In this issue of Neoplasia, Nicol Keith's laboratory [1] has carefully mapped the gene for the catalytic subunit of human telomerase reverse transcriptase (hTERT) to chromosome 5p15.33. Their findings have potentially important implications about the activation of telomerase in cancer. Telomerase is a cellular reverse transcriptase that uses its integral RNA (hTR) as a template for adding telomere repeats to the ends of chromosomes [2]. Due to the incomplete replication of linear chromosomes, cells lose telomeric sequences with each division while telomerase compensates by adding sequences back. Although reproductive cells and many types of stem cells retain the ability to express telomerase, it is repressed in most adult tissues. In all proliferative somatic tissues, including stem cells of renewal tissues, telomeres are shorter in older compared to younger individuals [3]. The idea that telomeres are a timing or clocking mechanism is now well established. It is believed that telomeres, acting as a cellular “replicometer”, may have evolved in long-lived species to limit the number of divisions and thus reduce the probability of cell immortalization, a potentially rate limiting step in cancer progression. By limiting the number of divisions, there may be a reduction in the accumulation of mutations: the vast majority of cells become senescent before they accumulate sufficient changes to become cancerous. With improvement in sanitation and the discovery of antibiotics, vaccines and modern pharmaceuticals, humans are living longer and as a consequence the leading cause of cancer is now increased age. Thus replicative senescence acts as an important, but not impassable, barrier to human cancer development.
Telomerase is up-regulated or re-activated as part of cancer progression [4] and the underlying mechanism is an active area of investigation. It is generally thought that the catalytic protein (hTERT) is the limiting component of the telomerase holoenzyme and that it is transcriptionally repressed in many normal cells [2,5]. During cancer progression, genes in this repression pathway maybe lost, resulting in the activation or up-regulation of telomerase. There is good evidence for existence of repressors of transcription of hTERT on chromosome 3 and this has led to a search for the identity of these genes [6]. Microcell mediated transfer of chromosome 3 results in telomerase inhibition by repression of hTERT transcription [7], resulting in gradual reduction of telomere length, and after a period of time that is related to the initial length of the telomeres of the tumor cells, growth arrest [6].
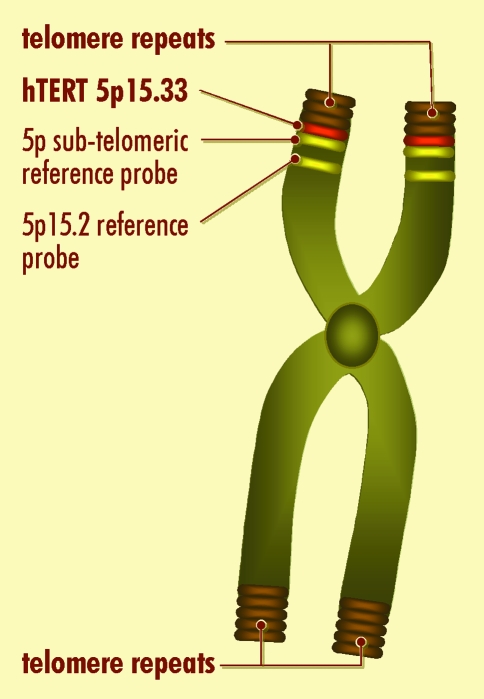
The hTERT gene had been previously mapped to the distal part of chromosome 5p15.33 (Geron Corporation accession #AF015950, and [8]). The striking finding in the present paper by Nicol Keith's group is that the location of the single-copy hTERT gene (with an estimated size of about 40kb) is very close to the telomere, at the extreme terminus of chromosome 5p. Indeed, there are no known more distal markers. The hTERT gene either co-localizes or is more distal to the subtelomeric probe (Chromoprobe T 5ptel) which is 300kb from the telomere (see Figure 1).
Figure 1.
Diagrammatic representation of the localization of hTERT to the tip of chromosome 5p. The precise localization of hTERT to chromosome 5p15.33 was achieved by direct mapping using DAPI banding on the fluorescence in situ hybridized slides to get a precise band position; mapping relative to a reference Cri-du-chat probe at 5p15.2; and mapping relative to 5ptel a sub-telomeric reference probe 300kb from the telomeric repeats. Under the resolution of the fluorescence microscope, the hTERT probe co-localized with the 5ptel probe, suggesting that hTERT lies very dose to the telomere on chromosome 5p15.33.
