Abstract
The proapoptotic gene bax is one of the downstream effectors of p53. The p53 binding site in the bax promoter is less responsive to p53 than the one in the growth arrest mediating gene p21. We introduced the bax gene under the control of 13 copies of a strong p53 responsive element into two ovarian cancer cell lines. The clones expressing bax under the control of p53 obtained from the wild-type (wt) p53-expressing cell line A2780 were much more sensitive (500- to 1000-fold) to the anticancer agent taxol than the parent cell line, with a higher percentage of cells undergoing apoptosis after drug treatment that was clearly p53-dependent and bax-mediated. Xenografts established in nude mice from one selected clone (A2780/C3) were more responsive to taxol than the parental line and the apoptotic response of A2780/C3 tumors was also increased after treatment. Introduction of the same plasmid into the p53 null SKOV3 cell line did not alter the sensitivity to taxol or the induction of apoptosis. In conclusion, driving the p53 response (after taxol treatment) by activating the bax gene rather than the p21 gene results in induction of massive apoptosis, in vitro and in vivo, and greatly enhances sensitivity to the drug.
Keywords: apoptosis, p53, anticancer agents, tumor xenografts, transcription
Introduction
The product of the tumor suppressor gene p53 is a key protein with multiple functions inside the cells. The majority of the functions of p53 are related to its ability to bind DNA and activate the transcription of different genes [1,2]. After treatment with different damaging agents, the p53, which normally has a short half-life, is stabilized with an increase in its levels [3] and is activated inducing the transcription of downstream genes. Many important genes are activated by p53 including WAF1, GADD45, mdm2 and bax. Schematically, depending on the type and extent of the damage and the cell type, the increase in p53 causes cell cycle arrest (in either G1 or G2) or apoptosis [4–6]. Although the cell cycle arrest is thought to be mainly mediated by p21WAF1, a potent inhibitor of cyclin-dependent kinases (cdks) [7,8], the apoptosis induced by p53 seems to involve the activation of different genes, the proapoptotic gene bax being one — although not the only one — of the key players [9,10]. In some cell lines the cell cycle arrest induced by p21 has a protective effect to the treatment of anticancer agents [6,11–14].
Introduction of the proapoptotic gene bax under the control of exogenous promoters into cancer cells increases apoptosis and the cellular response to anticancer drugs [15–18]. The possibility of driving the action of p53 by activating bax rather than the p21 gene should therefore increase the cancer cell's susceptibility to drug action. The p53 responsive element in the p21 promoter is much more potent in activating its transcription than the one in the bax promoter (Hardy-Bessard AC and Soussi T, personal communication) and this is probably one reason for the lower levels of bax than p21 observed in some wild-type (wt) p53-expressing cancer cell lines after drug treatment [19,20].
We here report that introducing into a human ovarian cancer cell line expressing wt p53 (A2780) the bax gene under the control of 13 copies of a strong p53 responsive element, results in massive induction of apoptosis and increases sensitivity to taxol. The same plasmid introduced into a human ovarian cancer cell line not expressing p53 did not change the sensitivity to taxol, indicating that the bax-induced apoptotic effect in the A2780 transfected cells is p53-dependent.
Materials and Methods
Cells and Constructs
The human ovarian cancer cell lines A2780 and SKOV3, expressing respectively wt p53 and no p53, were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS).
To construct the plasmid containing the bax gene under the control of 13 copies of a strong p53 responsive element, the HindIII/EcoRI fragment of PG13luc (kindly supplied by Dr. B. Vogelstein) was inserted in the HindIII/EcoRI digested pBluescript II SK. This construct was digested with SmaI/XbaI and ligated with the SmaI/XbaI fragment of pUHD10.3Bax (kindly supplied by Dr. S. Bergmann) to generate pSK13BAX. The correct insertion was verified by DNA sequencing.
For stable transfection A2780 and SKOV3 cells were seeded 24 hours before transfection. Cells were cotransfected with the pSK13BAX and pSV2neo plasmids using the calcium phosphate precipitation method. pSV2neo contained a neomycin-resistant gene that allowed neomycin-resistant colonies to be selected approximately 14 days after transfection in G418-supplemented (500 µg/ml) medium.
