Abstract
β-Catenin has a key role in the formation of adherens junction through its interactions with E-cadherin and α-catenin. We show here that interaction of β-catenin with α-catenin is regulated by the phosphorylation of β-catenin Tyr-142. This residue can be phosphorylated in vitro by Fer or Fyn tyrosine kinases. Transfection of these kinases to epithelial cells disrupted the association between both catenins. We have also examined whether these kinases are involved in the regulation of this interaction by K-ras. Stable transfectants of the K-ras oncogene in intestinal epithelial IEC18 cells were generated which show little α-catenin-β-catenin association with respect to control clones; this effect is accompanied by increased Tyr-142 phosphorylation and activation of Fer and Fyn kinases. As reported for Fer, Fyn kinase is constitutively bound to p120 catenin; expression of K-ras induces the phosphorylation of p120 catenin on tyrosine residues increasing its affinity for E-cadherin and, consequently, promotes the association of Fyn with the adherens junction complex. Yes tyrosine kinase also binds to p120 catenin but only upon activation, and stimulates Fer and Fyn tyrosine kinases. These results indicate that p120 catenin acts as a docking protein facilitating the activation of Fer/Fyn tyrosine kinases by Yes and demonstrate the role of these p120 catenin-associated kinases in the regulation of β-catenin-α-catenin interaction.
Cell-cell contacts among epithelial cells have a crucial function in organized tissues and are mainly mediated by adherens junctions and desmosomes. In adherens junctions, although the extracellular domain of E-cadherin is essential for connecting cells through homophilic interactions, its intracellular domain is required for regulating cell-cell adhesion. The latter domain is indirectly associated with the actin cytoskeleton through either β-catenin or plakoglobin and α-catenin. These interactions are essential for proper cell adhesion (3, 28, 36). Another catenin, p120, also binds to the cytosolic region of E-cadherin through a different subdomain (11, 37, 48).
Tyrosine phosphorylation of the cadherin-catenin complex has been implicated in the regulation of adhesion (12, 14, 23). Indeed, stimulation of growth factor receptors or oncogenic Src kinases is implicated in the negative regulation of intercellular contacts (6, 26, 31, 41, 42). On the other hand, ectopic expression of phosphotyrosine (PTyr) phosphatases strengthens cell-cell adhesion (27, 45). Two components of the adherens junction complex have been considered the main targets of tyrosine kinases/phosphatases: β-catenin and p120 catenin. p120 catenin is highly phosphorylated by Src tyrosine kinase (25) and phosphorylation by this kinase increases the affinity of p120 catenin for E-cadherin (39). However, the exact role of p120 catenin in the regulation of adherens junction is not clear since different authors have suggested negative and positive effects (reviewed in reference 4). On the other hand, increased tyrosine phosphorylation of β-catenin is associated with adherens junction disruption (22; see references 12 and 23 for reviews). Using direct in vitro measurements, we have reported that phosphorylation of β-catenin by Src kinase decreases the interaction of this protein with E-cadherin. The modified residue was identified as Tyr-654 (39), which contributes to E-cadherin binding by establishing an ionic pair with E-cadherin Asp-667 (19). Although Src kinase can phosphorylate Tyr-654, it does it inefficiently, indicating that other tyrosine kinases are responsible for this modification in vivo. Indeed, the epidermal growth factor receptor and its homologue erbB2 both phosphorylate and interact with β-catenin (17, 42) and share the same binding domain, i.e., the C-terminal armadillo repeats of β-catenin, where Tyr-654 is located. Moreover, other tyrosine kinases such as Fer, Fyn, or Yes, interact with several members of the adhesion complex (21, 38, 46).
Besides the interaction of β-catenin with E-cadherin, the binding to α-catenin is also regulated by tyrosine phosphorylation. For instance, addition of the tyrosine phosphatase inhibitor peroxyvanadate to several cell lines disrupts β-catenin-α-catenin association (18, 32). The α-catenin-binding site in β-catenin has been assigned to a short sequence (amino acids 118 to 146) placed between the N-tail and the first armadillo repeat (1). This sequence contains only one tyrosine, Tyr-142, which is essential for the interaction with α-catenin (2). This residue is required for the stabilization of the β-catenin structure involved in this binding: the aromatic ring of Tyr-142 forms van der Waals contacts with several residues of α-catenin (35). Moreover, the hydroxyl group of Tyr-142 lies very close to β-catenin Asp-144 and Glu-147. We hypothesized that phosphorylation of this residue may interfere with β-catenin-α-catenin association.
We report here that Tyr-142 can be phosphorylated by the nonreceptor tyrosine kinases Fer or Fyn. As expected, modification of this amino acid disrupts β-catenin-α-catenin binding. Phosphorylation of Tyr-142 occurs in experimental conditions that decrease the β-catenin-α-catenin interaction, such as after K-ras transfection. Fer and Fyn kinases are normally found associated with p120 catenin; phosphorylation of this catenin on Tyr residues increases the binding of Fer/Fyn-p120 catenin complex to E-cadherin. This interaction is increased by K-ras transfection. These results suggest a role for p120 catenin as a regulatory protein in adherens junctions by recruiting to the complex tyrosine kinases that can modulate α-catenin-β-catenin interaction.
MATERIALS AND METHODS
Expression of recombinant proteins in Escherichia coli.
Expression and purification of full-length murine β-catenin and point mutants Tyr→86Phe, Tyr→654Phe, Tyr→86/654Phe as glutathione S-transferase (GST) fusion proteins were performed as described previously (39). β-Catenin point mutants Tyr→142Glu and Tyr→142Phe were obtained using the QuikChange site-directed mutagenesis kit (Stratagene). A PCR was performed using Pfx polymerase, pGEX-6P3-βcatenin as template, and oligonucleotide primers containing each mutation. Sense primers used for generation of Tyr→142Glu and Tyr→142Phe mutants were, respectively, 5′-TTGATTAACGAGCAGGATGAC-3′ and 5′-TTGATTAACTTTCAGGATGAC-3′. Changes are indicated in boldface type. After amplification, the product was treated with DpnI, which digests the parental construct. Finally the nicked plasmid was transformed and sequenced. Wild-type or mutant β-catenin cDNAs were inserted in the BamHI site of pcDNA3.1His(C) plasmid. A SalI-NotI DNA fragment corresponding to the full-length murine p120 catenin (provided by A. Reynolds, Vanderbilt University, Nashville, Tenn.) was cloned into SalI-NotI sites of pGEX-6P2 (Amersham Pharmacia Biotech). The expressed fusion GST-p120 catenin and the GST-cytosolic domain of murine E-cadherin (cytoEcadh) were purified as described (39).
Protein binding assays.
