Abstract
Transcriptional and allosteric regulation of ADP-Glc pyrophosphorylase (AGPase) plays a major role in the regulation of starch synthesis. Analysis of the response after detachment of growing potato tubers from the mother plant revealed that this concept requires extension. Starch synthesis was inhibited within 24 h of tuber detachment, even though the catalytic subunit of AGPase (AGPB) and overall AGPase activity remained high, the substrates ATP and Glc-1-P increased, and the glycerate-3-phosphate/inorganic orthophosphate (the allosteric activator and inhibitor, respectively) ratio increased. This inhibition was abolished in transformants in which a bacterial AGPase replaced the potato AGPase. Measurements of the subcellular levels of each metabolite between Suc and starch established AGPase as the only step whose substrates increase and mass action ratio decreases after detachment of wild-type tubers. Separation of extracts on nonreducing SDS gels revealed that AGPB is present as a mixture of monomers and dimers in growing tubers and becomes dimerized completely in detached tubers. Dimerization led to inactivation of the enzyme as a result of a marked decrease of the substrate affinity and sensitivity to allosteric effectors. Dimerization could be reversed and AGPase reactivated in vitro by incubating extracts with DTT. Incubation of tuber slices with DTT or high Suc levels reduced dimerization, increased AGPase activation, and stimulated starch synthesis in vivo. In intact tubers, the Suc content correlated strongly with AGPase activation across a range of treatments, including tuber detachment, aging of the mother plant, heterologous overexpression of Suc phosphorylase, and antisense inhibition of endogenous AGPase activity. Furthermore, activation of AGPase resulted in a stimulation of starch synthesis and decreased levels of glycolytic intermediates.
INTRODUCTION
ADP-Glc pyrophosphorylase (AGPase) catalyzes the conversion of Glc-1-P and ATP to ADP-Glc and inorganic pyrophosphate (PPi), which is the first committed step in the pathway of starch synthesis (Figure 1) (Preiss, 1988; Martin and Smith, 1995; Smith et al., 1997). The higher plant enzyme is a heterotetramer, consisting of two “regulatory” subunits (AGPS; 51 kD) and two slightly smaller “catalytic” subunits (AGPB; 50 kD) (Okita et al., 1990). AGPase plays a major role in the regulation of starch synthesis. Studies with an Arabidopsis AGPS mutant have demonstrated that AGPase is a key site for the control of starch synthesis in leaves (Neuhaus and Stitt, 1990). In potato tubers expressing an antisense AGPB construct, starch synthesis is decreased when activity is decreased to <50% of the wild-type level (Müller-Röber et al., 1992; Geigenberger et al., 1999a).
Figure 1.
Pathway of Suc-to-Starch Conversion and Its Subcellular Compartmentation in Potato Tubers.
1, Suc synthase; 2, UDP-Glc pyrophosphorylase; 3, fructokinase; 4, cytosolic phosphoglucomutase; 5, phosphoglucoisomerase; 6, plastidic phosphoglucomutase; 7, ADP-Glc pyrophosphorylase; 8, alkaline pyrophosphatase; 9, granule-bound starch synthase; 10, soluble starch synthase; 11, branching enzyme; 12, hexose phosphate translocator; 13, triose phosphate translocator; 14, adenylate translocator. TCA, tricarboxylic acid.
Two mechanisms are known to regulate AGPase activity. First, AGPase is subject to transcriptional regulation, with expression being increased by sugars (Salanoubat and Belliard, 1989; Müller-Röber et al., 1990; Sokolov et al., 1998) and decreased by nitrate (Scheible et al., 1997) and phosphate (Nielsen et al., 1998). This may allow starch accumulation to respond to changes in the carbon and nutritional status (Scheible et al., 1997; Stitt and Krapp, 1999). Second, AGPase is exquisitely sensitive to allosteric regulation, being activated by glycerate-3-phosphate (3PGA) and inhibited by Pi (Sowokinos, 1981; Sowokinos and Preiss, 1982; Preiss, 1988).
Increasing levels of phosphorylated intermediates typically lead to a marked increase of the 3PGA/Pi ratio. Therefore, activation of AGPase by an increasing 3PGA/Pi ratio allows the rate of starch synthesis to be adjusted in response to changes in the balance between photosynthesis and Suc synthesis in leaves (Heldt et al., 1977; Herold, 1980; Stitt et al., 1987) and to changes in the balance between Suc breakdown and respiration in nonphotosynthetic tissues (Stark et al., 1992; Hajirezaei et al., 1994; Geigenberger et al., 1997, 1998a, 2000; Jenner et al., 2001).
Several situations have been reported in which starch synthesis changes independently of overall AGPase activity and reciprocally to the levels of phosphorylated intermediates (Geigenberger et al., 1994; Geiger et al., 1998; Trethewey et al., 1998, 2001; Geigenberger and Stitt, 2000). This fact indicates that there may be important gaps in our understanding of the regulation of starch synthesis. One such situation formed the starting point for the experiments described here. Detachment of growing potato tubers from the mother plant leads within 24 h to a threefold decrease of ADP-Glc and a 50% inhibition of starch synthesis, even though AGPase activity remains unaltered and the overall levels of hexose phosphates and 3PGA increase (Geigenberger et al., 1994).
Because only the overall metabolite levels were measured, there are various explanations for these observations. One is that AGPase is being inhibited by a novel mechanism. Another is that the substrate supply for AGPase may be changing as a result of regulation of the envelope hexose phosphate:phosphate transporter (Kammerer et al., 1998), the plastidic phosphoglucomutase (PGM) (Tauberger et al., 2000), or the envelope adenylate translocator (Tjaden et al., 1998; Geigenberger et al., 2001). These are required to transport the carbon substrates and ATP from the cytosol to the plastid, where AGPase is located (Figure 1). Another possibility is that AGPase activity may be responding to changes in plastid 3PGA/Pi ratio caused by changes in the activity of the envelope triose phosphate:phosphate translocator. This transporter is required to transmit changes of the 3PGA/Pi ratio from one compartment to the other (Borchert et al., 1989; Schott et al., 1995) (Figure 1).
Here, we show that the inhibition of starch synthesis after tuber detachment occurs via a mechanism that depends on the properties of the plant AGPase; additionally, we used nonaqueous fractionation to investigate the response of cytosolic and plastidic metabolite levels and define a unique crossover point at AGPase. We also demonstrate that detachment does not lead to a decrease of the plastid 3PGA/Pi ratio and has no effect on AGPB protein or overall AGPase activity; instead, it leads to post-translational inactivation of AGPase via a reversible mechanism that involves redox-dependent dimerization of the ABPB subunits. Finally, we present evidence that this novel mechanism contributes to the regulation of starch synthesis in response to a range of treatments that modify the Suc level in tubers.