Evaluation of Taxol-Induced Cytotoxicity
Cytotoxicity was evaluated by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay in 96-well plates (Nunc) at different times after treatment with different concentrations of taxol (obtained from Bristol-Meyers, dissolved in DMSO at a concentration of 1 mM and stored at -20°C). Drug concentrations inhibiting the growth by 50% (IC50) were calculated after 72 hours of recovery in drug-free medium after 24 hours taxol treatment.
Evaluation of Apoptosis
To detect apoptotic cells, cells were seeded on glass coverslips in 24-well plates (25 000 cells/ml) and treated with the drug at different concentrations. After 24, 48 and 72 hours, attached cells were fixed in 70% ethanol, air dried and stained with DAPI (4′,6-diamino-2-phenylindole) and sulphorhodamine B [19]. Percentage of cells with characteristic apoptotic morphology was determined by counting different fields, each consisting of at least 100 cells.
Western Blotting Analysis
Total cell extracts were prepared from untreated or treated cells at different times after drug exposure, according to standard procedures [21]. One hundred micrograms of proteins for each sample were electrophoresed through 12% polyacrylamide-SDS gels and electroblotted onto nitrocellulose membrane (Schleicher and Schull, Germany) in transfer buffer (50 mM Tris, 100 mM glycine, 0.01% SDS, 20% methanol) for 2 hours at 50 V. Filters were stained with Ponceau red, hybridized with monoclonal antibody against p53 (clone DO-1) or polyclonal antibodies against bax and p21 (Santa Cruz Biotechnology) and detected with the ECL (enhanced chemiluminiscence) system (Amersham). The experiments were repeated at least twice in all cell lines.
Tumor Transplantation and Drug Treatment
A2780 and A2780/C3 were grown subcutaneously (s.c.) in nude mice (female NCr-nu/nu, Animal Production Colony, NCI-FCRC, Frederick, MD) for one passage to unify their growth in vivo. Two 3-mm tumor fragments were then implanted s.c. in the flank of nude mice and treatment (six randomized mice per group) started when the tumor reached approximately 300 mg in size. The tumor diameters were measured every 3 days with a caliper and the weights in milligrams were calculated as [length x (width)2/2]. Taxol (dissolved in Chremophor EL) was given intravenously every 4 days for three injections at doses of 3, 6 or 12 mg/kg. Changes in tumor weight from the start of treatment (Wo) until the value at any given time (Wt) were calculated for each tumor and for each day of measurement and expressed as relative tumor weights (RTW=Wt/Wo). The optimal growth inhibition is defined as the lowest ratio of RTW of treated over control tumorsx100. With a T/C≤50% the treatment is considered active [22].
Three mice per group were killed 24 hours after treatment, the tumor was removed and immediately deep frozen for Southern blotting analysis and for TUNEL staining, which was performed on tissue sections using an in situ cell-death detection kit (Boehringer, Italy) following the manufacturer's instructions. Stained, apoptotic cells were visualized by fluorescence microscopy.
Results
In Vitro Cytotoxicity Induced by Taxol
After transfection with the pSK13BAX plasmid, different clones were obtained from A2780 and SKOV3 cells.
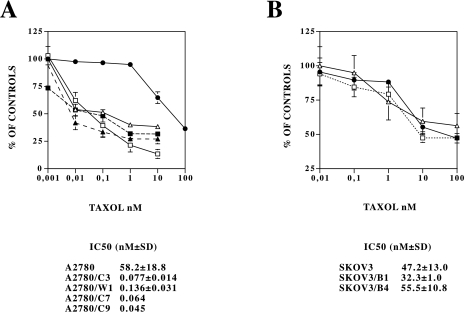
We first analyzed taxol-induced cytotoxicity, using the MTT assay after 24 hours treatment with different taxol concentrations (Figure 1A). In A2780 parental cells, cell number was significantly reduced only at taxol concentrations greater than 10 nM, whereas in the clones obtained from A2780 (A2780/C3, A2780/W1, A2780/C7 and A2780/C9) 0.01 nM of taxol already had significant activity. The taxol IC50 values, calculated from three independent experiments were 58.2±18.8 nM for A2780 cells and 0.077±0.014, 0.136±0.031, 0.064 and 0.045 nM respectively for the three clones A2780/C3, W1, C7 and C9. In contrast, (Figure 1, panel B) taxol had similar activity in the SKOV3 cell line (not expressing p53) and in the SKOV3 clones (B1 and B4) obtained after transfection with the same p53-dependent, bax expressing plasmid. For further characterization, two clones (A2780/C3 and SKOV3/B1) were selected.