Pull down assays were performed incubating 6.7 pmol of GST-β-catenin (wild type or point mutants) with 40 μg of RWP1 or IEC18 K-ras total cell extracts in binding buffer (50 mM Tris-HCl [pH 7.3], 150 mM NaCl, 3 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 0.1% [wt/vol] Triton X-100) in a final volume of 200 μl for 30 min at 25°C. When indicated, 15 pmol of GST-cytoEcadh was incubated, in the presence or absence of 5.4 pmol of p120 catenin phosphorylated or not by pp60c-src, with 40 μg of IEC18 K-ras total cell extracts. Conditions for binding assays with recombinant proteins were previously described (34, 39). Protein complexes were isolated by incubation with 40 μl of a 50% (wt/vol) suspension of glutathione-Sepharose 4B (Amersham Pharmacia Biotech) for 30 min at 25°C. Beads were collected by brief centrifugation and washed three times with binding buffer. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the presence of bound proteins in the complex was analyzed by Western blot with specific monoclonal antibodies (MAbs). Reanalysis of the blots with a different antibody was performed after stripping the membranes as described (43). Monoclonal antibodies against β-catenin, α-catenin, E-cadherin, p120 catenin, Fyn, Yes, and PTyr were from Transduction Laboratories; Src was from Upstate Biotechnology; pan-Ras was from Calbiochem; and anti-Xpress was from Invitrogen. Polyclonal antibody against GST was from Amersham Pharmacia Biotech.
Stable and transient transfections.
Cell lines were routinely grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. For the generation of stably transfected IEC K-ras cells, Val12-mutant human K-ras, cloned in pCEFL plasmid (a kind gift of J. M. Rojas, Instituto Carlos III, Madrid, Spain) was transfected to IEC18 cells using LipofectAMINE (Life Technologies) and following the instructions of the manufacturer. Individual clones were isolated after three weeks selection in G418 and expression of the oncogene was verified by Western blot. All the clones analyzed presented the same phenotype. As a control, empty pCEFl plasmid was transfected to the same cells. Transient expression of ectopic proteins was achieved in 50% confluent RWP1, IEC18 or IEC18 K-ras transfecting pEF-Bos-Fyn plasmid (provided by A. Carrera, Centro Nacional de Biotecnología, Madrid, Spain) pMIK-Neo-Yes kinase (provided by M. Sudol, Mount Sinai Medical Center, New York, N.Y.), pcDNA3.His-Fer or the indicated β-catenin forms in pcDNA3.His. Cells were analyzed 48 h after transfection.
Immunoprecipitations.
Cell extracts were prepared from confluent or transfected cultured cells resuspended in lysis buffer (50 mM Tris HCl, pH 7.6, 200 mM NaCl, 5 mM MgCl2, 0.1% Nonidet P-40, 1 mM dithiothreitol, 0.1 mM sodium orthovanadate, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride, leupeptin [10 μg/ml] and aprotinin [10 μg/ml]) in ice for 30 min. Lysates were centrifuged at 12,000 × g in a microcentrifuge for 15 min at 4°C. Cell extracts (300 μg) were incubated with a 4-μg/ml concentration of antibody for 16 h at 4°C. Insoluble material was removed by centrifugation at 12,000 × g; and supernatant was incubated for 90 additional min with 30 μl of protein A-agarose (Sigma). Immunoprecipitates were washed twice with lysis buffer and bound proteins were directly eluted with electrophoresis sample buffer and analyzed by Western blot or used for kinase assays. When indicated, genistein (Calbiochem) (100 μM) was added to the cell medium 20 h before preparing the extracts.
Kinase assays.
pp60c-src kinase was purchased from Upstate Biotechnology. Fer kinase was cloned in pcDNA3-His(C) (Invitrogen), expressed as anti-Xpress and polyhistidine-tagged protein in cells and purified with nickel nitrilotriacetic acid-agarose (Qiagen). Purification was achieved by incubating 250 μg of cell extracts in a final volume of 300 μl with 20 μl of a 50% (wt/vol) suspension of nickel-agarose for 1 h at 4°C. Beads were washed twice with lysis buffer, and occasionally, bound proteins were eluted with lysis buffer supplemented with 200 mM imidazole. Fyn or Yes tyrosine kinases were purified from cell extracts by immunoprecipitation. Phosphorylation assays were performed in a final volume of 30 μl of kinase buffer (25 mM Tris-HCl [pH 6.8], 25 mM MgCl2, 5 mM MnCl2, 0.5 mM EGTA, 1 mM dithiothreitol, 0.25 mM sodium orthovanadate, 0.1 mM ATP) for 1 h at 22°C (Src assays) or 30°C (Fer, Fyn, or Yes assays).
RESULTS
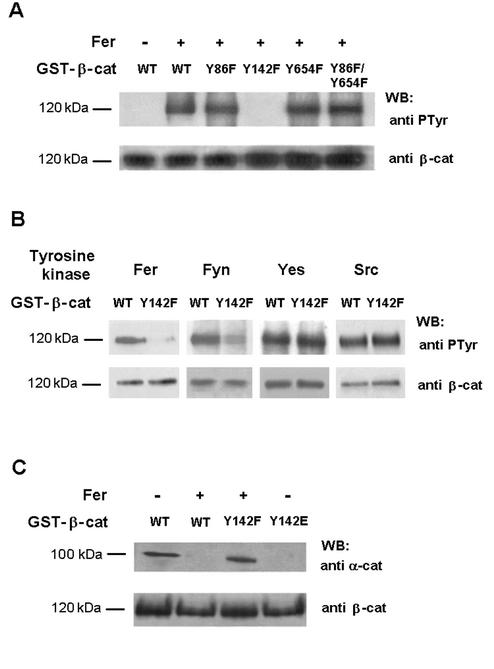
We have tested various nonreceptor tyrosine kinases related to the Src family, searching for a kinase that could specifically phosphorylate β-catenin within a domain involved in α-catenin binding. The region of β-catenin involved in the interaction with α-catenin has been identified between amino acids 118 to 146 (2). Within this region, Tyr-142 was a plausible candidate to be phosphorylated. Therefore, Tyr-142 was mutated to Phe and this mutant and the wild-type form of β-catenin tested for phosphorylation. Other β-catenin mutants, Tyr-86→Phe and Tyr-654→Phe, which can be phosphorylated by tyrosine kinase pp60v-src (39), were also analyzed. The wild type and these tyrosine-β-catenin mutants were phosphorylated in vitro by a purified preparation of Fer (Fig. 1A), a protein tyrosine kinase which binds to adherens junction complexes (38). Fer phosphorylated efficiently wild-type β-catenin; replacement of Tyr-142 by Phe affected β-catenin phosphorylation by this kinase (Fig. 1A, lanes 2 and 4). Phosphorylation of β-catenin Tyr-86→Phe, Tyr-654→Phe, or the double mutant did not differ from that of wild-type β-catenin (Fig. 1A, lanes 2, 3, 5, and 6). Other tyrosine kinases of the Src family were also tested for phosphorylation. Although Src and Yes phosphorylated β-catenin, only the action of Fyn was sensitive to Tyr-142 mutation (Fig. 1B), indicating that only Fyn mimics the effect of Fer on the modification of this residue.
FIG. 1.
Fer and Fyn kinases phosphorylate β-catenin Tyr-142, and this phosphorylation decreases α-catenin-β-catenin interaction. (A) GST-β-catenin fusion proteins (6.7 pmol, 0.8 μg) were phosphorylated with Fer kinase purified from transfected RWP1 cells as described in Materials and Methods. Phosphorylation was analyzed by Western blotting with anti-PTyr MAb. The membrane was stripped and reanalyzed against β-catenin to check that similar levels of GST-β-catenin were phosphorylated in all cases. (B) Wild-type GST-β-catenin or Tyr-142→Phe mutant (Y142F) was phosphorylated under the conditions indicated, either with Fer purified from transfected RWP1 cells, with Fyn or Yes kinases immunoprecipitated from transfected RWP1 cells, or with recombinant pp60c-src. Samples were analyzed by Western blotting with anti-PTyr MAb and reblotted with anti-β-catenin. (C) GST-β-catenin fusion proteins were phosphorylated with Fer as above. Pull down assays were then performed incubating the GST proteins with 40 μg of total cell extracts from RWP1. The amount of associated α-catenin was determined using specific MAb. WT, wild type β-catenin; Y142F and Y142E correspond to β-catenin mutants Tyr-142→Phe and Tyr-142→Glu, respectively. The estimated molecular masses of the bands detected with each antibody are indicated.
