Abstract
Phagosomal biogenesis is central for microbial killing and antigen presentation by leukocytes. However, the molecular mechanisms governing phagosome maturation are poorly understood. We analyzed the role and site of action of phosphatidylinositol 3-kinases (PI3K) and of Rab GTPases in maturation using both professional and engineered phagocytes. Rab5, which is recruited rapidly and transiently to the phagosome, was found to be essential for the recruitment of Rab7 and for progression to phagolysosomes. Similarly, functional PI3K is required for successful maturation. Remarkably, inhibition of PI3K did not preclude Rab5 recruitment to phagosomes but instead enhanced and prolonged it. Moreover, in the presence of PI3K inhibitors Rab5 was found to be active, as deduced from measurements of early endosome antigen 1 binding and by photobleaching recovery determinations. Though their ability to fuse with late endosomes and lysosomes was virtually eliminated by wortmannin, phagosomes nevertheless recruited a sizable amount of Rab7. Moreover, Rab7 recruited to phagosomes in the presence of PI3K antagonists retained the ability to bind its effector, Rab7-interacting lysosomal protein, suggesting that it is functionally active. These findings imply that (i) dissociation of Rab5 from phagosomes requires products of PI3K, (ii) PI3K-dependent effectors of Rab5 are not essential for the recruitment of Rab7 by phagosomes, and (iii) recruitment and activation of Rab7 are insufficient to induce fusion of phagosomes with late endosomes and lysosomes. Accordingly, transfection of constitutively active Rab7 did not bypass the block of phagolysosome formation exerted by wortmannin. We propose that Rab5 activates both PI3K-dependent and PI3K-independent effectors that act in parallel to promote phagosome maturation.
Engulfment of pathogens into phagocytic vacuoles is an essential component of the innate immune response (1, 36). Following formation, the vacuoles or nascent phagosomes acquire microbicidal properties by a maturation process that involves sequential fusion events with compartments of the endocytic pathway, culminating with the formation of a hybrid organelle, the phagolysosome (12, 13, 37). The factors that initiate and coordinate the maturation process are poorly understood. By analogy with the progression of the endocytic pathway, it is thought that Rab GTPases orchestrate the sequence of fusion events. Accordingly, Rab5 has been detected in early phagosomes, where it resides transiently (2, 12, 20, 33) and Rab7 is present at later stages of maturation (13). In addition, proteomic analysis identified also Rab2, Rab3, Rab10, Rab11, and Rab14 as components of the phagosomal membrane (17).
Rab5 directs the fusion of early endosomes. It is recruited to endocytic vesicles and is also present in sorting endosomes (5, 18, 26). Bridging between the two compartments, which leads to their fusion, is thought to be the role of early endosome antigen 1 (EEA1), an effector that contains two spatially separate Rab5-binding domains (7, 34). While Rab5 is constitutively associated with sorting endosomes, it is scarce at the plasmalemma, and it is unclear what mediates its recruitment to newly formed endocytic vesicles, though a role for RIN1 has been suggested (35). Even less is known about the mechanism leading to Rab5 association with nascent phagosomes, which are equivalent to endocytic vesicles in the maturation sequence. It is likely that signals emanating from phagocytic receptors direct the recruitment of Rab5 to the nascent phagosome. Prominent among these is the activation of phosphatidylinositol 3-kinases (PI3K), which have been recently reported to be essential for proper phagosomal maturation (16, 33, 37). Phagosomes formed by cells treated with wortmannin had a reduced content of EEA1 (16, 37), consistent with the notion that Rab5, which serves to anchor it to the membrane, may not be properly recruited to phagosomes when PI3K is impaired. However, in addition to its dual Rab5-binding domains, EEA1 has a FYVE domain that binds to phosphatidylinositol 3-phosphate [PI(3)P], a product of the class III PI3K. Because these kinases are also sensitive to wortmannin, loss of EEA1 from the phagosomes may have resulted from depletion of PI(3)P and is not necessarily an indication of Rab5 density or activity at the membrane. For these reasons, the role of PI3K in the recruitment and activation of Rab5 remains to be defined.
Rab7 appears on the phagosomal membrane after Rab5 has been recruited and likely resides downstream in the maturation sequence. Indeed, the sole known effector of Rab7, termed Rab7-interacting lysosomal protein (RILP), is thought to mediate fusion of late endosomes with lysosomes (8, 21), and preliminary evidence indicates that it also functions in phagolysosome formation (R. E. Harrison, C. Bucci, and S. Grinstein, unpublished observations). Because fusion of phagosomes with lysosomes is precluded by wortmannin, it is conceivable that Rab7 fails to bind or become activated at the phagosomal membrane, either because Rab5 recruitment is defective under these conditions or, alternatively, because the putative connection between Rab5 and the acquisition of Rab7 has been disrupted. These predictions were tested experimentally in the present report. Specifically, we used fluorescent chimeric constructs of Rab5, Rab7, and RILP, in combination with fluorescence recovery after photobleaching (FRAP), to define the kinetics of association and the state of activation of the GTPases in control and wortmannin-treated phagosomes. Contrary to our predictions, we found that both Rab5 and Rab7 are recruited and activated in phagosomes of wortmannin-treated cells, implying that PI3K is not required for these events and is instead involved in parallel and/or downstream events that are essential for phagosomal maturation.
MATERIALS AND METHODS
Reagents, antibodies, and constructs.
Dulbecco's minimal Eagle's medium and fetal bovine serum were from Wisent Inc. HEPES-buffered RPMI, wortmannin, and human immunoglobulin G (IgG) were from Sigma. The 3-μm-diameter latex beads were from Bangs Laboratories. Sheep red blood cells (RBC) and rabbit anti-RBC IgG were from ICN-Cappel. N-(3-Triethylammoniumpropyl)-4-(6-[4-{diethylamino}phenyl]hexatrienyl)pyridinium dibromide (FM4-64) and tetramethylrhodamine dextran (10,000 kDa) were from Molecular Probes. Anti-lysosomal associated membrane protein 1 (anti-LAMP-1) antibodies were from the Developmental Studies Hybridoma Bank (Iowa City, Iowa). Anti-c-Myc antibody and goat anti-EEA1 antibody were from Santa Cruz Biotechnology. The monoclonal anti-hemagglutinin (anti-HA) antibody was from BabCo (Berkeley, Calif.). The antibodies to lysobisphosphatidic acid (LBPA) have been described earlier (22, 23). Fluorochrome-conjugated secondary anti-human, anti-mouse, anti-rat, anti-rabbit, and anti-goat antibodies were all from Jackson ImmunoResearch Laboratories.
Cell culture and transfection.
Culture conditions for macrophage RAW 264.7 and Chinese hamster ovary cells stably transfected with FcγRIIA receptors (CHO-IIA) have been previously described (37). Where specified, the cells were treated with 100 nM wortmannin for 30 min at 37°C.
The vector directing the expression of a fusion of syntaxin 13 with green fluorescent protein (GFP) was described by Collins et al. (10a). The plasmid encoding RN-tre-GFP was described in reference 24. The vector encoding RILP-HA was generated by Cantalupo et al. (8). The generation of the plasmids used for expression of wild-type and mutant forms of either epitope-tagged or GFP-conjugated Rab5 (S34N and Q79L) and Rab7 (Q67L) is described in detail elsewhere (6, 32). In all cases the cells were transiently transfected using FuGENE 6 (Roche Molecular Biochemicals) as suggested by the manufacturer.
Phagocytosis assays and wortmannin treatment.
Fresh or fixed sheep RBC were opsonized with rabbit anti-sheep RBC antibody (1:50). Latex beads (3-μm diameter) were opsonized by incubation with human IgG (1 mg/ml) for 1 h at room temperature or overnight at 4°C. Before phagocytosis the cells were treated with or without wortmannin in the absence of serum. The cells were incubated at 37°C with either RBC or latex beads for the specified time, excess particles were washed away with phosphate-buffered saline (PBS), and the cells were incubated further in culture medium at 37°C for the indicated times. To identify adherent opsonized RBC or opsonized latex beads that were not internalized, the cells were incubated for 10 min at 4°C in HEPES-buffered medium containing Cy5-labeled donkey anti-rabbit IgG or anti-human IgG (1:1,000). Where indicated, the onset of phagocytosis was synchronized by allowing the particles to bind to cells on ice for 5 min, and ingestion was then initiated by incubation at 37°C.