Figure 1.
Dose-dependent growth inhibition, measured by the MTT assay, induced by taxol in A2780 (•), A2780/C3 (□), A2780/W1 (▵), A2780/C7 (▴) and A2780/C9 (▪) cells (panel A) and SKOV3 (•), SKOV3/B1 (□) and SKOV3/B4 (▵) cells (panel B) treated with different taxol-concentrations for 24 hours. The IC50 values are reported for each clone as the mean±SD of at least three independent experiments except for A2780/C7 and A2780/C9 where the IC50 is the mean of two experiments.
Induction of Apoptosis and Expression of Bax
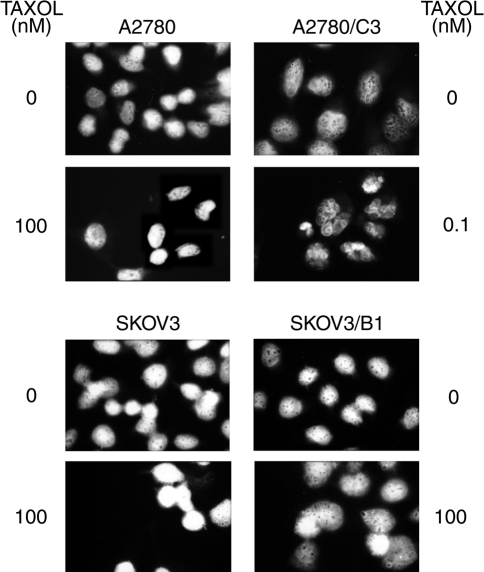
Microscopic examination of cells (after DAPI-sulphorhodamine staining) treated for 24 hours with different concentrations of taxol (Figure 2) revealed the typical morphology of apoptosis in A2780/C3 cells even at taxol concentrations as low as 0.1 nM, whereas in the parental A2780 cell line no significant apoptosis was evident even at 1000 times higher drug concentrations. The percentage of apoptotic cells in untreated A2780 cells was 2.0±1.4% and slightly increased to 13.1±8.5% 24 hours after treatment with 100 nM of taxol. By contrast, in A2780/C3 cells the percentage of cells undergoing apoptosis strongly increased from 2.8±2.4% (in untreated cells) to 38.7±1.7% 24 hours after treatment with a dose as low as 0.1 nM of taxol. In SKOV3 cells and in the clone SKOV3/B1 treated for 24 hours with different taxol concentrations, there were no differences between the two cell lines and no evidence of apoptosis was found even at the highest taxol concentration (100 nM). In this experimental system the percentage of cells with apoptotic morphology was always lower than 1%.
Figure 2.
DAPI-sulphorhodamine staining of A2780, A2780/C3 (upper panel), SKOV3 and SKOV3/B1 cells (lower panel) treated for 24 hours with different taxol concentrations, as indicated.
