocytosis. Biol. Reprod. 64:148-156. [DOI] [PubMed] [Google Scholar]
- 15.Laird, P. W., A. Zijderveld, K. Linders, M. A. Rudnicki, R. Jaenisch, and A. Berns. 1991. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 19:4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozahic, S., D. Christiansen, S. Manie, D. Gerlier, M. Billard, C. Boucheix, and E. Rubinstein. 2000. CD46 (membrane cofactor protein) associates with multiple beta1 integrins and tetraspans. Eur. J. Immunol. 30:900-907. [DOI] [PubMed] [Google Scholar]
- 17.Lu, Q., M. Gore, Q. Zhang, T. Camenisch, S. Boast, F. Casagranda, C. Lai, M. K. Skinner, R. Klein, G. K. Matsushima, H. S. Earp, S. P. Goff, and G. Lemke. 1999. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398:723-728. [DOI] [PubMed] [Google Scholar]
- 18.Lu, Q., and B. D. Shur. 1997. Sperm from beta 1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development 124:4121-4131. [DOI] [PubMed] [Google Scholar]
- 19.Miwa, T., M. Nonaka, N. Okada, S. Wakana, T. Shiroishi, and H. Okada. 1998. Molecular cloning of rat and mouse membrane cofactor protein (MCP, CD46): preferential expression in testis and close linkage between the mouse Mcp and Cr2 genes on distal chromosome 1. Immunogenetics 48:363-371. [DOI] [PubMed] [Google Scholar]
- 20.Naito, K., Y. Toyoda, and R. Yanagimachi. 1992. Production of normal mice from oocytes fertilized and developed without zonae pellucidae. Hum. Reprod. 2:281-285. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi, T., M. Ikawa, S. Yamada, M. Parvinen, T. Baba, Y. Nishimune, and M. Okabe. 1999. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 449:277-283. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi, T., M. Ikawa, S. Yamada, K. Toshimori, and M. Okabe. 2001. Alkalinization of acrosome measured by GFP as a pH indicator and its relation to sperm capacitation. Dev. Biol. 237:222-231. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura, H., C. Cho, D. R. Branciforte, D. G. Myles, and P. Primakoff. 2001. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev. Biol. 233:204-213. [DOI] [PubMed] [Google Scholar]
- 24.Nomura, M., A. Tsujimura, K. Shida, M. Matsumoto, Y. Matsuda, K. Toyoshima, and T. Seya. 1999. Membrane and secretory forms of mouse membrane cofactor protein (CD46) generated from a single gene through alternative splicing. Immunogenetics 50:245-254. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka, M. I., G. Wang, T. Mori, H. Okada, and M. Nonaka. 2001. Novel androgen-dependent promoters direct expression of the C4b-binding protein alpha-chain gene in epididymis. J. Immunol. 166:4570-4577. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi, K., F. Saji, M. Kato, T. Tsutsui, T. Tomiyama, and O. Tanizawa. 1995. Acrobeads test: a new diagnostic test for assessment of the fertilizing capacity of human spermatozoa. Fertil. Steril. 63:625-630. [PubMed] [Google Scholar]
- 27.Ohta, R., M. Imai, Y. Fukuoka, T. Miwa, N. Okada, and H. Okada. 1999. Characterization of mouse DAF on transfectant cells using monoclonal antibodies which recognize different epitopes. Microbiol. Immunol. 43:1045-1056. [DOI] [PubMed] [Google Scholar]
- 28.Okabe, M., M. Nagira, Y. Kawai, S. Matzno, T. Mimura, and T. Mayumi. 1990. A human sperm antigen possibly involved in binding and/or fusion with zona-free hamster eggs. Fertil. Steril. 54:1121-1126. [DOI] [PubMed] [Google Scholar]
- 29.Okabe, M., X. Ying, M. Nagira, M. Ikawa, Y. Kohama, T. Mimura, and K. Tanaka. 1992. Homology of an acrosome-reacted sperm-specific antigen to CD46. J. Pharmacobio-dyn 15:455-459. [DOI] [PubMed] [Google Scholar]
