Abstract
The genesis of phyllotaxis, which often is associated with the Fibonacci series of numbers, is an old unsolved puzzle in plant morphogenesis. Here, we show that disruption of an Arabidopsis topoisomerase (topo) I gene named TOP1α affects phyllotaxis and plant architecture. The divergence angles and internode lengths between two successive flowers were more random in the top1α mutant than in the wild type. The top1α plants sporadically produced multiple flowers from one node, and the number of floral organ primordia often was different. The mutation also caused the twisting of inflorescences and individual flowers and the serration of leaf margins. These morphological abnormalities indicate that TOP1α may play a critical role in the maintenance of a regular pattern of organ initiation. The top1α mutant transformed with the RNA interference construct for TOP1β, another topo I gene arrayed tandemly with TOP1α, was found to be lethal at young seedling stages, suggesting that topo I activity is essential in plants.
INTRODUCTION
Shoot systems of higher plants show a variety of patterns of leaf and flower arrangement (phyllotaxis), which result from the positioning of primordia at the shoot apex. One of the typical phyllotactic patterns is a spiral form in which the divergence angle between successive primordia approaches 137.5°, the Fibonacci angle. Many mathematical, physical, and chemical models have been proposed to explain how such phyllotactic patterns are generated (reviewed by Steeves and Sussex, 1989; Callos and Medford, 1994; Jean, 1994; Lyndon, 1998). However, the molecular mechanisms by which regular patterns of the position and the timing of primordium initiation are determined remain obscure. Furthermore, regardless of the phyllotactic patterns in leaves and flowers, protrusive patterns of floral organ primordia usually are whorled, but the mechanism underlying the shift to a whorled pattern also is unknown.
Analyses of mutants with abnormal shoot development have provided evidence that various factors affect phyllotactic patterns. Disorganization of the spiral phyllotaxis in clavata (clv1, clv2, and clv3), fasciata (fas1 and fas2), fully fasciated (fuf), and mgoun (mgo1 and mgo2) mutants of Arabidopsis appears to be caused by the enlargement of shoot apical meristems, suggesting the importance of meristem size in the maintenance of a defined pattern (Leyser and Furner, 1992; Medford et al., 1992; Clark et al., 1993, 1995; Laufs et al., 1998). Studies on the Arabidopsis pin-formed1 (pin1) mutants have revealed that the local distribution of auxin determines the radial positioning of flowers (Okada et al., 1991; Gälweiler et al., 1998; Vernoux et al., 2000). Microapplication of auxin to the naked inflorescence apex of pin1 induces flower formation (Reinhardt et al., 2000).
The Arabidopsis SERRATE (SE) gene, whose mutation affects phyllotaxis and the elaboration of leaves (Clarke et al., 1999), encodes a zinc finger protein and has been implicated in the regulation of changes in gene expression via chromatin modification (Prigge and Wagner, 2001). Unlike these mutants, which show defects in the regularity of the pattern, the abphyl1 mutant of maize shows a shift from an alternate phyllotaxis to a decussate one in which leaves are initiated in opposite pairs (Greyson and Walden, 1972; Jackson and Hake, 1999). The abphyl1 mutant apex also is enlarged. The ABPHYL1 gene remains to be identified.
To further address this classic puzzle in plant morphogenesis, additional factors associated with the control of phyllotaxis must be identified. We have analyzed a mutation at the TOP1α locus that affects spiral phyllotaxis and organ architecture in Arabidopsis. The TOP1α gene encodes a type-I DNA topoisomerase (topo I). Topo I promotes the relaxation of supercoiled DNA by introducing a transient single-strand break in the duplex and acts in a number of different DNA metabolisms, such as DNA replication, transcription, and repair and chromatin compaction (reviewed by Wang, 1996; Champoux, 2001).
The topo I function has been shown to be essential in animal systems (Lee et al., 1993; Morham et al., 1996). Analysis of topo I–deficient mutants of Drosophila with a conditional rescue construct has revealed that topo I plays critical roles in stages actively engaged in cell proliferation (Zhang et al., 2000). On the other hand, from the first identification of plant topo I enzyme activity in wheat germ (Dynan et al., 1981), reports on plant topo I enzymes have concerned primarily their biochemical characterization and gene cloning. Our study provides a new clue to the role of topo I in plant morphogenesis and suggests that topo I enzymes are essential for plant survival.
RESULTS
Phenotype of the top1α Mutant
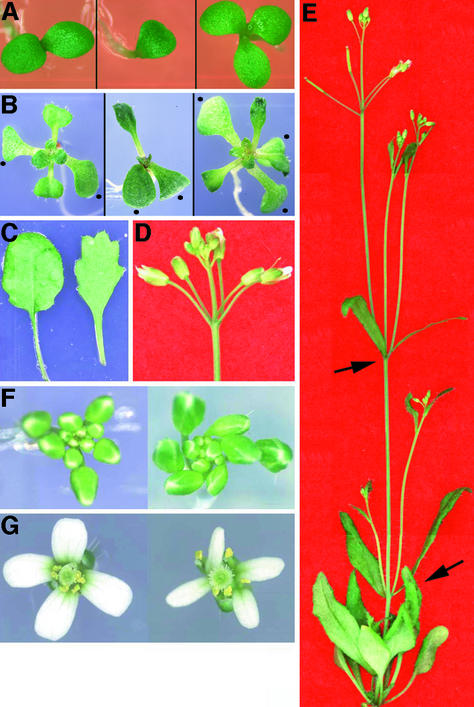
Wild-type Arabidopsis plants have two cotyledons and show a typical spiral phyllotaxis of leaves and flowers (Leyser and Furner, 1992; Callos and Medford, 1994). The top1α-1 mutant exhibits irregular patterns of leaf and flower initiation. Under our growth conditions, ∼2% of the mutant seedlings had either one or three cotyledons (Figure 1A). Figure 1B shows examples of the top1α-1 mutant with a defect in the initiation of the second leaf. More than one leaf of the same size develops, suggesting the simultaneous initiation of multiple leaf primordia. In addition, mutant leaf margins were serrated (Figure 1C).