RESULTS
Inhibition of Starch Synthesis in Response to Tuber Detachment Is Abolished in Transgenic Tubers That Express a Heterologous AGPase
We used two independent approaches to determine the step or steps in the pathway of starch synthesis that are the targets for the novel mechanism that inhibits starch synthesis when tubers are detached from the mother plant. The first approach asked whether specific regulatory features of potato tuber AGPase are essential for the inhibition of starch synthesis. To answer this question, the response was compared in wild-type tubers and in transformants in which endogenous AGPase was replaced largely by a nonplant AGPase.
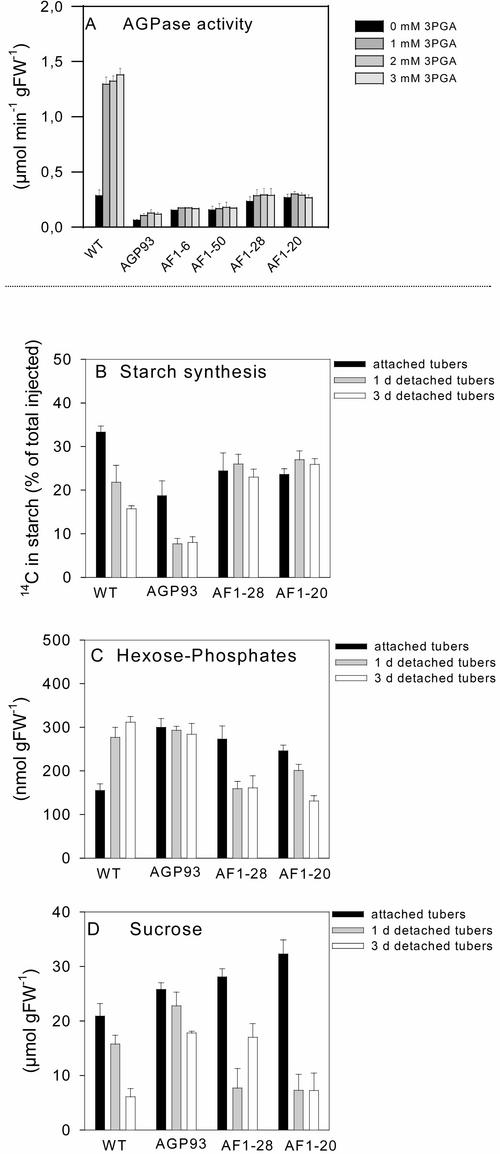
The introduced construct encodes a form of the monomeric Escherichia coli enzyme (glgC16) that is different in its kinetic properties from the plant enzyme (Stark et al., 1992). It was introduced (Lloyd et al., 1999) into the AGPase antisense line AGP93, which has low activity of the higher plant AGPase (Müller-Röber et al., 1992). AGPase activity in wild-type tubers was strongly dependent on 3PGA (Figure 2A). AGPase activity in the antisense line AGP93 was reduced by >90% and remained dependent on 3PGA. AGPase activity in the double-transformed lines AF1-28 and AF1-20 was threefold higher than that in AGP93 and was independent of 3PGA (Figure 2A) and Pi (data not shown) (Lloyd et al., 1999).
Figure 2.
Inhibition of Starch Synthesis in Response to Tuber Detachment Is Abolished in Transgenic Tubers Expressing the glgC16 Gene in an AGPB Antisense Background.
(A) AGPase activity in wild-type tubers (WT), the parental antisense AGPB line (AGP93), and four independent transgenic AF1 lines expressing the glgC16 gene in an antisense AGPase (AGP93) background. AGPase activity was assayed using a standard protocol (Müller-Röber et al., 1992) in the absence (black bars) or the presence of 1 mM (dark-gray bars), 2 mM (medium-gray bars), or 3 mM (light-gray bars) 3PGA.
(B) to (D) Changes in the rate of starch synthesis and metabolite levels after tuber detachment. Tubers from 8-week-old wild-type potato plants, AGPase antisense plants (AGP93), and supertransformed plants expressing glgC16 (AF1-28 and AF1-20) were analyzed either directly (black bars) or 1 day (gray bars) or 3 days (white bars) after detachment from the plant. To measure the rate of starch synthesis (B), U-14C-Glc of high specific activity (∼40 to 50 kBq per tuber) was injected into a fine borehole of an otherwise intact tuber. The tubers then were incubated for 1 h, and a concentric core of material around the borehole was extracted and analyzed to determine 14C incorporation into starch. The data are expressed as a percentage of the total label injected. The same samples were used to measure the levels of hexose phosphates (sum of Glc-6-P, Fru-6-P, and Glc-1-P) (C) and Suc (D) by enzymatic analysis. Results are means ± se of four tubers from different plants.
FW, fresh weight.
To measure the rate of starch synthesis, low concentrations of 14C-Glc were injected into a fine borehole in growing tubers that were attached to the mother plant or 1 and 3 days after detaching them from the mother plant by severing the stolon (for details, see Geigenberger et al., 1994). After 1 h, the area around the injected label was removed and analyzed to determine how much of the injected label had been converted to starch (Figure 2B). Metabolite levels were measured in the same material (Figures 2C and 2D). Similar results were obtained in an independent experiment in which 14C-Glc was supplied for 30 min to discs cut from attached or detached tubers (data not shown).
In wild-type tubers, 34% of the label was incorporated into starch in attached tubers, decreasing to 21 and 15% in tubers that had been detached for 1 and 3 days, respectively (Figure 2B). The inhibition of starch synthesis was accompanied by an increase of hexose phosphates (Figure 2C) and a slight increase of 3PGA (data not shown). Suc decreased gradually, declining by 24 and 70% after 1 and 3 days, respectively (Figure 2D). This finding confirms the results described previously (Geigenberger et al., 1994).
In the antisense line AGP93, 19% of the injected label was incorporated into starch in attached tubers, decreasing to 8% in detached tubers (Figure 2B). Hexose phosphates were high in attached tubers and remained high after detachment (Figure 2C), and Suc decreased even more slowly than in wild-type tubers (Figure 2D).
