Abstract
We hypothesized that the requirement for Ca2+-dependent exocytosis in cell-membrane repair is to provide an adequate lowering of membrane tension to permit membrane resealing. We used laser tweezers to form membrane tethers and measured the force of those tethers to estimate the membrane tension of Swiss 3T3 fibroblasts after membrane disruption and during resealing. These measurements show that, for fibroblasts wounded in normal Ca2+ Ringer's solution, the membrane tension decreased dramatically after the wounding and resealing coincided with a decrease of ∼60% of control tether force values. However, the tension did not decrease if cells were wounded in a low Ca2+ Ringer's solution that inhibited both membrane resealing and exocytosis. When cells were wounded twice in normal Ca2+ Ringer's solution, decreases in tension at the second wound were 2.3 times faster than at the first wound, correlating well with twofold faster resealing rates for repeated wounds. The facilitated resealing to a second wound requires a new vesicle pool, which is generated via a protein kinase C (PKC)-dependent and brefeldin A (BFA)-sensitive process. Tension decrease at the second wound was slowed or inhibited by PKC inhibitor or BFA. Lowering membrane tension by cytochalasin D treatment could substitute for exocytosis and could restore membrane resealing in low Ca2+ Ringer's solution.
INTRODUCTION
Disruptions of plasma membranes are widespread, common, and normal events in many animal tissues, and cells survive these disruptions by restoring the integrity of the cell membrane (McNeil and Steinhardt, 1997). The repair of a disrupted cell membrane requires that the lipid bilayer be resealed. This had been thought to be a passive process in which the removal of hydrophobic domains of phospholipid molecules from the aqueous environment was a spontaneous energetically favored event. However, it has been demonstrated recently that cell-membrane repair is an active process that requires Ca2+-dependent exocytosis. The disruption of the plasma membrane evokes a Ca2+-dependent exocytosis that utilizes vesicle docking/fusion SNARE proteins, and this exocytotic response has been shown to be essential for rapid cell-membrane repair in invertebrate embryos and mammalian cells (Steinhardt et al., 1994; Bi et al., 1995; Miyake and McNeil, 1995; Bi et al., 1997; Togo et al., 1999). In a previous study, we also found that the rate of membrane resealing with repeated wounds is facilitated and that this response is dependent on a new vesicle pool generated via a protein kinase C (PKC)-dependent and brefeldin A (BFA)-sensitive process (Togo et al., 1999).
The resealing of artificial lipid bilayers has been studied extensively by using liposomes. These studies have established that pores in lipid bilayers will close rapidly if membrane tension is low. With increased membrane tension, the rate of resealing will be slowed, and, for large enough tensions, pores can grow and lyse the liposome (Taupin et al., 1975; Zhelev and Needham, 1993; Moroz and Nelson, 1997). Since the existence of a cortical cytoskeleton in cells greatly increases membrane tension compared with simple lipid bilayers (Sheetz and Dai, 1996; Dai and Sheetz, 1999), cells may have to decrease membrane tension to successfully close a membrane disruption. It has been shown that stimulation by Ca2+-dependent exocytosis decreases membrane tension (Dai et al., 1997). It has been shown also that other treatments that expand membrane area will lower tension (Raucher and Sheetz, 2000) and facilitate membrane resealing (Togo et al., 1999). Therefore, we hypothesized that the requirement for Ca2+-dependent exocytosis in cell-membrane repair is to provide an adequate lowering of membrane tension to allow resealing of the lipid bilayer.
The purpose of the present study was to evaluate the relationship between decreases in membrane tension and the ability of cell membranes to reseal. To study the change in apparent membrane tension during membrane resealing, membrane tethers were formed from Swiss 3T3 fibroblasts with IgG-coated beads held by laser tweezers, and the tether force was measured by the extent of displacement of the bead in the laser trap (Dai and Sheetz, 1998).
MATERIALS AND METHODS
Cell Preparation
Swiss 3T3 fibroblasts were cultured in DMEM (Life Technologies, Grand Island, NY) containing 8% fetal bovine serum (Atlanta Biologicals, Norcross, GA) and 50 μg/ml gentamicin (Life Technologies). Cells for experiments were plated on glass coverslip-inserted plastic dishes and were grown for 1 or 2 d before use. During experiments, the cells were maintained in Ringer's solution. Ca2+-free Ringer's solution contained 138 mM NaCl, 2.7 mM KCl, 1.06 mM MgCl2, 5.6 mM glucose, and 12.4 mM HEPES (pH 7.25). A stock solution of 100 mM CaCl2 was used to adjust the concentration of Ca2+. Normal Ringer's solution contained 1.8 mM Ca2+.