Figure 1.
Phenotype of top1α-1 Plants.
(A) Examples of top1α-1 mutant seedlings grown for 3 days after germination.
(B) Examples of 10-day-old wild-type (left) and mutant (center and right) seedlings. Note the lack of a second true leaf (center) and the simultaneous growth of three true leaves (right). Dots indicate cotyledons, which are increased in the mutant seedling at right.
(C) Leaf morphology of the wild type (left) and the mutant (right).
(D) An inflorescence of the mutant bearing multiple flowers from a node.
(E) Top views of wild-type (left) and mutant (right) inflorescences.
(F) Top views of wild-type (left) and mutant (right) flowers.
(G) An adult flowering plant of the mutant. Arrows indicate the development of two lateral shoots from a node.
At the reproductive stage, the top1α-1 mutant produced multiple flowers from one node and bifurcated lateral shoots with a high frequency (Figures 1D and 1E, Table 1). All mutant inflorescences and individual flowers were twisted spirally (Figures 1F and 1G). The percentages of plants with clockwise twisting and those with counterclockwise twisting were found to be equal (data not shown). Although mutant flowers had a normal whorled phyllotaxy of floral organs, those with extra sepals and carpels and those with fewer petals and stamens were observed (Table 1).
Table 1.
Numbers of Flowers per Node and Floral Organs per Flower
| Frequency of Numbers of Flowers (%)a
|
Average Number of Floral Organsb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plants | 1c | 2 | 3 | 4 | 5 | Sepal | Petal | Stamen | Carpel |
| Wild type | 97.4 | 2.4 | 0.2 | 0 | 0 | 4.0 | 4.0 | 6.0 | 2.0 |
| top1α-1 | 49.4 | 33.4 | 12.0 | 4.6 | 0.6 | 4.1 | 3.7 | 5.6 | 2.1 |
Values were scored on 500 flower-bearing nodes of 25 plants and are shown as percentages.
Average numbers of 100 flowers.
Number of flowers per node.
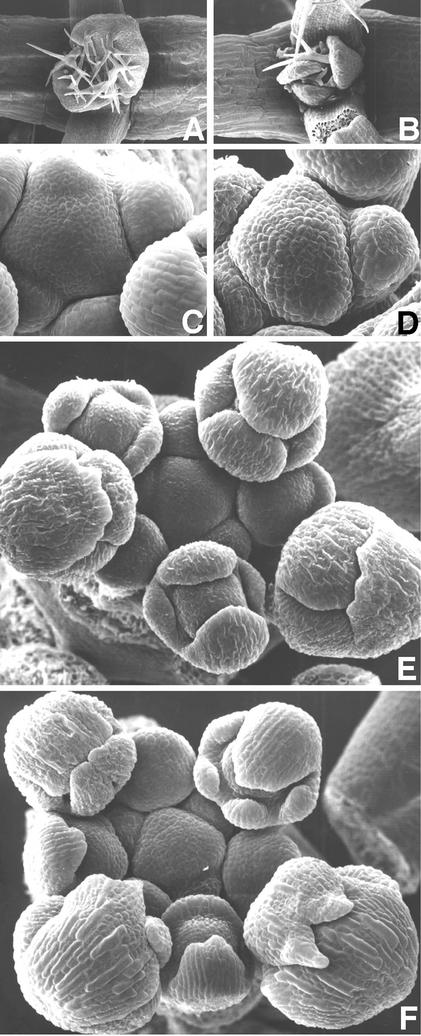
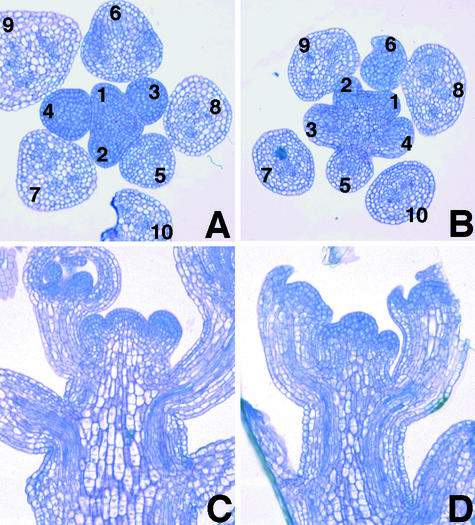
Scanning electron microscopy revealed abnormal leaf primordia initiation in the top1α-1 seedling (Figures 2A and 2B). At the reproductive stage, the apical inflorescence meristem of the mutant appeared to protrude more than that of the wild type (Figures 2C and 2D). Interestingly, all of the examined mutant sepals in the dorsal position initiated as two primordia and became fused during elaboration (Figures 2E and 2F). Sections of inflorescences revealed that the top1α-1 mutation resulted in an irregular phyllotactic arrangement of flower primordia and a more protruding apical dome shape compared with the wild type (Figure 3). The mutation also caused a decrease in the diameter of inflorescence stems to ∼80% of that of the wild type (Figures 3C and 3D).