In the double transformants AF1-20 and AF1-28, 24 to 26% of the label was incorporated into starch in attached tubers (Figure 2B). Detachment did not lead to a rapid inhibition of starch synthesis in these lines (Figure 2B) but instead led to a marked decline of hexose phosphates (Figure 2C) and a rapid 70 to 80% decrease of Suc during the first day after detachment (Figure 2D). There were no substantial changes in the specific activities of the internal hexose phosphate pools in the various genotypes after detachment (data not shown), demonstrating that the different response in the double transformants is not attributable to isotopic dilution of the incoming label by internal pools. These results provide strong genetic evidence that starch synthesis is inhibited after detachment by a regulatory mechanism that requires the presence of native potato tuber AGPase.
Separation of Tuber Material into Subcellular Compartments
Regulated enzymes can be identified by perturbing the flux through a metabolic pathway and measuring the resulting changes in metabolite levels to identify the step or steps at which the substrate concentration(s) changes reciprocally to the flux through the pathway (Rolleston, 1972). To allow the unbiased identification of the step(s) at which starch synthesis is inhibited after the detachment of wild-type tubers, we investigated changes in the subcellular levels of every metabolite from Suc to starch.
This was performed by nonaqueous fractionation, a technique developed to analyze the subcellular compartmentation of metabolites in leaves (Gerhardt and Heldt, 1984; Stitt et al., 1989) and adapted recently for use with potato tubers (Farré et al., 2001). Tubers are frozen in liquid nitrogen to quench metabolism, homogenized in liquid nitrogen, lyophilized at low temperature, and resuspended in heptane. Enzymic reactions are blocked for the reminder of the fractionation procedure because water is absent. During lyophilization, metabolites and proteins from a particular region of the cell aggregate. The suspension then is ultrasonicated to generate particles that are partially enriched for different material from subcellular compartments and that can be separated by nonaqueous density gradient centrifugation.
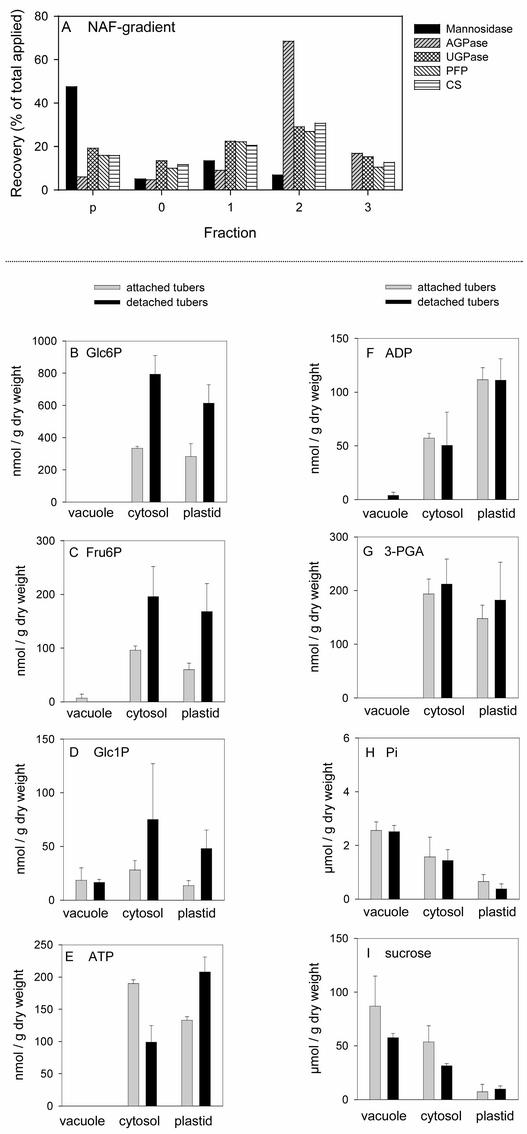
Marker enzyme activities are measured to reveal how materials from different cellular compartments distribute across the gradient (Figure 3A). The vacuolar marker mannosidase (Boller and Kende, 1979) was highly enriched in the pellet, the cytosolic markers UDP-Glc pyrophosphorylase (UGPase; Kleczkowsky, 1994) and pyrophosphate:Fru-6-P1 phosphotransferase (MacDonald and Preiss, 1986) and the mitochondrial marker citrate synthase were enriched in fractions 0 and 1, and the plastidic marker AGPase (Kim et al., 1989) was enriched in the lightest fractions (fractions 2 and 3) (Figure 3A) (Farré et al., 2001). This effect resembles the distribution in gradients of leaf material (Stitt et al., 1989); in that earlier study, it also was shown that the distribution of enzymes tracks the distribution of metabolites that are known to be restricted to a particular compartment.
Figure 3.
Subcellular Analysis of Metabolite Levels in Attached and 1-Day-Detached Wild-Type Tubers.
(A) Marker enzyme distribution in a typical nonaqueous gradient of lyophilized wild-type tuber tissues. The values represent the enzyme activity found in each fraction as a percentage of the total applied to the gradient. The marker enzyme distribution in the fractions of each gradient was used to calculate the distribution of metabolites in the subcellular compartments. CS, citrate synthase; NAF, nonaqueous fractionation; PFP, pyrophosphate:Fru-6-P1 phosphotransferase.
(B) to (I) Compartmentation of metabolites in potato tuber attached to the plant (gray bars) or after 1 day of detachment (black bars). Metabolites in the fractions of the nonaqueous gradients were extracted with trichloroacetic acid before analysis of Glc-6-P (B), Fru-6-P (C), Glc-1-P (D), ATP (E), ADP (F), 3PGA (G), Pi (H), and Suc (I). The subcellular compartmentation of the metabolites was calculated by more-dimensional regression analysis (best-fit method). The values are standardized to tissue dry weight and give the mean of three separate gradients (means ± se, n = 3).
Metabolites are measured in each fraction, and their subcellular distribution are estimated by more-dimensional linear regression. The estimated distribution can be compared with the overall content to estimate the metabolite content in each compartment (per gram of total dry weight) and with empirically determined values for the volume of each compartment (Farré et al., 2001) to estimate the subcellular concentrations. This analysis yields estimates for the vacuole, plastids, and cytosol. The estimated values for the cytosol include the mitochondrial metabolites because the cytosol and mitochondria are not separated (see above).