Laser Tweezers Manipulations
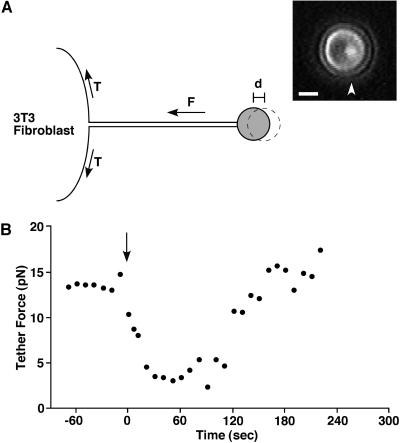
Fluoresbrite plain YG 2.0-micron microspheres (Polysciences, Warrington, PA) were coated with antimouse IgG (Sigma, St. Louis, MO) as described previously (Togo et al., 1999). The trapping beam from an 899 Ring CW titanium:sapphire laser (Coherent, Santa Clara, CA) at 820 nm, sent through an Axiovert 135 M inverted microscope (Zeiss, Thornwood, NY), was used to both trap the bead and to excite two-photon fluorescence at the center of the trap. To form a membrane tether from the 3T3 fibroblast, an IgG-coated bead was trapped with 60- or 80-mW laser power, measured at the objective, held on the cell surface for 2 s, and pulled away from the surface by moving the cell 4–5 μm to one side (Figure 1A). During experiments, the bead was held at least 2 μm above the coverslip to eliminate viscous coupling to the glass surface. The cell and bead were imaged by a CCD camera (model ZVS-47E, Optronics, Goleta, CA) and were recorded on videotape for later analysis. The tether method for estimating membrane tension can report changes continuously, allowing for the assessment of rapid changes.
Figure 1.
(A) Schematic drawing of a tether force measurement from a 3T3 fibroblast. An IgG-coated bead, held by the laser tweezers was attached to the plasma membrane, and a membrane tether was formed by moving the cell to one side. Tether force (F) can be estimated from the displacement of the bead (d) from the center of laser trap. T signifies the apparent membrane tension. The bright fluorescent spot (arrowhead) in the bead image shows the center of the laser trap. The distance between the fluorescent spot, which marks the center of the laser trap, and the geometric center of the spherical bead indicates the distance of bead displacement. Tether force squared is proportional to the apparent membrane tension. Bar = 1 μm. (B) Tether force changes after wounding in 1.8 mM Ca2+ Ringer's solution. The cell membrane was cut by laser scissors at 0 s (arrow).
Tether Force Calibration
The force on the bead can be calculated from the displacement of the bead from the center of the laser trap. To calibrate trap stiffness, a viscous force was generated by moving the microscope stage at a known velocity, and the distance of bead displacement was measured. The distance between the fluorescent spot, which marks the center of the laser trap, and the geometric center of the spherical bead indicates the distance of bead displacement (see Figure 1). The viscous force on the bead was calculated through Stokes' Law (Dai and Sheetz, 1998). The calibration showed a linear force-displacement relationship in the range used. In our system, a 0.1-μm displacement of the bead was equivalent to 3.34 pN and 4.49 pN, respectively, for 60 mW and 80 mW laser power measured at the objective.
Wounding Procedure
For tests of membrane resealing, cells were wounded by laser scissors or a glass needle. Laser wounding was performed by a frequency-doubled (532 nm) Q-switched Nd:YAG laser (SureLite I, Continuum, Santa Clara, CA) emitting 4- to 6-ns pulses. These pulses were focused just below the plasma membrane and delivered energy equal to 2–5 μJ per pulse. Mechanical wounding was performed using a 1-micron-tipped, solid glass needle controlled by a micromanipulator (model 5170, Eppendorf Scientific, Westbury, NY) and a microinjector (model 5242, Eppendorf Scientific), as described previously (Steinhardt et al., 1994; Togo et al., 1999). All wounding experiments were performed at 25°C.
FM 1–43 Destaining
3T3 cells were starved for 2 h in DMEM without serum and were incubated with fresh culture medium containing 20 μM FM 1–43 (Molecular Probes, Eugene, OR) for 30 min. Each dish was washed with 1.8 mM or 0.1 mM Ca2+ Ringer's solution just before the experiment. Image data acquisition was performed as described previously (Togo et al., 1999). Local fluorescent intensity from a circular region around the wounding site (5-μm diameter) was measured at 4-s intervals.
Estimation of Resealing Rate
The resealing rate was defined as the inverse of resealing time measured in seconds. For cells that failed to reseal, the rate was defined as zero. The resealing time was determined as follows. Fura-2 was loaded into the cells by AM ester loading as described previously (Togo et al., 1999). The cells were wounded with a glass needle, and Fura-2 fluorescence was monitored. A persistent decrease of the calcium-insensitive 357-nm excited fluorescent intensity (as an indicator of dye loss) together with a persistent increase of the ratio of fluorescent intensity excited by 385/357-nm light (as an indicator of increasing intracellular Ca2+ concentration) indicated resealing failure. A transient decrease of 357 nm excited fluorescent intensity indicated successful resealing. The interval between wounding and when the signal reached a constant value (stopped declining) was defined as the resealing time.
