Abstract
A docking protein, Gab2, is recruited to the vicinity of the TCR complex and inhibits downstream signaling by interaction with negative regulators. However, the molecular mechanisms of this recruitment remain unclear. We have found that Gab2 associates with LAT upon TCR stimulation and that LAT is essential for Gab2 phosphorylation. By analysis of several Gab2 mutants, the c-Met binding domain (MBD) of Gab2 was found to be both necessary and sufficient for stimulation-induced LAT binding. Within the MBD domain, a novel Grb2 SH3 binding motif, PXXXR, is critical for constitutive association with Gads/Grb2. Through this association, Gab2 is recruited to the lipid raft after TCR ligation and exerts inhibitory function. The in vivo significance of this association is illustrated by the fact that T-cell responses are impaired in transgenic mice expressing wild-type Gab2 but not in mice expressing mutant Gab2 lacking the motif. Furthermore, T cells from Gab2-deficient mice showed enhanced proliferative responses upon TCR stimulation. These results indicate that Gads/Grb2-mediated LAT association is critical for the inhibitory function of Gab2, implying that Gab2 induced in stimulated T cells may exert an efficient negative feedback loop by recruiting inhibitory molecules to the lipid raft and competing with SLP-76 through Gads binding.
T cells recognize antigens presented by the major histocompatibility complex on antigen-presenting cells (APCs). An antigen recognition signal is then transmitted to the cytoplasm via the T-cell antigen receptor (TCR) complex and induces rapid tyrosine phosphorylation of intracellular substrates, leading to a variety of effector functions (15). The regulation of tyrosine phosphorylation is determined by the balance between protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs). In the immune system, the precise and coordinated regulation of this equilibrium enables rapid responses to foreign antigens, whereas an imbalance between PTKs and PTPs may have pathological consequences, including autoimmunity, immunodeficiency, and malignancy. In contrast to the positive regulation by PTKs, however, the precise mechanism of the negative regulation of T cells has not yet been widely characterized. Recently, it was highlighted that PTPs play critical roles in modulating phosphotyrosine levels in resting T cells, during T-cell activation, and during inhibition of immune response (11).
The Gab family molecules function as scaffold proteins by interacting with multiple signaling molecules, such as SHP-2, p85 phosphatidylinositol (PI) 3-kinase (PI3K), and Grb2, and are involved in biological activities through a variety of growth factors and cytokines (12). Gab1 and Gab2 are tyrosine phosphorylated upon stimulation through T- and B-cell antigen receptors as well as cytokine receptors (12). In a previous study, we found that Gab2 is phosphorylated by ZAP-70, is associated with the TCR signaling complex, and acts as an inhibitory adaptor molecule via recruitment of SHP-2 upon TCR engagement (34). However, the precise molecular mechanisms for the recruitment of Gab2 to the vicinity of the TCR complex remain unclear. On the other hand, we have also found that Gab2 is associated with LAT in a stimulation-dependent manner. LAT is a membrane adaptor protein and is detected as a prominent 36- to 38-kDa tyrosine-phosphorylated protein upon TCR engagement. LAT is a ZAP-70 substrate and plays critical roles in the recruitment of downstream signaling molecules such as phospholipase C-γ1 (PLC-γ1) and Gads/Grb2 to membrane proximity. LAT contains a transmembrane domain and palmitoylation sites, which are required for localization into the glycolipid-enriched microdomains or lipid raft (GEMs/raft) (8). These observations imply that the interaction between LAT and Gab2 may be responsible for the recruitment of Gab2 to the vicinity of the TCR complex and then for Gab2-mediated T-cell suppression (34).
Grb2 family molecules containing Grb2, Gads, and Grap consist of a central SH2 domain flanked by two SH3 domains. Despite the similarity in structure, however, the binding specificities and/or affinities of the SH3 domains between Gads and Grb2 indicate their distinct functions (2, 18). The SH3 domain of Grb2 binds to Sos or Vav and is critical for downstream signaling such as Ras activation (7). As for Gads, a hematopoiesis-specific Grb2 family protein, its SH3 domain is exclusively required for binding to SLP-76 and connecting to LAT and it is considered to be critical for pre-TCR/TCR signaling (36).
In the present study, we show that Gab2 constitutively interacts with Gads/Grb2 and is recruited to the lipid raft by association with LAT through the Gads/Grb2-Gab2 assembly and that the Gads/Grb2-Gab2 interaction may compete with the SLP76-Gads complex for LAT association. The recruitment of Gab2 to LAT induces the recruitment of inhibitory molecules into the raft for efficient negative regulation. From these results, a representative suppressive mechanism of T-cell activation by inhibitory adaptor molecules through competitive recruitment of positive versus negative adaptors to the membrane proximity, particularly the lipid raft, can be proposed.
MATERIALS AND METHODS
Cells and reagents.
Jurkat T cells and ecotropic viral receptor-expressing Jurkat (J.EcoR) cells were maintained in 10% fetal calf serum-supplemented RPMI 1640 (34). J.CaM2.5, a LAT-deficient Jurkat cell line, and J.14, an SLP-76-deficient Jurkat cell line, were kindly provided by A. Weiss (University of California, San Francisco) and G. Koretzky (University of Pennsylvania), respectively. 293T cells and a retrovirus packaging cell line, phoenix, kindly provided by G. P. Nolan (Stanford University), were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 100 μg of l-glutamine/ml, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 50 μM 2-mercaptoethanol.
Abs.
The antibody (Ab) used for TCR stimulation of Jurkat cells was OKT3, an anti-CD3ɛ monoclonal Ab (MAb). Abs used for blots and immunoprecipitation were as follows: anti-Flag M2 MAb (Sigma Chemical Co.); anti-myc MAb (9E10; Santa Cruz); rabbit anti-myc Ab (MBL); hamster anti-CD3ζ MAb, H146 (provided by R. Kubo, BD Biosciences); rabbit anti-LAT serum (provided by L. Samelson, National Institutes of Health); sheep anti-SLP-76 serum (provided by G. Koretzky, University of Pennsylvania School of Medicine); anti-ZAP-70 MAb, 2F3.2 (provided by M. Iwashima, Medical College of Georgia); anti-Lck MAb, Mol171 (provided by Y. Koga, Kyushu University); anti-Sos MAb, 4G10, and anti-PI3K p85 MAb (Upstate Biotechnology); rabbit anti-Gads serum (19); rabbit anti-Grb2 Ab, anti-SHP-2 serum (Santa Cruz); rabbit anti-Gab2 serum (Upstate Biotechnology) (27); and anti-Grb2 MAb (Transduction Laboratories). Secondary reagents used were horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) and goat anti-rabbit IgG (Upstate Biotechnology).
cDNA constructs.
Expression constructs for the wild-type, Y614/643F, and Y452/476/584F alleles of Flag-Gab2 were previously described (27, 34). Deletion mutants Gab2.ΔMBD, encoding a deletion of the c-Met binding domain (MBD) (amino acids 454 to 525), and Gab2.ΔPro, encoding a deletion of the PxxxR motif (amino acids 510 to 515), were generated by PCR by using Flag-Gab2 DNA as template. All PCR-generated constructs were verified by sequencing. Gads cDNA was kindly provided by K. Sugamura (Tohoku University). Grb2 cDNA was provided by T. Iwahara (Osaka Bioscience Institute). ZAP-70 and Lck cDNAs were kindly provided by M. Iwashima. LAT and LAT 4YF mutant cDNAs were kindly provided by L. Samelson (37). SLP-76 cDNA was a kind gift from G. Koretzky. pMX-IRES-GFP, pMX-puro, and the ecotropic viral receptor cDNA were generously provided by T. Kitamura (University of Tokyo).
Cell transfection and retrovirus-mediated gene transfer.