Figure 2.
Scanning Electron Micrographs of Wild-Type and top1α-1 Mutant Shoot Apices.
(A) and (B) Vegetative shoot apices of 7-day-old seedlings of the wild type (A) and the mutant (B). Three leaf primordia are seen in the mutant, whereas only two leaf primordia are seen in the wild type.
(C) and (D) Inflorescence apices of the wild type (C) and the mutant (D). Note that the apical dome of the mutant protrudes more than that of the wild type. See also sections in Figures 3C and 3D.
(E) and (F) Flower development in the wild type (E) and the mutant (F). Note the development of five sepal primordia in the mutant.
Figure 3.
Sections of Wild-Type and top1α-1 Mutant Inflorescences.
(A) and (B) Cross-sections of inflorescences of the wild type (A) and the mutant (B). Flower primordia are labeled in order of presumptive increasing age.
(C) and (D) Longitudinal sections of inflorescences of the wild type (C) and the mutant (D).
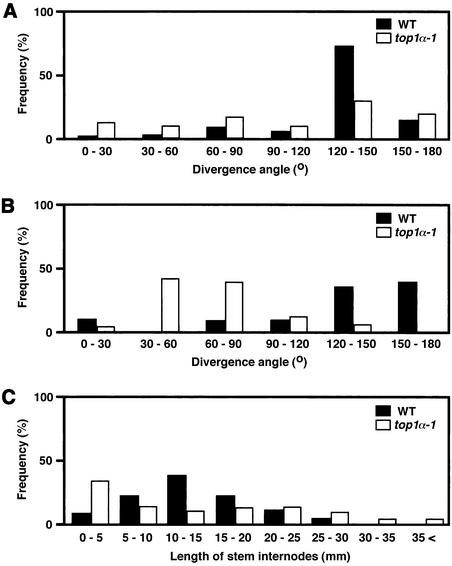
Measurement of the angle between two successive flower pedicels revealed that the angle in the mutant deviated significantly from that in the wild type, which probably approached 137.5°, the angle estimated in spiral systems of the Fibonacci series (Figure 4A). We found that the angle between two pedicels borne on one node tended to be small in top1α-1, whereas wild-type plants rarely bore two flowers on one node, and they seemed to be positioned away from each other (Figure 4B). Furthermore, although the total number of flowers and plant height in top1α-1 were comparable to those in wild-type plants (data not shown), the internode lengths between two successive flowers were dispersed in the mutant (Figure 4C).
Figure 4.
Distribution of Divergence Angles and Lengths of Stem Internodes between Two Flowers of Wild-Type and top1α-1 Mutant Plants.
(A) Divergence angles between two successive flowers that were separated by internodes. The data were scored on 200 pairs of flowers excluding those borne on one node. WT, wild type.
(B) Divergence angles between two successive flowers borne on one node. The data were scored on 15 pairs of wild-type flowers and 50 pairs of mutant flowers.
(C) Lengths of stem internodes between flowers. The data were scored on the first five pairs of each plant of 50 individuals grown for 40 days.
Molecular Cloning of the TOP1α Locus
The mutant allele named top1α-1 was identified originally from a pool of T-DNA insertion lines generated in our laboratory (Matsuhara et al., 2000). Because the mutant phenotype was linked to the T-DNA insertion and segregated as a single recessive mutation (data not shown), we cloned the flanking genome DNA of the insertion in the top1α-1 allele and found that the insertion occurred in a type-I DNA topoisomerase (topo I) gene that was cloned previously as TOP1 by Kieber et al. (1992). Database searches revealed that the Arabidopsis genome has another putative topo I gene located tandemly with the mutated one on chromosome 5 (Figure 5A). Therefore, we renamed the mutated locus TOP1α and the other TOP1β.
Figure 5.
Identification of TOP1α and TOP1β.
(A) Genomic structure of TOP1α and TOP1β. Black bars represent exons.
(B) Comparison of the TOP1α and TOP1β deduced amino acid sequences. Identical matches are shaded. Domains are defined according to the human TOP1 (Stewart et al., 1996a).
(C) RNA gel blot analysis of TOP1α and TOP1β expression. Total RNA samples were prepared from roots (R), flowers (F), rosette leaves (L), stem internodes (S), and pods (P) of wild-type plants. Expression in wild-type (WT) and top1α-1 10-day-old seedlings (Sd) also was compared.
A full-length TOP1α cDNA was cloned by reverse transcription–PCR from wild-type plants and fused with its own promoter of 1.1 kb to make a minimal T-DNA construct for complementation. The construct was introduced into wild-type plants and then transferred to top1α-1 by genetic crosses. In the F2 progeny, all of the homozygous top1α-1 plants carrying the minimal construct showed a phenotype indistinguishable from that of the wild type (data not shown), confirming that the mutant phenotype described above was caused by the T-DNA insertion into the TOP1α gene.
The deduced amino acid sequence of TOP1α shares 66% identity with TOP1β and 57 and 40% identity with topo I enzymes of other plant species and mammals, respectively. Sequence comparison (Figure 5B) revealed that the domain organization proposed previously for the human topo I enzyme (Stewart et al., 1996a) is conserved within TOP1α and TOP1β. The similarity of the N-terminal domains of TOP1α and TOP1β is lower than that of other domains. Topo I enzyme activity of the TOP1α gene product has been reported previously (Kieber et al., 1992).