This raises the intriguing possibility that telomere positional effects could be involved in the regulation of the hTERT gene. For example in yeast, genes placed near telomeres are often repressed [9]. Since yeast telomeres are heterochromatic, it is possible that heterochromatin spreads into the subtelomeric DNA and silences nearby genes. If a similar situation exists in vertebrate telomeres, it might lead to the repression of the hTERT gene when telomeres are sufficiently long [10]. This may have implications for the regulation of telomere length in the human germline, where telomerase is expressed, to ensure that telomeres do not over elongate. In contrast, the mouse telomerase catalytic component maps to an internal location on mouse chromosome 13, far from the telomere [11]. Mouse telomeres are much longer than human telomeres and it is possible that this might reflect a lack of telomere positional repression of internally located mouse mTERT gene.
The other interesting finding in the Bryce et al paper [1] is that there are several unusually large numbers of hTERT-copies [8–10] in some tumor types. Most tumor cells lines had 2–4 copies of hTERT, which is consistent with the general aneuploidy associated with most cancers cell lines that have been passaged for long times. However 60% (3/5) cervical carcinoma cell lines had 6–11 copies. This suggests that dosage effects could be contributing to the initial telomerase activity that is detected in some cancers. As the authors suggested [1], increasing copies of the hTERT gene could titrate out repressors or compensate for low levels of transcriptional activators, thus allowing tumors to increase the transcription of hTERT. Another possibility is that the disregulation of the hTERT gene by translocation could also contribute to the up-regulation of telomerase activity. Most cervical carcinomas express human papillomavirus (HPV). It has been shown that high-risk strains of HPV abrogate both p53 and pRb G1 checkpoint pathways allowing for extended proliferation and genomic instability. Perhaps the HPV-induced genomic instability induces c-myc, which is thought to positively regulate hTERT [12–14], or the genomic instability increases the copy number of hTERT, permitting telomerase activity up-regulation and continued cell growth. Interestingly, early cervical lesions (in situ carcinoma) are often telomerase positive [15,16]. It is also interesting that 5p is one of the most common targets for amplification in non-small lung cancers occurring in about 70% of cases [17,18]. Obviously, additional experiments will need to be conducted to verify this general observation.
Dyskerin is a protein that associates with hTR, the functional RNA component of telomerase [19]. Mutations in dyskerin result in the X-linked form of the human disease dyskeratosis congenita. Sufferers have defects in highly regenerative tissues such as skin, bone marrow and a predisposition to develop certain types of malignancies. Several of the features are consistent with a compromised telomerase function, and cells from these patients exhibit unusually short telomeres. This raises the interesting possibility that other human disorders may be associated with reduced telomere maintenance. There are human disorders that are associated with loss of the distal portion of chromosome 5p, and thus these individuals would be hemizygous in the hTERT gene, potentially leading to reduced telomere maintenance. For example, Cri-du-chat syndrome (also know as cat cry syndrome) is one of the most common human deletion syndromes, with the incidence varying between 1 in 20,000 to 1 in 50,000. At birth children are characterized by a high pitched cry, low birth weight, poor muscle tone, microcephaly, and a number of other morphological features such as facial dysmorphology including a thin narrow face with a prominent nasal bridge [20]. While 5p12.2 seems to be the location of most deletions associated with this syndrome (encoding genes such as semaphorin F implicated in axonal path-finding, and delta-catenin which interacts with presenilin-1), many children have lost the entire distal end of chromosome 5p [21]. The 5p12.2 deletions probably accounts for the severe mental retardation in Cri-du-chat syndrome, but subtle effects of a hemizygous state of telomerase on developmental abnormalities may contribute to some of phenotypes and would be an interesting avenue to investigate.
In summary, detailed mapping of the human telomerase gene to the most distal end of chromosome 5p adds new layers of interest to the role of 5p telomere length in regulating telomerase and the biological effects of 5p alterations in human genetic diseases including cancer.