For transient transfection in 293T cells, 106 cells were transfected with various combinations of expression constructs for Flag-Gab2, Lck, ZAP-70, and LAT by using Lipofectamine according to the manufacturer's instructions (Gibco BRL). Thirty-six hours after transfection, cells were harvested and lysed in 1% NP-40 containing protease and phosphatase inhibitors as described below.
Retrovirus-mediated gene transfer was performed by using phoenix packaging cells as previously described (34). Transgene-positive cells were collected for the expression of green fluorescent protein (GFP) by using FACStar plus (Becton Dickinson).
Immunoprecipitation and Western blot analysis.
Spleen cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4/8 MAb and anti-FITC microbeads (Miltenyi Biotec). CD4+ and CD8+ cells were isolated by positive selection with the high-gradient magnetic cell separation system MACS (Miltenyi Biotec). The cells were lysed in 1% NP-40 lysis buffer (1% NP-40, 50 mM Tris, 150 mM NaCl, 5 mM EDTA, 10 μg of aprotinin/ml, 12.5 μg of chymostatin/ml, 50 μg of leupeptin/ml, 25 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride, and 2 mM Na3VO4). Immunoprecipitation was carried out by using protein A-Sepharose (Pharmacia Biotech) conjugated with each Ab. Western blots were carried out with the enhanced chemiluminescence assay according to the manufacturer's recommendations (Pierce).
GST pulldown assay.
The PvuI-ClaI fragment encoding the MBD region of human Gab2 was subcloned into pGEX2TK (Amersham Pharmacia Biotech) to generate glutathione S-transferase (GST)-Gab2.MBD. GST fusion proteins were expressed in Escherichia coli (25) and were purified on glutathione-agarose (Amersham Pharmacia Biotech). For pulldown assay, lysates were incubated for 1 h at 4°C with glutathione-agarose bound to GST alone or to GST-Gab2.MBD. Beads were rapidly washed five times with lysis buffer, and bound proteins were eluted by boiling the beads in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer.
Isolation of GEM fractions using equilibrium density gradients.
For each GEM preparation, 108 cells were either left unstimulated or stimulated via TCR, followed by lysis at 4°C for 30 min in 1 ml of morpholineethanesulfonic acid (MES)-buffered saline (25 mM MES, pH 6.5, 150 mM NaCl) containing 1% Triton X-100, 50 mg of aprotinin/ml, 10 mg of leupeptin/ml, 50 mg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride, 400 mM sodium vanadate, 10 mM sodium fluoride, and 10 mM sodium pyrophosphate (14). The lysates were then mixed with 1 ml of 80% sucrose in MES-buffered saline and were transferred to ultracentrifugation tubes. The samples were overlaid with 6.5 ml of 30% sucrose in MES-buffered saline, followed by 3.5 ml of 5% sucrose in MES-buffered saline. Triton-insoluble fractions were separated from cell lysates by ultracentrifugation for 16 h at 45,000 rpm in a Beckman SW55 Ti rotor at 4°C. One-milliliter fractions were removed sequentially from the top of the gradient.
Confocal microscopy analysis.
J.EcoR cells were infected with Gab2.WT and Gab2.ΔPro by using pMX-puro retrovirus vector and were selected with 0.8 μg of puromycin/ml. T cells were incubated with staphylococcal enterotoxin E (SEE)-pulsed Raji cells at 37°C for 30 min as previously described. Immunological synapse formation was analyzed by using a confocal microscope (LSM510; Carl Zeiss, Oberkochen, Germany).
Mice.
Gab2-deficient mice were established and used with a mixed genetic background of 129 and C57BL/6 as previously described (26). Transgenic (Tg) mice expressing Gab2.WT and Gab2.ΔPro were generated as previously described (6). Briefly, Flag-tagged human Gab2 was cloned into a human CD2 minigene cassette. The CD2 promoter directs the expression to the T-cell compartment (38). DNA was injected into C57BL/6 mice (Nihon SLC). Two founder mice for each construct, respectively, were identified by PCR from tail DNA. Among these lines, WT#20 and ΔPro#46, which showed approximately 27-fold expression of Gab2 mRNA compared to endogenous Gab2 in splenic T cells, were subjected to further analysis.
KLH immunization.
Mice were immunized with 80 μg of keyhole limpet hemocyanin (KLH) with colonization factor antigen (WAKO) intradermally in both hind footpads for analysis of lymph node cells. Seven days after immunization, whole lymph node cells from each popliteal draining lymph node from the immunized mice were stimulated with graded amounts of KLH in vitro.
RESULTS
Requirement of LAT for the phosphorylation of Gab2 following TCR engagement.
Although Gab2 was coimmunoprecipitated with several TCR proximal signaling molecules, such as CD3ζ, LAT, and ZAP-70, only LAT showed phosphorylation-dependent binding to Gab2 in a heterologous reconstitution system (34). Since LAT is exclusively localized in the lipid raft and TCR is also colocalized following TCR stimulation (16, 24), it is hypothesized that Gab2-LAT interaction is responsible for the recruitment of Gab2 to the vicinity of the TCR complex.
To assess this hypothesis, first, we examined whether LAT is required for Gab2 phosphorylation upon TCR engagement. LAT-deficient Jurkat cells, J.CaM2.5, were stimulated with anti-CD3 MAb, and the cell lysates were immunoprecipitated with anti-Gab2 Ab and immunoblotted with antiphosphotyrosine (anti-PY) MAb. Whereas J.CaM2.5 cells expressed Gab2 at a level similar to that expressed by wild-type Jurkat cells, the phosphorylation of Gab2 was significantly impaired in J.CaM2.5 (Fig. 1A, top panel, lane 4). SLP-76 was not necessary for the phosphorylation of Gab2, since strong phosphorylation was observed in the SLP-76-deficient Jurkat cell line J.14. The intensity of the anti-Gab2 blot tended to decrease upon stimulation particularly in wild-type Jurkat cells and J.14, as previously described (27), suggesting that stimulation-dependent modification such as serine/threonine or tyrosine phosphorylation may disturb the recognition by anti-Gab2 Abs. In these three cell lines, CD3ζ-associated ZAP-70 was similarly phosphorylated, indicating that TCR proximal signaling was triggered to the same extent by TCR stimulation (Fig. 1A, bottom panel) (8). Upon TCR engagement, raft-localized LAT becomes heavily phosphorylated and associates with several signaling molecules. In addition, TCR is also recruited to the lipid raft upon its engagement, which leads to further recruitment of ZAP-70 to the vicinity of LAT (24). In fact, the phosphorylation of Gab2 upon TCR stimulation was restored in J.CaM2.5 cells reconstituted with myc-LAT (Fig. 1A, lane 10). Taken together, LAT is required for the phosphorylation of Gab2, possibly as a platform upon which Gab2 comes close to ZAP-70.
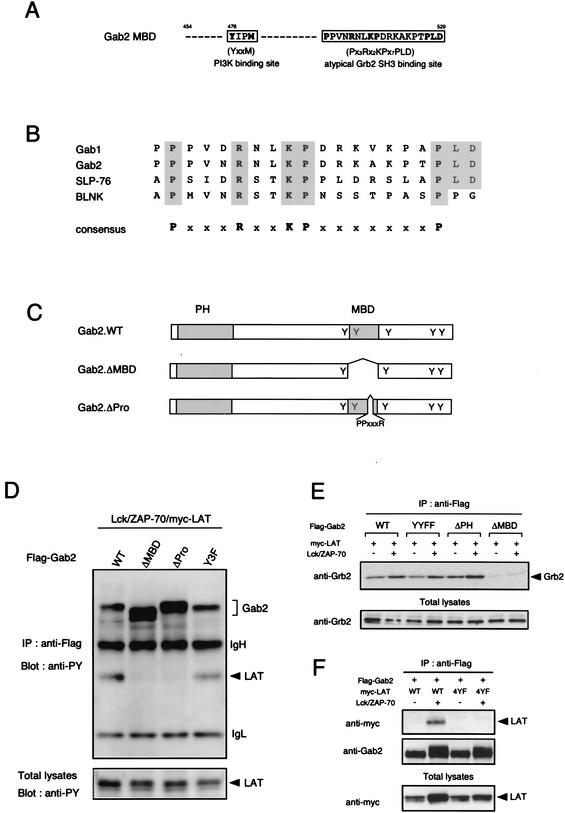
FIG. 1.