Immunofluorescence and confocal microscopy.
Prior to immunostaining the cells were fixed for 60 min in 4% formaldehyde in PBS, permeabilized by incubation in 0.1% Triton X-100 (vol/vol) in PBS for 20 min, and blocked for 30 min with 5% donkey serum (Gibco-BRL) in PBS (vol/vol). All steps were carried out at room temperature. For LAMP-1 staining, which is incompatible with the above protocol, CHO-IIA cells were fixed and permeabilized with methanol. Permeabilized cells were incubated with primary antibody for 1 h at room temperature, washed extensively, and then incubated with secondary antibodies for 1 h at room temperature and washed again. The following primary antibodies were used at the indicated dilutions: EEA1, 1:50; LAMP-1, 1:4; Myc, 1:200; and HA, 1:1,000. LBPA immunostaining was performed using a 1:400 dilution, as previously described (23). Coverslips were then mounted and fixed onto glass slides using Dako mounting reagent (Dako Corporation). Both live and fixed cells were analyzed by confocal microscopy using a LSM 510 laser scanning confocal microscope (Zeiss) with a 100× oil immersion objective. Digital images were prepared using Adobe Photoshop 6 and Adobe Illustrator 10 (Adobe Systems Inc.). Quantification of immunofluorescence was performed using NIH Scion Image software.
To estimate FRAP CHO-IIA cells transfected with WT-Rab5-GFP or CA-Rab5-GFP were allowed to internalize opsonized 3-μm-diameter beads. The selected phagosome was photobleached using the 488-nm laser line of the Zeiss LSM 510 confocal microscope at full power, resulting in a 50 to 80% reduction in the fluorescence intensity. The bleached area was 4 μm in diameter, exceeding the diameter of the phagosome (3 μm). The recovery of fluorescence was then monitored over time by scanning the bleached area at the conventional (low) laser power to minimize photobleaching during sampling. To analyze the rate of recovery, we compared the fluorescence of the bleached area to that of an adjacent unbleached area of the same cell with similar fluorescence intensity. For each time point, the fluorescence of the bleached area was normalized to that of the corresponding control (unbleached) area to correct for possible drift of the focal plane or photobleaching incurred during the low light sampling. A similar protocol was used to measure FRAP in endosomes. All FRAP measurements were performed at 37°C.
Uptake of FM4-64.
Two types of experiments were performed using the solvochromic dye FM4-64. In one case FM4-64 was used to determine whether early endosomes had access to preformed phagosomes. For these experiments cells were allowed to internalize IgG-opsonized particles for 20 min. After phagocytosis, the cells were washed to remove extracellular particles and then cooled and treated with fluorescein isothiocyanate-labeled donkey anti-human IgG (1:1,000) for 10 min to identify adherent, incompletely internalized beads. Next, the cells were pulsed with FM4-64 (1.5 μM) for 10 min and immediately analyzed by fluorescence microscopy.
In the second type of experiment, the lysosomes of RAW or CHO-IIA cells were prelabeled by incubation with FM4-64 (1.5 μM) for 1 h, and this was followed by a 3-h chase in the absence of the dye. The cells were then challenged with IgG-opsonized particles. After 20 min at 37°C, the cells were washed, cooled, and treated as described above to label extracellular beads. Finally, the phagosomes were allowed to mature by a further incubation for 30 or 60 min at 37°C. As before, the cells were analyzed immediately by fluorescence microscopy to determine the percentage of FM4-64-positive phagosomes.
All quantitative data are presented as means ± standard errors (SE) of the number of experiments indicated.
RESULTS
Effect of wortmannin on fusion of phagosomes with late endosomes and lysosomes.
Two model systems were employed throughout this manuscript to analyze phagosome maturation: RAW264.7 cells, a murine line of monocytes/macrophages, and CHO-IIA cells. RAW cells were chosen because they are closely related to primary macrophages, which are professional phagocytes. However, myeloid cells are notoriously difficult to transfect, limiting the number of transfected cells available for analysis. To overcome this problem we also used CHO-IIA cells that, unlike RAW cells, can be transfected efficiently and express higher levels of ectopic gene products. CHO-IIA is a line of engineered phagocytes generated by stable transfection of cDNA encoding the human FcγIIA receptor into Chinese hamster ovary cells. As described earlier (14), expression of Fcγ receptors on the surface of nonphagocytic cells confers onto them the ability to internalize IgG-coated particles (19). Moreover, the resulting phagosomes proceed to mature in a manner that is indistinguishable from that of professional phagocytes (14).
We tested the effect of wortmannin on the maturation of phagosomes in CHO-IIA cells. Although phagocytosis is known to be impaired by wortmannin (4, 11), the effect is directly proportional to the size of the particles used (11) so that phagocytosis of beads with diameters of ≤3 μm proceeds at sufficiently high rates to permit analysis of maturation. We therefore used 3-μm-diameter latex beads opsonized with IgG for all our studies and identified fully internalized beads by their inaccessibility to anti-IgG antibodies. Lysosomes were labeled by pulsing the cells with FM4-64, and this was followed by a prolonged chase period. FM4-64 is an impermeant solvochromic dye that fluoresces brightly when associated with biological membranes and can be used to label components of the endocytic pathway. Lysosomes stained with FM4-64 could be readily seen to merge with phagosomes within 30 min in control cells (not shown). By contrast, a profound inhibition of phagolysosomal fusion was noted in cells pretreated with wortmannin. After 60 min only 13% ± 6.4% of the phagosomes contained the lysosomal marker. The maturation process was seemingly arrested at a comparatively early stage, because wortmannin also blocked the recruitment to phagosomes of LBPA, a marker of late endosomes (22, 23). These results resemble those obtained using professional phagocytes (16, 37), validating the use of CHO-IIA cells to study the role of PI3K in maturation. It is noteworthy that, while primary monocytes and macrophages express three types of Fcγ receptors (I, IIB, and III), the engineered CHO-IIA line expresses only one (FcγIIA). Nevertheless, all the aspects of maturation analyzed to date in both systems are, at least qualitatively, very similar. These include phagosomal acquisition of Rab5, PI(3)P, EEA1, Hrs, Rab7, RILP, LAMP-1, LBPA, and other markers; vacuolar ATPase-mediated luminal acidification; and centripetal motion along microtubules; etc. This implies that a virtually identical maturation program is triggered by engagement of different Fcγ receptors and confirms the usefulness of CHO-IIA cells in the study of phagosome maturation.
Effect of wortmannin on acquisition of Rab5 by phagosomes.
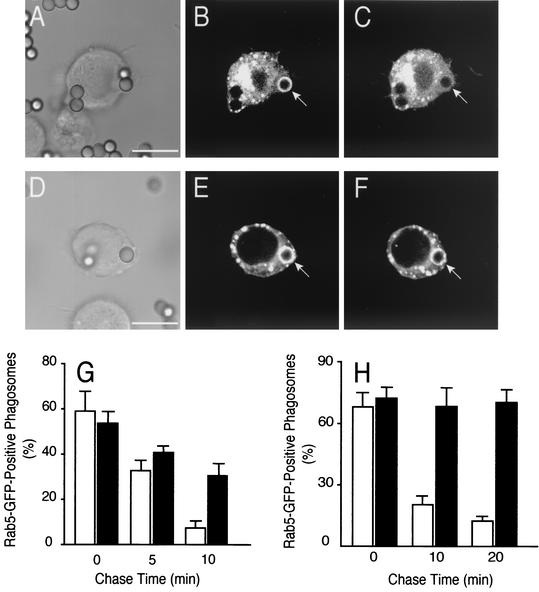
The role of PI3K in the phagosomal acquisition and activation of Rab5 was studied next, by transient transfection of a fluorescent fusion protein, Rab5-GFP. As illustrated in Fig. 1A and C and reported earlier (31), in otherwise-untreated cells Rab5-GFP can be seen to associate transiently with early phagosomes. In RAW cells ∼60% of the phagosomes displayed Rab5 following a 10-min phagocytosis pulse, but less than 10% were stained following a 10-min chase (Fig. 1G). The kinetics of disappearance was slightly slower in CHO-IIA cells (Fig. 1H).