RESULTS
Membrane Tension of the Cell Decreases After Wounding
To estimate apparent membrane tension, we used the laser tweezers method (Dai and Sheetz, 1998). A membrane tether was formed by first placing an IgG-coated bead trapped by the laser tweezers on the surface of a Swiss 3T3 fibroblast and then pulling out a tether of attached plasma membrane a distance of 4–5 μm. The bead was held at least 2 μm above the coverslip to minimize viscous coupling to the glass surface during experiments. As illustrated in Figure 1A, apparent membrane tension can be estimated from the force exerted by the membrane tether on the bead in the trap. The displacement of the bead from the center of the laser trap was digitally analyzed to calculate the tether force. This force is dependent on both the membrane tension in the bilayer and the adhesion energy between the plasma membrane and the cortical cytoskeleton. The tether force squared is proportional to the apparent membrane tension (Dai and Sheetz, 1998).
For 3T3 cells in 1.8 mM Ca2+ (normal Ca2+) Ringer's solution, the static membrane tether force was 12.1 ± 0.6 pN (n = 13) before wounding (Table 1). After wounding the cells with either a laser scissors or a glass needle controlled by a programmable micromanipulator, similar responses were observed. A typical change in tether force is shown in Figure 1B. After the wounding, the tether force started to decrease at 4.3 ± 1.7 s (n = 9) after the membrane was cut, and the average rate of decrease in tether force was −0.3 ± 0.04 pN/s (Tables 1 and 2). Membrane resealing was completed, on average, 24.5 ± 2.5 s (n = 108) after an initial cell-membrane disruption (Togo et al., 1999). The average tether force 24 s after wounding was 4.7 ± 0.5 pN, a drop of ∼60% from the predisruption level. Tether force continued to decrease and reached minimum values (2.1 ± 0.5 pN, n = 13) at 32.8 ± 4.1 s (n = 9) (Tables 1 and 2). After the tether force reached minimum values, the force gradually increased and recovered initial static values at 78.3 ± 7.2 s after the wounding (n = 9) (Table 2).
Table 1.
Tether force values during membrane resealinga
| Condition | First
Wound
|
Second Wound
|
||||||
|---|---|---|---|---|---|---|---|---|
| Before wounding (pN) | Rate of decreaseb (pN/sec) | Minimum value (pN) | n | Before wounding (pN) | Rate of Decrease† (pN/sec) | Minimum value (pN) | n | |
| 1.8 mM Ca2+ Ringer's Solution | 12.1 ± 0.6 | −0.3 ± 0.04 | 2.1 ± 0.5 | 13 | 13.2 ± 1.3 | −0.7 ± 0.2 | 3.6 ± 0.7 | 8 |
| 0.1 mM Ca2+ Ringer's Solution | 10.6 ± 0.9 | −0.07 ± 0.01 | 7.5 ± 0.8 | 8 | — | — | — | — |
| 1μM Gö-6976 | 12.3 ± 0.4 | −0.3 ± 0.03 | 3.2 ± 0.3 | 5 | 11.7 ± 3.6 | −0.08 ± 0.04 | 7.6 ± 1.0 | 5 |
| 50 μM brefeldin A | 10.6 ± 1.1 | −0.4 ± 0.2 | 3.1 ± 0.6 | 4 | 14.4 ± 0.4 | −0.07 ± 0.04 | 9.8 ± 3.3 | 4 |
Values are given as mean ± SE, unless otherwise indicated.
The amplitude of tether force decrease was divided by the duration of tether force decrease.
Table 2.
Sequence of membrane events after woundinga
| Event | Time(s) |
|---|---|
| Duration of FM 1-43 destainingb | 3.4 ± 0.8 (n = 19) |
| Onset of tether force decrease | 4.3 ± 1.7 (n = 9) |
| Completion of membrane resealingb | 24.5 ± 2.5 (n = 108) |
| Tether force reaches minimum value | 32.8 ± 4.1 (n = 9) |
| Tether force recovers initial value | 78.3 ± 7.2 (n = 9) |
Values are mean ± SE.
The Onset of Exocytosis Precedes the Decrease in Membrane Tension
To observe exocytosis after wounding, the fluorescent dye FM 1–43 was transiently loaded by endocytosis and then cells were monitored for changes of fluorescent intensity after the wounding. The styryl dye FM 1–43 intercalates into the outer leaflet of lipid bilayers but cannot cross the bilayer, and FM 1–43 is much more fluorescent in hydrophobic than in hydrophilic environments (Angleson and Betz, 1997; Cochilla et al., 1999). When cells are incubated with the dye and later washed, the remaining dye in the plasma membrane rapidly diffuses away, leaving only dye that is trapped in the luminal leaflet of endocytosed vesicle membranes. Subsequent delivery of the labeled endosomes into the plasma membrane by exocytosis allows diffusion of the dye into the aqueous solution and results in a loss of cellular fluorescence.