Gab2 associates with LAT through the MBD. (A) LAT is essential for Gab2 phosphorylation following TCR ligation. Left panels (lanes 1 to 6): 3 × 107 Jurkat wild type (WT), J.CaM2.5 (LAT−/−), and J.14 (SLP-76−/−) cells were stimulated (+) by anti-CD3 MAb for 2 min or were left unstimulated (−). Lysates were immunoprecipitated (IP) with anti-Gab2 Ab, followed by immunoblotting with anti-PY MAb (top panel). The same membrane was also blotted with anti-Gab2 Ab to confirm that equal amounts of proteins were precipitated (middle panel). Cell lysates were also immunoprecipitated with anti-CD3ζ MAb and blotted with anti-PY MAb (bottom panel). Right panels (lanes 7 to 10): J.CaM2.5 cells (LAT−/−) were infected with vector alone (Mock) or pMX-puro myc-LAT (myc-LAT). After drug selection, cells were stimulated and analyzed as described above. The expression of myc-LAT was confirmed by anti-LAT blotting (lower panel). (B) MBD of Gab2 is required for LAT binding. 293T cells were transiently transfected with 0.5 μg of pcDNA3.Flag-Gab2 and three mutants (YYFF, SHP-2-binding site mutant; ΔPH, PH domain-deletion mutant; ΔMBD, MBD deletion mutant) together with pME-Lck, pME-ZAP-70 (Lck/ZAP-70), or pCEFL-myc-LAT (myc-LAT) as indicated. Lysates were immunoprecipitated with anti-Flag MAb and blotted with Abs against PY, myc, Gab2, SHP-2, and PI3K (left panel). Total lysates were blotted with indicated Abs as controls (right panel). (C) MBD is sufficient for LAT binding. Jurkat T cells (n = 107) were left unstimulated (−) or stimulated with anti-CD3 MAb and sheep anti-hamster Ig Abs (+), and each lysate was incubated with GST control (GST) or GST-fused Gab2-MBD (GST-MBD) as described in Materials and Methods. Total lysates (Total) and immunoprecipitates were blotted with anti-PY (upper panel) and anti-LAT (lower panel) Abs.
LAT association with Gab2 through MBD.
It has been previously shown that phosphorylated LAT is detected in anti-Gab2 immunoprecipitates following TCR stimulation in splenic T cells as well as Jurkat T cells (34). It has also been demonstrated that the LAT-Gab2 association was induced in a heterologous expression system in the presence of responsible kinases—Lck and ZAP-70. However, whether the interaction between LAT and Gab2 is direct or mediated by another molecule(s) remains unclear. To analyze the molecular mechanism underlying this interaction, several mutants defective in various domains of Gab2—the SHP-2-binding region (YYFF), the PH domain (ΔPH), and the MBD (ΔMBD)—were tested for their LAT binding ability in a system where Flag-tagged Gab2 was transfected with myc-LAT together with Lck and ZAP-70. As shown in Fig. 1B, YYFF and ΔPH mutants could associate with LAT in a phosphorylation-dependent manner, demonstrating that the SHP-2-binding tyrosine residues and the PH domain were not necessary for LAT binding (Fig. 1B, lanes 4 and 6). Only the ΔMBD mutant lost LAT binding ability even in the presence of Lck-activated ZAP-70, although phosphorylation of LAT and mutant Gab2 per se was normally induced (Fig. 1B, upper right panel, lane 8). This was in concordance with a reciprocal immunoprecipitation experiment that used anti-myc MAb followed by anti-Gab2 blotting, which also showed MBD-dependent association of Gab2 and LAT (data not shown). Total phosphorylation level (Fig. 1B, upper right panel, lane 8) and inducible interaction with SHP-2 and PI3K (Fig. 1B, left panels, lane 8) were not impaired in ΔMBD mutant cells, suggesting that this mutation does not grossly disrupt the overall structure of Gab2. These results indicate that MBD of Gab2 is critical for LAT binding.
Reciprocally, to test if MBD is sufficient for LAT binding, a pulldown assay using GST-MBD fusion protein was performed. Lysates from unstimulated or stimulated T cells were incubated with immobilized GST-MBD or GST as control, and the bound phosphoproteins were analyzed by immunoblotting with anti-PY MAb. Figure 1C shows that GST-MBD but not GST control could pull down the 36- to 38-kDa phosphoprotein from T-cell lysates in a stimulation-dependent manner (upper panel). Reblot analysis revealed that this phosphoprotein corresponded to LAT (Fig. 1C, lower panel). Collectively, these results indicate that MBD of Gab2 is both necessary and sufficient for LAT binding.
PXXXR motif of Gab2 is responsible for LAT binding.
We further focused on determining the precise region within MBD responsible for the association with LAT. MBD contains Y476, one of the three PI3K-binding tyrosine residues, and a PXXXR sequence, an atypical SH3 binding motif (Fig. 2A) (22). The PXXXR motif was also found in several adaptor molecules that are critical for TCR or BCR signaling, as shown in Fig. 2B. To define the domain responsible for the interaction with LAT, Gab2 mutants lacking the PI3K-binding sites (Y452/467/584F; Y3F) (34) or the PXXXR region (PP511PVNR515; ΔPro) (Fig. 2C) were analyzed. Figure 2D demonstrates that ΔPro but not Y3F lost the capacity of LAT binding. The PXXXR motif is reported to associate with the C-terminal SH3 domain of Grb2 (22, 30). On the other hand, Grb2 has been shown to interact with phospho-LAT via its SH2 domain after TCR ligation (31). When these data are taken together, it is likely that an endogenous Grb2 family protein possessing both SH2 and SH3 domains couples Gab2 to LAT. Indeed, reblotting analysis of the same membrane in Fig. 1B (left panel) with anti-Grb2 MAb confirmed that only Gab2 mutants possessing LAT binding ability could constitutively associate with Grb2 (Fig. 2E, lanes 1 to 6). To confirm this model, the requirement of tyrosine phosphorylation of the Grb2/Gads binding sites of LAT for the LAT-Gab2 complex formation was analyzed. LAT 4YF (Y132/171/191/226F), which lacks Grb2/Gads binding sites (37), was introduced with Gab2 in the presence of Lck and ZAP-70. Figure 2F clearly shows that the Gab2-LAT binding was completely abolished by LAT 4YF, demonstrating that the phosphorylation of the four tyrosine residues of LAT, including the Grb2/Gads binding sites, is required for the association with Gab2.
FIG. 2.
PXXXR motif of Gab2 is responsible for LAT binding. (A) Amino acid sequence of MBD of Gab2. An atypical Grb2 SH3 binding motif and Y476VKM, one of the PI3K-binding consensus motifs, are boxed. (B) Conserved PXXXR motif within adaptor proteins Gab1/2, SLP-76, and BLNK. (C) Structures of Gab2 (WT) and the mutants lacking MBD (ΔMBD) or PXXXR motif (ΔPro). (D) PXXXR motif of Gab2 is required for LAT binding. 293T cells were transfected with wild-type Gab2 (WT), mutant with MBD deletion (ΔMBD), mutant with deletion of PXXXR motif(ΔPro), and Y452/476/584F (Y3F), all of which were Flag tagged, together with indicated plasmids. Lysates were immunoprecipitated (IP) with anti-Flag and blotted with anti-PY MAb. Arrowheads show phospho-LAT. Total lysates were blotted with anti-PY (lower panel) and anti-myc (data not shown) Abs as control. (E) MBD is required for constitutive binding to Grb2. The same membrane as in the left panel in Fig. 1B was stripped and reblotted with anti-Grb2 MAb (upper panel). Total lysates were also blotted as control (lower panel). (F) Grb2/Gads binding sites of LAT are required for the recruitment of Gab2. 293T cells were transiently transfected with myc-LAT or myc-LAT (4YF) together with Flag-Gab2, Lck, and ZAP-70. Cell lysates were immunoprecipitated with anti-Flag MAb and blotted with indicated Abs.