FIG. 1.
Effect of wortmannin on Rab5 acquisition by phagosomes. (A to F) Time-lapse recordings of the distribution of Rab5-GFP during phagosome maturation. RAW cells were transiently transfected with Rab5-GFP. Untreated (A to C) or wortmannin-pretreated (D to F) cells were allowed to internalize IgG-opsonized beads while fluorescence images were acquired at regular intervals. The arrows in panels B and E point to phagosomes that had formed within 1 min of acquisition of the image. (A and D) Corresponding differential interference contrast images. (C and F) Images of the corresponding fields acquired after an additional 3 min. Scale bars = 10 μm. (G and H) Quantification of the fraction of Rab5-positive phagosomes in control (open bars) and wortmannin-treated (black bars) cells as a function of time after phagosome sealing in RAW (10-min phagocytosis pulse [G]) and CHO-IIA cells (20-min phagocytosis pulse [H]). Note that the time scales are different in these panels. Data are means + SE (error bars) of four separate experiments each with at least 100 individual phagosomes from ∼30 different cells expressing low levels of Rab5-GFP counted per condition.
We used the irreversible inhibitor, wortmannin, to address the role of PI3K in Rab5 recruitment. As shown in Fig. 1D to F, Rab5 also associated with phagosomes formed by wortmannin-treated cells. In fact, the fluorescence intensity was routinely greater in these cells than in untreated control cells (compare Fig. 1B and E). More strikingly, Rab5 remained associated with phagosomes for much longer periods in wortmannin-treated than in control cells (Fig. 1E to H). After a 10-min chase 30% ± 5.5% of the phagosomes were Rab5 positive in wortmannin-treated RAW cells, while only 7% ± 2.5% were labeled in the controls. The effect was even more pronounced in CHO-IIA cells, where little disappearance was apparent even after a 20-min chase. Thus, the inhibition of phagosome maturation induced by wortmannin cannot be attributed to the failure to recruit Rab5 and may instead be due to differences in its state of activation.
Rab5 is active in phagosomes of wortmannin-treated cells.
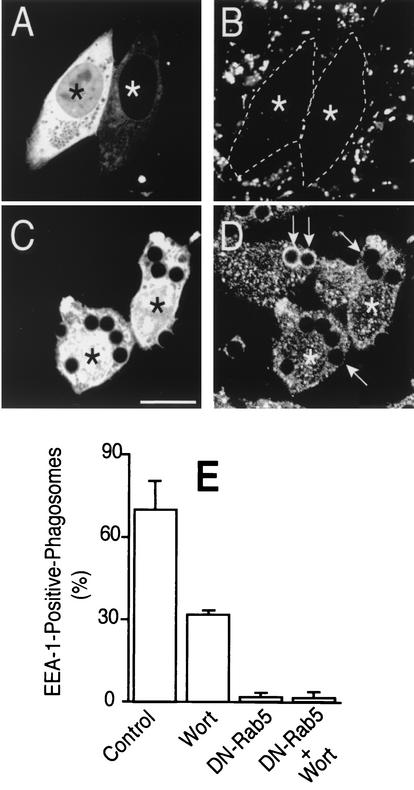
Rab proteins exist in active (GTP-bound) and inactive (GDP-bound) states. We investigated whether wortmannin prevents the activation of the Rab5 detected on the phagosomal membrane. Several criteria were applied to assess the state of Rab5 activation in situ. First, we measured the interaction of EEA1 with phagosomes. Because the Rab5-binding domains of EEA1 recognize only the GTP-bound form of the protein, the extent of association can be used to monitor the state of activation of Rab5. It must be borne in mind, however, that EEA1 can attach to PI(3)P on the phagosomal membrane via its FYVE domain. However, elimination of PI(3)P by inhibition of PI3K results only in partial displacement of EEA1 (Fig. 2E), implying that the latter has additional sites of attachment, likely via active Rab5. This assumption was tested by transfecting cells with Rab5(S34N), a dominant-negative form of the GTPase (DN-Rab5). The effectiveness of this inhibitory construct was first validated by monitoring the rate of fluid phase endocytosis, which is known to require active Rab5 (25). Expression of DN-Rab5 prevented uptake of labeled dextran (Fig. 2A and B) yet had no discernible effects on phagocytic efficiency; in four experiments the phagocytic index was statistically indistinguishable in control and DN-Rab5 expressing cells (362 ± 41 and 359 ± 35 particles/100 cells, respectively). More importantly, the dominant-negative Rab5 also eliminated the wortmannin-resistant fraction of EEA1 binding to phagosomes (Fig. 2C, D, and E), implying that this component is mediated by the active, GTP-bound form of Rab5. Similar effects were noted 10 and 20 min after phagocytosis. The effects of DN-Rab5 could be studied only in CHO-IIA cells, because the low levels of expression attained in RAW cells were insufficient to prevent fusion of endosomes with phagosomes or the clathrin-mediated endocytosis of aggregated IgG, which is blocked by the dominant-negative construct in CHO-IIA cells (not illustrated). These findings stress the usefulness of CHO-IIA in instances where high levels of ectopic expression of a protein are required.
FIG. 2.
Effect of dominant-negative Rab5 on fluid phase uptake and EEA1 acquisition. CHO-IIA cells were transiently transfected with dominant-negative Rab5-GFP (DN-Rab5). (A and B) The cells were incubated for 30 min in medium containing rhodamine-dextran (100 μg/ml) to assess fluid phase intake. (C and D) The cells were allowed to internalize IgG-opsonized beads for 20 min, and this was followed by fixation and EEA1 immunostaining. (A and C) GFP fluorescence; (B) fluorescence of rhodamine-dextran; (D) distribution of EEA1. Asterisks identify transfected cells. Arrows point to phagosomes. In panel B, transfected cells are outlined to facilitate identification of their boundaries. Scale bar = 10 μm. (E) Quantification of the effects of DN-Rab5 and wortmannin (Wort) on EEA1 acquisition by phagosomes in CHO-IIA cells. Cells were allowed to internalize beads for 20 min, fixed, and immunostained for EEA1. Where indicated, cells were pretreated with 100 nM wortmannin for 30 min. The cells were analyzed microscopically, and phagosomes lined by clearly observable EEA1 were scored. Data are means + SE (error bars) of four separate experiments, each with at least 100 individual phagosomes from 30 to 40 different cells counted per condition.
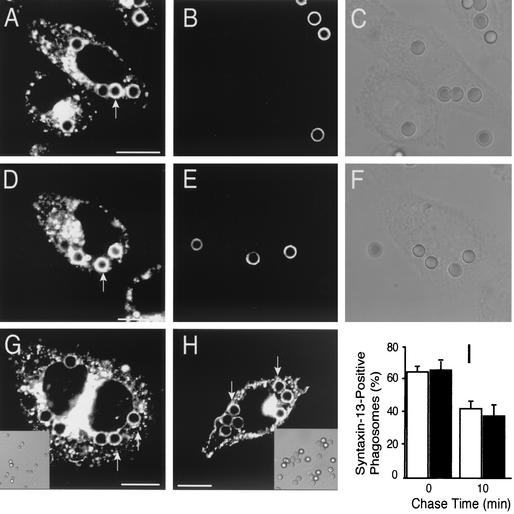
The ability of wortmannin-treated phagosomes to bind EEA1 in a DN-Rab5-sensitive manner suggests that Rab5 is at least partly active in cells treated with the inhibitor. In an attempt to confirm this notion, we measured the ability of phagosomes to fuse with early endosomes in control and wortmannin-treated cells. Because Rab5 is thought to mediate fusion of phagosomes with early endosomes (2, 20), we reasoned that delivery to the phagosomal lumen of a marker trapped in early endosomes could be used as an index of Rab5 activity. Such experiments are illustrated in Fig. 3. The cells were initially allowed to internalize opsonized latex beads for 20 min. Next, beads that were bound but not yet internalized were identified by addition of anti-IgG at 4°C (e.g., Fig. 3B and E). This step was essential to differentiate delivery of endosomal markers to a preformed phagosome, from merely trapping the fluid-phase marker during phagosome formation. Then, early endosomes were loaded with FM4-64 and fusion of newly formed endosomes with preformed phagosomes was monitored by confocal microscopy for the next 10 min. Fusion of early endosomes with phagosomes, revealed by the appearance of a rim of FM4-64 fluorescence around the phagocytic particle, was readily detectable in control cells (Fig. 3A to C) but also in wortmannin-treated cells (Fig. 3D to F). Similar results were obtained using RAW cells (not illustrated). That fusion of phagosomes with early endosomes persists after treatment with wortmannin was also demonstrated by monitoring the distribution of the early endosomal marker syntaxin 13 (28). Cells were transfected with a GFP fusion of this early endosomal SNARE and analyzed after ingestion of opsonized beads. As illustrated in Fig. 3G and H and summarized in Fig. 3I, endosomes containing syntaxin 13 merged with phagosomes to a similar extent and comparable kinetics in both control and wortmannin-treated cells. Very similar results were obtained with RAW cells (not shown). Together, these results suggest that fusion-competent, presumably GTP-bound Rab5 is present on the membrane of wortmannin-treated phagosomes.