Changes in Subcellular Levels of Its Substrates Reveal That AGPase Is Involved in the Inhibition of Starch Synthesis
The immediate substrates for AGPase are the pools of Glc-1-P and ATP in the plastid (Figure 1). Suc is degraded in the cytosol via Suc synthase (SuSy), UGPase, and PGM to form Glc-6-P, which is imported into the plastid via the Glc-6-P/Pi transporter and converted back to Glc-1-P by the plastidic PGM (Figure 1). Glc-6-P, Fru-6-P, and Glc-1-P were distributed between the plastid and cytosol in attached tubers (Figures 3B to 3D) (Farré et al., 2001). The vacuole contained negligible hexose phosphates, except for traces of Glc-1-P (Figure 3D) (Farré et al., 2001). Detachment led to an increase of Glc-6-P, Fru-6-P, and Glc-1-P in the cytosol and the plastid. The error bars are large for Glc-1-P because this metabolite is present at low levels, leading to analytic errors that sum during the calculations. The estimated average plastid Glc-1-P concentration increased fourfold from 17 to 64 μM (Table 1).
Table 1.
Estimated Cytosolic and Plastidic Concentrations of Metabolites Involved in the Pathway of Suc to Starch
| Metabolite | Attached Tubers | Detached Tubers |
|---|---|---|
| Cytosol | ||
| Suc | 82.4 ± 22.9 | 47.9 ± 4 |
| Fru | 1.37 ± 0.72 | 1.79 ± 0.38 |
| UDP-Glc | 571 ± 17 | 627 ± 54 |
| Glc-1-P | 42.7 ± 13 | 115 ± 79 |
| Glc-6-P | 510 ± 18 | 1212 ± 177 |
| Fru-6-P | 147 ± 12 | 299 ± 85 |
| ATP | 292 ± 9 | 151 ± 39 |
| ADP | 87.3 ± 7 | 77 ± 47 |
| UTP | 238 ± 8 | 121 ± 8 |
| UDP | 52.4 ± 3 | 37.1 ± 4 |
| PPi | 12.0 ± 0.7 | 6.9 ± 2.9 |
| Pi | 2400 ± 1120 | 2200 ± 620 |
| 3PGA | 296 ± 43 | 324 ± 72 |
| Plastid | ||
| Glc-6-P | 379 ± 108 | 822 ± 155 |
| Glc-1-P | 17.4 ± 6.7 | 64 ± 22 |
| ADP-Glc | 21.5 ± 2 | 11.2 ± 1 |
| ATP | 179 ± 7 | 279 ± 31 |
| ADP | 149 ± 14 | 148 ± 27 |
| PPi | 2.36 ± 1.43 | 0.68 ± 0.68 |
| Pi | 870 ± 350 | 500 ± 260 |
| 3PGA | 198 ± 33 | 244 ± 95 |
The cytosolic and plastidic metabolite levels, obtained after nonaqueous fractionation, were divided by the fractional volumes of the cytosolic (0.606 mL/g dry wt) and plastidic compartments (0.747 mL/g dry wt) to derive the respective cytosolic and plastidic concentrations (Farré et al., 2001). Data are means ± se (n = 3) and are expressed in μM, except for Suc and Fruc, which are expressed in mM.
ATP is imported from the cytosol via the envelope adenylate exchanger (Figure 1). A substantial proportion of the adenine nucleotides are located in the plastid (Figures 3E and 3F), whereas uridine and guanidine nucleotides are located mainly in the cytosol of tubers (data not shown) (Farré et al., 2001), as is found for leaves (Dancer et al., 1990; Riens et al., 1991). Detachment led to a 50% increase of ATP in the plastid and a 50% decrease of ATP in the cytosol (Figure 3E). ADP levels were not affected substantially after detachment (Figure 3F).
The ATP/ADP ratio was lower in the plastid than in the cytosol in attached tubers (1.2 compared with 3.3; data calculated from Figures 3E and 3F), again resembling the ratio in leaves (Stitt et al., 1982). The value attributed to the cytosol underestimates the actual cytosolic value, because significant amounts of adenine nucleotides also are present in the mitochondria, and the ATP/ADP ratio in the mitochondria is lower than that in the cytosol (Stitt et al., 1982). After detachment, the ATP/ADP ratio in the plastid increased to a value (1.9) similar to that estimated for the cytosol plus the mitochondria (2.0). The estimated plastid ATP concentration increased after detachment from 179 to 279 μM (Table 1).
The estimated plastidic concentrations of Glc-1-P and ATP were in the range of the values determined in vitro for the substrate affinity S0.5 Glc-1-P (40 to 140 μM) and S0.5 ATP (120 to 190 μM) of AGPase (Sowokinos and Preiss, 1982; Ballicora et al., 1995). Detachment led to an increase of the levels of both substrates, whereas the rate of starch synthesis decreased. These results show that the inhibition of starch synthesis involves a mechanism that acts on AGPase.
Estimation of Mass-Action Ratios for Each Enzyme-Catalyzed Reaction and Transport Step between Suc and Starch Identifies AGPase as the Unique Site Involved in the Inhibition of Suc-to-Starch Interconversion after Tuber Detachment
The cytosolic concentrations of Suc, Fru, UDP-Glc, Glc-1-P, Glc-6-P, Fru-6-P, ATP, ADP, UTP, UDP, PPi, Pi, and 3PGA, as well as the plastidic concentrations of Glc-1-P, Glc-6-P, ADP-Glc, ATP, ADP, PPi, Pi, and 3PGA, are summarized in Table 1. The variation for some metabolites, including Glc-1-P and PPi in the plastid and ADP in the cytosol, was high. The variation for Glc-1-P was discussed above. The variation in PPi was caused by the low level of PPi and by the fact that only a very small fraction of the total PPi was located in the plastid.
These results (Table 1) were used to calculate the ratio between the in vivo concentrations of the products and the substrates (termed the mass-action ratio) for every step between Suc and ADP-Glc (Table 2). The theoretical equilibrium constant (Keq; the ratio of product and substrate concentrations at which the reaction is at its thermodynamic equilibrium and net flux is zero) is listed for comparison. Keq for the transport steps is set at unity. In attached tubers, the mass-action ratios of the reactions catalyzed by SuSy, UGPase, and cytosolic PGM, the transport exchanges catalyzed by the Glc-6-P/Pi and ATP/ADP transporters, and the reaction catalyzed by plastidic PGM are close to their Keq. The mass-action ratios of fructokinase, AGPase, and inorganic pyrophosphatase are displaced from their Keq (Table 2). These results resemble those reported previously for cytosol and plastids in leaves (Stitt et al., 1982, 1989) and for phloem sap (Geigenberger et al., 1993). Thus, our results provide evidence for the reliability of the fractionation technique and subsequent calculations.
Table 2.