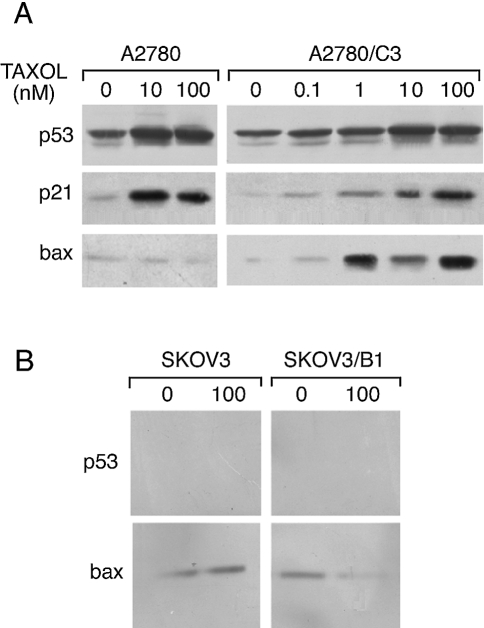
Western blot analysis of p53 and bax expression in A2780, A2780/C3, SKOV3 and SKOV3/B1 cells (Figure 3, A and B) demonstrated that both A2780-derived cell lines showed an increase in the levels of p53 after treatment with taxol. bax, however, was almost undetectable in the parental A2780 cells, whereas in the A2780/C3 cells the levels of this protein were strongly induced at all the taxol concentrations tested (panel A). The selective induction of bax in A2780/C3 but not in A2780 cells was already evident 6 hours after taxol treatment, whereas at 3 hours we did not detect any p53 induction and consequently any bax induction in both cell lines (data not shown). In these treatment conditions, p21 induction was similar in A2780 and A2780/C3 cells.
Figure 3.
Western blotting analysis of p53, bax and p21 expression in A2780 and A2780/C3 (panel A), SKOV3 and SKOV3/B1 (panel B) cells treated for 24 hours with different taxol concentrations, as indicated.
In SKOV3 and SKOV3/B1 cells (panel B) p53 was, as expected, undetectable and the levels of bax in the parental SKOV3 cell line were not different from those in SKOV3/B1 cells and did not change after taxol treatment in both lines.
In Vivo Activity and Induction of Apoptosis by Taxol in p53-Dependent Bax-Expressing Clones
Both A2780 and A2780/C3 cells were transplantable in nude mice giving tumors in all the implanted animals and by Southern blot analysis we could confirm the presence of pSK13BAX in the A2780/C3 clone growing in vivo in nude mice.
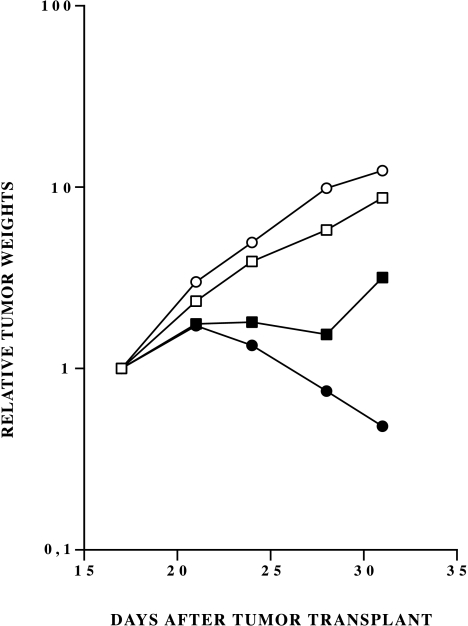
In vehicle-treated mice, A2780 and A2780/C3 tumors grew at similar rates (reaching 1 g tumor weight in 23 and 24 days, respectively). The activity of taxol was clearly dose-dependent in the A2780/C3 tumors treated with the optimal schedule of one i.v. injection every four days for three times. The highest tested dose of 12 mg/kg gave a significant growth inhibition (T/C 4%) with 50% of tumors in partial regression (greater than 50% reduction in tumor mass) (Figure 4). At day 31, mean tumor weights in control and treated groups were 2.2±0.57 and 0.30±0.11 g respectively.
Figure 4.
Tumor growth in nude mice implanted with A2780 (▪, □) or A2780/C3 (•, ○) cells and treated with vehicle (□, ○) or 12 mg/kg (▪, •) of taxol every four days for three times.
The dose of 6 mg/kg caused delay of tumor growth with a T/C of 24%, whereas the dose of 3 mg/kg showed a borderline efficacy (T/C=48%) (data not shown).
On A2780 parental tumors, taxol caused a moderate inhibition (T/C=27%) at the highest dose of 12 mg/kg (Figure 4) and it was not active (T/C-55%) at 6 mg/kg (data not shown).