To verify the relevance of Tyr-142 phosphorylation, the ability of β-catenin phosphorylated in this residue to bind α-catenin was examined in vitro and compared with that of the unmodified β-catenin. Recombinant GST-β-catenin was phosphorylated with purified Fer, and binding to α-catenin was determined by pull down assays. As shown in Fig. 1C, very little α-catenin was bound to the phosphorylated form of β-catenin (lane 2) compared to the unmodified protein (lane 1). This decrease in α-catenin association was not observed when a Tyr-142→Phe β-catenin mutant was incubated with Fer (lane 3). Tyr-142 was also mutated to Glu in order to mimic the effect of the negative charge introduced by phosphorylation of this residue. Pull down experiments indicate that β-catenin-α-catenin interaction was completely blocked in this mutant (lane 4). Interaction of the mutants with E-cadherin was unaffected (data not shown). The results were the same when Fyn tyrosine kinase was used instead of Fer (not shown). Thus, these results suggest that Fer and Fyn kinases can modulate α-catenin-β-catenin binding through phosphorylation of Tyr-142.
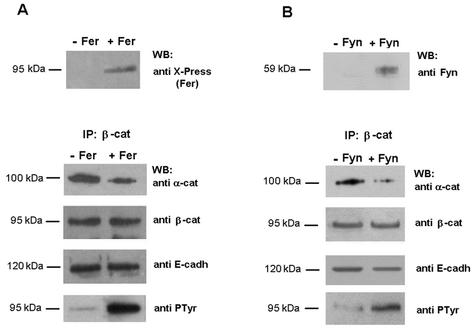
To verify this conclusion, RWP1 cells were transiently transfected with Fer or Fyn and α-catenin-β-catenin association was determined by coimmunoprecipitation. As shown in Fig. 2A and B, less α-catenin was detected in the β-catenin immunoprecipitates of cells transiently transfected with either Fer or Fyn than in nontransfected cells, which correlates with an increased proportion of tyrosine phosphorylated β-catenin (Fig. 2, lower panel). Contrarily, the association of β-catenin to E-cadherin was not altered by Fer or Fyn transfection (Fig. 2).
FIG. 2.
Overexpression of Fer or Fyn kinases in RWP1 induces β-catenin phosphorylation and reduces α-catenin-β-catenin interaction. RWP1 cells were transfected with 5 μg of either pcDNA3-His-Fer (A), pEF-Bos-Fyn (B), or empty vector as control. After 48 h, cell extracts were prepared. (A) As shown in the upper panel, to verify Fer- kinase transfection, 5 μg of untransfected or transfected RWP1 total cell extracts was analyzed by SDS-PAGE and Western blot with anti-Xpress antibody corresponding to a tag that labels the transgene. As shown in the lower panel, 300 μg of the above-mentioned transfected-cell extracts was immunoprecipitated with anti-β-catenin followed by immunoblotting with antibodies against α-catenin, β-catenin, E-cadherin, and PTyr. The estimated molecular masses of the bands detected with each antibody are indicated. (B) Same as in (A) except that cells were transfected with pEF-Bos-Fyn and analyzed with an anti-Fyn MAb.
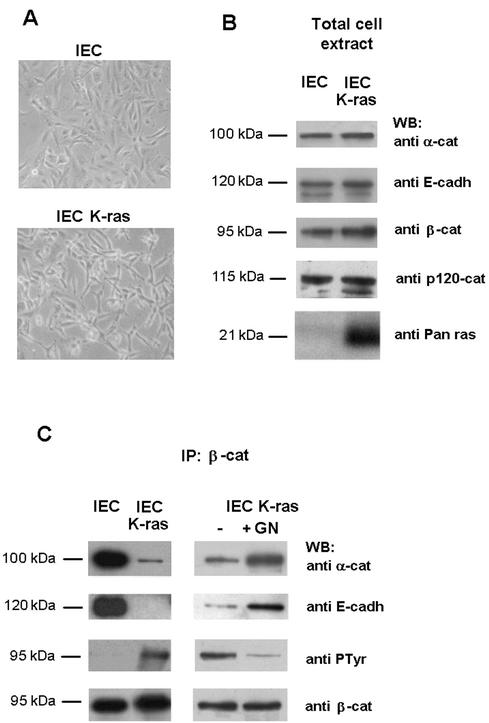
We also investigated whether Tyr-142 phosphorylation and the activity of tyrosine kinases was raised in conditions that cause loss of cell-cell contacts. Expression of activated forms of ras has been reported to disrupt intercellular adhesion (22). Therefore, we generated stable transfectants of K-ras (Val12) oncogene in IEC18 intestinal epithelial cells. Transfection of this oncogene loosened cell-cell contacts and altered the shape of the cell (Fig. 3A). N- or H-ras also produced similar alterations (not shown). While similar amounts of α-catenin and E-cadherin were found in normal and K-ras cell extracts, slightly higher levels of β-catenin were detected in K-ras cells (Fig. 3B). No remarkable changes in the content of another component of the adherens junction, p120 catenin, were detected (Fig. 3B). Coimmunoprecipitation experiments showed that β-catenin interaction with α-catenin and E-cadherin was disrupted in IEC-K-ras cells (Fig. 3C). These changes correlate with an increased PTyr content of β-catenin in the K-ras transfectants (Fig. 3C). The relevance of Tyr phosphorylation in the disassembly of β-catenin complexes was confirmed treating IEC-K-ras cells with the tyrosine kinase inhibitor genistein, a compound that has been used to inhibit src-family kinases in epithelial cells (8, 15). Addition of this compound markedly restored the adhesion of β-catenin to α-catenin and E-cadherin, and concomitantly decreased the PTyr content of β-catenin (Fig. 3C). Cells incubated with genistein presented a phenotype similar to parental IEC18 cells (not shown).
FIG. 3.
IEC18 K-ras: an in vivo cell system with reduced α-catenin-β-catenin and E-cadherin-β-catenin interaction due to increased tyrosine phosphorylation. (A) Morphology of IEC18 and IEC18 K-ras cell lines. (B) IEC18 or IEC18 K-ras total cell extracts (5 μg) were analyzed by SDS-PAGE and immunoblotting with antibodies against α-catenin, E-cadherin, β-catenin, p120 catenin, or Pan ras to check the endogenous levels of each protein. (C) Extracts (300 μg) prepared from the indicated cells were immunoprecipitated with anti-β-catenin antibody, and this was followed by immunoblotting with the indicated MAbs. When indicated, genistein (100 μM) (GN) was added to the IEC-K-ras cell medium for 20 h before preparing the extracts.