Estimated in Vivo Mass-Action Ratios of Enzyme and Transport Reactions Involved in Suc-to-Starch Conversion
| Molar Mass-action Ratio
|
||||
|---|---|---|---|---|
| Reaction | Formula | Attached Tubers | Detached Tubers | Theoretical Equilibrium Constant (Keq) |
| SuSy (cytosol) |
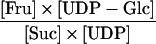 |
0.181 | 0.632 | 0.15 to 0.56a |
| UGPase (cytosol) |
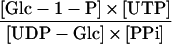 |
1.48 | 3.21 | 3.2b |
| Fructokinase (cytosol) |
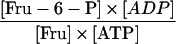 |
0.032 | 0.085 | 851c |
| Phosphoglucoisomerase (cytosol) |
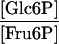 |
3.47 | 4.05 | 2c |
| PGM (cytosol) |
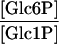 |
11.9 | 10.5 | 19c |
| Glc-6-P/Pi translocator |
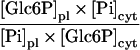 |
2.04 | 2.97 | 1d |
| 3PGA/Pi translocator |
 |
1.85 | 3.31 | 1d |
| Adenylate translocator |
 |
0.359 | 0.961 | 1d |
| PGM (plastid) |
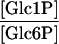 |
0.046 | 0.078 | 0.053c |
| AGPase (plastid) |
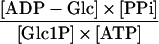 |
1.6 × 10−2 | 4 × 10−4 | 1e |
| Inorganic pyrophosphatase (plastid) |
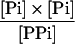 |
0.323 | 0.373 | 1000b |
The data from Table 1 were used to calculate the in vivo molar mass-action ratios (ratio of product to substrate concentration) for each reaction. The theoretical equilibrium constant (ratio of product/substrate concentration) at which the net flux is zero) of each reaction is shown for comparison.
Estimated value.
The inhibition of starch synthesis after detachment was accompanied by a 40-fold decrease of the mass-action ratio for AGPase (Table 2). This was the result of an increase in the concentration of the substrates Glc-1-P and ATP and a decrease in the concentration of the products ADP-Glc and PPi in the plastid (Table 1) (Geigenberger et al., 1994). The mass-action ratios for all of the other steps were unaltered or even increased slightly after tuber detachment (Table 2). This finding provides strong biochemical evidence that AGPase is the only site at which significant regulation occurs after detachment.
Inhibition of Starch Synthesis in Response to Tuber Detachment Does Not Involve Changes in the Plastidic Concentrations of 3PGA and Pi
We next investigated the possibility that a decrease of the plastidic 3PGA/Pi ratio is responsible for the inhibition of AGPase activity after detachment. The overall level of 3PGA increased slightly after detachment (342 and 394 nmol/g dry weight in attached and 1-day-detached tubers; data not shown) (Geigenberger et al., 1994). This increase included a slight nonsignificant increase of 3PGA in the plastid (Figure 3G, Table 1). In attached tubers, more than half of the Pi was located in the vacuole, 33% was located in the cytosol, and 14% was located in the plastid. Detachment led to a decrease of Pi in the plastid and the cytosol (Figure 3H). The estimated concentration in the plastid decreased from 974 to 502 μM (Table 1).
To explain why there is a 30 to 50% inhibition of starch synthesis after tuber detachment (Figure 2B) (Geigenberger et al., 1994) even though there is an increase in the substrates and an increase in the 3PGA/Pi ratio, it is necessary to postulate either (1) a very large decrease in expression or (2) a novel regulatory mechanism acting on AGPase.
Changes in AGPase Expression Are Not Reflected Rapidly at the Protein Level and Do Not Explain the Inhibition of Starch Synthesis in Wild-Type Tubers after 1 Day of Detachment
Although the levels of the AGPB and AGPS1 transcripts decreased markedly in the first 24 h after detachment (Figure 4A), this did not lead to a marked change of protein or AGPase activity (Figures 4B and 4C). AGPase protein was determined by preparing extracts from attached and detached tubers in the presence of DTT before SDS-PAGE and protein gel blot analysis with an antibody raised against the maize AGPB (brittle-2) protein (Giroux and Hannah, 1994) (Figure 4B). A single band at ∼50 kD was present at a similar intensity in extracts from attached and 1-day-detached tubers.
Figure 4.
AGPB and AGPS Expression in Attached and 1-Day-Detached Wild-Type Tubers.
Attached (t0) and 1-day-detached (1 d) tubers were analyzed for steady state mRNA levels of AGPB and AGPS1 (A), AGPase protein after reducing SDS-PAGE and immunoblot analysis with antiserum raised against the homologous maize brittle-2 protein (Giroux and Hannah, 1994) (B), and overall activity of AGPase using a standard protocol (Müller-Röber et al., 1992) including DTT in the extraction and assay buffers (C). Representative samples are shown in (A) and (B); in (C), results are means ± se (n = 4 extracts from separate tubers). FW, fresh weight.
AGPase activity was measured using a standard assay in which DTT was included in the extraction and assay buffer and activity was determined in the presence of saturating substrate levels. AGPase activity was unaltered at 1 day after detachment and started to decrease at 3 days after detachment (Figure 4C and data not shown) (Geigenberger et al., 1994). These results show that the inhibition of starch synthesis during the first day after tuber detachment is not the result of changes in AGPase expression.
Tuber Detachment Leads to Reversible Oxidation and Dimerization of AGPase
In a series of experiments that were performed to produce heterotetrameric higher plant AGPase in E. coli, Preiss and co-workers (Iglesias et al., 1993; Ballicora et al., 1995; Fu et al., 1998) found that to obtain high activity it was necessary to incubate the overexpressed enzyme with DTT to reduce an intermolecular disulfide bond that forms in E. coli at the Cys-12 position in the heterologously expressed small subunits. This Cys is located in a QTCL motif and corresponds to Cys-82 of the potato full-length mRNA for AGPB. Breakage of the disulfide bond can be monitored by subjecting AGPase to SDS-PAGE in nonreducing conditions and monitoring the disappearance of the dimerized 100-kD band and the appearance of the 50-kD monomer (Iglesias et al., 1993; Ballicora et al., 1995; Fu et al., 1998). The intramolecular disulfide bond also increases the heat stability of the heterotetrameric plant enzyme (Ballicora et al., 1999).