PXXXR-motif-dependent association of Gab2 with both Gads and Grb2.
In T cells, Gads also associates with LAT upon T-cell activation. Previous observations have suggested that Grb2 and Gads possess different binding specificities despite their structural similarity (36). Accordingly, we compared the preferences of Gab2 for binding to Gads and to Grb2. Since it has been shown that the isolated Grb2 SH3 domain sometimes loses binding specificity (19), we examined the affinity of Gab2 for binding to Grb2 and Gads by using the entire molecule. 293T cells were transfected with myc-tagged Grb2 and Gads together with graded amounts of Flag-tagged Gab2, and the Gads-Gab2 and Grb2-Gab2 complexes were analyzed by anti-Flag immunoprecipitation. Figure 3A shows that similar amounts of Gads and Grb2 were bound to Gab2 at an equal dose dependency when compared with the total amount (Fig. 3A, lanes 1 to 6 [Total]), demonstrating that Gads and Grb2 bind to Gab2 with similar affinity. These constitutive associations between Gab2 and endogenous Gads/Grb2 were also observed in T cells. J.EcoR cells were transfected with the vector alone (Mock), Gab2.WT (WT), or Gab2.ΔMBD (ΔMBD) by retrovirus infection, and sorted bulk transfectants were lysed and immunoprecipitated with anti-Flag MAb. The relative ratios of the precipitated Gads/Grb2 (Gab2-bound [Fig. 3B, lane 5]) to total Gads/Grb2 (input [Fig. 3B, lane 2]) were similar, respectively. In contrast, neither Gads nor Grb2 could associate with the Gab2 mutant with deletion of MBD at all (Fig. 3B, lanes 3 and 6). To confirm these complexes with endogenous proteins in normal T cells, the lysates of resting splenic T cells were immunoprecipitated with anti-Grb2 or anti-Gads Abs and were blotted with Abs against Gab2, Sos, and SLP-76. While Sos and SLP-76 were exclusively associated with Grb2 and Gads as reported earlier (19, 36), respectively, Gab2 could be detected in the immunoprecipitates with anti-Grb2 and anti-Gads Ab (Fig. 3C, lanes 3 and 4). These data confirm the association of Gab2 with both Grb2 and Gads in normal T cells. To further characterize the binding of Gab2 with Grb2 or Gads, several Gab2 mutants were tested. Although Gab2 possesses several potential SH3 binding motifs (typical proline-rich sequences) outside MBD, only Gab2.ΔMBD and Gab2.ΔPro almost completely lost their ability to bind to Gads and Grb2 (Fig. 3D). These results were obtained by immunoprecipitation in both directions with anti-Flag and anti-myc MAbs, followed by blotting with anti-myc and anti-Gab2 Abs, respectively.
FIG. 3.
PXXXR-motif-dependent Gads/Grb2 binding of Gab2. (A) Gads and Grb2 associated with Gab2 with similar affinity. 293T cells were transfected with 1 μg of myc-Gads and myc-Grb2 together with increasing amounts of Flag-Gab2. Lysates were immunoprecipitated (IP) with anti-Flag MAb, and total cell lysates (lanes 1 to 6) and immunoprecipitates (lanes 7 to 12) were blotted with rabbit anti-myc Ab. (B) Gab2 is constitutively associated with Gads and Grb2 in T cells. J.EcoR cells were infected with vector alone (Mock), Gab2.WT (WT), or Gab2.ΔMBD (ΔMBD) by retroviral gene transfer. Transgene-expressing bulk populations were sorted by means of bicistronic GFP expression and analyzed. Cells (n = 2 × 107) were immunoprecipitated with anti-Flag MAb and blotted with anti-Gads (19) (middle panel) and rabbit anti-Grb2 (lower panel) Abs. Anti-Gab2 blot is also shown as control (upper panel). (C) Endogenous Gab2 is coprecipitated with Gads and Grb2 in normal T cells. Lysates of 2 × 107 splenic T cells were immunoprecipitated with anti-Gads and rabbit anti-Grb2 Abs and blotted with anti-Gab2 Ab (top panel). The same membranes were also blotted with Abs against Sos, SLP-76, Gads, and Grb2 (MAb). (D) Gab2 associates with Grb2 and Gads in a PXXXR-motif-dependent manner. 293T cells were transfected with 1 μg of various Flag-Gab2 mutants together with 1 μg of myc-Grb2 (left panels) or myc-Gads (right panels). Cell were immunoprecipitated and blotted with indicated Abs.
Taken together, these results indicate that the PXXXR motif of Gab2 is exclusively required for the interaction of Gab2 with Grb2 and Gads, probably through the SH3 domain, as previously shown (30).
PXXXR-motif-dependent recruitment of Gab2 into GEM after TCR ligation.
Since LAT is known to be localized in lipid raft/GEMs through its palmitoylation, we examined whether the cellular localization of Gab2 was affected by the association with LAT. SEE strongly induces the activation of Jurkat cells in the presence of Raji B cells as APCs (35). When Gab2.WT-transfected J.EcoR cells were stimulated by Raji and SEE, GM1, a raft marker, and LAT were strongly accumulated at the interface between T and Raji cells (Fig. 4A, rows b to d) (14). Although Gab2 showed diffuse membrane localization in unstimulated Jurkat T cells (Fig. 4A, row a) (34), it was also accumulated at the interface and colocalized with GM1 and LAT upon stimulation (Fig. 4A, rows c and d). This suggests that Gab2 is translocated into lipid raft/GEMs upon TCR stimulation. The translocation of Gab2 into a lipid raft was further analyzed by biochemical analysis. J.EcoR cells expressing Gab2.WT or Gab2.ΔPro were stimulated, and the lysates were fractionated by sucrose gradient ultracentrifugation as previously described (14). As shown in Fig. 4B, a significant amount of Gab2.WT was detected in the raft fractions after TCR ligation (upper panel, lanes 5 and 6). Ponceau S staining revealed that these raft fractions (Fig. 4B, fractions 4 to 6) contained only 3% of total proteins in the lysates (data not shown), indicating specific recruitment of Gab2 to these fractions. In contrast, Gab2 was barely detected in the raft fraction in Gab2.ΔPro mutant-expressing cells, even though the raft fraction contained similar amounts of Lck or LAT. These data indicate that Gab2 is recruited to the lipid raft upon TCR stimulation in a PXXXR-motif-dependent manner.
FIG. 4.
Localization of Gab2 in the lipid raft upon T-cell activation. (A) Gab2 is colocalized with GM1 and LAT upon superantigen stimulation. J.EcoR cells expressing Gab2.WT were cocultured with Raji cells in the absence (a) or presence (b to d) of 200 ng of SEE/ml for 30 min. Cells were fixed on a coverslip coated with poly-l-lysine and were stained with cholera toxin B-FITC (CTx, a to c), biotinylated anti-Flag MAb (a, c, and d), control MAb (b), anti-LAT Ab (c), and Cy3-labeled streptavidin (a to d) or Alexa488-labeled anti-rabbit IgG Ab (c), followed by confocal microscopy analysis (LSM510; Carl Zeiss). (B) Recruitment of Gab2 to GEMs after TCR ligation. J.EcoR cells and transfectants were either left unstimulated (−) or stimulated (Stim.) (+) with anti-CD3 MAb for 3 min, followed by lysis in MES lysis buffer plus protease and phosphatase inhibitors. Lysates were subjected to sucrose gradient ultracentrifugation for GEM purification. Sequential fractions from the top of the gradient are indicated by numbers. Each fraction was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by blotting with anti-Gab2, anti-Lck, and anti-LAT Abs.