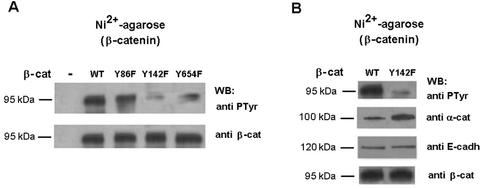
To examine whether the disassembly of β-catenin-α-catenin complexes in the IEC-K-ras cells was a direct result of Tyr-142 phosphorylation of β-catenin, these cells were transiently transfected with wild-type β-catenin or the β-catenin mutants Tyr-142→Phe, Tyr-654→Phe or Tyr-86→Phe, labeled with a polyhistidine tag. Transfected forms of β-catenin were purified by Ni2+-agarose chromatography and their phosphorylation status was examined. As shown in Fig. 4A, phosphorylation of β-catenin Tyr-142→Phe and Tyr-654→Phe in these cells was much lower than that of wild-type β-catenin or the Tyr-86→Phe mutant. Thus, in K-ras transfected cells, β-catenin Tyr-142 and Tyr-654 are being modified (Fig. 4A). In order to verify the relevance of Tyr-142 modification in the disruption of α-catenin-β-catenin interaction detected in Fig. 3, IEC-K-ras cells were transfected with wild-type and Tyr-142→Phe mutant. Again, this mutant incorporated much lower levels of PTyr (Fig. 4B). The amount of α-catenin that copurified with β-catenin was higher in the Tyr-142→Phe mutant than in the wild-type β-catenin (Fig. 4B), indicating that blocking Tyr-142 phosphorylation restored α-catenin-β-catenin binding. No differences were observed in the levels of E-cadherin associated with β-catenin (Fig. 4B).
FIG. 4.
In IEC18 K-ras β-catenin, Tyr-142 and Tyr-654 are phosphorylated. Phosphorylation of Tyr-142 modulates the α-catenin-β-catenin interaction in vivo. (A) IEC18 K-ras cells were transfected with 5 μg of pcDNA3.1His-β-catenin (wild type or the indicated mutant forms) or empty vector as control. After 48 h, cell extracts were prepared, His-tagged β-catenin was purified by chromatography on nickel-agarose, and the level of phosphorylation was analyzed by Western blotting with PTyr MAb. Membrane was reanalyzed against β-catenin to check that similar levels of expression were obtained in all the cases. Y86F, Y142F, and Y654F correspond to β-catenin mutants Tyr-86→Phe, Tyr-142→Phe, and Tyr-654→Phe, respectively. (B) IEC18 K-ras cells were transfected with 5 μg of pcDNA3.1His-β-catenin (wild type or Tyr-142→Phe). His-tagged β-catenin complexes were analyzed with the indicated MAbs.
The activity of Fer and Fyn kinases was examined in our cellular system. To test whether Fer kinase was activated in the K-ras cells, IEC and IEC-K-ras cells were transiently transfected with poly-His-tagged Fer. The activity of purified Fer kinase on β-catenin was greater in IEC-K-ras than in control cells (Fig. 5B). The PTyr content of Fer was also higher (Fig. 5A), further supporting the conclusion that the kinase is activated following transformation by K-ras. Fyn β-catenin kinase activity (Fig. 5D) and PTyr content (Fig. 5C) were also increased in IEC-K-ras. In this case the analysis was performed on the endogenous tyrosine kinase immunoprecipitated with a specific MAb.
FIG. 5.
Fer and Fyn kinases are activated in IEC18 K-ras. (A) IEC18 or IEC18 K-ras cells were transfected with 5 μg of pcDNA3-His-Fer or empty vector as control. After 48 h, cell extracts were prepared and His-tagged Fer kinase was purified. Proteins bound to the nickel-agarose were subjected to SDS-PAGE and Western blotting with anti-Xpress antibody to verify that similar levels of the transgene were expressed. Membrane was stripped and reblotted to analyze the PTyr content of Fer. (B) The activity of purified Fer was determined by adding 0.4 μg of GST-β-catenin under the phosphorylation conditions indicated in Materials and Methods for 1 h at 30°C. Samples were subjected to SDS-PAGE and Western blotting with antibodies against PTyr, β-catenin, and Xpress (to check that similar amounts of β-catenin and Fer were present in the reactions). (C and D) Assays were carried out as in panels A and B except that IEC18 or IEC18 K-ras cells were transfected with 5 μg of pEF-Bos-Fyn and extracts immunoprecipitated with anti-Fyn antibody. Determination of the β-catenin kinase activity present in the immunoprecipitate was performed as in panel B. The position of immunoglobulin G (IgG) heavy chains is indicated.
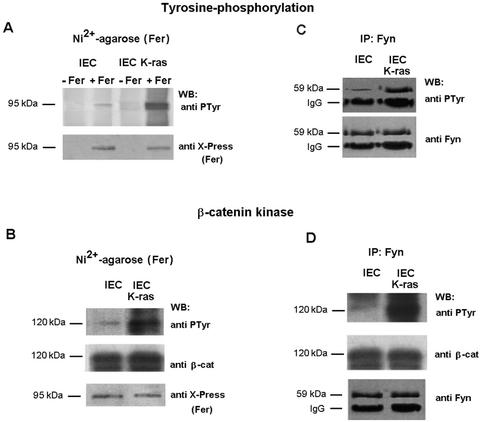
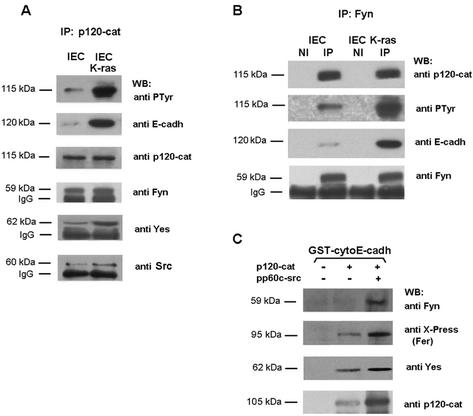
Fer tyrosine kinase is constitutively associated with p120 catenin (21, 38; also data not shown). We examined whether Fyn was also bound to p120 catenin and if this interaction was affected by K-ras. Experiments of immunoprecipitation with anti-p120 catenin (Fig. 6A) or anti-Fyn antibodies (Fig. 6B) evidenced that these two proteins formed part of the same complex. K-ras induced the tyrosine phosphorylation of p120 catenin (Fig. 6A), without affecting the total levels of this protein (Fig. 6A and 3B). Tyrosine-phosphorylated p120 catenin bound E-cadherin better than the unmodified protein (Fig. 6A), in accordance with previous reports (22, 39, 43). Similar amounts of Fyn were present in the p120 catenin-immunoprecipitates independently of the p120 catenin phosphorylation state (Fig. 6A). Thus, p120 phosphorylation had no effect on Fyn-p120 catenin interaction. Results were similar when Fyn was immunoprecipitated. Essentially the same levels of p120 catenin were bound to Fyn in both control IEC and K-ras cells (Fig. 6B, upper panel). Therefore, as a consequence of the better binding of tyrosine-phosphorylated p120 catenin to E-cadherin, higher amounts of this cadherin were detected in the Fyn immunoprecipitates in K-ras transformed cells (Fig. 6B, third panel). These results suggest that K-ras regulates E-cadherin association with Fyn through the tyrosine phosphorylation of p120 catenin.