FIG. 3.
Effect of wortmannin on fusion of nascent phagosomes with early endosomes. (A to F) CHO-IIA cells that were untreated (A to C) or pretreated with wortmannin (D to F) were allowed to internalize IgG-opsonized latex beads for 20 min. After washing excess beads, the cells were incubated briefly on ice with anti-human antibody conjugated with fluorescein isothiocyanate to identify adherent, incompletely internalized beads (B and E). The cells were then incubated for an additional 10 min at 37°C with FM4-64.The red fluorescence of FM4-64 is shown in A and D (arrows). C and F are the differential interference contrast images corresponding to A and D, respectively. (G to I) CHO-IIA cells transiently transfected with syntaxin 13-GFP were treated with wortmannin (H and black columns in I) or left untreated (G and empty columns in I) and allowed to internalize particles for 20 min. Adherent, incompletely internalized beads were then stained as above using Texas red-conjugated antibodies. Lastly, the cells were chased for the indicated times and fixed, and confocal fluorescence microscopy was used to visualize the distribution of syntaxin 13. (G and H) representative fluorescence images, with corresponding differential interference contrast insets. Arrows point to phagosomes. Scale bars =10 μm. (I) Quantitation of the effect of wortmannin on syntaxin 13 acquisition by phagosomes. Data are means + SE (error bars) of three separate experiments, each with at least 100 individual phagosomes from 30 to 50 different cells counted.
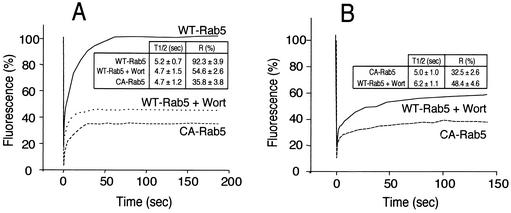
FRAP was used as a final criterion to assess the state of activation of Rab5 on wortmannin-treated phagosomes. Because the rate of dissociation of Rab GTPases from membranes is thought to be determined by the conversion of the GTP-bound to the GDP-bound forms, measurements of the rate of Rab5 exchange from the phagosome should provide an indication of its activation state. Dissociation rates can be inferred from the rate of fluorescence recovery after an organelle expressing a labeled protein has been photobleached. This approach was recently used successfully to assess the effect of RILP on the activation state of Rab7 (21). We therefore compared the rate and extent of FRAP of Rab5 in phagosomes and endosomes. The latter were used to validate the sensitivity of the assay. Cells were transfected with GFP fusions of either wild-type Rab5-GFP or Rab5(Q79L), a constitutively active mutant (CA-Rab5). Typical FRAP measurements in endosomes are illustrated in Fig. 4A. Following bleaching of wild-type Rab5, recovery was fast (half-life = 5.2 s) and nearly complete (fractional recovery = 92.3% ± 4%; see Fig. 4A). This implies that most of the endosomal Rab5 turns over rapidly, dissociating when GTP is hydrolyzed and reassociating when GDP is exchanged for GTP. Accordingly, when constitutively active Rab5-GFP was expressed, recovery after photobleaching was very poor. Only 35.8% ± 4% recovery was achieved after 200 s, the longest time studied. Having validated the use of FRAP, we proceeded to analyze the behavior of phagosomes treated with wortmannin (normal phagosomes could not be studied because the short time of residence of wild-type Rab5 in untreated cells precludes FRAP studies). As shown in Fig. 4B, the recovery of fluorescence was poor in wortmannin-treated phagosomes (48.4% ± 5%), implying that Rab5 is largely in the active state. In accordance with this interpretation, the extent of the recovery was only slightly greater than that of constitutively active Rab5 (32.5% ± 3%). In both instances, part of the recovery is likely due to lateral diffusion of a fraction of fluorescent Rab5 that remains unbleached at the poles of the phagosome, which lie outside the focal plane of the bleaching laser. Under the conditions used, a depth of only about 1.8 μm, less than the diameter of the phagosome, was effectively bleached (unpublished observations). Of interest, treatment with wortmannin also greatly reduced the fraction of endosomal Rab5 that recovered after photobleaching (Fig. 4A), implying that products of PI3K are also required for deactivation of this GTPase in endosomes.
FIG. 4.
Rab5-GFP FRAP. CHO-IIA cells were transfected with either wild-type (WT) or constitutively active (CA) Rab5-GFP. Where indicated, the cells had been pretreated with wortmannin (Wort). (A) Labeled endosomes were identified and their initial fluorescence quantified and defined as 100%. Bleaching was then performed and fluorescence recovery monitored as described in Materials and Methods. (B) Cells transfected with WT-Rab5 were treated with wortmannin, while cells transfected with CA-Rab5 were left untreated. The cells were then allowed to ingest opsonized beads, fluorescent phagosomes were identified, and FRAP was measured as above. Representative experiments are shown in the main panels. The inset tables show the corresponding times for 50% recovery (T1/2) and the fractional recovery (R). Data in the tables are means + SE (error bars) of 8 to 10 determinations, each involving 10 different cells.
Jointly, the EEA1, FM4-64, syntaxin 13, and FRAP experiments indicate that Rab5 is largely in the active state in phagosomes formed by wortmannin-treated cells.
Overexpression of DN-Rab5 arrests phagosome maturation.
While earlier evidence had demonstrated that wortmannin blocks phagolysosome formation (16, 37) (see above), the experiments summarized above showed that Rab5 is active in the membrane of phagosomes treated with the PI3K inhibitor. Two explanations may account for these observations: (i) the PI3K-dependent step may lie downstream or on an arm of the fusogenic pathway that is parallel to that involving Rab5, or (ii) Rab5 may not be part of the pathway required for phagosome fusion with lysosomes. While Rab5 is known to be an essential component of progression along the endocytic pathway (5; for a review, see reference 39), the role of Rab5 in phagosome maturation has not been addressed directly.