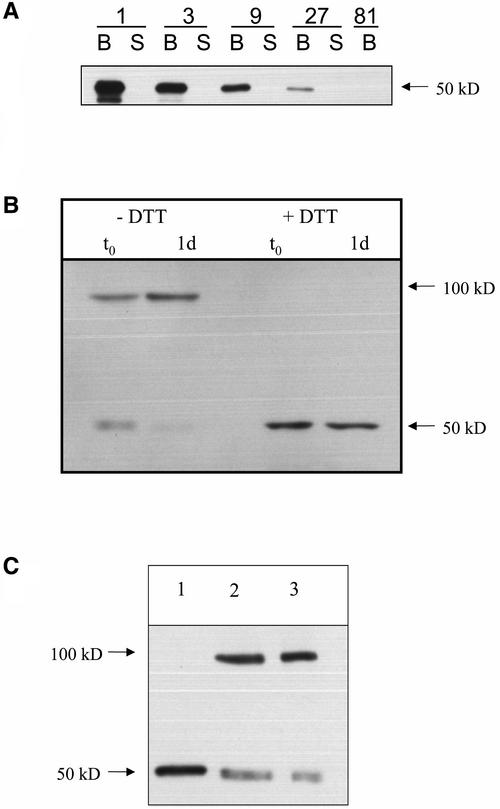
To determine whether analogous changes occur in planta, growing potato tubers were extracted in degassed SDS extraction buffer, separated immediately by nonreducing SDS-PAGE to maintain the in vivo redox status of the enzyme, and immunoblotted with antibodies raised against maize or potato AGPB. To ensure the specificity of the antibodies, His-tagged potato AGPB and AGPS were overexpressed in E. coli and purified; their identities were confirmed by matrix-assisted laser-desorption ionization time-of-flight analysis. Then, they were applied in a concentration series, separated by SDS-PAGE, and immunoblotted. The antibody raised against maize brittle-2 was highly specific for the potato AGPB subunit (Figure 5A).
Figure 5.
Analysis of the Dimerization of AGPase.
(A) Demonstration of the specificity of the antiserum raised against the homologous maize brittle-2 protein (Giroux and Hannah, 1994) for the potato AGPB subunit. Similar amounts of heterologously overexpressed His-tagged AGPB (B) and AGPS1 (S) proteins were applied in a concentration series (diluted 1 to 81 times), separated by SDS-PAGE in the presence of 4 mM DTT, and immunoblotted.
(B) Detachment leads to the dimerization of AGPase. Total protein was extracted from wild-type tubers, separated by SDS-PAGE, and examined by immunoblot analysis using maize brittle-2 protein antibody. Samples from attached tubers (t0; lanes 1 and 3) and 1-day-detached tubers (1 d; lanes 2 and 4) were prepared under anaerobic conditions in SDS and analyzed by SDS-PAGE in the absence of DTT (lanes 1 and 2) or in the presence of 4 mM DTT (lanes 3 and 4). Each lane contained protein from ∼3 mg fresh weight of tuber tissue.
(C) Effect of different extraction methods on the dimerization of AGPase after nonreducing SDS-PAGE and immunoblot analysis with maize brittle-2 protein antibody. Lane 1, AGPase extracted using a standard protocol as described by Müller-Röber et al. (1992); lane 2, AGPase extracted using a modified protocol omitting DTT (see Methods); lane 3, tissue extracted in SDS. Electrophoresis samples of the different extracts were prepared in an argon atmosphere by mixing 100 μL of the respective extract (directly after its preparation) with 100 μL of argon-treated 2 × sample buffer lacking DTT and additional supplements. The sample was boiled immediately for 5 min. Every lane contained the proteins originating from 1.5 mg fresh weight of tuber tissue. Representative sample blots using tubers from 8-week-old wild-type plants are shown.
Extracts from tubers that were attached to the plant gave immunopositive signals with the AGPB-specific maize antibody at 50 and 100 kD (Figure 5B, lane 1). When 1-day-detached tubers were analyzed (Figure 5B, lane 2), the 50-kD band disappeared almost completely and the 100-kD band became more intense. Incubation of the extracts in vitro with DTT before SDS-PAGE completely converted the 100-kD band to the 50-kD band (Figure 5B, cf. lanes 3 and 4 with lanes 1 and 2). Similar results were obtained in repeated experiments over a period of months with separate batches of tubers. These results reveal that AGPB is present as a mixture of dimer and monomer in growing tubers and becomes completely dimerized when the tubers are detached and Suc import from the mother plant is interrupted.
Establishment of a New Protocol to Extract and Measure AGPase Activity Reveals That the Changes in Redox State Are Accompanied by Changes in Activity and Kinetic Properties
We next investigated whether AGPase activity is altered when AGPB becomes dimerized in planta. The standard protocol (Sowokinos, 1981; Müller-Röber et al., 1992; Ballicora et al., 1995) for the extraction and assay of AGPase gave only small and variable differences of activity between extracts from attached and detached tubers (data not shown). The solutions used in this protocol include DTT, and only the 50-kD band was found when aliquots from these extracts were subjected to nonreducing SDS-PAGE (Figure 5C, lane 1).
To detect the large changes of activity of redox-modulated Calvin cycle enzymes that occur when chloroplasts or leaves are illuminated or darkened, it was necessary to develop very fast assay and extraction procedures (Laing et al., 1981; Wirtz et al., 1982). Therefore, we modified the protocol for the extraction and assay of AGPase by omitting all redox agents, presaturating all extraction and assay media with nitrogen, and decreasing the time between tissue disruption and transfer into the assay to <1 min.
When an extract from growing tubers was prepared in this way and subjected to nonreducing SDS-PAGE, a mixture of bands at 50 and 100 kD (Figure 5C, lane 2) was found that resembled a parallel sample that was extracted in SDS (Figure 5C, lane 3). Large differences in AGPase activity were found between extracts from attached tubers and tubers that had been detached for 1 day (Figure 6). The differences were stable for at least 10 min in the assay conditions (data not shown) but were lost rapidly if extract was incubated in the absence of substrate (data not shown).
Figure 6.
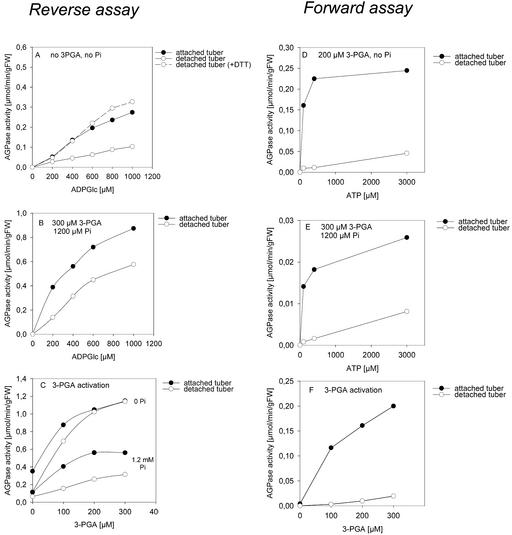
Enzyme Kinetics of AGPase in Extracts from Attached or 1-Day-Detached Wild-Type Tubers Prepared Using the Modified Rapid Protocol.