FIG. 6.
p120 catenin recruits Fer and Fyn kinases to the junctional complex. IEC18 or IEC18 K-ras total cell extracts (300 μg) were immunoprecipitated with anti-p120 catenin (A) or anti-Fyn (B) antibodies, and this was followed by immunoblotting with the indicated MAbs. Lane NI corresponds to the result of an immunoprecipitation performed with an irrelevant antibody. (C) GST-cytoE-cadherin (15 pmol) was incubated or not with full-length p120 catenin (prephosphorylated with pp60c-src when indicated). Forty micrograms of total extract prepared from IEC18 K-ras transfected with 5 μg of pcDNA3-His-Fer was added. Protein complexes were pelleted down by affinity on glutathione-Sepharose beads, and proteins bound to the complex were analyzed by SDS-PAGE and Western blotting with MAbs against Fyn, the Xpress epitope, Yes, and p120 catenin. The estimated molecular masses of the bands detected with each antibody are indicated as well as the position of immunoglobulin G (IgG) heavy chains.
To verify this conclusion pull down experiments were performed using GST-cytoE-cadherin as a bait, in the presence of unphosphorylated or phosphorylated p120 catenin. Fer or Fyn were only detected associated to E-cadherin when p120 catenin was previously bound (Fig. 6C, compare lane 1 with 2 and 3). In both cases, and particularly for Fyn, tyrosine phosphorylation of p120 catenin significantly enhanced the association of the kinases with cytoE-cadherin (Fig. 6C, lanes 2 and 3). These results further support our conclusion that p120 catenin mediates Fer and Fyn association to E-cadherin.
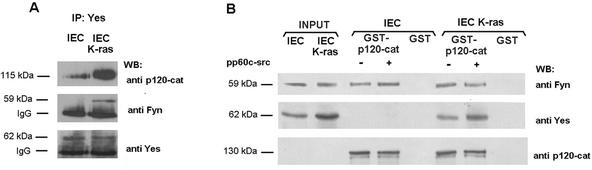
The possible roles of other nonreceptor tyrosine kinases were also examined. Both Yes and Src proteins were coimmunoprecipitated with p120 catenin (Fig. 6A, lower panels). At least in these cells, Yes was present at higher levels than Src in p120 catenin immunoprecipitates. Yes-p120 catenin interaction was highly dependent on K-ras transfection; in IEC K-ras the association of these two proteins was much more evident than in control cells. Evidence of Yes-p120 catenin association was also provided by experiments of Yes immunoprecipitation (Fig. 7A).
FIG. 7.
Yes is associated with p120 catenin in IEC-K-ras cells. (A) IEC18 or IEC18 K-ras total cell extracts (300 μg) were immunoprecipitated with anti-Yes antibody, and the immune complexes were analyzed by sequential immunoblotting with antibodies against p120 catenin, Fyn, and Yes. Position of immunoglobulin G (IgG) heavy chains is indicated. (B) Eleven picomoles of GST or GST-p120 catenin fusion proteins was phosphorylated by pp60c-src under the conditions indicated in Materials and Methods. Pull down assays were performed with incubation of the GST proteins with 50 μg of total cell extracts prepared from IEC or IEC-K-ras. Presence of Fyn, Yes, or p120 catenin in the complex was analyzed by sequential analysis with specific MAbs. In the lane Input, a sample corresponding to 10% of the total cell extract used for the assay was loaded. The estimated molecular masses of the bands detected with each antibody are shown.
The ability of Yes to interact with p120 catenin, which is constitutively associated with Fyn, suggests that both tyrosine kinases should be detected in the same complex. As shown in Fig. 7A (middle panel), Fyn is present in anti-Yes immunoprecipitates, but only in IEC K-ras cells. The interaction of Yes with E-cadherin was also observed in pull down assays; in this case the requirement of p120 catenin was clearly evidenced since no binding was detected in the absence of this catenin (Fig. 6C).
The differences observed in Yes-p120 catenin association between IEC and IEC K-ras cells suggest that modifications in one or both proteins are required for binding. In order to answer this question, pull down experiments were performed with GST-p120 catenin. Endogenous Yes kinase from IEC cells did not bind to p120 catenin, irrespective of whether p120 catenin had been prephosphorylated by Src (Fig. 7B, middle panel). However, Yes kinase from IEC-K-ras was efficiently retained by GST-p120 catenin (Fig. 7B, middle panel). Phosphorylation of p120 catenin slightly increased the amount of Yes bound to p120 catenin. On the other hand, Fyn behaved differently; as expected from our previous results, no significant changes in the amount of Fyn bound to p120 catenin were detected in any of the conditions analyzed (Fig. 7B, upper panel).
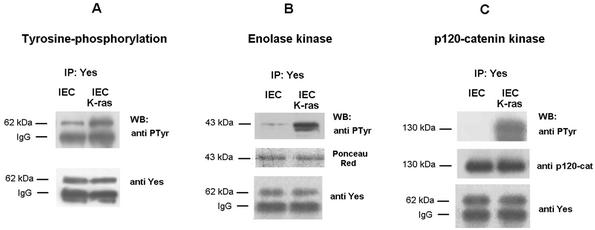
Therefore, modifications in the Yes protein induced after transfection of K-ras are necessary for binding to p120 catenin. The PTyr content of Yes was increased in IEC-K-ras respect to IEC cells indicating that this kinase had been activated (Fig. 8A). Moreover, the activity of Yes kinase was higher when this protein was immunoprecipitated from IEC-K-ras than from control cells. Similar results were obtained when enolase (Fig. 8B), p120 catenin (Fig. 8C), or β-catenin (not shown) was used as the substrate. These results also evidenced that p120 catenin is a good substrate of Yes. Accordingly, the binding to p120 catenin takes place preferentially to activated Yes.
FIG. 8.
Yes kinase is activated in IEC18 K-ras cells. IEC18 or IEC18 K-ras total cell extracts (300 μg) was immunoprecipitated with anti-Yes antibody. The immune complexes were analyzed with the anti-PTyr MAb (A) or subjected to an in vitro kinase assay with 0.5 μg of enolase (B) or GST-p120 catenin (C) as exogenous substrates. Samples were analyzed by SDS-PAGE and Western blotting with antibody against PTyr. Membranes were reblotted with MAb against Yes. To verify that similar amounts of substrates were used in both cases, Ponceau S staining (B) (central panel) and the anti-p120 blot (C) (central panel) are shown.
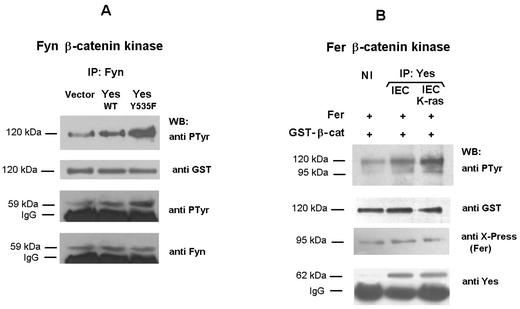
Since active Yes binds to p120 catenin that is constitutively associated to Fer and Fyn, it was of interest to examine whether Yes was involved in the activation of these two kinases. IEC18 cells transfected with the wild-type form of Yes modestly stimulated the β-catenin kinase activity of Fyn, or the PTyr content of this kinase, respect to cells transfected with the empty vector (Fig. 9A). The stimulation was much more evident when a constitutively activated form of Yes (Tyr-535→Phe) was used (Fig. 9A), indicating that active Yes causes Fyn activation. The ability of Yes to directly activate Fer was also analyzed. Purified Fer was incubated with Yes immunoprecipitates, separated by centrifugation and analyzed in phosphorylation assays using β-catenin as substrate (Fig. 9B). Yes increased the β-catenin kinase activity of Fer (Fig. 9B, compare lanes 1 and 2); when Yes was immunopurified from IEC-K-ras cells the stimulation of Fer activity was higher (Fig. 9B, lanes 1 and 3).