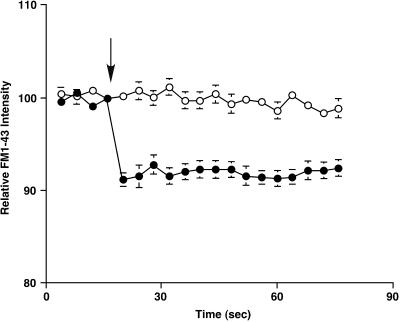
The cells were wounded by a glass needle, and the FM 1–43 fluorescent intensity near the wounding site (5-μm diameter) was measured at 4-s intervals. When cells were wounded in normal Ca2+ Ringer's solution, the relative fluorescent intensity around the wounding site dropped to 91.2 ± 0.7% of the initial value (n = 9) at 4 s after wounding, and then the destaining stopped (Figure 2). This result indicates that FM 1–43 destaining after the wound is a transient event. This is in agreement with our previous report, which showed that the FM 1–43 destaining started immediately after the wounding and that the duration of destaining was 3.4 ± 0.8 s (n = 19) (Togo et al., 1999) (Table 2). Therefore, FM 1–43 destaining precedes the onset of a decrease in tether force (Table 2). These results suggest that, after a cell-membrane wound, a process associated with exocytosis decreases membrane tension and is followed by subsequent endocytosis that leads to recovery of static values of tension within 2 min after the initial disruption.
Figure 2.
FM 1–43 destaining after wounds in 1.8 mM Ca2+ Ringer's solution (filled circles) and 0.1 mM Ca2+ Ringer's solution (open circles). The local fluorescent intensity around the disruption site was acquired at 4-s intervals. Each cell was wounded by a glass needle just after fourth image acquisition (arrow). Values are mean ± SE.
Low External Ca2+ Concentration Inhibits Both Decrease in Tension and Exocytosis
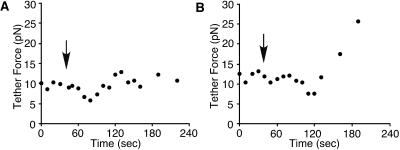
We next wounded the cells in 0.1 mM Ca2+ (low Ca2+) Ringer's solution and measured tether force (Figure 3). This level of Ca2+ is at or below the threshold required for membrane resealing (Steinhardt et al., 1994). The average static tether force before wounding was 10.6 ± 0.9 pN (n = 8), which was not significantly different from the static tether force in normal Ca2+ Ringer's solution (Student's t test, p = 0.1523). Tether force decreased slightly and slowly after wounding in low Ca2+ Ringer's (Figure 3A). The average rate of decrease was −0.07 ± 0.01 pN/s (n = 8) (Table 1), which was significantly slower than the rate of decrease in normal Ca2+ Ringer's solution (Student's t test, p = 0.0008). Tether force reached minimum values (7.5 ± 0.8 pN, n = 8) at 71.3 ± 14.7 s (n = 8) after the wounding, however, the minimum values were much higher than those in normal Ca2+ Ringer's solution (Student's t test, p < 0.0001). In these experiments, all cells wounded in low Ca2+ Ringer's solution appeared to be dead when inspected several minutes after wounding. The cells wounded in low Ca2+ Ringer's solution did not show FM 1–43 destaining (Figure 2). Therefore, the cells that failed to reseal in low Ca2+ Ringer's solution were inhibited both in the rate of decrease in membrane tension and in the rate of exocytosis, suggesting that the rapid decrease in apparent membrane tension after the wounding was initiated by exocytosis. Furthermore, these results in low Ca2+ Ringer's solution provide additional evidence that membrane disruption itself does not significantly decrease tether force.
Figure 3.
Tether force changes during wounding experiments in low Ca2+ Ringer's solution containing 0.1 mM Ca2+. The cells were wounded by a glass needle at the times indicated by the arrows. Changes of tether force were slow and small. A late abrupt increase of tether force was observed in four of eight cells (B).
For four of eight cells wounded in low Ca2+ Ringer's solution, an increase of tether force was observed 80.0 ± 14.1 s after the wounding (Figure 3B), suggesting that endocytosis was not inhibited at this Ca2+ concentration. The Ca2+ concentration required for endocytosis may be lower than that required for maximal exocytosis, as was observed previously in neurons (Marks and McMahon, 1998).
The Rate of Decrease in Membrane Tension Can Be Related to the Rate of Membrane Resealing
As reported previously, a repeated wound at the same site reseals more rapidly than the initial wound (Togo et al., 1999), and this facilitated response is inhibited by Gö-6976, a specific inhibitor of Ca2+-dependent PKC isozymes (Martiny-Baron et al., 1993), and by BFA, a fungal metabolite that inhibits the binding of ADP-ribosylation factor to the Golgi (Klausner et al., 1992). This inhibition of resealing and the results of our previous studies of FM 1–43 destaining suggest that facilitation of membrane resealing requires a new vesicle pool generated via a PKC-dependent and BFA-sensitive process (Togo et al., 1999). To investigate further the relationship between a decrease in apparent membrane tension and membrane resealing, we wounded cells twice and followed the changes of apparent membrane tension during double wounding.