(A) to (C) Reverse assay coupling Glc-1-P formation in real time to NADP+ reduction.
(A) ADP-Glc saturation kinetics with or without DTT.
(B) ADP-Glc saturation kinetics in the presence of 300 μM 3PGA and 1200 μM Pi.
(C) 3PGA activation kinetics in the presence of 400 μM ADP-Glc with and without 1200 μM Pi.
(D) to (F) Forward assay using a stopped assay and determination of ADP-Glc by HPLC.
(D) ATP saturation kinetics in the presence of 200 μM 3PGA.
(E) ATP saturation kinetics in the presence of 300 μM 3PGA and 1200 μM Pi.
(F) 3PGA activation kinetics in the presence of 100 μM ATP.
Results are from determinations with an extract from three pooled tubers for each condition. Similar results were obtained with extracts from other pools of tubers. Closed circles, attached tubers; open circles, 1-day-detached tubers. FW, fresh weight.
For routine determination of AGPase activity, the assay was performed in the reverse direction (Figures 6A to 6C) using PGM and Glc-6-P dehydrogenase to couple Glc-1-P formation in real time to NADP+ reduction. Detachment led to a decrease of AGPase activity and the affinity for ADP-Glc when activity was assayed in the absence of allosteric effectors (Figure 6A). This inhibition was reversed completely when 5 mM DTT was included during extraction and assay (Figure 6A).
Detachment also led to a decrease of activity and the affinity for ADP-Glc when activity was assayed in the presence of 300 μM 3PGA and 1200 μM Pi (Figure 6B), which lie in the range of estimated concentrations in amyloplasts (Table 1). When assayed with limiting substrate concentrations, AGPase from detached tubers was strongly dependent on the presence of 3PGA and was not activated until high concentrations of 3PGA were present (Figure 6C). It also was more sensitive to inhibition by Pi than AGPase extracted from attached tubers (Figure 6C). Maximum activity in the presence of high 3PGA and zero Pi was similar for attached and detached tubers.
We also investigated whether AGPase activity was modified when it was assayed in the ADP-Glc–producing direction. A stopped assay was used, in which each individual sample was analyzed by HPLC to determine ADP-Glc (Figures 6D to 6F). Control experiments showed that ADP-Glc was not degraded during the incubation. Detachment led to a very marked inhibition of activity. The affinity for ATP was decreased when assayed in the presence of 200 μM 3PGA (Figure 3D) or 300 μM 3PGA plus 1200 μM Pi (Figure 6E). AGPase from detached tubers showed a strongly reduced sensitivity to 3PGA activation at physiological concentrations (Figure 6F).
To provide a routine microplate-compatible test for changes in redox regulation in subsequent experiments, AGPase activity was measured in the ADP-Glc–cleaving direction in the presence of 600 μM ADP-Glc in the absence (Vsel) or presence of 5 mM DTT (Vred). Similar results were obtained at a range of ADP-Glc concentrations between 200 and 1000 μM (Figure 6A and data not shown). The ratio between the activities in these two assays (Vsel/Vred) is termed “activation.”
Incubation of Tuber Discs with DTT to Redox Activate AGPase Leads to a Stimulation of Starch Synthesis and a Decrease of Phosphorylated Metabolites
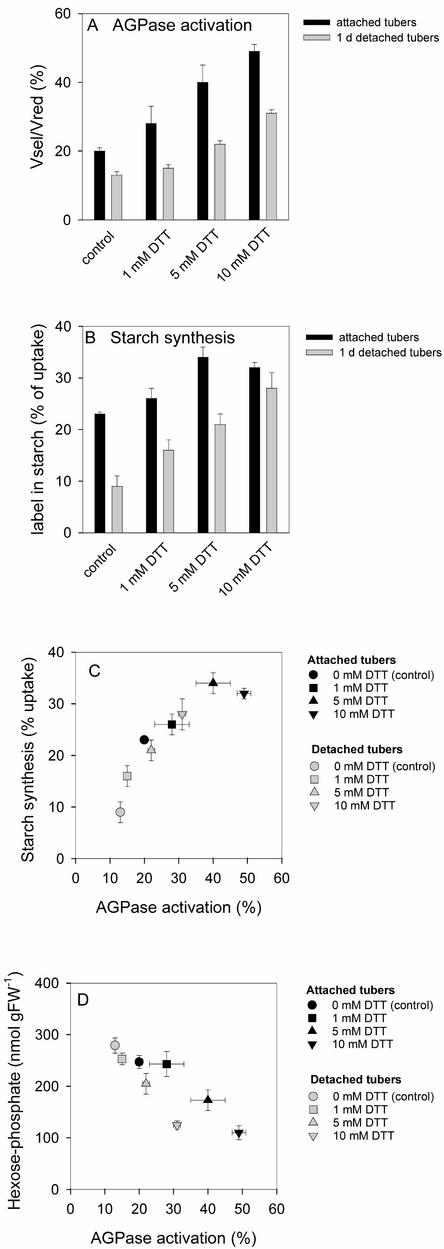
To provide independent evidence that redox modulation of AGPase can lead to large changes in the rate of starch synthesis, freshly cut discs from attached and 1-day-detached tubers were incubated for 2 h in medium containing 0, 1, 5, or 10 mM DTT. To prevent postextraction modification of AGPase, the discs were washed before extraction to prevent carryover of DTT into the extraction mixture. To measure the rate of starch synthesis, 2 mM 14C-Glc was included in the incubation.
Increasing concentrations of DTT led to a progressive increase of AGPase activation (Figure 7A) and the proportion of AGPB that was present as a monomer (data not shown). There was a parallel increase of label incorporation into starch (Figure 7B). AGPase activation (Figure 7A) and starch synthesis (Figure 7B) were lower in discs from detached tubers than in discs from attached tubers in the absence of DTT, and this difference was reversed partly by DTT. When the results for all of the treatments were combined, a strong correlation was found between AGPase activation and label incorporation into starch (Figure 7C).
Figure 7.
Redox Inactivation of AGPase and Inhibition of Starch Synthesis Both Can Be Reversed in Vivo by External Feeding of DTT to Wild-Type Tuber Discs for 2 h.
Discs were taken from attached tubers (black bars, black symbols) or 1-day-detached tubers (gray bars, gray symbols) and were incubated with 0 mM (circles), 1 mM (squares), 5 mM (triangles), or 10 mM (inverted triangles) DTT. Results are means ± se; n = 5 separate incubations with discs from separate tubers.