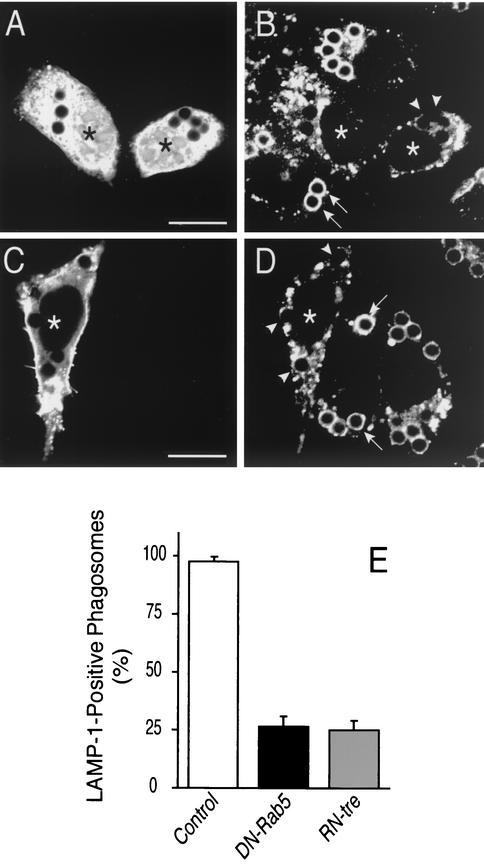
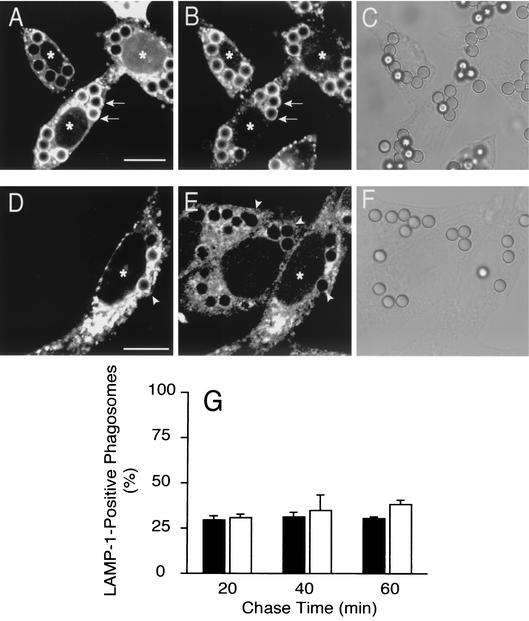
The requirement for Rab5 in phagosome maturation was tested by overexpression of DN-Rab5. CHO-IIA cells were used for these studies, to attain higher levels of the inhibitory protein. As shown in Fig. 5A and B incorporation of LAMP-1, a late endosomal/lysosomal marker, into the phagosomal membrane was greatly reduced by expression of DN-Rab5. In 3 experiments, inhibition averaged 72% ± 3.9% (Fig. 5E).
FIG. 5.
Role of Rab5 in fusion of phagosomes with late endocytic compartments. CHO-IIA cells were transfected with DN-Rab5-GFP (A, B and black column in E) or with RN-tre-GFP (C, D, and gray column in E). Transfected cells (labeled by asterisks) are identifiable by their green fluorescence (A and D). The cells were allowed to internalize opsonized beads for 20 min. After washing unbound beads, incubation at 37°C proceeded for 30 more min. The cells were then fixed, permeabilized with methanol at −20°C, and stained for LAMP-1 (B, D, and E). Arrows and arrowheads point to LAMP-1-positive and -negative phagosomes, respectively. Scale bars = 10 μm. (E) Quantification of the effect of DN-Rab5 (black column) and RN-tre (gray column) overexpression on LAMP-1 acquisition by phagosomes, compared to untransfected control cells (open bar). Results shown are means + SE (error bars) of three separate experiments, each with at least 100 phagosomes from 20 to 40 different cells counted.
Rab7 associates with phagosomes following acquisition of Rab5 and, by analogy with the endosomal pathway, is likely involved in directing fusion with lysosomes. We therefore anticipated that recruitment of Rab7 by phagosomes would require the prior activation of Rab5. This prediction was verified in the experiments summarized in Fig. 6. Cells were cotransfected with myc epitope-tagged Rab7 and either GFP or DN-Rab5-GFP. Rab7 association with late phagosomes was readily detectable in cells transfected with GFP (Fig. 6B), while those expressing the inhibitory Rab5 had much reduced staining (Fig. 6E). In four separate experiments, Rab7 recruitment was depressed by ≥66% at all the times analyzed (Fig. 6G). Therefore, Rab5 seems to be essential for normal phagosome maturation.
FIG. 6.
Effect of dominant-negative Rab5 on Rab7 acquisition. CHO-IIA cells were cotransfected with wild-type Rab7-myc and either GFP (A to C and empty columns in G) or with DN-Rab5-GFP (D to F). The cells were incubated for 20 min with IgG-opsonized beads, chased for the indicated times, and then fixed, permeabilized, and immunostained. (A and D) Green fluorescence; (B and E) distribution of Rab7, revealed by immunostaining the myc epitope; (C and F) corresponding differential interference contrast images. Arrows and arrowheads point to Rab7-myc positive and -negative phagosomes, respectively. Scale bars = 10 μm. (G) quantification of the effect of DN-Rab5 on Rab7 recruitment by phagosomes, from experiments like those in panels A to F. Empty columns, control; black columns, cells transfected with DN-Rab5-GFP. Results shown are means + SE (error bars) of four separate experiments, each with at least 100 individual phagosomes counted per condition in cells with high expression levels of DN-Rab5.
Overexpression of a Rab5-GTPase activating protein arrests phagosome maturation.
The role of Rab5 in maturation was confirmed using RN-tre, a Rab5 GTPase-activating protein (GAP) (24). Overexpression of a GTPase activator is expected to minimize the ability of Rab5-GTP to interact with its effectors. CHO-IIA cells were transfected with RN-tre-GFP and successful expression was verified by direct fluorescence microscopy (Fig. 5C). Fluid phase pinocytosis was depressed in cells expressing RN-tre, implying that the concentrations attained were sufficient to inhibit Rab5 (not illustrated). Expression of RN-tre-GFP did not affect the phagocytic efficiency and the GTPase was detectable on the membrane of nascent phagosomes. Importantly, overexpression of RN-tre-GFP profoundly depressed the ability of phagosomes to acquire LAMP-1 (Fig. 5D and E). The extent of inhibition was similar to that observed in DN-Rab5 transfected cells (Fig. 5E). Jointly, these findings imply that active Rab5 is required for fusion of phagosomes with late endosomes and/or lysosomes.
Effect of wortmannin on Rab7 acquisition.
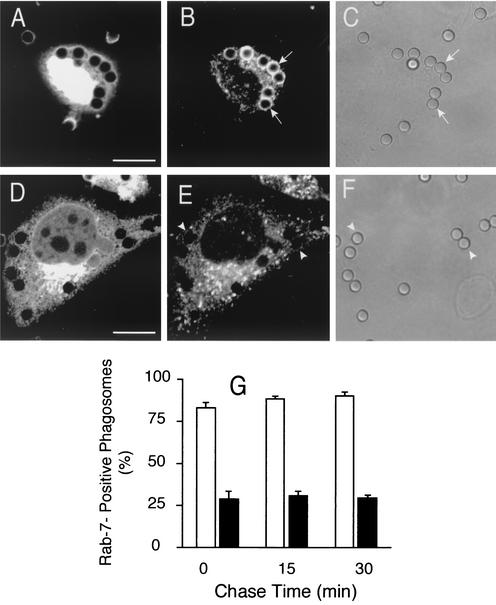
As Rab5 acquisition and activation are not dependent on the presence of PI(3)P on the phagosome, we next determined whether the recruitment of Rab7 to the phagosome is affected by wortmannin. RAW and CHO-IIA cells were transiently transfected with a construct encoding Rab7-GFP. When appropriate levels of the fusion protein were expressed, the cells were either treated with wortmannin or left untreated and next challenged with opsonized beads. The presence of the fluorescent chimera on phagosomes was monitored by confocal microscopy. Rab7 could be readily detected on the membrane of control phagosomes (Fig. 7A). In CHO-IIA cells 79% ± 6.4% of the phagosomes were Rab7-positive 20 min after initiation of ingestion and this number increased, approaching 100% over the next 30 min (Fig. 7E). Very similar results were obtained in RAW cells (Fig. 7F). As illustrated in Fig. 8C and summarized in Fig. 7E and F (black columns), wortmannin treatment attenuated, but did not prevent, the acquisition of Rab7 by the phagosome. Over 50% of phagosomes were Rab7 positive immediately after the ingestion pulse (a 40% inhibition compared to control), and this fraction changed only marginally thereafter in both CHO-IIA (Fig. 7E) and RAW cells (Fig. 7F).
FIG. 7.
Effect of wortmannin on Rab7 acquisition by phagosomes. CHO-IIA cells (A to E) or RAW cells (F) were transfected with wild-type Rab7-GFP. The cells were either left untreated (A, B, and empty columns in E and F) or were treated with 100 nM wortmannin for 30 min (C, D, and black columns in E and F). The cells were allowed to internalize IgG-opsonized beads and the distribution of Rab7 was monitored by confocal microscopy at the indicated times. (A and C) Phagocytosis was for 20 min, without chase. The arrows and arrowheads point to Rab7-GFP-positive and -negative phagosomes, respectively. (B and D) Corresponding differential interference contrast images. Scale bars = 10 μm. (E and F) Quantification of the effect of wortmannin on Rab7-GFP acquisition by phagosomes in CHO-IIA (E) and RAW cells (F). Empty columns, control; black columns, cells treated with wortmannin. Data are means + SE (error bars) of four separate experiments, each with at least 100 individual phagosomes from 30 to 40 different cells expressing low levels of Rab7-GFP counted per condition.