- 30.Qian, Y. M., X. Qin, T. Miwa, X. Sun, J. A. Halperin, and W. C. Song. 2000. Identification and functional characterization of a new gene encoding the mouse terminal complement inhibitor CD59. J. Immunol. 165:2528-2534. [DOI] [PubMed] [Google Scholar]
- 31.Scofield, V. L., J. M. Schlumpberger, L. A. West, and I. L. Weissman. 1982. Protochordate allorecognition is controlled by a MHC-like gene system. Nature 295:499-502. [DOI] [PubMed] [Google Scholar]
- 32.Seya, T., T. Hara, M. Matsumoto, H. Kiyohara, I. Nakanishi, T. Kinouchi, M. Okabe, A. Shimizu, and H. Akedo. 1993. Membrane cofactor protein (MCP, CD46) in seminal plasma and on spermatozoa in normal and “sterile” subjects. Eur. J. Immunol. 23:1322-1327. [DOI] [PubMed] [Google Scholar]
- 33.Seya, T., M. Nomura, Y. Murakami, N. A. Begum, M. Matsumoto, and S. Nagasawa. 1998. CD46 (membrane cofactor protein of complement, measles virus receptor): structural and functional divergence among species. Int. J. Mol. Med. 1:809-816. [DOI] [PubMed] [Google Scholar]
- 34.Shamsadin, R., I. M. Adham, K. Nayernia, U. A. Heinlein, H. Oberwinkler, and W. Engel. 1999. Male mice deficient for germ-cell cyritestin are infertile. Biol. Reprod. 61:1445-1451. [DOI] [PubMed] [Google Scholar]
- 35.Shida, K., M. Nomura, M. Matsumoto, Y. Suzuki, K. Toyoshima, and T. Seya. 1999. The 3′-UT of the ubiquitous mRNA of human CD46 confers selective suppression of protein production in murine cells. Eur. J. Immunol. 29:3603-3608. [DOI] [PubMed] [Google Scholar]
- 36.Simpson, K. L., and C. H. Holmes. 1994. Differential expression of complement regulatory proteins decay-accelerating factor (CD55), membrane cofactor protein (CD46) and CD59 during human spermatogenesis. Immunology 81:452-461. [PMC free article] [PubMed] [Google Scholar]
- 37.Spicer, A. P., M. F. Seldin, and S. J. Gendler. 1995. Molecular cloning and chromosomal localization of the mouse decay-accelerating factor genes. Duplicated genes encode glycosylphosphatidylinositol-anchored and transmembrane forms. J. Immunol. 155:3079-3091. [PubMed] [Google Scholar]
- 38.Sundstrom, S. A., B. S. Komm, H. Ponce-de-Leon, Z. Yi, C. Teuscher, and C. R. Lyttle. 1989. Estrogen regulation of tissue-specific expression of complement C3. J. Biol. Chem. 264:16941-16947. [PubMed] [Google Scholar]
- 39.Taylor, C. T., and P. M. Johnson. 1996. Complement-binding proteins are strongly expressed by human preimplantation blastocysts and cumulus cells as well as gametes. Mol. Hum. Reprod. 2:52-59. [DOI] [PubMed] [Google Scholar]
- 40.Toshimori, K., I. Tanii, and S. Araki. 1995. Intra-acrosomal 155,000 dalton protein increases the antigenicity during mouse sperm maturation in the epididymis: a study using a monoclonal antibody anti-MC101. Mol. Reprod. Dev. 42:72-79. [DOI] [PubMed] [Google Scholar]
- 41.Toyoda, Y., M. Yokoyama, and T. Hoshi. 1971. Studies on the fertilization of mouse egg in vitro. Jpn. J. Anim. Reprod. 16:147-151. [Google Scholar]
- 42.Tsujimura, A., K. Shida, M. Kitamura, M. Nomura, J. Takeda, H. Tanaka, M. Matsumoto, K. Matsumiya, A. Okuyama, Y. Nishimune, M. Okabe, and T. Seya. 1998. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem. J. 330:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tybulewicz, V. L., C. E. Crawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 44.Xu, C., D. Mao, V. M. Holers, B. Palanca, A. M. Cheng, and H. Molina. 2000. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 287:498-501. [DOI] [PubMed] [Google Scholar]
- 45.Yanagimachi, R. 1994. Mammalian fertilization, 2nd ed. Raven Press, Ltd., New York, N.Y.