RNA gel blot analysis performed with gene-specific probes revealed that both TOP1α and TOP1β transcripts were present ubiquitously in all organs examined and that the transcript level of TOP1β was not altered in top1a-1 plants (Figure 5C).
Effect of Camptothecins on the Growth of Arabidopsis Seedlings
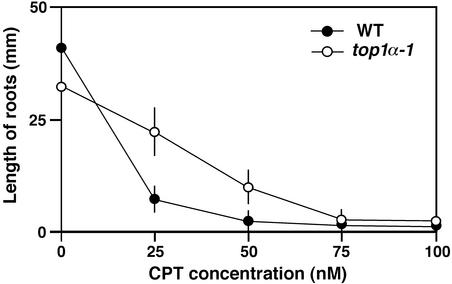
Topo I is known to be the target of an anticancer alkaloid, camptothecin (CPT), that transforms topo I into a DNA-damaging agent (Wang, 1996). The loss of topo I function results in reduced CPT sensitivity in yeast cells (Nitiss and Wang, 1988). Therefore, we examined the effects of exogenous application of CPT on the growth of wild-type and top1α-1 mutant seedlings. The primary root length of top1α-1 grown on normal nutrient agar plates was slightly less than that of the wild type (Figure 6), although cell files and morphology were indistinguishable between wild-type and mutant root tissues. The addition of >50 nM CPT to the medium resulted in the abortion of both roots and shoots of the wild type at young seedling stages, but top1α-1 seedlings clearly showed reduced sensitivity to CPT (Figure 6).
Figure 6.
Effects of CPT on the Growth of Wild-Type and top1α-1 Mutant Roots.
Plants were grown for 10 days on Murashige and Skoog (1962) agar medium containing the indicated concentrations of CPT. WT, wild type.
RNA Interference Analysis of the TOP1βGene
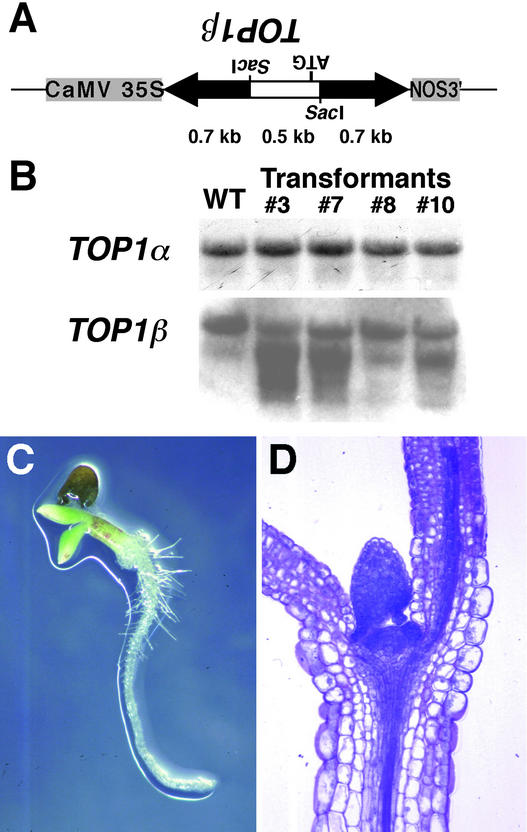
To determine the functional relationship between TOP1α and TOP1β, the effect of double-stranded RNA interference (RNAi) (Voinnet et al., 1998; Waterhouse et al., 1998; Chuang and Meyerowitz, 2000) with the TOP1β gene was investigated. The hairpin construct corresponding to a part of the N-terminal coding region of TOP1β was placed under the control of the 35S promoter of Cauliflower mosaic virus (Figure 7A) and introduced into the wild-type genome. RNA gel blot analysis revealed that signals possibly representing the hairpin transcripts and the degrading TOP1β mRNA were detected in transgenic plants carrying the construct, whereas no alterations in TOP1α expression were detected (Figure 7B). However, these transgenic lines showed no morphological abnormalities.
Figure 7.
RNAi Analysis of the TOP1β Gene.
(A) Hairpin construct used to study the effect of double-stranded RNAi with TOP1β expression. Arrows represent a part of the TOP1β coding region predicted to form a double-stranded RNA stem as a result of the expression. An open bar corresponds to its upstream part, which contains the TOP1β start codon and is predicted to form a single-stranded RNA loop. CaMV, Cauliflower mosaic virus.
(B) RNA gel blot analysis of TOP1α and TOP1β expression in transformants. Transgenic lines 3, 7, and 10 showed the seedling-lethal phenotype in the top1α-1 mutant background, whereas line 8 showed no phenotype. WT, wild type.
(C) Seedling-lethal phenotype of a plant that was homozygous for the top1α-1 mutant allele and that carried the TOP1β RNAi construct. A 10-day-old seedling segregated in the F2 progeny from the cross between the top1α-1 mutant and transgenic line 3 is shown. See Figure 1B for control wild-type and top1α-1 seedlings.
(D) Section of the lethal seedling shown in (C), which displays normal cellular morphology.