FIG. 8.
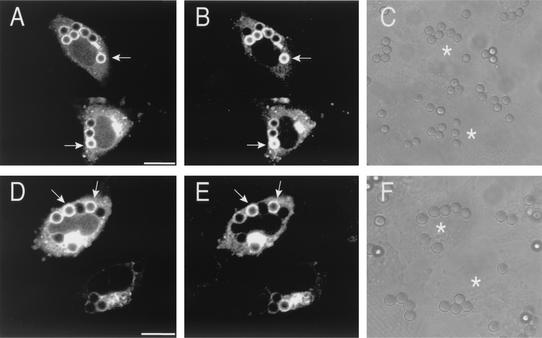
RILP recruitment to phagosomes. CHO-IIA cells were cotransfected with wild-type Rab7-GFP and RILP-HA. (D to F) Cells were treated with wortmannin 30 min before phagocytosis. Phagocytosis was allowed to proceed for 20 min, and this was followed by a 30-min chase. The cells were then fixed and immunostained for the HA epitope. (A and D) Distribution of Rab7-GFP; (B and E) the distribution of RILP-HA; (C and F) corresponding differential interference contrast images. The arrows point to phagosomes positive for both Rab7 and RILP. Asterisks identify transfected cells. Bars = 10 μm. Results are representative of four similar experiments, each with ∼30 cells counted.
PI3K activity is not necessary for Rab7 activation.
The modest decrease in Rab7 recruitment documented in Fig. 7 cannot fully explain the much greater (∼90%) inhibition of lysosomal fusion produced by wortmannin. Two possible mechanisms could explain this quantitative discrepancy: products of PI3K may be required for Rab7 activation, independently of its recruitment, or alternatively, the kinase may be required for a separate event that, in parallel with Rab7, may be essential for successful phagolysosome formation.
To differentiate these hypotheses, we tested the state of activation of Rab7 in cells treated with wortmannin. Members of the Rab family bind their effectors only when bound to GTP, i.e., in their active configuration. We therefore determined whether Rab7 is capable of recruiting effectors following treatment with the PI3K inhibitor. To date, only one effector of Rab7, termed RILP, has been identified (8, 21). Experiments analyzing the distribution of RILP are depicted in Fig. 8. CHO-IIA and RAW cells were cotransfected with Rab7-GFP and with an epitope-tagged form of RILP and their distribution was monitored by confocal microscopy. As reported (8, 21), RILP accumulated in a juxtanuclear complex, near the microtubule-organizing center. In addition, in cells that had ingested particles but were otherwise untreated, RILP was found in the vast majority of phagosomes that contained Rab7 (Fig. 8A to C). Of note, RILP was also found in most of the Rab7-positive phagosomes in wortmannin-treated cells (Fig. 8D to F). This observation implies that PI3K is not essential for the activation of Rab7 and that the large fraction of Rab7 recruited to phagosomes in wortmannin-treated cells is capable of binding effectors.
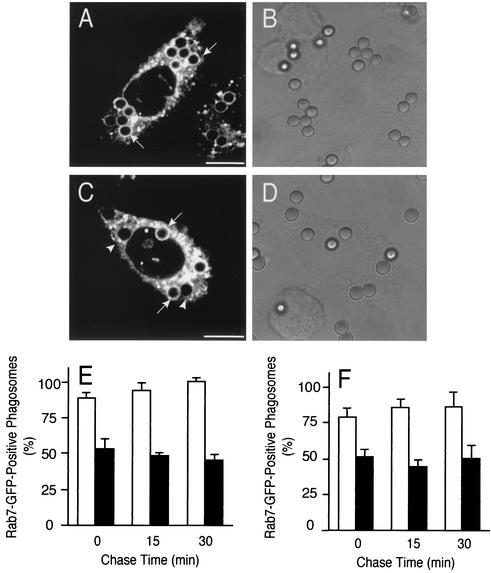
The preceding data imply that the site of action of wortmannin is at a step that occurs in parallel with, or after activation of Rab7. This conclusion was validated using Rab7(Q67L), a GTPase-deficient and therefore constitutively active form of Rab7 (CA-Rab7). We predicted that if wortmannin prevents phagolysosome formation by interfering with the activation of Rab7, expression of the constitutively active mutant would bypass the inhibition. Conversely transfection of CA-Rab7 would be without effect if PI3K acts after or in parallel with Rab7. The experiments addressing these alternatives are illustrated in Fig. 9. The CA-Rab7 was found to associate with phagosomes in both control and, to a slightly lesser extent in wortmannin-treated cells (Fig. 9A and D). Expression of the active mutant did not interfere with the recruitment of LAMP-1 by phagosomes in control cells (Fig. 9B) and, importantly, it failed to overcome the inhibitory effects of wortmannin on phagolysosome formation. As shown in Fig. 9G, expression of CA-Rab7 had no detectable effect on LAMP-1 acquisition after 20 and 40 min and produced a marginal stimulation at 60 min. Importantly, similar results were obtained using RAW macrophages (not shown). These findings confirm the notion that PI3K activity is required for a step(s) other than Rab7 activation.
FIG. 9.
Effect of constitutively active Rab7 on LAMP-1 acquisition by phagosomes. CHO-IIA cells were transfected with CA-Rab7-GFP and either left untreated (A to C and empty columns in G) or treated with wortmannin (D to F and black columns in G) prior to phagocytosis. After 20 min of phagocytosis the unbound particles were removed and the cells were incubated for 30 more min to allow phagosome maturation. The cells were then fixed, permeabilized, and immunostained with anti-LAMP-1 antibodies. (A and D) Distribution of CA-Rab7-GFP; (B and E) distribution of LAMP-1. Arrows and arrowheads point to phagosomes that were positive or negative, respectively, for Rab7 or LAMP-1, as appropriate. Asterisks identify transfected cell. Scale bars = 10 μm. (C and F) Corresponding differential interference contrast images. (G) Quantitation of LAMP-1 acquisition by phagosomes in cells treated with wortmannin. The graph compares cells that were untransfected (black bars) with CA-Rab7-GFP-transfected cells (open bars). Data are means + SE (error bars) of four separate experiments, each with at least 100 individual phagosomes from 20 to 40 different cells counted per condition.
DISCUSSION
Treatment of macrophages with gamma interferon was found to induce the biosynthesis of Rab5 and, in parallel, to accelerate the intracellular killing of pathogens like Listeria monocytogenes (3). In addition, Duclos and colleagues (15) reported that heterologous expression of CA-Rab5 resulted in the formation of enlarged phagosomes. On the basis of these observations, and by analogy with the endosomal pathway, a role for Rab5 in phagosome maturation had been suspected, but it was not demonstrated. Here we present direct evidence that active Rab5 is required for successful phagolysosome formation (Fig. 5). Association of active Rab5 with phagosomes precedes and is essential for the recruitment of Rab7, which in turn precedes and is required for the fusion of phagosomes with late endocytic compartments (R. E. Harrison and S. Grinstein, unpublished observations). While sequential recruitment of active Rab5 and Rab7 to the phagosome is necessary, it is not sufficient for completion of maturation. This is best illustrated in cells treated with wortmannin, where phagosomes contain increased amounts of active Rab5 (Fig. 1) and upwards of 60% of the normal complement of active Rab7 (Fig. 7) yet fail to fuse with late endosomes and lysosomes to a significant degree. Clearly, one or more additional PI3K-dependent events are also required for successful generation of phagolysosomes.