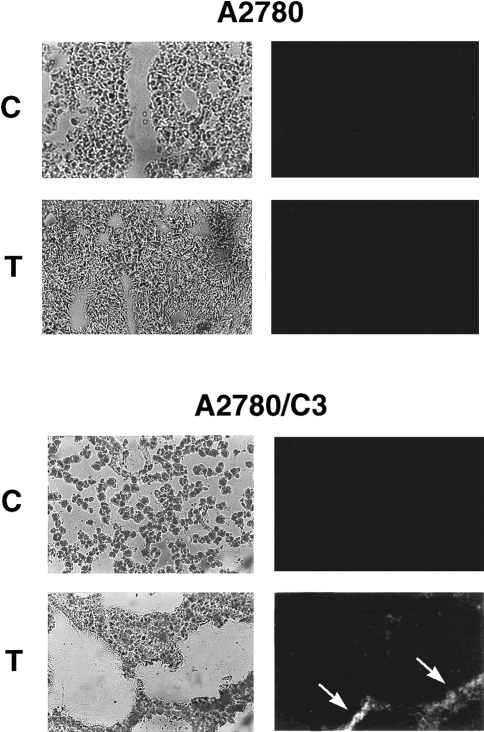
Direct evidence of in vivo taxol-induced apoptosis was obtained by TUNEL analysis of tumor tissues obtained 24 hours after a single treatment with 12 mg/kg of taxol: staining was seen only in A2780/C3 tumors (but not in A2780 tumors) treated with taxol (Figure 5). Positive TUNEL staining was also observable in A2780/C3 tumors treated with 6 mg/kg of taxol (data not shown).
Figure 5.
TUNEL staining of A2780 and A2780/C3 tumor sections obtained 24 hours after treatment with vehicle (C) or 12 mg/kg of taxol (T). Photomicrographs of phase contrast (left panels) and the corresponding TUNEL fluorescence (right panels) are reported. Arrows indicate areas of fluorescence (white) corresponding to apoptotic cells.
Discussion
p53 is an important determinant of cancer-cell susceptibility to drug treatment, although its role as prognostic factor is still controversial. Its activation, mainly through posttranscriptional stabilization, can lead to cell cycle arrest or to apoptosis. The cellular context and the extent and type of damage induced dictate whether cell death or cell cycle arrest will happen [4–6]. In some cell types the cell damage dependent p53 induction leads mainly to cell cycle arrest [11,19]. This is particularly true for ovarian cancer cells where p53-dependent apoptosis is often very limited after drug treatment [19,20] with one of the reasons being the high p53-dependent expression of p21 but not of bax. The low p53-dependent transcription of bax might partly be explained by the imperfect p53 binding site in the promoter of bax [23] and in fact this promoter is less responsive to p53 than the p21 promoter (Hardy-Bessard, AC and Soussi, T., personal communication). The present study was aimed at elucidating whether in a human ovarian cancer cell line expressing wt p53 (A2780) the forced p53-dependent expression of bax could direct the response toward cell death rather than cell cycle arrest and whether this shift was accompanied by an increased sensitivity to taxol. bax plays an important role in determining the sensitivity to drugs in cells overexpressing this protein and, in general, an increase in bax levels is associated with a greater induction of cell death after DNA damage [16,17,24–26]. In these systems, however, the high bax levels were not a consequence of drug treatment, but were already present in untreated cells. The present study employed a system in which bax can only be activated by p53 as a consequence of damage in the cell, as clearly demonstrated in the SKOV3/B1 cell line not expressing p53, where the construct does not respond to drug-induced damage, but fully responds to cotransfection with a wt p53 expressing plasmid (data not shown).
We selected taxol for two reasons: first because it is one of the most effective drugs in the treatment of ovarian cancer [27], and second because it is particularly sensitive to the presence of wt p53 and in cells (including A2780) where the induction of p53-dependent cell cycle arrest is predominant, an increase in sensitivity to taxol is observed after disruption of p53 [11,12]. The striking increase in sensitivity to taxol in the A2780 transfected clones strongly supports findings obtained so far and stress the importance of the cell “decision” to arrest cell cycle or to undergo apoptosis in response to damage. That the balance between p21 and bax is decisive for cellular response was indirectly shown in wt p53, p21 -/- cells, which were more susceptible to DNA damage in vitro or in vivo than the corresponding wt p53, p21 +/+ cells [6,14]. The mechanism by which bax promotes apoptosis in these cells is not clear. A release of cytochrome c from mitocondria was observed either after short or long taxol treatment in A2780/C3 cells but not in A2780 parental cells (data not shown) and this could be important for the induction of apoptosis and confirmed the bax-dependent release of cytochrome c from mitochondria previously described in other systems [18,28]. This mechanism, and the consequent plausible activation of caspases, is under study in these cell systems.