Competitive function of Gab2 with SLP-76 for Gads/Grb2 binding.
The PXXXR motif is also found in SLP-76, and this motif is involved in the region responsible for Gads binding (15). Gads plays a key role in bringing SLP-76 to the membrane-bound LAT, which is a critical step in the process of enabling SLP-76 to transmit downstream signals (36). LAT is therefore required for Gab2 phosphorylation (Fig. 1) and SLP-76 function (8). Our finding of the formation of the LAT-Gads-Gab2 ternary complex and its homology to the LAT-Gads-SLP-76 complex led us to the hypothesize that positive (SLP-76) and negative (Gab2) substrates of ZAP-70 might compete for the Gads/Grb2 binding. To test this hypothesis, we first compared the Grb2/Gads binding affinity of Gab2 with that of SLP-76. 293T cells were transiently transfected with SLP-76 and Gab2 with various amounts of Grb2 or Gads. Figure 5A demonstrates that Gab2 and SLP-76 bind to Gads with similar affinity, as the Gads-bound/input intensity ratios are similar between Gab2 and SLP-76 for various amounts of Gads (Fig. 5A, right panels). However, in the case of Grb2 binding, Gab2 showed a higher affinity than did SLP-76 under the condition of a limited amount of Grb2 (Fig. 5A, left panels). This is consistent with the observation that SLP-76 has a higher affinity for Gads than for Grb2 (1, 2). Since the expression of Gab2 is strongly up-regulated upon TCR stimulation (9), it is possible that the induced Gab2 may replace (compete with) SLP-76 from Gads, at least partly, to regulate excessive activation. Indeed, increased amounts of Gab2 prevented SLP-76 binding to Gads as well as to Grb2 in a dose-dependent manner in heterologous expression systems (Fig. 5B). To further assess this Gab2-mediated suppressive mechanism in vivo, Gab2-Tg mice under the human CD2 promoter were established and analyzed for the constitutive binding molecule with Gads. In splenic T cells from non-Tg mice, a large amount of SLP-76 was constitutively associated with Gads. However, in Gab2-Tg mice, the amount of Gads-bound SLP-76 was reduced by approximately 40% while a significant amount of Gab2 interacted with this Gads (Fig. 5C). Control immunoprecipitation did not precipitate any detectable Gads or Grb2 in Gab2.WT-expressing cells (data not shown), proving the specific coimmunoprecipitation. This implies that, in vivo, the induced Gab2 potentially competes with SLP-76 for Gads binding, and this may play a role in the efficient negative regulation of TCR signaling through Gab2.
FIG. 5.
Competitive function of Gab2 with SLP-76 for Gads/Grb2 binding. (A) Quantitative analysis of Gab2 competitive function in SLP-76 binding to Gads/Grb2. 293T cells were transfected with Flag-SLP-76 and myc-Grb2 together with increasing amounts of Flag-Gab2. Cell lysateswere immunoprecipitated (IP) with anti-myc MAb. Total cell lysates (lanes 1 to 6) and immunoprecipitates (lanes 7 to 12) were blotted with anti-Flag, anti-SLP-76, rabbit anti-myc, and anti-Gads Abs as indicated. (B) Gab2 competes for Gads/Grb2 binding with SLP-76. 293T cells were transfected with Flag-SLP-76 and myc-Grb2/myc-Gads together with increasing amounts of Flag-Gab2. Total lysates or immunoprecipitates with anti-myc Abs were blotted with anti-Flag-MAb to detect both Gab2 and SLP-76 simultaneously. The intensity of each band was quantitated and shown as the ratio of SLP-76 to Gab2 for input (Total lysates) or Grb2/Gads-bound (IP: anti-myc) protein. (C) Impaired SLP-76 binding of Gads in Gab2-Tg mice. Splenic T cells from non-Tg and Gab2-Tg mice were lysed and immunoprecipitated by anti-Gads Abs. Total cell lysates and immunoprecipitates were blotted with anti-Gab2, anti-SLP-76, and anti-Gads Abs as indicated. In the right panel, the relative amount of Gads-associated SLP-76 is shown as the ratio of SLP-76 in anti-Gads precipitates to SLP-76 in total lysates when the same ratio for non-Tg mice is 1.0. The amount of Gads-bound SLP-76 in Gab2-Tg (Gab2 Tg) mice was significantly (P < 0.05) low compared with that of littermate control (non Tg). Data are presented as means plus or minus standard deviations from three independent experiments.
LAT binding is critical for both phosphorylation and inhibitory activity of Gab2.
Next, the role of Gab2-LAT binding in phosphorylation, complex formation with other proteins, and the inhibitory effect on TCR signaling was investigated. As shown in Fig. 6A, the phosphorylation level of Gab2 was dramatically decreased by ΔMBD mutation in Jurkat cells. Furthermore, LAT binding was also specifically abolished (Fig. 6A, top panel, lane 6), although total LAT phosphorylation was not reduced in ΔMBD (data not shown), consistent with the fact that LAT is required for the phosphorylation of Gab2 (Fig. 1A) and that Gab2-LAT binding is mediated by Grb2/Gads (Fig. 2D). Phosphorylation-dependent SHP-2 binding was also impaired in ΔMBD (Fig. 6A, bottom panel, lane 6).
FIG. 6.
LAT binding is crucial for phosphorylation and inhibitory function of Gab2. (A) Interaction with LAT is critical for the optimal phosphorylation of Gab2. J.EcoR cells infected with vector alone (Mock), Gab2.WT, or Gab2.ΔMBD were stimulated with anti-CD3 MAb and sheep anti-mouse Ig Ab for 2 min. Flag-Gab2 was immunoprecipitated (IP) from total cell lysates with anti-Flag MAb and blotted with anti-PY MAb (top panel). The same membrane was also reblotted with anti-Gab2 (middle panel) and anti-SHP-2 Abs (bottom panel). (B) LAT binding is critical for the inhibitory function of Gab2. Each transfected cell line described for panel A was stimulated with 50 μg of soluble anti-CD3 MAb (upper panel) or 10 ng of SEE/ml (lower panel) in the presence of Raji cells as APC for 24 h. The levels of IL-2production were determined by enzyme-linked immunosorbent assay and were expressed as a percentage of maximum response induced by PMA plus ionomycin. Data are presented as means plus or minus standard deviations of triplicate assays. Similar results were obtained from three independent experiments.
The functional effects of Gab2.WT and Gab2.ΔMBD on interleukin 2 (IL-2) production upon TCR ligation were analyzed. As we have previously reported, Gab2 suppressed IL-2 production upon stimulation with anti-TCR or SEE plus APC. In contrast, the ΔMBD mutant completely lost inhibitory activity (Fig. 6B). Since ΔMBD retained the same PI3K-binding ability as WT (Fig. 1B), the suppression failure is not due to the lack of one of three putative PI3K-binding sites present within MBD. This is also confirmed by the observation that Gab2.ΔPro containing all three PI3K-binding sites also lacks inhibitory activity (shown below). These results indicate that the impairment of the inhibitory activity of ΔMBD or ΔPro mutant is due to the lack of Grb2/Gads binding and that the Gads/Grb2-Gab2 assembly is responsible for all important signaling events, including LAT binding, raft localization, phosphorylation, and inhibitory function.
LAT binding is critical for the inhibition of T-cell responses in vivo.