The experiments reported here indicate that treatment with wortmannin prevented neither the recruitment of Rab5 nor its activation on the phagosomal membrane (Fig. 1 to 4), while it completely eliminated PI(3)P from the phagosomal membrane, as detected using FYVE or PX domain chimeras (not illustrated). This implies that class I or III PI3K does not provide the signal that targets Rab5 to the phagosome. In fact, the amount of Rab5 detectable on phagosomes was greater in wortmannin-treated cells than in controls, and its dwelling time on the phagosomal membrane was prolonged. Three mechanisms could account for these observations. (i) The activity or availability of Rab5 anchors and activators on the phagosomal membrane may increase upon inhibition of PI3K. This mechanism, however, is not consistent with the FRAP determinations, which indicate that the primary cause of accumulation is reduced dissociation, rather than increased association of Rab5 with the membrane. (ii) PI3K may be required for the activation and/or recruitment of Rab5 GAPs to the phagosome. At least three types of Rab5-GAPs have been described: tuberin (38), Ras-GAP (27), and RN-tre (24). Of these, Ras-GAP was reported to be directly inhibited by wortmannin by a process that is independent of PI3K (10). While this proposed mechanism would explain many of our data, we found that in cells treated with the chemically unrelated PI3K inhibitor LY294002, Rab5 accumulates in phagosomes in a manner that closely mimics the effects of wortmannin (unpublished observations). We therefore believe that depletion of products of PI3K, rather than direct inhibition of Ras-GAP, is responsible for the altered behavior of Rab5. Of the GAPs listed above, only RN-tre is specific towards Rab5 and therefore of the greatest interest. RN-tre was found to localize primarily to the plasma membrane and to reside in phagosomes only briefly (less than 5 min). The temporal pattern of association of RN-tre with the phagosome was not visibly altered by treatment of the cells with wortmannin (O. V. Vieira, unpublished observations). Hence, PI3K is not required for its targeting or maintenance in phagosomes. Alternatively, other GAPs or Rab-GDI (guanine nucleotide dissociation inhibitor) may account for the protracted activation of Rab5 in wortmannin-treated cells; (iii) The removal of Rab5 from the phagosome may occur by fission of vesicles containing the GTPase, rather than by the canonical nucleotide hydrolysis and solubilization of the protein. In this scenario, PI3K may be required for fission to occur and its inhibition would lead to Rab5 accumulation.
Products of PI3K are also required to recruit and/or activate effectors of Rab5, such as EEA1 and rabenosyn 5 (29, 30, 34). It is likely that impairment of one or more of these effectors contributes to the inability of the early phagosome to fuse with late endosomes and phagosomes. Regardless of the mode of action of wortmannin on Rab5, our data suggest that the role of PI3K is not limited to this step of the maturation cascade. Because association of Rab7 follows that of Rab5, and because inhibition of Rab7 impairs maturation, we suspected that recruitment of Rab7 was the alternate target of PI3K. However, we found that Rab7 acquisition by phagosomes was only partially (∼40%) diminished by wortmannin (Fig. 7). We speculate that phagosomal Rab7 is acquired from 2 sources: one that is independent of PI3K and may be the cytosolic pool or an unidentified wortmannin-insensitive vesicular pool (purple oval in Fig. 10), while another wave of Rab7 may merge with the phagosomes when the latter fuse with late endosomes or lysosomes, which are well established to carry the GTPase. To the extent that fusion is precluded by wortmannin, the latter component would be lacking in phagosomes treated with the inhibitor.
FIG. 10.
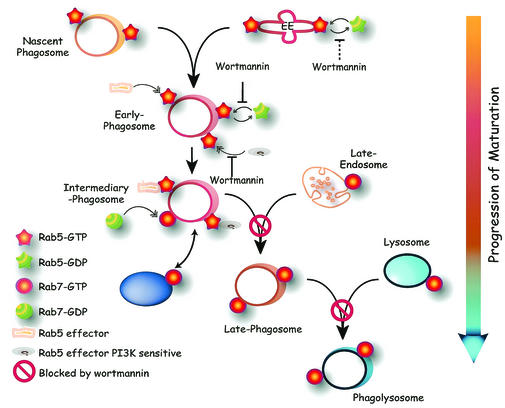
Schematic representation of phagosome maturation, highlighting putative sites of action of PI3K. Nascent phagosomes formed upon fission from the plasma membrane contain small amounts of Rab5. Additional Rab5 is acquired by fusion with early endosomes. Inhibition of PI3K prevents the dissociation of active Rab5 from early phagosomes and presumably also from early endosomes (dotted line). Recruitment or activation of (some) Rab5 effectors also requires products of PI3K. Intermediary phagosomes are formed upon acquisition of Rab7 from either the soluble pool or from a PI3K-independent vesicular compartment. Late phagosomes form by fusion with late endosomes, which themselves bear Rab7. This step is blocked by wortmannin. Late phagosomes become phagolysosomes upon fusion with lysosomes. Wortmannin also prevents this step. A putative Rab7-bearing organelle that can still fuse with phagosomes despite the presence of PI3K inhibitors is indicated by the purple oval (lower left).
The sizable fraction of Rab7 that associates with the phagosome in wortmannin-treated cells appears to be functionally active, as indicated by its ability to recruit RILP, an effector thought to interact only with the GTP-bound form of Rab7 (8). It would therefore appear that active Rab7 is not, per se, sufficient to mediate fusion of the phagosomes with late endosomes and lysosomes. Accordingly, the expression of CA-Rab7 failed to bypass the block exerted by wortmannin (Fig. 9). One could conclude from these observations that wortmannin is effecting the block of maturation downstream of Rab7. However, it is equally possible that two parallel inputs are required to trigger proper fusion of endosomes or lysosomes with the phagosome: one mediated by Rab7 and another that includes the PI3K-dependent event, likely the activation of Rab5 effectors, yet does not involve Rab7. Because RILP was recently shown to bridge between dynein and Rab7-containing cargo, it is possible that the Rab7 arm is involved in mechanically positioning phagosomes in the vicinity of late endosomes/lysosomes, while another, PI3K dependent step is required for the actual fusion event. In this context, it is noteworthy that, at least in yeast, the association of SNAREs like Vam7 with membranes of the endocytic pathway requires interaction with PI(3)P, a product of class III PI3K (9). Moreover, SNAREs can associate with activated Rab5 in mammalian systems (28). These concepts are synthesized in diagrammatic form in Fig. 10. Nascent phagosomes are shown to contain some Rab5, present originally in the invaginating plasma membrane and/or recruited there by activated Fc receptors. Additional Rab5 is acquired by fusion with early endosomes, but mostly from the soluble pool, since experiments using Rab5-GFP showed rapid gain of phagosomal fluorescence that was not accountable by fusion with labeled vesicular compartments (31). Wortmannin is shown to interfere with the dissociation of the active (GTP-bound) form of Rab5 from early phagosomes and presumably also from early endosomes. Rab5 is shown in the diagram to recruit two types of effectors: some that are PI3K independent and others that require PI3K products for recruitment and/or activation. Rab5-enriched early phagosomes then proceed to become intermediary phagosomes by shedding Rab5 and acquiring Rab7. We propose that the PI3K-independent effectors of Rab5 take part in the recruitment of Rab7. Rab7 is recruited initially from the cytosol and/or from an unidentified vesicular compartment distinct from late endosomes and lysosomes. Next, the Rab7-enriched phagosome fuses with late endosomes by a 3′-phosphoinositide-dependent process, perhaps involving Rab5 effectors, generating late phagosomes. These, in turn coalesce with lysosomes by a process that also requires PI3K. It is not clear whether this is a distinct PI3K-dependent step, or the manifestation of the inability of the phagosome to reach the late stage, which may be essential for fusion with lysosomes. Merger with late endosomes and lysosomes delivers to the phagosome additional Rab7 that is constitutively present in these organelles. The latter component of Rab7 recruitment is therefore wortmannin sensitive, accounting for the partial effects of the inhibitor. The ultimate stage of the process is the formation of phagolysosomes, which have optimal microbicidal properties. An essential feature of this model is the parallel operation of two types of effectors, some that are PI3K dependent and others that are independent.
Acknowledgments
We thank Pier Paolo di Fiore for his advice and for generously sharing his reagents.
This work was supported by the Canadian Institutes of Health Research, the Canadian Arthritis Society and the National Sanatorium Association. O. V. Vieira is the recipient of a postdoctoral fellowship from PRAXIS XX1 (BPD/22039/99). S.G. is supported in part by the Pitblado Chair in Cell Biology.