FIG. 9.
Yes kinase activates Fer and Fyn. (A) IEC18 cells were transfected with 5 μg of pMIK-Neo-Yes kinase (wild type or the constitutively active form Tyr-535→Phe) or empty vector. After 48 h, cell extracts were prepared and 200 μg of total cell extracts were immunoprecipitated with anti-Fyn antibody. The immune complexes were analyzed with an anti-PTyr MAb or subjected to an in vitro kinase assay with 0.5 μg of GST-β-catenin as exogenous substrate and analyzed by sequential immunoblotting with antibodies against PTyr and Fyn. The position of immunoglobulin G (IgG) heavy chains is indicated. Very little phosphorylation was obtained when a similar assay was performed using GST-β-catenin (Y142F) as substrate (not shown). (B) IEC18 cells were transfected with 5 μg of pcDNA3-His-Fer kinase. After 48 h, cell extracts were prepared and His-tagged Fer kinase was purified by chromatography on Ni2+-agarose and eluted with 60 μl of lysis buffer plus 200 mM imidazole. In parallel, 300 μg of IEC18 or IEC18 K-ras total cell extracts was immunoprecipitated with anti-Yes antibody. The immune complexes were mixed with 20 μl of eluted-Fer kinase and incubated 1 h at 30°C in conditions indicated for kinase assays. Supernatants containing Fer were separated from the immunocomplexes spinning 30 s in a microcentrifuge and subjected to a GST-β-catenin kinase assay as mentioned above. The extent of tyrosine phosphorylation was analyzed blotting with an anti-kinase PTyr MAb. As control to verify that similar amounts of Yes were immunoprecipitated, immunocomplexes were also analyzed by Western blotting with anti-Yes antibody. Lane NI corresponds to the β-catenin phosphorylation obtained when the 20 μl of eluted-Fer was incubated with an IEC18 K-ras cell extract immunoprecipitated with an irrelevant antibody. No phosphorylation of β-catenin was observed when Fer kinase was omitted from the reaction or when GST-β-catenin (Y142F) was used as substrate. The molecular masses of 120 and 95 kDa correspond to GST-β-catenin and Fer, respectively.
DISCUSSION
Altered cell adhesion is associated with the disruption of the interaction of β-catenin with both E-cadherin (22, 27, 39) and α-catenin (15, 16, 17). Disassembly of these complexes is accompanied by increased tyrosine phosphorylation of β-catenin and is required for β-catenin to be transported to the nucleus and act as a transcriptional coactivator (13, 30, 40). In previous works we have evidenced that phosphorylation of β-catenin Tyr-654 is important for the modulation of E-cadherin-β-catenin interaction both in vitro and in vivo (7, 39). In this study we identify a different tyrosine residue in β-catenin, Tyr-142, involved in the regulation of β-catenin-α-catenin association. Tyr-142 is the only tyrosine located in the β-catenin domain involved in α-catenin interaction and is necessary for the stabilization of the structure of this domain. The position of its hydroxyl group, in close vicinity to two acidic residues, makes it highly probable that the introduction of a negative charge disrupts the α-catenin binding domain, thus impeding α-catenin binding, as demonstrated by our results.
We have identified two nonreceptor tyrosine kinases that can phosphorylate this residue: Fer and Fyn. Both kinases are widely expressed in epithelial tissues (46) and have been implicated in β-catenin phosphorylation and regulation of cell-cell contacts (9, 20, 31, 38). We show here that conditions that cause the disruption of both β-catenin-E-cadherin and β-catenin-α-catenin interactions (like K-ras transformation) increase the activity of Fer and Fyn kinases and promote the phosphorylation of Tyr-142 and Tyr-654. Phosphorylation of these two residues regulates the interaction with the two β-catenin partners differently: whereas modification of Tyr-142 inhibits α-catenin binding, phosphorylation of Tyr-654 alters E-cadherin association (see above). Fer does not phosphorylate Tyr-654, and Fyn does so with very low efficiency (data not shown), suggesting that these kinases do not directly regulate β-catenin-E-cadherin interaction. However, we cannot discard that they may indirectly alter this association in other cell systems. Both enzymes in vitro phosphorylate p120 catenin and increase its binding to E-cadherin (data not shown). Therefore, Fer or Fyn may facilitate the interaction of p120 catenin-associated kinases to E-cadherin and the phosphorylation of β-catenin Tyr-654 by these still unidentified enzymes. This would explain why β-catenin-E-cadherin interaction is lost in other cell systems after overexpression of Fer kinase (38). This hypothetical p120 catenin associated kinase specific for β-catenin Tyr-654 does not correspond to any of the Src family members studied in this article, since in our assays Yes does not phosphorylate this residue (not shown) and Src does it with low efficiency (39). We consider more likely the possibility that the epidermal growth factor receptor or its homologue erbB2, both of which phosphorylate Tyr-654 (data not shown), might interact with the tyrosine-phosphorylated form of p120 catenin. For the same reason we cannot totally discard the notion that another p120 catenin-associated kinase, different from Fer or Fyn, might be responsible for Tyr-142 phosphorylation in this or another cell system.
Our results also show that Fyn and Fer kinase mutually complement their ability to regulate cell adhesion. These data explain why mice deficient for Fyn or Fer tyrosine kinase activities present such mild phenotypes (10, 44). Other protein kinases from the Fer or Src families, e.g., Fps, may also show the same specificity for β-catenin or p120 catenin. At this respect, in vitro assays performed in our lab indicate that p120 catenin can be directly phosphorylated by several tyrosine kinases (not shown).
Our data suggest that p120 catenin is essential for the regulation of the adherens complex. This protein, initially described as a heavily tyrosine phosphorylated protein after Src transformation, binds to the cytoplasmic domain of E-cadherin, to a different sequence than β-catenin (12, 37, 48). Tyrosine phosphorylation of p120 catenin enhances binding to E-cadherin (22, 39, 43); however, the role of this increased association in the regulation of E-cadherin functionality is still a matter of discussion. Although most authors report negative effects of p120 overexpression on cell-cell adhesion (4, 5, 29, 33), positive actions have also been described (4, 47). Our results suggest that p120 catenin is normally associated to the tyrosine kinases Fer and Fyn, and also binds Yes when this kinase is active. Yes can also activate Fyn and Fer, indicating that p120 catenin may facilitate the activation of these kinases, increasing the effective concentration of active Yes in their vicinity. It is possible that the role of Yes is exerted in other cell lines by similar kinases such as Src.