Therefore, we crossed these plants with top1α-1 mutants. Although all of the F1 progeny plants were morphologically normal, the F2 generation from the cross between line 3 and top1α-1 segregated lethal seedlings (Figure 7C) at a ratio of 54:316 and segregated plants that survived and showed the top1α-1 phenotype at a ratio of 18:316. These ratios likely correspond to the ratios of 3:16 and 1:16 expected from the recessive homozygous top1α-1 allele (one-fourth of the F2 progeny) and the dominant TOP1β RNAi allele (three-fourths of the F2 progeny), respectively, by Mendelian segregation.
Genotyping by PCR revealed that all of the lethal seedlings were homozygous for the top1α-1 allele and contained the TOP1β RNAi construct and that none of the adult plants with the top1α phenotype contained the TOP1β RNAi construct, suggesting that topo I is essential for plant survival. However, we observed no obvious abnormalities in terms of the shape and cell files of the vegetative shoot meristem and leaf primordia of the lethal seedling (Figure 7D). Similar lethal seedlings segregated in the F2 progeny from crosses of top1α-1 with three independent transgenic lines (lines 3, 7, and 10) that showed reduced transcript levels of the endogenous TOP1β gene (Figure 7B).
DISCUSSION
Topo I activity in yeast is not essential for viability and has been suggested to play an auxiliary role to type-II DNA topoisomerases (topo II) (Thrash et al., 1984; Uemura and Yanagida, 1984), which modify DNA topology by introducing a double-strand break (reviewed by Wang, 1996; Champoux, 2001). On the other hand, genetic studies of topo I–deficient mutants in fruit flies and mice have shown that topo I is essential for the growth and development of these multicellular organisms (Lee et al., 1993; Morham et al., 1996). Although plant topo I genes have been cloned from some species, including Arabidopsis (Kieber et al., 1992), pea (Reddy et al., 1998), and carrot (Balestrazzi et al., 2000), their significance in growth and development has remained unclear.
The results of the present study have provided genetic evidence that disruption of a topo I gene affects normal morphogenesis in higher plants. Because the T-DNA insertion in top1α-1 disrupted a region corresponding to the central core domain of the topo I enzyme, and no TOP1α transcripts corresponding to the conserved C-terminal domain were detected in the mutant plants (Figure 5), morphological abnormalities of top1α-1 most likely represent a null mutant phenotype. Although animal topo I genes have been identified previously as single genes, at least two genes encoding topo I enzymes are present in the carrot (Balestrazzi et al., 2000) and Arabidopsis genomes. The fact that a likely null allele of the TOP1α locus, top1α-1, displayed the recessive phenotype described here suggests that TOP1α plays a unique role that cannot be complemented by TOP1β, although we detected no difference in the organ specificity of expression between the two genes (Figure 5C).
Like the two topo I enzymes in the carrot (Balestrazzi et al., 2000), TOP1α and TOP1β share reduced similarity in the N-terminal domain (Figure 5B). The N-terminal domain of the animal topo I enzymes also is poorly conserved between species and is dispensable for activity in vitro, but it is likely to be important for targeting the enzyme to its sites of action within the nucleus of the cell (Alsner et al., 1992; Stewart et al., 1996b). It has been shown to interact with helicases and other proteins in human cells (reviewed by Champoux, 2001). Stimulation of topo I activity by a DNA helicase and their direct interaction also have been reported for pea enzymes (Pham et al., 2000). Together, these findings suggest that the enzyme activities of TOP1α and TOP1β are exerted differently by the specific interaction at their N-terminal domains with helicases or other regulatory cofactors. However, the possibility cannot be excluded that TOP1α and TOP1β are functionally equivalent and that the top1α-1 phenotype is attributable to a threshold effect of knocking one of the pair out.
Because we expected difficulty in making double mutants of the tandemly arrayed TOP1α and TOP1β genes, we used RNAi for TOP1β suppression to study the functional relationship between these genes (Figure 7). The lack of evident phenotypes in wild-type transformants with the TOP1β RNAi construct might be explained by a functional redundancy between TOP1α and TOP1β and/or by insufficient suppression of endogenous TOP1β expression. On the other hand, the seedling lethality of top1α-1 plants with the TOP1β RNAi construct suggests that topo I function is essential for survival.
But if this is the case, why do these plants not abort during embryogenesis? Again, a likely reason is that TOP1β expression cannot be suppressed sufficiently in the embryo by the RNAi construct that we used. Alternatively, topo I function might be substituted by other topoisomerases such as topo II in early embryos. How topo I and topo II are specified functionally in plants and in animal systems is an open question. Although isolation of the knockout allele of TOP1β clearly is required to verify the functional significance of this gene, our results suggest that RNAi can be used as a powerful approach to examine genetic interactions between tandemly duplicated genes.
Considering the top1α mutant phenotype and recent data on Drosophila topo I showing its importance in many developmental stages active in cell proliferation (Zhang et al., 2000), TOP1α may play a critical role in the active proliferation of cells of apical meristems. The precise sequence of events leading to the initiation of leaf and flower primordia on the flanks of the shoot apical meristem remains unknown. Sporadic alterations in the timing and the number of primordium protrusions in top1α-1 raise the possibility that, although the basic mechanism generating a regular pattern of spiral phyllotaxis still works, the mutation causes local fatal damage, such as the accumulation of DNA torsional tensions and incomplete chromosome condensation in some of the actively proliferating cells, by which a subset of initial cells committed as an organ primordium may cease to proliferate or may be separated into two or more primordia. Serration of leaves and twisting of inflorescences and flowers also might be attributable to local and sporadic disturbance of the coordination of cell proliferation.