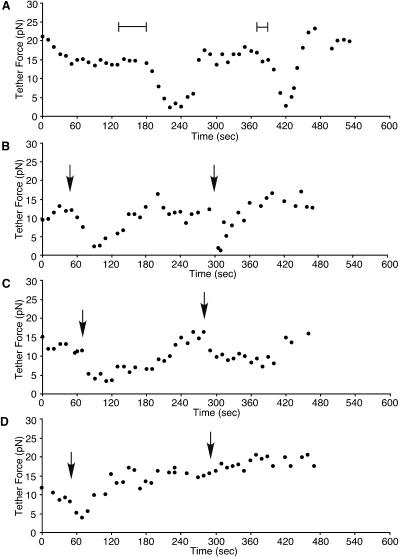
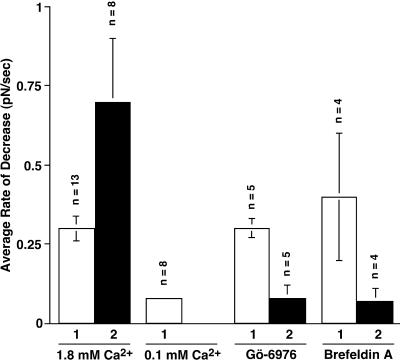
When the cells were wounded twice in normal Ca2+ Ringer's solution control experiments, decreases in tether force at the second wound were 2.3 times faster than the one at the initial wound (Figures 4A, B, and 5, Table 1). This result is consistent with our previous report, which showed membrane resealing after repeated wounding was two times faster than at the initial wound (Togo et al., 1999) and that faster membrane resealing is correlated with a faster decrease in tether force. In each case, the second wound was inflicted at a time when membrane tension had recovered to control values, and the facilitated response was not due to a residue of added membrane and to persistently low values of membrane tension.
Figure 4.
Tether force changes during double-wounding experiments. External Ca2+ concentrations were 1.8 mM. (A) The cell was wounded twice by laser scissors. Laser pulses were applied during the time indicated by bars. (B) The cell was wounded twice with a glass needle at the arrows. The same reaction was observed in both wounding methods. The cells were treated with 1 μM Gö-6976 (C) or 50 μM BFA (D) and were wounded twice by a glass needle. The arrows indicate the time of wounding.
We next treated the cells with either 1 μM Gö-6976 or 50 μM BFA to inhibit the facilitated response of membrane resealing and then wounded the cells twice. The average rate of decrease in tether force at an initial wound was −0.3 ± 0.03 pN/s (n = 5) and −0.4 ± 0.2 pN/s (n = 4), respectively, and there was no significant difference from previous control values (−0.3 ± 0.04 pN/s) (Table 1). Tether force reached 3.2 ± 0.3 pN at 38.0 ± 7.4 s in Gö-6976-treated cells and 3.1 ± 0.6 pN at 27.8 ± 8.4 s in BFA-treated cells. Therefore, decreases of tether force to the initial wound were not affected by these treatments. Although BFA is known to be involved in endosomal traffic (Klausner et al., 1992), it has been shown previously that the rate of membrane resealing also is not affected by these treatments (Togo et al., 1999). Furthermore, exocytosis after the initial wounding, as measured by FM 1–43 destaining, was not affected by BFA treatment. The average destaining of total fluorescence after the initial wound was 1.62%, which was not significantly different from control values (Togo et al., 1999).
As shown in Figure 4C, the recovery of tether force after the initial wounding for cells treated with 1 μM Gö-6976 was significantly slower (167.0 ± 23.3 s, n = 5) than for cells in normal Ca2+ Ringer's solution (Figure 4, A and B) (78.3 ± 7.2 s, n = 9). In contrast, BFA had no effect on the recovery of tether force (data not shown).
Contrary to the initial wounding, decreases in tether force to repeated wounds were inhibited or slowed in the presence of Gö-6976 (Figure 4C) or BFA (Figure 4D). The average rate of decrease in tether force at a second wounding was −0.08 ± 0.04 pN/s (n = 5) or −0.07 ± 0.04 pN/s (n = 4), respectively (Figure 5, Table 1). These values are almost identical with those in low Ca2+ Ringer's solution (−0.07 ± 0.01 pN/s). In a previous study, we showed that the membrane resealing was slowed or inhibited at repeated wounding by these reagents (Togo et al., 1999). Therefore, these results indicate that, at repeated wounds, both facilitated membrane resealing and the more rapid decrease of apparent membrane tension are dependent on a PKC activity and a BFA-sensitive process.
Figure 5.