Then, to assess the function of the PXXXR motif within Gab2 in normal T cells, Tg mice expressing Gab2.WT or Gab2.ΔPro under the human CD2 promoter were established and analyzed. Western blot analysis of thymocytes (data not shown) and splenic T cells (Fig. 7A) from these Tg mice showed similar levels of Gab2 protein expression. Gab2 protein was not detectable in T-cell-depleted splenocytes from both Tg lines, indicating that both Gab2 proteins were expressed specifically in T-cell lineage as expected (data not shown). These mice showed normal development and cellularity of thymocytes and splenic T cells (data not shown). However, when crossed with TCR-Tg mice, the impairment of both positive and negative selections was observed in WT-Tg but not in ΔPro-Tg (unpublished data). To test the effect of Gab2 and Gab2.ΔPro on mature T-cell activation, splenic T cells were stimulated with anti-CD3 and anti-CD28 Abs (Fig. 7B). Splenic T cells from Gab2.WT-Tg mice showed decreased proliferative response upon stimulation compared to the littermate non-Tg control. However, the Gab2.ΔPro-transgene did not induce any significant inhibition of T-cell response. It should be noted that phorbol myristate acetate (PMA) plus ionomycin induced similar proliferation, supporting our previous observation that Gab2 inhibits TCR proximal signaling (34).
FIG.7.
LAT binding is critical for the inhibition of T-cell response upon TCR stimulation in vivo. (A) Establishment of Tg mice expressing Gab2.WT and Gab2.ΔPro. Splenic T cells were lysed and analyzed by Western blotting with anti-Gab2 Abs. It should be noted that a similar level of Gab2 was expressed in both lines. Arrowhead indicates Gab2. LM, littermate. (B) Gab2.WT but not Gab2.ΔPro exhibits inhibitory effect on T-cell response. Splenic T cells (2 × 105 cells) from Gab2.WT-Tg (closed circles) or Gab2.ΔPro-Tg (gray triangles) mice or each control littermate (open circles and open triangles) were stimulated with indicated concentrations of immobilized anti-CD3 MAb and 5 μg of anti-CD28 MAb/ml (left panels) or were stimulated with PMA plus ionomycin (right panel). Cells were cultured for 42 h, pulsed with [3H]thymidine, and harvested after 6 h. Data are presented as means plus or minus standard deviations of triplicate in an experiment that is representative of three separate experiments. (C) In vivo KLH-specific T-cell responses of Gab2-Tg mice. Each mouse was immunized with KLH and colonization factor antigen intradermally in each hind footpad. Seven days after immunization, whole lymph node cells (2 × 105 cells) were prepared from popliteal draining lymph nodes and were stimulated with graded amounts of KLH. Cells were cultured for 48 h, pulsed with [3H]thymidine, and harvested after 16 h. Open symbols, non-Tg littermate control; closed symbols, Tg mice. Data are presented as means plus or minus standard deviations of triplicate assays on each individual mouse. Data represent two independent experiments. (D) Cytokine production of T cells from Gab2-Tg mice upon antigen-specific stimulation. Culture supernatants of the same culture as shown in panel C were harvested at 48 h, and IL-2 concentration was determined by enzyme-linked immunosorbent assay. Data are presented as means of two individual mice for triplicate assays. Data represent two independent experiments.
Antigen-dependent secondary T-cell responses were also examined in these mice. Figure 7C and D show that the in vitro KLH-specific T-cell response after in vivo priming was reduced in Gab2.WT-Tg mice but not in Gab2.ΔPro-Tg mice in terms of both proliferation and IL-2 production, respectively. These results also demonstrated that the PXXXR motif is critical for the inhibitory function of Gab2 in normal peripheral T cells in vivo.
Enhanced T-cell response in Gab2-deficient mice.
Finally, to examine the physiological contribution of Gab2-mediated inhibitory signals to T-cell responses, T cells from Gab2-deficient mice were analyzed (10, 26). In Gab2−/− mice, the cellularity of thymocytes and peripheral T cells was similar to that of littermate control (data not shown). However, mature T cells isolated from Gab2−/− mice exhibited enhanced proliferation in response to TCR stimulation, compared with those from wild-type mice (Fig. 8, left and middle panels). Similar to the case of Tg mice (Fig. 7B), Gab2−/− T cells showed the same response to PMA plus ionomycin (Fig. 8, right panel). These results revealed that Gab2 mediates inhibitory function upon TCR stimulation in T cells under physiological conditions.
FIG. 8.
Gab2-deficient mice show increased T-cell responses. Lymph node T cells were purified by reacting with anti-Thy-1 MAb from wild-type mice (WT) and Gab2-deficient mice (KO) by using MACS. Cells (n = 105) were stimulated with anti-CD3 and anti-CD28 MAbs as indicated or also with 10 ng of PMA/ml and 1 μM ionomycin as control (right panel). Cells were cultured for 42 h, pulsed with [3H]thymidine, and harvested after 6 h. Data were means plus or minus standard deviations of triplicates in a representative from three independent experiments. All experiments showed similar results.
DISCUSSION
In the present study, we found that Gab2 binds to LAT by associating with Grb2/Gads and that this complex formation is required to realize the inhibitory activity of Gab2, based on the analysis of Gab2-expressing Tg mice and transfected T-cell lines.
The requirement of LAT for Gab2 phosphorylation upon TCR stimulation was found from the observation that Gab2 phosphorylation was greatly diminished in J.CaM2.5, LAT-deficient T cells. Indeed, the defect of Gab2 phosphorylation in this cell line was restored by LAT transfection. Since Gab2 lacks a classical phosphotyrosine binding domain, several mutants of Gab2 were tested to explore the possible mechanism underlying this interaction. Among them, the MBD of Gab2 was found to be both necessary and sufficient for LAT binding. Since MBD was initially reported to be a direct binding site for phosphorylated c-Met (32), we initially assumed that the MBD of Gab2 might directly bind to phospho-LAT. However, Schaeper et al. recently reported that, although the MBD of Gab2 is closely related to that of Gab1, Gab2 lacks a short motif responsible for direct c-Met binding, called the c-Met binding sequence, which is found in the MBD of Gab1 (30). Instead, the MBD of Gab2 contains an atypical Grb2 binding site (PXXXR) and one tyrosine residue of three PI3K-binding consensus motifs. When a large portion of the PXXXR sequence was deleted (Gab2.ΔPro), phosphorylation-dependent LAT binding was completely abolished, whereas the PI3K-binding-defective mutant had no effect (Fig. 4A). Gab2.ΔPro could not interact with Gads or Grb2 (Fig. 4C) in spite of the normal association with SHP-2 (data not shown). Supporting this observation, Grb2 was found to link Gab2 and several phosphorylated receptors, such as c-Met and EGF receptor. As for cytokine signaling, the receptors for IL-2 and IL-3 do not possess a Grb2 binding site, and thus Shc links the receptors and the Grb2-Gab2 complex (21). Taken together, since Gab2 does not possess a phosphotyrosine binding domain, it utilizes an SH3-SH2-SH3-containing adaptor(s) that is recruited to phospho-LAT as well as various phosphoreceptors. Indeed, LAT mutant lacking the Grb2/Gads binding sites failed to associate with Gab2 in the presence of kinases.
Although Gads and Grb2 are similar in terms of protein structure, the binding specificity of the C-terminal SH3 domain is quite different. For example, SLP-76 binds to Gads more strongly than to Grb2, while Sos interacts with Grb2 but not with Gads (1, 19). In our biochemical analysis, Gab2 is capable of binding to both Gads and Grb2 in T cells. A recent report indicated that Gads interacts with Gab3 but not with Gab2 in a myeloid progenitor cell line (5). Although it is possible that the abundant expression of Gab3 potentially deprives Gab2 of Gads in this cell line or that the difference is dependent on cell type, the reason for this divergence is not clear at present.