The results in nude mice bearing p53-dependent, bax-transfected tumor support the hypothesis that p53-induced apoptosis can indeed increase human tumor sensitivity to chemotherapy. It is worth noting that a significant response of A2780/C3 xenografts was obtained on advanced stage tumors (treatment starting on 300 mg tumors) and at doses of taxol lower than those described as optimal in similar tumor models [29]. The clear demonstration in these cellular systems that the increase in apoptosis is p53-dependent and bax-mediated supports bax as one of the mediators of p53-induced apoptosis in vitro and in vivo, and open up the possibility to study possible modifications of p53 structure which could preferentially activate bax rather than p21. This possibility is not remote as changes in phosphorylation of p53 could indeed influence its transcriptional activity [30–32]. In addition, DNA damage-induced phosphorylation of the p53 N-terminus has been reported [33,34] and studies in cells with selective alterations of putative kinases responsible for these phosphorylations (DNA-PK, CK, ATM and so on) could help in clarifying whether these posttranslational modifications are also important for p53 to decide which downstream effector has to be mainly activated. Although at present entirely speculative, it would also be important to understand whether kinase-specific inhibitors/activators could be used as p53 drivers. Moreover, induction of conformational changes in p53 mutants (which would allow specific gene transcriptional activation, i.e., bax and not p21) would be an interesting area of research in which to seek highly selective anticancer drugs.
Acknowledgments
This work was partially supported by the project No. ICS120/RF98/73 of the Italian Ministry of Health and by the “CNR progetto strategico apoptosi No. 96.01026.ST74”. The generous contribution of the Italian Association for Cancer Research (AIRC) and Italian Foundation for Cancer Research (FIRC) is gratefully acknowledged. Paola De Feudis is a “Famiglie Belloni e Guglielmetti” fellow.
References
- 1.Ko LJ, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;56:2649–2654. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 2.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 3.Oren M, Prives C. p53: upstream, downstream and off stream. Review of the 8th p53 workshop (Dundee, July 5–9, 1996) Biochim Biophys Acta. 1996;1288:R13–R19. doi: 10.1016/s0304-419x(96)00030-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Ko LJ, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 5.Bates S, Vousden KH. p53 in signaling checkpoint arrest or apoptosis. Curr Opin Genet Dev. 1996;6:12–18. doi: 10.1016/s0959-437x(96)90004-0. [DOI] [PubMed] [Google Scholar]
- 6.Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J. Cell-cycle arrest versus cell death in cancer therapy [see comments] Nat Med. 1997;3:1034–1036. doi: 10.1038/nm0997-1034. [DOI] [PubMed] [Google Scholar]
- 7.el Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 8.Harper JW, Adamy GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 9.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 10.Lill NL, Grossman SR, Ginsberg D, DeCaprio J, Livingston DM. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 11.Vikhanskaya F, Vignati S, Beccaglia P, Ottoboni C, Russo P, D'Incalci M, Broggini M. Inactivation of p53 in a human ovarian cancer cell line increases the sensitivity to paclitaxel by inducing G2/M arrest and apoptosis. Exp Cell Res. 1998;241:96–101. doi: 10.1006/excr.1998.4018. [DOI] [PubMed] [Google Scholar]
- 12.Wahl AF, Donaldson KL, Fairchild C, Lee FY, Foster SA, Demers GW, Galloway DA. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat Med. 1996;2:72–79. doi: 10.1038/nm0196-72. [DOI] [PubMed] [Google Scholar]