The rate of decrease in the tether force after a first and second wound. The cells were wounded by laser scissors or with a glass needle. The amplitude of the tether force decrease was divided by the duration of the tether force decrease. Values are mean ± SE.
Cytochalasin D Can Substitute for Exocytosis in Exocytosis-inhibited Cells
If membrane resealing requires the lowering of membrane tension, one would predict that supplying lower cell-membrane tension by artificial means could effectively substitute for the active exocytotic response required for resealing. Apparent membrane tension can be decreased artificially by expanding the membrane area or by decreasing the adhesion energy between the plasma membrane and the cortical cytoskeleton. In a previous study, we found that artificial reduction of the membrane tension by a surfactant, pluronic F68 NF, or by cytochalasin D facilitated membrane resealing and restored membrane resealing even if exocytosis was inhibited (Togo et al., 1999). If exocytosis was specifically inhibited by tetanus toxin, resealing was nearly abolished. When Pluronic F68 NF was added, resealing was restored to the toxin-treated cells. Similarly, when cytochalasin D was present, exocytosis was greatly inhibited but the rate of resealing was facilitated, to a rate nearly twice as fast as in normal Ca2+ Ringer's solution. Both Pluronic F68 NF and cytochalasin D very significantly lowered apparent membrane tension (Togo et al., 1999). Cytochalasin D (20 μM) decreased tether force > 60% to the levels that we found here coincide with membrane resealing. Therefore, we predicted that cytochalasin D should restore resealing to cells that could not otherwise lower membrane tension when exocytosis was inhibited by low Ca2+ Ringer's solution (Figure 3). This prediction was confirmed. The results in Table 3 show that the rate of resealing in 0.1 mM Ca2+ Ringer's solution is increased 10-fold for cytochalasin D-treated cells and that the cell survival for these treated cells is close to control values for cells in 1.8 mM Ca2+ Ringer's solution. We conclude that lowering membrane tension is the critical contribution of the exocytotic response for cell-membrane repair.
Table 3.
Effect of cytochalasin D on membrane resealing in low Ca2+ Ringer's solution
| Condition | Resealing (%) | Resealing ratea | n |
|---|---|---|---|
| DMSO (control) | 25 | 0.003 ± 0.002 | 12 |
| 20 μM Cytochalasin D | 76.5 | 0.03 ± 0.007 | 17 |
Cells were treated with DMSO or 20 μM cytochalasin D for 1 h and wounded. Membrane sealing was monitored by photometric measurement of fura-2 fluorescence and visual inspection (see MATERIALS AND METHODS).
The resealing rate was defined as the inverse of the resealing time in seconds. For cells that failed to reseal, the rate was defined as zero. Values are mean ± SE.
DISCUSSION
Our results suggest that a decrease in membrane tension is required for membrane resealing. We found that membrane disruption induced a decrease in apparent membrane tension and that both membrane resealing and the decrease in membrane tension were inhibited in low Ca2+ (Figures 1 and 3). Our results here demonstrate that successful resealing is always preceded by a decrease in membrane tension. The rate of decrease in apparent membrane tension after the wounding can be related to the rate of membrane resealing. When cells were wounded twice, the rate of decrease in membrane tension at the second wounding was faster than at the initial wounding. However, the more rapid decrease in membrane tension at the second wounding was inhibited in the cells treated with Gö-6976 and BFA (Figure 5). These results are correlated with those of a previous study (Togo et al., 1999) that showed membrane resealing at repeated wounds was faster than at the initial wounds and that this facilitated membrane resealing was inhibited by these reagents. We also found that an artificial decrease in membrane tension by cytochalasin D could restore membrane resealing even in low Ca2+ (Table 3). Therefore, we concluded that decreases in membrane tension after the wounding were necessary and sufficient for membrane resealing.
We have previously shown that Ca2+-dependent exocytosis is required for the successful repair of disrupted plasma membrane. In sea urchin embryos, Botulinum neurotoxins A, B, and C1 and tetanus toxin inhibit both membrane repair and exocytosis at the sites of membrane disruption (Steinhardt et al., 1994; Bi et al., 1995). In Swiss 3T3 fibroblasts, Botulinum neurotoxins A and B and tetanus toxin inhibit membrane repair (Steinhardt et al., 1994; Togo et al., 1999), and tetanus toxin has been shown to inhibit exocytosis after the disruption of plasma membrane (Togo et al., 1999). Since each of these neurotoxins specifically proteolyses one of the SNARE proteins that are required for vesicle fusion (Schiavo et al., 1992; Blasi et al., 1993; Schiavo et al., 1993; Binz et al., 1994; Bi et al., 1995), vesicle fusion with the plasma membrane is apparently essential for normal membrane resealing.