REFERENCES
- 1.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Dominguez, C., A. M. Barbieri, W. Beron, A. Wandinger-Ness, and P. D. Stahl. 1996. Phagocytosed live. Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 271:13834-13843. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Dominguez, C., and P. D. Stahl. 1998. Interferon-gamma selectively induces Rab5a synthesis and processing in mononuclear cells. J. Biol. Chem. 273:33901-33904. [DOI] [PubMed] [Google Scholar]
- 4.Araki, N., M. T. Johnson, and J. A. Swanson. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucci, C., R. G. Parton, I. H. Mather, H. Stunnenberg, K. Simons, B. Hoflack, and M. Zerial. 1992. The small, GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70:715-728. [DOI] [PubMed] [Google Scholar]
- 6.Bucci, C., P. Thomsen, P. Nicoziani, J. McCarthy, and B. van Deurs. 2000. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11:467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan, J., A. Simonsen, J. M. Gaullier, B. H. Toh, and H. Stenmark. 1999. The endosome fusion regulator early-endosomal autoantigen 1 (EEA1) is a dimer. Biochem J. 338:539-543. [PMC free article] [PubMed] [Google Scholar]
- 8.Cantalupo, G., P. Alifano, V. Roberti, C. B. Bruni, and C. Bucci. 2001. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheever, M. L., T. K. Sato, T. de Beer, T. G. Kutateladze, S. D. Emr, and M. Overduin. 2001. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat. Cell Biol. 3:613-618. [DOI] [PubMed] [Google Scholar]
- 10.Chen, X., and Z. Wang. 2001. Regulation of epidermal growth factor receptor endocytosis by wortmannin through activation of Rab5 rather than inhibition of phosphatidylinositol 3-kinase. EMBO Rep. 2:842-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Collins, R. F., A. D. Schreiber, S. Grinstein, and W. S. Trimble. 2002. Syntaxins 13 and 7 function at distinct steps during phagocytosis. J. Immunol. 169:3250-3256. [DOI] [PubMed] [Google Scholar]
- 11.Cox, D., C. C. Tseng, G. Bjekic, and S. Greenberg. 1999. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274:1240-1247. [DOI] [PubMed] [Google Scholar]
- 12.Desjardins, M., J. E. Celis, G. van Meer, H. Dieplinger, A. Jahraus, G. Griffiths, and L. A. Huber. 1994. Molecular characterization of phagosomes. J. Biol. Chem. 269:32194-32200. [PubMed] [Google Scholar]
- 13.Desjardins, M., L. A. Huber, R. G. Parton, and G. Griffiths. 1994. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 124:677-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downey, G. P., R. J. Botelho, J. R. Butler, Y. Moltyaner, P. Chien, A. D. Schreiber, and S. Grinstein. 1999. Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcγRIIA receptors. J. Biol. Chem. 274:28436-28444. [DOI] [PubMed] [Google Scholar]
- 15.Duclos, S., R. Diez, J. Garin, B. Papadopoulou, A. Descoteaux, H. Stenmark, and M. Desjardins. 2000. Rab5 regulates the kiss and run fusion between phagosomes and endosomes and the acquisition of phagosome leishmanicidal properties in RAW 264.7 macrophages. J Cell Sci. 113:3531-3541. [DOI] [PubMed]
- 16.Fratti, R. A., J. M. Backer, J. Gruenberg, S. Corvera, and V. Deretic. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garin, J., R. Diez, S. Kieffer, J. F. Dermine, S. Duclos, E. Gagnon, R. Sadoul, C. Rondeau, and M. Desjardins. 2001. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152:165-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorvel, J. P., P. Chavrier, M. Zerial, and J. Gruenberg. 1991. rab5 controls early endosome fusion in vitro. Cell 64:915-925. [DOI] [PubMed] [Google Scholar]
- 19.Indik, Z. K., J. G. Park, S. Hunter, and A. D. Schreiber. 1995. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood 86:4389-4399. [PubMed] [Google Scholar]
- 20.Jahraus, A., T. E. Tjelle, T. Berg, A. Habermann, B. Storrie, O. Ullrich, and G. Griffiths. 1998. In vitro fusion of phagosomes with different endocytic organelles from J774 macrophages. J. Biol. Chem. 273:30379-30390. [DOI] [PubMed] [Google Scholar]
- 21.Jordens, I., M. Fernandez-Borja, M. Marsman, S. Dusseljee, L. Janssen, J. Calafat, H. Janssen, R. Wubbolts, and J. Neefjes. 2001. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11:1680-1685. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi, T., F. Gu, and J. Gruenberg. 1998. Lipids, lipid domains and lipid-protein interactions in endocytic membrane traffic. Semin. Cell. Dev. Biol. 9:517-526. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, T., M. H. Beuchat, M. Lindsay, S. Frias, R. D. Palmiter, H. Sakuraba, R. G. Parton, and J. Gruenberg. 1999. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 24.Lanzetti, L., V. Rybin, M. G. Malabarba, S. Christoforidis, G. Scita, M. Zerial, and P. P. Di Fiore. 2000. The Eps8 protein coordinates EGF receptor signalling through. Rac and trafficking through Rab5. Nature 408:374-377. [DOI] [PubMed] [Google Scholar]
- 25.Li, G., C. D'Souza-Schorey, M. A. Barbieri, J. A. Cooper, and P. D. Stahl. 1997. Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J. Biol. Chem. 272:10337-10340. [PubMed] [Google Scholar]
- 26.Li, G., M. A. Barbieri, M. I. Colombo, and P. D. Stahl. 1994. Structural features of the GTP-binding defective Rab5 mutants required for their inhibitory activity on endocytosis. J. Biol. Chem. 269:14631-14635. [PubMed] [Google Scholar]
- 27.Liu, K., and G. Li. 1998. Catalytic domain of the p120 Ras GAP binds to RAb5 and stimulates its GTPase activity. J. Biol. Chem. 273:10087-10090. [DOI] [PubMed] [Google Scholar]
- 28.McBride, H. M., V. Rybin, C. Murphy, A. Giner, R. Teasdale, and M. Zerial. 1999. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell 98:377-386. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen, E., S. Christoforidis, S. Uttenweiler-Joseph, M. Miaczynska, F. Dewitte, M. Wilm, B. Hoflack, and M. Zerial. 2000. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol. 151:601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patki, V., J. Virbasius, W. S. Lane, B. H. Toh, H. S. Shpetner, and S. Corvera. 1997. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 94:7326-7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts, R. L., M. A. Barbieri, J. Ullrich, and P. D. Stahl. 2000. Dynamics of rab5 activation in endocytosis and phagocytosis. J. Leukoc. Biol. 68:627-632. [PubMed] [Google Scholar]
- 32.Roberts, R. L., M. A. Barbieri, K. M. Pryse, M. Chua, J. H. Morisaki, and P. D. Stahl. 1999. Endosome fusion in living cells overexpressing GFP-rab5. J. Cell Sci. 112:3667-3675. [DOI] [PubMed] [Google Scholar]
- 33.Scott, C. C., P. Cuellar-Mata, T. Matsuo, H. W. Davidson, and S. Grinstein. 2002. Role of 3-phosphoinositides in the maturation of Salmonella-containing vacuoles within host cells. J. Biol. Chem. 277:12770-12776. [DOI] [PubMed] [Google Scholar]
- 34.Simonsen, A., R. Lippe, S. Christoforidis, J. M. Gaullier, A. Brech, J. Callaghan, B. H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494-498. [DOI] [PubMed] [Google Scholar]
- 35.Tall, G. G., M. A. Barbieri, P. D. Stahl, and B. F. Horazdovsky. 2001. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1:73-82. [DOI] [PubMed] [Google Scholar]
- 36.Underhill, D. M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825-852. [DOI] [PubMed] [Google Scholar]
- 37.Vieira, O. V., R. J. Botelho, L. Rameh, S. M. Brachmann, T. Matsuo, H. W. Davidson, A. Schreiber, J. M. Backer, L. C. Cantley, and S. Grinstein. 2001. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao, G. H., F. Shoarinejad, F. Jin, E. A. Golemis, and R. S. Yeung. 1997. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J. Biol. Chem. 272:6097-6100. [DOI] [PubMed] [Google Scholar]
- 39.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]