It also is possible that the morphological abnormalities in top1α-1 result from aberrant expression patterns of some specific genes. Topo I has been shown to be involved actively in the transcription process in animal systems (Merino et al., 1993; Shykind et al., 1997; Shaiu and Hsieh, 1998). Furthermore, the top1α-1 mutant phenotype also seems to be in line with recent genetic data that have highlighted the importance of chromatin remodeling and assembly in the control of plant morphogenesis (Verbsky and Richards, 2001). The Arabidopsis FAS1 and FAS2 genes, whose mutants show pleiotropic phenotypes, including fasciated stems, serrated leaves, and disturbed phyllotaxis (Leyser and Furner, 1992), encode two of the three subunits of chromatin assembly factor-1 (Kaya et al., 2001).
Antisense expression of an Arabidopsis histone deacetylase gene, HDA1, which also is involved in chromatin modifications, leads to pleiotropic abnormalities such as serrated leaves, aerial rosettes, delayed flowering, multiple shoot branching, and early senescence (Tian and Chen, 2001). In addition, a few genes have been found to be expressed ectopically in these mutants and transformants, suggesting the importance of proper epigenetic control of gene expression during development (Kaya et al., 2001; Tian and Chen, 2001). Identification of genes affected by the top1α-1 mutation will be the next step in the further investigation of the involvement of topo I function in chromatin-based control of plant gene expression and the molecular basis underlying the homeostatic control of plant morphogenesis.
The phenotype observed in top1α-1 resembles that reported for Arabidopsis se mutants (Clarke et al., 1999; Prigge and Wagner, 2001), whose mutated gene encodes a putative transcription factor with a single zinc finger (Prigge and Wagner, 2001). However, we found that the SE expression level in inflorescences was not affected by the top1α-1 mutation (data not shown). An intriguing possibility is that the SE transcription factor may regulate TOP1α expression, although it remains to be determined whether TOP1α expression is altered in se mutants. Based on a synergistic double mutant phenotype of se fas1 plants, the SE protein has been suggested to be involved in regulating changes in gene expression via chromatin modification (Prigge and Wagner, 2001). It is possible as well that SE is associated with actively transcribed regions of chromatin in cooperation with TOP1α.
Our results on the effects of CPT (Figure 6) indicate that CPT has a toxic effect on plant growth, probably through the trapping of topo I–DNA cleavage complexes, as in other eukaryotes (Champoux, 2001). The availability of CPT together with top1α mutant plants provides a starting point for the further analysis of the relationship between topo I function and plant development.
METHODS
Plant Materials
All plants used in this study were Arabidopsis thaliana ecotype Columbia. The top1α-1 mutant has been identified in T-DNA–tagged lines generated in our laboratory (Matsuhara et al., 2000). Plants were grown in a growth chamber under continuous illumination at 22°C. For growth of seedlings and root tissues, seeds were surface-sterilized and placed on Murashige and Skoog (1962) plates as described previously (Takahashi et al., 1995). For treatment of plants with camptothecin (Sigma), camptothecin was dissolved in DMSO and added to Murashige and Skoog (1962) agar medium before solidification.
Microscopy
For light microscopy of tissue sections, plant material was fixed in 50% ethanol, 5% formaldehyde, and 5% acetic acid, dehydrated through an ethanol series, and embedded in Technovit 7100 resin (Kulzer, Wehrheim, Germany). Sections (10 μm) were cut with an LR-85 Microm microtome (Yamato-Koki, Asaka, Japan) and stained for 2 min in an aqueous 0.1% toluidine blue solution. Scanning electron microscopy of inflorescences was performed as described previously (Takahashi et al., 1995).
Molecular Analysis
The plant DNA flanking the T-DNA insertion in top1α-1 was cloned by inverse PCR as described previously (Matsuhara et al., 2000). For RNA gel blot analysis, total RNA was prepared by the SDS-phenol extraction method (Matsuhara et al., 2000), separated on formaldehyde-denatured agarose gels, and blotted onto GeneScreen nylon membranes (DuPont–New England Nuclear). Gene-specific probes for TOP1α and TOP1β were labeled with 32P by random priming of cDNA fragments that were amplified by reverse transcription–PCR and cloned into pGEM-T Easy vector (Promega). The primers used for PCR were 1αF (5′-CATGCATAC-AAAAGAGGACTTGAA-3′), 1αR (5′-TTGAGCTCTTTTGCCCTCCAA-TAC-3′), 1βF (5′-GAAAGAGGAACTTAACACCTGAAG-3′), and 1βR (5′-CTCTGTAAATGTATGGTGAATGGT-3′). Hybridizations and washes were performed according to the manufacturer's instructions.
Transgenic Plants and Genotyping
For complementation of the mutant, a genomic fragment encompassing the region from the first untranslated exon of TOP1α to 1140 bp upstream of a putative TOP1α transcription start site was amplified from wild-type genomic DNA by PCR with primers 1αF-ClaI (5′-ATCGATCCTAGGATCACTGTTGC-3′) and 1αR-XhoI (5′-CTCGAG-ATTGAAGAAATTGTCTAGC-3′), cloned into pGEM-T Easy vector, sequenced, and transferred as a ClaI-SacI fragment to a binary vector, pBI101 (Clontech, Palo Alto, CA), to replace the β-glucuronidase (GUS) gene. The full-length TOP1α coding region was amplified from total RNA prepared from wild-type flowers by reverse transcription–PCR with primers 1αF-XhoI (5′-CTCGAGAAGAAGTGGTCTGAG- CCT-3′) and 1αR-SpeI (5′-ACTAGTCCAAACACCCTAATTCAG-3′), cloned into pGEM-T Easy vector, sequenced, and transferred as an XhoI-SpeI fragment to the pBI101 derivative described above.