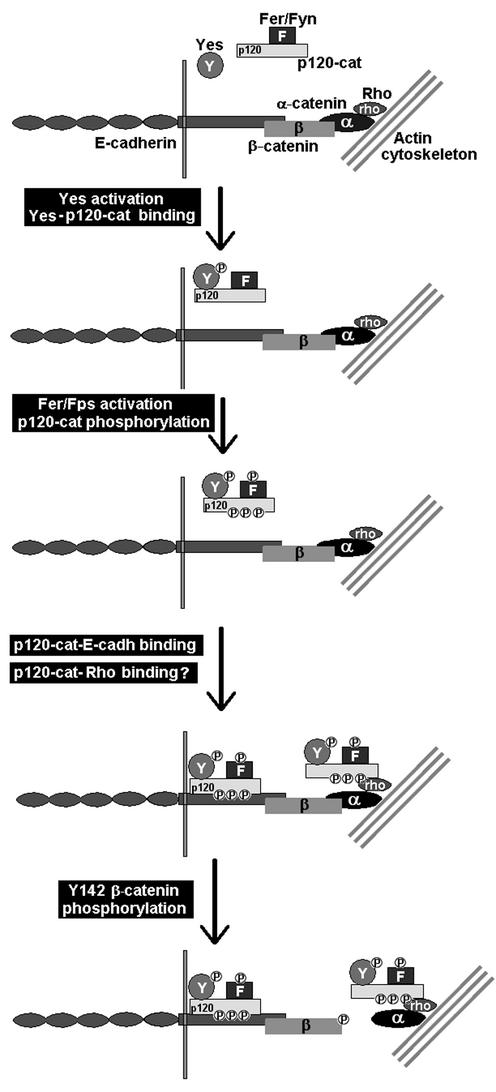
Our results also suggest a possible role of p120 catenin as a docking protein facilitating the interaction of Fer and Fyn with the adherens junction complex, and the subsequent phosphorylation of β-catenin Tyr-142. Since p120 catenin interacts with E-cadherin, the activity of the p120 catenin-associated Fer and Fyn kinases would be mainly restricted to β-catenin still bound to E-cadherin. This hypothesis suggests that phosphorylation of β-catenin Tyr-142 would precede that of Tyr-654 and disruption of β-catenin-α-catenin to that of β-catenin-E-cadherin. However, recent data suggest that this sequential model may not be entirely correct. In Drosophila melanogaster, p120 catenin has been reported to interact indirectly with α-catenin through the simultaneous binding of both proteins to Rho1 (24). Therefore, p120 catenin can associate with the adherens junction complex independently of E-cadherin and promote α-catenin-β-catenin disassembly even though β-catenin is not attached to E-cadherin. Our present view of the regulation of the adhesion complex is depicted in Fig. 10. However, many questions remain still unanswered, mainly concerning to the association of the p120 catenin complex to α-catenin. For instance, it is still not clear which forms of Rho associate with α-catenin and p120 catenin and whether this interaction is dependent on p120 catenin phosphorylation, either in tyrosine or serine residues. In any case, the results presented in this work open new perspectives on the function of p120 catenin and associated kinases in the regulation of adherens junctions.
FIG. 10.
Proposed model of regulation of α-catenin-β-catenin interaction. In canonical adherens junctions, E-cadherin contacts with the actin cytoskeleton through β-catenin and α-catenin. p120 catenin is associated to Fer or/and Fyn kinases and normally is not bound to the E-cadherin complex. Following activation, Yes binds to the p120-Fer/Fyn complex and activates Fer/Fyn kinases. Either Yes, Fer/Fyn, or both phosphorylate Tyr residues in p120 catenin and enhance its binding to E-cadherin. Alternatively, the Fer/Fyn/Yes-p120 catenin complex binds to α-catenin through the association to Rho. The presence of Fer/Fyn kinases in the adhesion complex promotes the phosphorylation of β-catenin Tyr-142 and subsequent disruption of α-catenin-β-catenin interaction. For the sake of clarity, Fer, Fyn, Yes, and Rho proteins have not been depicted bound to the membrane, where they are probably located. For the same reason, p120 catenin is not presented simultaneously bound to Rho and E-cadherin, a fact that cannot be discarded.
Acknowledgments
We thank Albert B. Reynolds, Ana Clara Carrera, José María Rojas, and Marius Sudol for providing plasmids and Alberto Muñoz for his help in the generation of the K-ras transfectant clones.
This work was supported by grants 01/045-00 (from Fundació La Caixa) to A.G.H.; PM-99-0132 and PM99-0064 (from Ministerio de Ciencia y Tecnología) to A.G.H. and M.D., respectively; and 2001SGR00410 and 2001SGR00197 (from Direcció General de Recerca). J.C. and S.M. were recipients of predoctoral fellowships from the Ministerio de Educación y Ciencia and UAB, respectively.
REFERENCES
- 1.Aberle, H., S. Butz, J. Stappert, H. Weisseig, R. Kemler, and H. Hoschuetzky. 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107:3655-3663. [DOI] [PubMed] [Google Scholar]
- 2.Aberle, H., H. Schwartz, H. Hoschuetzky, and R. Kemler. 1996. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to α-catenin. J. Biol. Chem. 271:1520-1526. [DOI] [PubMed] [Google Scholar]
- 3.Adams, C., and J. W. Nelson. 1998. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10:572-577. [DOI] [PubMed] [Google Scholar]
- 4.Anastasiadis, P. Z., and A. B. Reynolds. 2000. The p120 catenin family: complex roles in adhesion, signalling and cancer. J. Cell Sci. 113:1319-1334. [DOI] [PubMed] [Google Scholar]
- 5.Aono, S., S. Nagakawa, A. B. Reynolds, and M. Takeichi. 1999. p120ctn acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J. Cell Biol. 145:551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens, J., L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. Mareel, and W. Birchmeier. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonvini, P., W. G. An, A. Rosolen, P. Nguyen, J. Trepel, A. García de Herreros, M. Duñach, and L. M. Neckers. 2001. Geldanamycin abrogates erbB2 association with proteasome-resistant β-catenin in melanoma cells, increases β-catenin-E-cadherin association, and decreases β-catenin-sensitive transcription. Cancer Res. 61:1671-1677. [PubMed] [Google Scholar]
- 8.Calautti, E., S. Cabodi, P. Stein, M. Hatzfeld, N. Kedersha, and P. Dotto. 1998. Tyrosine phosphorylation and Src family kinases control keratinocyte cell-cell adhesion. J. Cell Biol. 141:1449-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calautti, E., M. Grossi, C. Mammucari, Y. Aoyama, M. Pirro, Y. Ono, J. Li, and P. Dotto. 2002. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion. J. Cell Biol. 156:137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig, A., R. Zirngibl, K. Williams, L. Cole, and P. Greer. 2001. Mice devoid of Fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol. Cell. Biol. 21:603-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel, J. M., and A. B. Reynolds. 1995. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol. Cell. Biol. 110:345-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel, J. M., and A. B. Reynolds. 1997. Tyrosine phosphorylation and cadherin/catenin function. Bioessays 19:883-891. [DOI] [PubMed] [Google Scholar]
- 13.Giannini, A., M. M. Vivanco, and R. M. Kypta. 2000. α-catenin inhibits β-catenin signaling by preventing formation of a β-catenin-T-cell factor · DNA complex. J. Biol. Chem. 275:21883-21888. [DOI] [PubMed] [Google Scholar]
- 14.Gumbiner, B. M. 2000. Regulation of cadherin adhesive activity. J. Cell Biol. 148:399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, X., J. N. Rao, L. Liu, M. Rizvi, D. J. Turner, and J. Y. Wang. 2002. Polyamines regulate β-catenin tyrosine phosphorylation via Ca2+ during intestinal epithelial cell migration. Am. J. Physiol. Cell Physiol. 283:C722-C734. [DOI] [PubMed] [Google Scholar]
- 16.Hazan, R. B., and L. Norton. 1998. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J. Biol. Chem. 273:9078-9084. [DOI] [PubMed] [Google Scholar]