Thus, a decrease in both membrane tension and vesicle fusion from exocytosis are required for membrane resealing. It has been shown that the expansion of membrane area by adding lipids can decrease membrane tension artificially (Raucher and Sheetz, 2000). Therefore, the simplest interpretation of our data is that exocytosis, which is stimulated by Ca2+ entry through the wound site, decreases the membrane tension by expanding the cell-surface area. Several lines of evidence support this interpretation. First is the relationship between the timing of exocytosis and a decrease in membrane tension after the membrane disruption. We found that wound-induced exocytosis, measured by FM 1–43 destaining, preceded the onset of the decrease in membrane tension (Table 2), and that low external Ca2+ inhibited both exocytosis and the decrease in membrane tension (Figures 2 and 3). Second, we found that an artificial decrease in membrane tension by adding a surfactant Pluronic F68 NF could restore membrane resealing even if exocytosis was inhibited by neurotoxins (Togo et al., 1999). Although Ca2+ enters into the cells through the disruption site and can activate various signaling pathways in addition to the step of membrane fusion, other Ca2+-dependent processes by themselves do not permit membrane resealing if vesicle fusion is blocked by neurotoxins (Steinhardt et al., 1994; Bi et al., 1995; Togo et al., 1999). Finally, the double-wounding experiments suggest that a decrease in the apparent membrane tension is dependent on the availability of a vesicle pool. When cells were wounded twice, the rate of decrease in apparent membrane tension at the repeated wounding was faster than at the initial wounding. However, the rapid decrease in apparent membrane tension at repeated wounding was inhibited in cells treated with Gö-6976 and BFA (Figure 4). Since the inhibition of membrane resealing at repeated wounding by these reagents implicates a new vesicle pool derived from the Golgi apparatus (Togo et al., 1999), the rapid decrease in apparent membrane tension appears to be related to the amount and the rate of membrane addition by exocytosis. Taken together, we conclude that wound-induced exocytosis is essential for membrane resealing because it lowers membrane tension by expanding membrane area. Since the membrane tension is largely determined by the membrane-cytoskeleton adhesion in living cells (Dai and Sheetz, 1999), we speculate that the insertion of new membrane, which is unattached to the cytoskeleton, decreases the adhesion energy between the cytoskeleton and plasma membrane.
In the present study, we monitored exocytosis during membrane resealing by measuring FM 1–43 fluorescence (Angleson and Betz, 1997; Cochilla et al., 1999) and showed that FM 1–43 destaining was a rapid and a transient event (Figure 2), whereas decreases in tether force continued after FM 1–43 destaining stopped (Table 2). Since FM 1–43 destaining can only monitor the fate of the prelabeled endocytotic compartment (Angleson and Betz, 1997; Cochilla et al., 1999), one possible explanation is that unlabeled vesicles continued to be exocytosed and contributed to the decrease in tether force. A good case for this interpretation can be made from studies of the increase in membrane capacitance from Ca2+-triggered exocytosis in CHO cells and NIH 3T3 fibroblasts. Using flash photolysis of caged-Ca2+ compounds in CHO cells and NIH 3T3 fibroblasts, it has been shown that membrane addition from Ca2+-dependent exocytosis increases capacitance rapidly during the first 20 s and then more slowly for an additional 30 s before capacitance peaks (Coorssen et al., 1996). These measurements of capacitance taken together with our results from FM 1–43 destaining suggest that FM 1–43 destaining only monitors the first few seconds of exocytosis. The total period of capacitative increase after the release of Ca2+ by photolysis in NIH 3T3 fibroblasts seems similar to our records of decreased apparent membrane tension after Ca2+ entry from wounds, but kinetics may differ because flash photolysis of caged-Ca2+ compounds should induce exocytosis at the entire surface of the cell (Coorssen et al., 1996). Capacitance measurements could not be applied to our system because it is impossible to measure capacitance when the cell membrane is disrupted.
In RBL cells, a decrease in tether force starts within 10 s after the stimulation by the antigen, but serotonin secretion is delayed (Dai et al., 1997). It has been known that serotonin secretion by an antigen occurs 30–60 s after the increase in intracellular Ca2+ caused by an antigen (Kim et al., 1997). The delay in serotonin secretion at first seems to imply that the drop in tension must have preceded membrane addition by exocytosis. However, capacitance measurements have shown that the expansion of membrane area starts immediately after an increase in intracellular Ca2+ concentration by flash photolysis of caged-Ca2+ compounds in RBL cells (Kasai, 1999). Therefore, the time course of membrane fusion events may be very similar to the time scale of the drop in tension in RBL cells. The capacitance data indicate that there are many nonserotonin vesicles the fusion of which is triggered without delay. The rate of decrease in membrane tension after antigen addition is very rapid and brief, just a few seconds, in RBL cells (Dai et al., 1997), whereas the decrease in membrane tension is relatively slow and continues for ∼30 s after a wound occurs in 3T3 fibroblasts. The more rapid transition of membrane tension in RBL cells may be because, in part, the antigen induces exocytosis from the entire surface of the cells, but a wound allows Ca2+ entry at only one site.