Acknowledgements
JWS is a Senior Scholar of the Ellison Medical Foundation. We thank Joe Baur for valuable discussions and Tom McGuire for graphics help.
References
- 1.Bryce LA, Morrison N, Hoare SF, Muir S, Keith WN. Mapping of the gene for the human reverse transcriptase, hTERT, to chromosome 5p15.3 by fluorescence in situ hybridization. Neoplasia. 2000:197–201. doi: 10.1038/sj.neo.7900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and humans. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 3.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with aging. Nature (London) 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 4.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 5.Bodnar AG, Ouellete M, Frolkis M, Holt SE, Chiu C-P, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of lifespan by introduction of telomerase in normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 6.Ohmura H, Tahara H, Suzuki M, Ide T, Shimizu M, Mitsuaki A, Yoshida A, Tahara E, Shay JW, Barrett JC, Oshimura M. Restoration of the cellular senescence program and repression of telomerase by human chromosome 3. Jpn J Cancer Res. 1995;86:899–904. doi: 10.1111/j.1349-7006.1995.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horikawa I, Oshimura M, Barrett JC. Repression of the telomerase catalytic subunit by a gene on human chromosome 3 that induces cellular senescence. Molecular Carcinogenesis. 1998;22:65–72. doi: 10.1002/(sici)1098-2744(199806)22:2<65::aid-mc1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddie SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 9.Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 10.Wright WE, Shay JW. Time, telomeres and tumours: is cellular senescence more than an anticancer mechanism? Trends in Cell Biology. 1995;5:293–297. doi: 10.1016/s0962-8924(00)89044-3. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg RA, O'Hagan RC, Deng HY, Xiao QR, Hann SR, Adams RR, Lichtsteiner S, Chin L, Morin GB, DePinho RA. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene. 1999;18:1219–1226. doi: 10.1038/sj.onc.1202669. [DOI] [PubMed] [Google Scholar]
- 13.Wu KJ, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, Dalla-Favera R. Direct activation of TERT transcription by c-MYC. Nature Genetics. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes & Development. 1998;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyo S, Takakura M, Ishikawa H, Sasagawa T, Satake S, Tateno M, Inoue M. Application of telomerase assay for the screening of cervical lesions. Cancer Research. 1997;57:1863–1867. [PubMed] [Google Scholar]
- 16.Kawai K, Yaginuma Y, Tsuruoka H, Griffin M, Hayashi H, Ishikawa M. Telomerase activity and human papillomavirus (HPV) infection in human uterine cervical cancers and cervical smears. European Journal of Cancer. 1998;34:2082–2086. doi: 10.1016/s0959-8049(98)00242-1. [DOI] [PubMed] [Google Scholar]
- 17.Petersen I, Bujard M, Petersen S, Wolf G, Goeze A, Schwendel A, Langreck H, Gellert K, Reichel M, Just K, du Manoir S, Cremer T, Dietel M, Ried T. Patterns of chromosomal imbalances in adenocarcinoma and squamous cell carcinoma of the lung. Cancer Res. 1997;57:2331–2335. [PubMed] [Google Scholar]
- 18.Ried T, Petersen I, Holtgreve-Grez H, Speicher MR, Schröck E, du Manoir S, Cremer T. Mapping of multiple DNA gains and losses in primary small cell lung carcinomas by comparative genomic hybridization. Cancer Res. 1994;54:1801–1806. [PubMed] [Google Scholar]
- 19.Mitchell JR, Wood E, Collin K. A telomerase component defective in the human disease dsykeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 20.Van Buggenhout GJCM, Pijkels E, Holvoet M, Schaap C, Hamel BCJ, Fryns JP. Cri du chat syndrome: changing phenotype in older patients. American J Medical Genetics. 2000;90:203–215. doi: 10.1002/(sici)1096-8628(20000131)90:3<203::aid-ajmg5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Medina M, Marinescu RC, Overhauser J, Kosik KS. Hemizygosity of delta catenin (CTNND2) is associated with severe mental retardation in cri-du-chat syndrome. Genomics. 2000;63:157–164. doi: 10.1006/geno.1999.6090. [DOI] [PubMed] [Google Scholar]