- 17.Hoschuetzky, H., H. Aberle, and R. Kemler. 1994. β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J. Cell Biol. 127:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu, P., E. J. O'Keefe, and D. S. Rubinstein. 2001. Tyrosine phosphorylation of human keratinocyte β-catenin and plakoglobin reversibly regulates their binding to E-cadherin and α-catenin. J. Invest. Dermatol. 117:1059-1067. [DOI] [PubMed] [Google Scholar]
- 19.Huber, A., and W. Weis. 2001. The structure of β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell 105:391-402. [DOI] [PubMed] [Google Scholar]
- 20.Kapus, A., C. Di Ciano, J. Sun, X. Zhan, L. Kim, T. W. Wong, and O. D. Rotstein. 2000. Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell-cell contact sites. J. Biol. Chem. 275:32289-32298. [DOI] [PubMed] [Google Scholar]
- 21.Kim, L., and T. W. Wong. 1995. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol. Cell. Biol. 15:4553-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinch, M. S., G. J. Clark, J. D. Channing, and K. Burridge. 1995. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J. Cell Biol. 130:461-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lilien, J., J. Balsamo, C. Arregui, and G. Xu. 2002. Turn-off, drop-out. Functional state switching of cadherins. Dev. Dyn. 224:8-29. [DOI] [PubMed] [Google Scholar]
- 24.Magie, C. R., D. Pinto-Santini, and S. M. Parkhurst. 2002. Rho1 interacts with p120ctn and a-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development 129:3771-3782. [DOI] [PubMed] [Google Scholar]
- 25.Mariner, D., P. Anastasiadis, H. Keilhack, F. D. Bohmer, J. Wang, and A. B. Reynolds. 2001. Identification of Src phosphorylation sites in the catenin p120ctn. J. Biol. Chem. 276:28006-28013. [DOI] [PubMed] [Google Scholar]
- 26.Matsuyoshi, N., M. Hamaguchi, S. Taniguchi, A. Nagafuchi, S. Tsukita, and M. Takeichi. 1992. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J. Cell Biol. 118:703-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller, T., A. Choidas, E. Reichmann, and A. Ullrich. 1999. Phosphorylation and free pool of β-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J. Biol. Chem. 274:10173-10183. [DOI] [PubMed] [Google Scholar]
- 28.Nagafuchi, A. 2001. Molecular architecture of adherens junctions. Curr. Opin. Cell Biol. 13:600-603. [DOI] [PubMed] [Google Scholar]
- 29.Ohkubo, T., and M. Ozawa. 1999. p120ctn binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J. Biol. Chem. 274:21409-21415. [DOI] [PubMed] [Google Scholar]
- 30.Orsulic, S., O. Huber, H. Aberle, S. Arnold, and R. Kemler. 1999. E-cadherin binding prevents β-catenin nuclear localization and β-catenin/LEF-1-mediated transactivation. J. Cell Sci. 112:1237-1245. [DOI] [PubMed] [Google Scholar]
- 31.Owens, D. W., G. W. McLean, A. W. Wyke, C. Paraskeva, E. K. Parkinson, M. C. Frame, and V. G. Brunton. 2000. The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell-cell contacts. Mol. Biol. Cell 11:1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozawa, M., and R. Kemler. 1998a. Altered cell adhesion activity by pervanadate due to the dissociation of alpha-catenin from the E-cadherin-catenin complex. J. Biol. Chem. 273:6166-6170. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa, M., and R. Kemler. 1998b. The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J. Cell Biol. 142:1605-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piedra, J., D. Martínez, J. Castaño, S. Miravet, M. Duñach, and A. García de Herreros. 2001. Regulation of β-catenin structure and activity by tyrosine phosphorylation. J. Biol. Chem. 276:20436-20443. [DOI] [PubMed] [Google Scholar]
- 35.Pokutta, S., and W. Weis. 2000. Structure of the dimerization and β-catenin-binding region of α-catenin. Mol. Cell 5:533-543. [DOI] [PubMed] [Google Scholar]
- 36.Provost, E., and D. L. Rimm. 1999. Controversies at the cytoplasmic face of the cadherin-based adhesion complex. Curr. Opin. Cell Biol. 11:567-572. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds, A. B., J. M. Daniel, P. D. McCrea, M. M. Wheelock, J. Wu, and Z. Zhang. 1994. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with cadherin complexes. Mol. Cell. Biol. 14:8333-8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosato, R., J. Veltmaat, J. Groffen, and N. Heisterkamp. 1998. Involvement of the tyrosine kinase Fer in cell adhesion. Mol. Cell. Biol. 18:5762-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roura, S., S. Miravet, J. Piedra, A. García de Herreros, and M. Duñach. 1999. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 274:36734-36740. [DOI] [PubMed] [Google Scholar]
- 40.Sadot, E., I. Simcha, M. Shtuman, A. Ben-Ze'ev, and B. Geiger. 1998. Inhibition of β-catenin-mediated transactivation by E-cadherin derivatives. Proc. Natl. Acad. Sci. USA 95:15339-15344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibamoto, S., M. Hayakawara, K. Takeuchi, T. Hori, N. Oku, K. Miyazawa, N. Kimamura, M. Takeichi, and F. Ito. 1994. Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes. Commun. 1:295-305. [DOI] [PubMed] [Google Scholar]
- 42.Shibata, T., A. Ochiai, Y. Kanai, S. Akimoto, M. Gotoh, N. Yasui, R. Machinami, and S. Hirohashi. 1996. Dominant negative inhibition of the association between β-catenin and c-erbB2 by N-terminally deleted β-catenin suppresses the invasion and metastasis of cancer cells. Oncogene 13:883-889. [PubMed] [Google Scholar]
- 43.Skoudy, A., M. M. Llosas, and A. García de Herreros. 1996. Intestinal HT-29 cells with dysfunction of E-cadherin show increased pp60src activity and tyrosine phosphorylation of p120-catenin. Biochem. J. 317:279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein, P. L., H. M. Lee, S. Rich, and P. Soriano. 1992. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell 70:741-750. [DOI] [PubMed] [Google Scholar]
- 45.Taddei, M. L., P. Chiarugi, P. Cirri, F. Buricchi, T. Fiaschi, E. Giannoni, D. Talini, G. Cozzi, L. Formigli, G. Raugei, and G. Ramponi. 2002. β-catenin interacts with low-molecular-weight protein tyrosine phosphates leading to cadherin-mediated cell-cell adhesion. Cancer Res. 62:6489-6499. [PubMed] [Google Scholar]
- 46.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 47.Thoreson, M. A., P. Z. Anastasiadis, J. M. Daniel, R. C. Ireton, M. J. Wheelock, K. R. Johnson, D. K. Hummingbird, and A. B. Reynolds. 2000. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell Biol. 148:189-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yap, A. S., C. M. Niessen, and B. M. Gumbiner. 1998. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell Biol. 141:779-789. [DOI] [PMC free article] [PubMed] [Google Scholar]