Since LAT is palmitoylated and specifically localized in the lipid raft, we supposed that Gab2 is recruited into the lipid raft through the assembly with LAT upon TCR stimulation. Indeed, although wild-type Gab2 is translocated into the lipid raft, Gab2.ΔPro is barely detected in the raft fraction. This observation also supports the model that Grb2/Gads links Gab2 to LAT. On the other hand, TCR is also recruited into the lipid raft upon its engagement, which brings ZAP-70 close to LAT (24). The close interaction between these two molecules allows ZAP-70 to phosphorylate Gab2. The inducible formation of the LAT-Gads/Grb2-Gab2 ternary complex is quite similar to that of the LAT-Gads-SLP-76 complex. SLP-76 also possesses the PXXXR motif and constitutively associates with Gads. The mutant with the deleted region containing this motif could not functionally reconstitute the defect of T-cell activation in SLP-76−/− cells (15). Furthermore, Boerth et al. demonstrated that targeting SLP-76 to the lipid raft was sufficient to reconstitute the functional defect of LAT-deficient J.CaM2 cells (4). Thus, both positive (SLP-76) and negative (Gab2) regulators are recruited into the lipid raft via similar mechanisms, and both are phosphorylated there by ZAP-70. This step is critical for exhibiting each effector (activatory versus inhibitory) function (Fig. 6A and B) through the recruitment of distinct downstream signaling molecules.
From these observations, we suppose that positive and negative substrates of ZAP-70 compete for raft recruitment through Grb2/Gads binding. The mechanisms controlling the selection of substrates for recruitment are important; however, they are as yet unknown. The PH domain of Gab2, which is absent in SLP-76, may confer an advantage to Gab2 for this competitive recruitment. Alternatively, the preferential recruitment may be simply determined by the molecular ratio of Gab2 to SLP-76. Interestingly, the expression of Gab2 in resting T cells is rather low but becomes markedly elevated with TCR stimulation, whereas the expression of SLP-76 remains constant (9). Furthermore, considering the recent report of SLP-76 being cleaved upon stimulation (3), the molecular ratio of SLP-76 to Gab2 may be altered to undergo overwhelming domination by Gab2 upon activation. The transfected cells used for Fig. 5B or T cells in Gab2-Tg mice may represent such activated T cells. Then, under this condition, Gads/Grb2 would bring Gab2 rather than SLP-76 to phospho-LAT following TCR stimulation. Consequently, ZAP-70 phosphorylates Gab2, a decoy substrate for SLP-76, which had been brought to the proximity of ZAP-70 by association with Gads/Grb2. Besides the Gads binding, Gab2-Grb2 interaction may result in blocking of the assembly with some other Grb2 SH3(C)-binding molecules such as Vav (28). Furthermore, tyrosine phosphorylation of Gab2 by ZAP-70 creates binding sites for several inhibitory molecules, such as SHP-2 and PI3K, which then leads to efficient negative regulation (29, 34). Consistently, Gab2 lacking Grb2/Gads binding ability did not exhibit an inhibitory function of T-cell activation in Tg mice as well as in Jurkat cells. Since Gab2.ΔMBD and ΔPro cannot be localized in the lipid raft and thus fail to be phosphorylated, the binding of negative regulators is greatly impaired. Thus, these loss-of-function mutations did not function as a dominant-negative mutant (Fig. 6B and Fig. 7B to D). A previous observation that Gab2 mutants unable to bind to SHP-2 or PI3K still exhibited weak inhibitory function without a dominant-negative effect leads us to assume the possibility of another inhibitory mechanism that has been described in a previous report (34). The present study demonstrates that, as a possible candidate for such a mechanism, PXXXR-mediated competition may partly contribute to Gab2-mediated suppressive function in the absence of SHP-2 or PI3K binding. Gab2 may utilize multiple domains for the efficient inhibitory activity; the relative contribution of each mechanism remains to be precisely defined. However, from present data showing that Gab2 mutant lacking LAT binding completely lost inhibitory activity, it should be stressed that PXXXR-mediated LAT binding is a key event for promoting all inhibitory machineries. Although Gab2 is involved in several cytokine receptor signals, Gab2.WT and Gab2.ΔPro had no effect on the T-cell- proliferative responses induced by IL-2, IL-6, and IL-7 in our Tg mice (unpublished observations), suggesting that the observed negative function of Gab2 on TCR signaling may dominate in T cells.
A few molecules other than Gab2 and SLP-76 contain the PXXXR motif. HPK1, hematopoietic progenitor kinase 1, was reported to interact with Grb2 and possesses the PXXXR motif (20). However, the major Grb2 binding site of HPK1 was reported to be a classical Pro-rich sequence, PXXP, rather than the atypical SH3 binding site, PXXXR (20, 23). AMSH also possesses the PXXXR motif, and a peptide encompassing this region can interact with the Grb2 SH3 domain in vitro (17). In spite of the striking phenotype in neurons, no apparent immunological disorder was observed in AMSH-deficient mice, suggesting that AMSH plays a minimal role in TCR signaling (13).
The analysis of T cells from Gab2−/− mice showed enhanced T-cell responses upon TCR stimulation, consistent with the analysis of transfectants and Tg mice. On the other hand, Gu et al. and a group have recently demonstrated that Gab2 plays a positive role in FcɛRI (10) and c-kit signaling (26) by using Gab2−/− mast cells. Although the mechanism for the apparently opposite function of Gab2 in mast cells and T cells has to be resolved, it is possible that “multitask receptor” TCR utilizes adaptors in a manner different from that of other receptors for fine regulation to various antigens.
Very recently, Gab3, a new member of the Gab family proteins, was identified and shown to be expressed in hematopoietic tissues (33). The unique and possibly redundant roles of each Gab family molecule in TCR signaling and immune responses remain unclear at this moment. Further extensive analyses of the expression levels and the cellular localization and phosphorylation status of each member, as well as the associated molecules of Gab family proteins in various T-cell stages and subsets, may clarify the individual and overall function of these molecules in the regulation of T-cell function.
Acknowledgments
We thank K. Sugamura for Gads cDNA, R. Shiina and E. Ishikawa for technical help, and H. Yamaguchi and Y. Kurihara for secretarial assistance.