For RNA interference experiments, an XbaI-SacI GUS gene fragment of pBI121 (Clontech) was replaced with an XbaI-SacI fragment of pUC18 to make pBI121ΔGUS. A 1.2-kb genomic fragment of the TOP1β N-terminal coding region was amplified by PCR with primers 1βF-KpnI (5′-GGTACCAAATGGTCATGACGGG-3′) and 1βR-SpeI (5′-ACTAGTTAAGTCAACAGGCTTTCC-3′), cloned into pGEM-T Easy vector, and sequenced. The latter half of this fragment was transferred to pBI121ΔGUS as a 0.7-kb SacI fragment. The 1.2-kb genomic fragment with KpnI and SpeI restriction sites was further introduced into KpnI and XbaI restriction sites of this clone to generate a TOP1β hairpin construct downstream of the 35S promoter of Cauliflower mosaic virus. These T-DNA constructs were introduced into wild-type Arabidopsis plants via Agrobacterium tumefaciens–mediated transformation (Clough and Bent, 1998).
Minipreparation of genomic DNA for PCR was performed according to the protocol of Konieczny and Ausubel (1993). PCR primer pairs used for genotyping were 1αF-EcoRI (5′-CCTATTAACCCAAAAGAATTCAAG-3′) and 1αR-SpeI for the wild-type TOP1α allele and the complementation construct, LB (5′-ACCCCAGTACATTAAAAACGTC-3′) and 1αR-SpeI for the top1α-1 allele, and 35S-90 (5′-GATGTGATATCTCCACTGACGT-3′) and 1βF-KpnI for the TOP1β RNA interference construct.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We thank Gen Takaku for advice regarding scanning electron microscopy. This work was supported in part by Grant 12740431 from the Japan Society for the Promotion of Science (JSPS) to T.T. M.A. was supported by a JSPS research fellowship for young scientists.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.001925.
References
- Alsner, J., Svejstrup, J.Q., Kjeldsen, E., Sorensen, B.S., and Westergaard, O. (1992). Identification of an N-terminal domain of eukaryotic DNA topoisomerase I dispensable for catalytic activity but essential for in vivo function. J. Biol. Chem. 267, 12408–12411. [PubMed] [Google Scholar]
- Balestrazzi, A., Chini, A., Bernacchia, G., Bracci, A., Luccarini, G., Cella, R., and Carbonera, D. (2000). Carrot cells contain two top1 genes having the coding capacity for two distinct DNA topoisomerases I. J. Exp. Bot. 51, 1979–1990. [DOI] [PubMed] [Google Scholar]
- Callos, J.D., and Medford, J.I. (1994). Organ position and pattern formation in the shoot apex. Plant J. 6, 1–7. [Google Scholar]
- Champoux, J.J. (2001). DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 70, 369–413. [DOI] [PubMed] [Google Scholar]
- Chuang, C.F., and Meyerowitz, E.M. (2000). Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 97, 4985–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1993). CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119, 397–418. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1995). CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121, 2057–2067. [Google Scholar]
- Clarke, J.H., Tack, D., Findlay, K., Van Montagu, M., and Van Lijsebettens, M. (1999). The SERRATE locus controls the formation of the early juvenile leaves and phase length in Arabidopsis. Plant J. 20, 493–501. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dynan, W.S., Jendrisak, J.J., Hager, D.A., and Burgess, R.R. (1981). Purification and characterization of wheat germ DNA topoisomerase I (nicking-closing enzyme). J. Biol. Chem. 256, 5860–5865. [PubMed] [Google Scholar]
- Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Greyson, R.I., and Walden, D.B. (1972). The ABPHYL syndrome in Zea mays. I. Arrangement, number and size of leaves. Am. J. Bot. 45, 466–472. [Google Scholar]
- Jackson, D., and Hake, S. (1999). Control of phyllotaxy in maize by the abphyl1 gene. Development 126, 315–323. [DOI] [PubMed] [Google Scholar]
- Jean, R.V. (1994). Phyllotaxis: A Systemic Study in Plant Morphogenesis. (New York: Cambridge University Press).
- Kaya, H., Shibahara, K.I., Taoka, K.I., Iwabuchi, M., Stillman, B., and Araki, T. (2001). FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104, 131–142. [DOI] [PubMed] [Google Scholar]
- Kieber, J.J., Tissier, A.F., and Signer, E.R. (1992). Cloning and characterization of an Arabidopsis thaliana topoisomerase I gene. Plant Physiol. 99, 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny, A., and Ausubel, F.M. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4, 403–410. [DOI] [PubMed] [Google Scholar]
- Laufs, P., Dockx, J., Kronenberger, J., and Traas, J. (1998). MGOUN1 and MGOUN2: Two genes required for primordium initiation at the shoot apical and floral meristems in Arabidopsis thaliana. Development 125, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Lee, M.P., Brown, S.D., Chen, A., and Hsieh, T. (1993). DNA topoisomerase I is essential in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 90, 6656–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser, H.M.O., and Furner, I.J. (1992). Characterization of three shoot apical meristem mutants of Arabidopsis thaliana. Development 116, 397–403. [Google Scholar]
- Lyndon, R.F. (1998). The Shoot Apical Meristem: Its Growth and Development. (Cambridge, UK: Cambridge University Press).