- 13.Fan S, Smith ML, Rivet DJ, Duba D, Zhan Q, Kohn KW, Fornace AJJ, O'Connor PM. Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res. 1995;55:1649–1654. [PubMed] [Google Scholar]
- 14.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 15.Sakakura C, Sweeney EA, Shirahama T, Igarashi Y, Hakomori S, Nakatani H, Tsujimoto H, Imanishi T, Ohgaki M, Ohyama T, et al. Overexpression of bax sensitizes human breast cancer MCF-7 cells to radiation-induced apoptosis. Int J Cancer. 1996;67:101–105. doi: 10.1002/(SICI)1097-0215(19960703)67:1<101::AID-IJC17>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 17.Strobel T, Swanson L, Korsmeyer S, Cannistra SA. BAX enhances paclitaxel-induced apoptosis through a p53-independent pathway. Proc Natl Acad Sci USA. 1996;93:14094–14099. doi: 10.1073/pnas.93.24.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossè T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 19.Debernardis D, Sire EG, De Feudis P, Vikhanskaya F, Valenti M, Russo P, Parodi S, D'Incalci M, Broggini M. p53 status does not affect sensitivity of human ovarian cancer cell lines to paclitaxel. Cancer Res. 1997;57:870–874. [PubMed] [Google Scholar]
- 20.De Feudis P, Debernardis D, Beccaglia P, Valenti M, Graniela Siré EA, Arzani D, Stanzione S, Parodi S, D'Incalci M, Russo P, et al. DDP-induced cytotoxicity is not influenced by p53 in nine human ovarian cancer cell lines with different p53 status. Br J Cancer. 1997;76:474–479. doi: 10.1038/bjc.1997.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Boven E, Winograd B, Berger DP, Dumont MP, Braakhuis BJ, Fodstad O, Langdon S, Fiebig HH. Phase II preclinical drug screening in human tumor xenografts: a first European multicenter collaborative study. Cancer Res. 1992;52:5940–5947. [PubMed] [Google Scholar]
- 23.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 24.Krajewski S, Blomqvist C, Franssila K, Krajewska M, Wasenius V, Niskanen E, Nordling S, Reed JC. Reduced expression of proapoptotic gene bax is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995;55:4471–4478. [PubMed] [Google Scholar]
- 25.Simonian PL, Grillot DA, Andrews DW, Leber B, Nunez G. Bax homodimerization is not required for Bax to accelerate chemotherapy-induced cell death. J Biol Chem. 1996;271:32073–32077. doi: 10.1074/jbc.271.50.32073. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi T, Ruan S, Clodi K, Kliche OK, Shiku H, Andreef M, Zhang W. Overexpression of bax gene sensitizes K562 erythroleukemia cells to apoptosis induced by selective chemotherapeutic agents. Oncogene. 1998;16:1587–1591. doi: 10.1038/sj.onc.1201681. [DOI] [PubMed] [Google Scholar]
- 27.Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Liu X, Bhalla K, Kim N, Ibrado AM, Cai J, Peng T, Jones DP, Wang X. Prevention of apoptosis by bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 29.Nicoletti MI, Lucchini V, Masazza G, Abbott BJ, D'Incalci M, Giavazzi R. Antitumor activity of taxol (NSC-125973) in human ovarian carcinomas growing in the peritoneal cavity of nude mice. Ann Oncol. 1993;4:151–155. doi: 10.1093/oxfordjournals.annonc.a058419. [DOI] [PubMed] [Google Scholar]
- 30.Adler V, Pincus MR, Minamoto T, Fuchs SY, Bluth MJ, Brandt Rauf PW, Friedman FK, Robinson RC, Chen JM, Wang XW, et al. Conformation-dependent phosphorylation of p53. Proc Natl Acad Sci USA. 1997;94:1686–1691. doi: 10.1073/pnas.94.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinke V, Lozano G. Differential activation of p53 targets in cells treated with ultraviolet radiation that undergo both apoptosis and growth arrest. Radiat Res. 1997;148:115–122. [PubMed] [Google Scholar]
- 32.Lohrum M, Scheidtmann KH. Differential effects of phosphorylation of rat p53 on transactivation of promoters derived from different p53 responsive genes. Oncogene. 1996;13:2527–2539. [PubMed] [Google Scholar]
- 33.Siciliano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shieh S-Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]