This work was supported by a Grant-in-Aid for Young Scientists (B) and Grant-in-Aid for Priority Area Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Asada, H., N. Ishii, Y. Sasaki, K. Endo, H. Kasai, N. Tanaka, T. Takeshita, S. Tsuchiya, T. Konno, and K. Sugamura. 1999. Grf40, a novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J. Exp. Med. 189:1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry, D., P. Nash, S. Liu, T. Pawson, and C. McGlade. 2002. A high-affinity Arg-X-X-Lys SH3 binding motif confers specificity for the interaction between Gads and SLP-76 in T cell signaling. Curr. Biol. 12:1336. [DOI] [PubMed] [Google Scholar]
- 3.Berry, D. M., S. J. Benn, A. M. Cheng, and C. J. McGlade. 2001. Caspase-dependent cleavage of the hematopoietic specific adaptor protein Gads alters signalling from the T cell receptor. Oncogene 20:1203-1211. [DOI] [PubMed] [Google Scholar]
- 4.Boerth, N. J., J. J. Sadler, D. E. Bauer, J. L. Clements, S. M. Gheith, and G. A. Koretzky. 2000. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J. Exp. Med. 192:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgin, C., R. P. Bourette, S. Arnaud, Y. Liu, L. R. Rohrschneider, and G. Mouchiroud. 2002. Induced expression and association of the Mona/Gads adapter and Gab3 scaffolding protein during monocyte/macrophage differentiation. Mol. Cell. Biol. 22:3744-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinster, R. L., H. Y. Chen, M. E. Trumbauer, M. K. Yagle, and R. D. Palmiter. 1985. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc. Natl. Acad. Sci. USA 82:4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burack, W. R., A. M. Cheng, and A. S. Shaw. 2002. Scaffolds, adaptors and linkers of TCR signaling: theory and practice. Curr. Opin. Immunol. 14:312-316. [DOI] [PubMed] [Google Scholar]
- 8.Finco, T. S., T. Kadlecek, W. Zhang, L. E. Samelson, and A. Weiss. 1998. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity 9:617-626. [DOI] [PubMed] [Google Scholar]
- 9.Gadina, M., C. Sudarshan, R. Visconti, Y. J. Zhou, H. Gu, B. G. Neel, and J. J. O'Shea. 2000. The docking molecule Gab2 is induced by lymphocyte activation and is involved in signaling by interleukin-2 and interleukin-15 but not other common gamma chain-using cytokines. J. Biol. Chem. 275:26959-26966. [DOI] [PubMed] [Google Scholar]
- 10.Gu, H., K. Saito, L. D. Klaman, J. Shen, T. Fleming, Y. Wang, J. C. Pratt, G. Lin, B. Lim, J. P. Kinet, and B. G. Neel. 2001. Essential role for Gab2 in the allergic response. Nature 412:186-190. [DOI] [PubMed] [Google Scholar]
- 11.Hermiston, M. L., Z. Xu, R. Majeti, and A. Weiss. 2002. Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. J. Clin. Investig. 109:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibi, M., and T. Hirano. 2000. Gab-family adapter molecules in signal transduction of cytokine and growth factor receptors, and T and B cell antigen receptors. Leuk. Lymphoma 37:299-307. [DOI] [PubMed] [Google Scholar]
- 13.Ishii, N., Y. Owada, M. Yamada, S. Miura, K. Murata, H. Asao, H. Kondo, and K. Sugamura. 2001. Loss of neurons in the hippocampus and cerebral cortex of AMSH-deficient mice. Mol. Cell. Biol. 21:8626-8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh, K., M. Sakakibara, S. Yamasaki, A. Takeuchi, H. Arase, M. Miyazaki, N. Nakajima, M. Okada, and T. Saito. 2002. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J. Immunol. 168:541-544. [DOI] [PubMed] [Google Scholar]
- 15.Koretzky, G. A., and P. S. Myung. 2001. Positive and negative regulation of T-cell activation by adaptor proteins. Nat. Rev. Immunol. 1:95-107. [DOI] [PubMed] [Google Scholar]
- 16.Kosugi, A., S. Saitoh, S. Noda, K. Yasuda, F. Hayashi, M. Ogata, and T. Hamaoka. 1999. Translocation of tyrosine-phosphorylated TCRzeta chain to glycolipid-enriched membrane domains upon T cell activation. Int. Immunol. 11:1395-1401. [DOI] [PubMed] [Google Scholar]
- 17.Lewitzky, M., C. Kardinal, N. H. Gehring, E. K. Schmidt, B. Konkol, M. Eulitz, W. Birchmeier, U. Schaeper, and S. M. Feller. 2001. The C-terminal SH3 domain of the adapter protein Grb2 binds with high affinity to sequences in Gab1 and SLP-76 which lack the SH3-typical P-x-x-P core motif. Oncogene 20:1052-1062. [DOI] [PubMed] [Google Scholar]
- 18.Liu, S. K., D. M. Berry, and C. J. McGlade. 2001. The role of Gads in hematopoietic cell signalling. Oncogene 20:6284-6290. [DOI] [PubMed] [Google Scholar]
- 19.Liu, S. K., and C. J. McGlade. 1998. Gads is a novel SH2 and SH3 domain-containing adaptor protein that binds to tyrosine-phosphorylated Shc. Oncogene 17:3073-3082. [DOI] [PubMed] [Google Scholar]
- 20.Liu, S. K., C. A. Smith, R. Arnold, F. Kiefer, and C. J. McGlade. 2000. The adaptor protein Gads (Grb2-related adaptor downstream of Shc) is implicated in coupling hemopoietic progenitor kinase-1 to the activated TCR. J. Immunol. 165:1417-1426. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Y., and L. R. Rohrschneider. 2002. The gift of Gab. FEBS Lett. 515:1-7. [DOI] [PubMed] [Google Scholar]
- 22.Lock, L. S., I. Royal, M. A. Naujokas, and M. Park. 2000. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 275:31536-31545. [DOI] [PubMed] [Google Scholar]
- 23.Ma, W., C. Xia, P. Ling, M. Qiu, Y. Luo, T. H. Tan, and M. Liu. 2001. Leukocyte-specific adaptor protein Grap2 interacts with hematopoietic progenitor kinase 1 (HPK1) to activate JNK signaling pathway in T lymphocytes. Oncogene 20:1703-1714. [DOI] [PubMed] [Google Scholar]
- 24.Montixi, C., C. Langlet, A. M. Bernard, J. Thimonier, C. Dubois, M. A. Wurbel, J. P. Chauvin, M. Pierres, and H. T. He. 1998. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17:5334-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano, H., T. Yamazaki, M. Ikeda, H. Masai, S. Miyatake, and T. Saito. 1994. Purification of glutathione S-transferase fusion proteins as a non-degraded form by using a protease-negative E. coli strain, AD202. Nucleic Acids Res. 22:543-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishida, K., L. Wang, E. Morii, S. J. Park, M. Narimatsu, S. Itoh, S. Yamasaki, M. Fujishima, K. Ishihara, M. Hibi, Y. Kitamura, and T. Hirano. 2002. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood 99:1866-1869. [DOI] [PubMed] [Google Scholar]
- 27.Nishida, K., Y. Yoshida, M. Itoh, T. Fukada, T. Ohtani, T. Shirogane, T. Atsumi, M. Takahashi-Tezuka, K. Ishihara, M. Hibi, and T. Hirano. 1999. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood 93:1809-1816. [PubMed] [Google Scholar]
- 28.Nishida, M., K. Nagata, Y. Hachimori, M. Horiuchi, K. Ogura, V. Mandiyan, J. Schlessinger, and F. Inagaki. 2001. Novel recognition mode between Vav and Grb2 SH3 domains. EMBO J. 20:2995-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt, J. C., V. E. Igras, H. Maeda, S. Baksh, E. W. Gelfand, S. J. Burakoff, B. G. Neel, and H. Gu. 2000. Cutting edge: gab2 mediates an inhibitory phosphatidylinositol 3′-kinase pathway in T cell antigen receptor signaling. J. Immunol. 165:4158-4163. [DOI] [PubMed] [Google Scholar]
- 30.Schaeper, U., N. H. Gehring, K. P. Fuchs, M. Sachs, B. Kempkes, and W. Birchmeier. 2000. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149:1419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber, J. R., S. Orstavik, K. M. Torgersen, N. C. Danbolt, S. F. Berg, J. C. Ryan, K. Tasken, J. B. Imboden, and J. T. Vaage. 1998. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J. Exp. Med. 187:1157-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidner, K. M., S. Di Cesare, M. Sachs, V. Brinkmann, J. Behrens, and W. Birchmeier. 1996. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384:173-176. [DOI] [PubMed] [Google Scholar]
- 33.Wolf, I., B. J. Jenkins, Y. Liu, M. Seiffert, J. M. Custodio, P. Young, and L. R. Rohrschneider. 2002. Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol. Cell. Biol. 22:231-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamasaki, S., K. Nishida, M. Hibi, M. Sakuma, R. Shiina, A. Takeuchi, H. Ohnishi, T. Hirano, and T. Saito. 2001. Docking protein Gab2 is phosphorylated by ZAP-70 and negatively regulates T cell receptor signaling by recruitment of inhibitory molecules. J. Biol. Chem. 276:45175-45183. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki, S., M. Tachibana, N. Shinohara, and M. Iwashima. 1997. Lck-independent triggering of T-cell antigen receptor signal transduction by staphylococcal enterotoxins. J. Biol. Chem. 272:14787-14791. [DOI] [PubMed] [Google Scholar]
- 36.Yoder, J., C. Pham, Y. M. Iizuka, O. Kanagawa, S. K. Liu, J. McGlade, and A. M. Cheng. 2001. Requirement for the SLP-76 adaptor GADS in T cell development. Science 291:1987-1991. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, W., R. P. Trible, M. Zhu, S. K. Liu, C. J. McGlade, and L. E. Samelson. 2000. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J. Biol. Chem. 275:23355-23361. [DOI] [PubMed] [Google Scholar]
- 38.Zhumabekov, T., P. Corbella, M. Tolaini, and D. Kioussis. 1995. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J. Immunol. Methods 185:133-140. [DOI] [PubMed] [Google Scholar]