- Matsuhara, S., Jingu, F., Takahashi, T., and Komeda, Y. (2000). Heat-shock tagging: A simple method for expression and isolation of plant genome DNA flanked by T-DNA insertions. Plant J. 22, 79–86. [DOI] [PubMed] [Google Scholar]
- Medford, J.I., Behringer, F.J., Callos, J.D., and Feldmann, K.A. (1992). Normal and abnormal development in the Arabidopsis vegetative shoot apex. Plant Cell 4, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino, A., Madden, K.R., Lane, W.S., Champoux, J.J., and Reinberg, D. (1993). DNA topoisomerase I is involved in both repression and activation of transcription. Nature 365, 227–232. [DOI] [PubMed] [Google Scholar]
- Morham, S.G., Kluckman, K.D., Voulomanos, N., and Smithies, O. (1996). Targeted disruption of the mouse topoisomerase I gene by camptothecin selection. Mol. Cell. Biol. 16, 6804–6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nitiss, J., and Wang, J.C. (1988). DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc. Natl. Acad. Sci. USA 85, 7501–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, X.H., Reddy, M.K., Ehtesham, N.Z., Matta, B., and Tuteja, N. (2000). A DNA helicase from Pisum sativum is homologous to translation initiation factor and stimulates topoisomerase I activity. Plant J. 24, 219–229. [DOI] [PubMed] [Google Scholar]
- Prigge, M.J., and Wagner, D.R. (2001). The Arabidopsis SERRATE gene encodes a zinc-finger protein required for normal shoot development. Plant Cell 13, 1263–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, M.K., Nair, S., and Tewari, K.K. (1998). Cloning, expression and characterization of a gene which encodes a topoisomerase I with positive supercoiling activity in pea. Plant Mol. Biol. 37, 773–784. [DOI] [PubMed] [Google Scholar]
- Reinhardt, D., Mandel, T., and Kuhlemeier, C. (2000). Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaiu, W.L., and Hsieh, T.S. (1998). Targeting to transcriptionally active loci by the hydrophilic N-terminal domain of Drosophila DNA topoisomerase I. Mol. Cell. Biol. 18, 4358–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shykind, B.M., Kim, J., Stewart, L., Champoux, J.J., and Sharp, P.A. (1997). Topoisomerase I enhances TFIID-TFIIA complex assembly during activation of transcription. Genes Dev. 11, 397–407. [DOI] [PubMed] [Google Scholar]
- Steeves, T.A., and Sussex, I.M. (1989). Patterns in Plant Development, 2nd ed. (New York: Cambridge University Press).
- Stewart, L., Ireton, G.C., and Champoux, J.J. (1996. a). The domain or-ganization of human topoisomerase I. J. Biol. Chem. 271, 7602–7608. [DOI] [PubMed] [Google Scholar]
- Stewart, L., Ireton, G.C., Parker, L.H., Madden, K.R., and Champoux, J.J. (1996. b). Biochemical and biophysical analyses of recombinant forms of human topoisomerase I. J. Biol. Chem. 271, 7593–7601. [DOI] [PubMed] [Google Scholar]
- Takahashi, T., Gasch, A., Nishizawa, N., and Chua, N.-H. (1995). The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes Dev. 9, 97–107. [DOI] [PubMed] [Google Scholar]
- Thrash, C., Voelkel, K., DiNardo, S., and Sternglanz, R. (1984). Identification of Saccharomyces cerevisiae mutants deficient in DNA topoisomerase I activity. J. Biol. Chem. 259, 1375–1377. [PubMed] [Google Scholar]
- Tian, L., and Chen, Z.J. (2001). Blocking histone deacetylation in Arabidopsis induces pleiotropic effects on plant gene regulation and development. Proc. Natl. Acad. Sci. USA 98, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura, T., and Yanagida, M. (1984). Isolation of type I and II DNA topoisomerase mutants from fission yeast: Single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 3, 1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbsky, M.L., and Richards, E.J. (2001). Chromatin remodeling in plants. Curr. Opin. Plant Biol. 4, 494–500. [DOI] [PubMed] [Google Scholar]
- Vernoux, T., Kronenberger, J., Grandjean, O., Laufs, P., and Traas, J. (2000). PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127, 5157–5165. [DOI] [PubMed] [Google Scholar]
- Voinnet, O., Vain, P., Angell, S., and Baulcombe, D.C. (1998). Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95, 177–187. [DOI] [PubMed] [Google Scholar]
- Wang, J.C. (1996). DNA topoisomerases. Annu. Rev. Biochem. 65, 635–692. [DOI] [PubMed] [Google Scholar]
- Waterhouse, P.M., Graham, M.W., and Wang, M.B. (1998). Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc. Natl. Acad. Sci. USA 95, 13959–13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C.X., Chen, A.D., Gettel, N.J., and Hsieh, T. (2000). Essential functions of DNA topoisomerase I in Drosophila melanogaster. Dev. Biol. 222, 27–40. [DOI] [PubMed] [Google Scholar]