Our results, however, do not rule out the possibility that membrane tension can be modulated by other pathways that may be activated in parallel with or after the step of membrane fusion. Recent studies have shown that rearrangement or disassembly of cortical actin filaments occurs during exocytosis (Muallem et al., 1995; Bernstein et al., 1998; Sullivan et al., 1999; Trifaróet al., 2000). Furthermore, it has been shown that the concentration of plasma membrane phosphatidylinositol 4,5-bisphosphate (PIP2) regulates the cytoskeletal structure and the adhesion between the cortical cytoskeleton and the plasma membrane (Raucher et al., 2000). If PIP2 changes follow from the vesicle membrane fusion step, these changes could contribute to the decrease in membrane tension.
Our current study strongly supports the hypothesis that the primary function of wound-induced exocytosis in membrane repair is to induce a decrease in membrane tension that allows the bilayer to reseal. We favor the view that the decrease in tension is a result of the addition of vesicle membrane during exocytosis, however, we cannot rule out that some other process linked to exocytosis is the critical step in producing the low tension required for resealing the bilayer.
ACKNOWLEDGMENTS
We thank Bruce J. Tromberg for providing access to the facilities of the Beckman Laser Institute and for insightful suggestions. This study was supported by the National Institutes of Health (R01AR44066 and P41RR01192). We also thank William and Patricia Baker of Corona Del Mar, CA, who generously opened their home to us to stay there during our work.
REFERENCES
- Angleson JK, Betz WJ. Monitoring secretion in real time: capacitance, amperometry and fluorescence compared. Trends Neurosci. 1997;20:281–287. doi: 10.1016/s0166-2236(97)01083-7. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, DeWit M, Bamburg JR. Actin disassembles reversibly during electrically induced recycling of synaptic vesicles in cultured neurons. Mol Brain Res. 1998;53:236–250. doi: 10.1016/s0169-328x(97)00319-7. [DOI] [PubMed] [Google Scholar]
- Bi G-Q, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G-Q, Morris RL, Liao G, Alderton JM, Scholey JM, Steinhardt RA. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis. J Cell Biol. 1997;138:999–1008. doi: 10.1083/jcb.138.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Südhof TC, Jahn R, Niemann H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem. 1994;269:1617–1620. [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1–43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Coorssen JR, Schmitt H, Almers W. Ca2+ triggers massive exocytosis in Chinese hamster ovary cells. EMBO J. 1996;15:3787–3791. [PMC free article] [PubMed] [Google Scholar]
- Dai J, Sheetz MP. Cell membrane mechanics. Methods Cell Biol. 1998;55:157–171. [PubMed] [Google Scholar]
- Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Ting-Beall HP, Sheetz MP. The secretion-coupled endocytosis correlates with membrane tension changes in RBL 2H3 cells. J Gen Physiol. 1997;110:1–10. doi: 10.1085/jgp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H. Comparative biology of Ca2+-dependent exocytosis: implications of kinetic diversity for secretory function. Trends Neurosci. 1999;22:88–93. doi: 10.1016/s0166-2236(98)01293-4. [DOI] [PubMed] [Google Scholar]
- Kim TD, Eddlestone GT, Mahmoud SF, Kuchtey J, Fewtrell C. Correlating Ca2+ responses and secretion in individual RBL-2H3 mucosal mast cells. J Biol Chem. 1997;272:31225–31229. doi: 10.1074/jbc.272.50.31225. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marmé D, Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz JD, Nelson P. Dynamically stabilized pores in bilayer membranes. Biophys J. 1997;72:2211–2216. doi: 10.1016/S0006-3495(97)78864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverio de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335:99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- Sheetz MP, Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996;6:85–89. doi: 10.1016/0962-8924(96)80993-7. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA, Bi G-Q, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Price LS, Koffer A. Rho controls cortical F-actin disassembly in addition to, but independently of, secretion in mast cells. J Biol Chem. 1999;274:38140–38146. doi: 10.1074/jbc.274.53.38140. [DOI] [PubMed] [Google Scholar]
- Taupin C, Dvolaizky M, Sauterey C. Osmotic pressure induced pores in phospholipid vesicles. Biochemistry. 1975;14:4771–4775. doi: 10.1021/bi00692a032. [DOI] [PubMed] [Google Scholar]
- Togo T, Alderton JM, Bi G-Q, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112:719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- Trifaró J-M, Rosé SD, Marcu MG. Scinderin, a Ca2+-dependent actin filament severing protein that controls cortical actin network dynamics during secretion. Neurochem Res. 2000;25:133–144. doi: 10.1023/a:1007503919265. [DOI] [PubMed] [Google Scholar]
- Zhelev DV, Needham D. Tension-stabilized pores in giant vesicles: determination of pore size and pore line tension. Biochim Biophys Acta. 1993;1147:89–104. doi: 10.1016/0005-2736(93)90319-u. [DOI] [PubMed] [Google Scholar]