(A) AGPase redox activation state (Vsel/Vred).
(B) Labeling of starch after incubation of discs in 2 mM 14C-Glc (specific activity of 18.5 kBq/μmol).
(C) Starch labeling in relation to AGPase activation.
(D) Hexose phosphate levels relative to AGPase activation. FW, fresh weight.
The levels of hexose phosphates and label in the phosphorylated intermediates were measured and used to calculate the specific activity of the hexose phosphate pool and the absolute rate of starch synthesis. This calculation confirmed that there is a strong correlation between AGPase activation and the rate of starch synthesis (data not shown). The stimulation of starch synthesis was accompanied by a marked decrease of the levels of hexose phosphates (Figure 7D) and 3PGA (data not shown). This finding shows that the stimulation of starch synthesis by DTT is not caused by an additional action besides redox activation of AGPase, such as a stimulation of Suc breakdown or a restriction of respiration.
Incubation of Tuber Discs Leads to a Decrease in AGPase Activation State, Which Is Prevented by Suc
Detachment of a tuber interrupts Suc import, leading to a decrease of Suc in the vacuole and the cytosol (Figure 3I). It appeared plausible that changes in the import or level of Suc might stimulate the post-translational inactivation of AGPase. Therefore, experiments were performed to determine whether redox modulation of potato tuber AGPase contributes to the regulation of starch synthesis in other conditions in which the supply or level of Suc changes.
Geiger et al. (1998) reported that the addition of Suc to tuber discs stimulates starch synthesis and decreases the levels of glycolytic intermediate. In our experiments, when freshly cut discs were incubated in the absence of Suc, their internal Suc content decreased by ∼30% during the first 90 min and then stabilized (Figure 8A). AGPase activation decreased between 30 and 120 min (Figure 8A). Inclusion of 200 mM Suc in the medium prevented the decrease of the internal Suc pool (Figure 8B) and the decrease of AGPase activation (Figure 8C). In a parallel experiment, 2 mM 14C-Glc was provided to tuber slices in the absence and presence of 200 mM Suc. The increase in AGPase activation in the presence of 200 mM Suc was accompanied by a 50% stimulation of label incorporation into starch and a decrease of hexose phosphates (Table 3).
Figure 8.
Redox Inactivation of AGPase in Wild-Type Tuber Discs Is Prevented by External Feeding of Suc.
(A) Decrease in Suc content (gray circles) and AGPase activation state (black circles) after cutting and incubating discs in buffer solution in the absence of sugars for up to 4 h.
(B) and (C) Suc levels (B) and AGPase redox activation (C) in discs incubated for 2 h with no sugars (gray bars) or 200 mM Suc (black bars). After 2 h of incubation, subsamples of the discs were either washed three times with Mes buffer before analyzing their Suc content or frozen directly in liquid nitrogen to measure AGPase activity under selective (Vsel; −DTT) and reductive (Vred; +DTT) assay conditions to calculate the activation state of the enzyme (Vsel/Vred). Suc levels and AGPase activation in tuber slices directly after cutting (white bars) are shown for comparison.
Results are means ± se; n = 4 replicates from separate tubers. FW, fresh weight.
Table 3.
Suc Feeding Leads to Increased AGPase Redox Activation, Increased Starch Synthesis, and Decreased Hexose Phosphate Levels in Discs from Growing Tubers
| Parameter | Control | 200 mM Suc |
|---|---|---|
| Suc content (μmol/g fresh wt) | 11.6 ± 0.4 | 22.2 ± 1.4 |
| AGPase activation [Vsel/Vred] (%) | 33 ± 2 | 49 ± 3 |
| Starch synthesis (% of total 14C absorbed) | 40 ± 3 | 59 ± 3 |
| Hexose phosphate level (nmol/g fresh wt) | 225 ± 20 | 148 ± 11 |
Tuber discs were cut from an intact growing tuber and incubated in buffer with and without 200 mM Suc for 2 h before they were either washed three times to analyze their Suc content or frozen immediately in liquid nitrogen to measure hexose phosphate levels and AGPase activity under selective (Vsel; −DTT) and reductive (Vred; +DTT) assay conditions. In parallel samples, 2 mM 14C-Glc kBq/μmol specific activity of 18.5) was provided to measure label incorporation into starch. Results are means ± se (n = 3 replicate measurements on different discs from the same tuber).
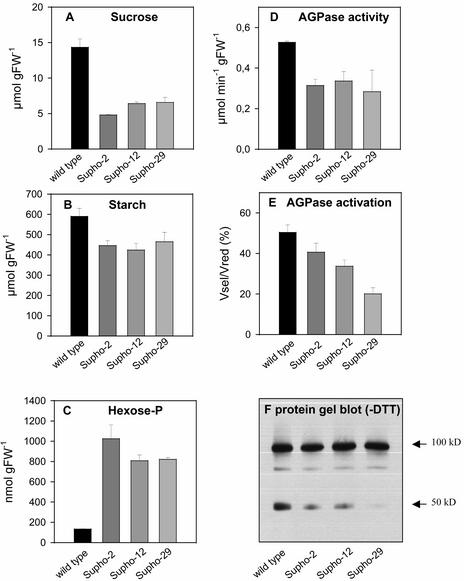
Inhibition of Starch Synthesis in Transgenic Tubers Overexpressing Suc Phosphorylase Involves the Inactivation of AGPase
In the second approach, heterologous expression of bacterial Suc phosphorylase in the cytosol was used as a tool to decrease Suc levels in planta (Trethewey et al., 2001). Suc phosphorylase converts Suc and Pi to Fru and Glc-1-P. This resembles the endogenous pathway for Suc breakdown via SuSy and UGPase in not producing Glc but differs because it does not require PPi or produce UDP-Glc as an intermediate. Critically, Suc phosphorylase has a far lower Km(Suc) (∼1 mM; Silverstein et al., 1967) than SuSy (40 to 200 mM; Avigad, 1982).
Introduction of Suc phosphorylase led to a threefold to fourfold decrease of Suc (Figure 9A). Glc did not change (data not shown) (Trethewey et al., 2001), UDP-Glc decreased slightly, PPi showed a slight and nonsignificant increase (data not shown), starch decreased (Figure 9B), and hexose phosphates (Figure 9C) and 3PGA (data not shown) (Trethewey et al., 2001) increased markedly. The inhibition of starch synthesis in the transformants was accompanied by a 30 to 50% decrease of overall AGPase activity (Figure 9D). There also was a marked decrease of the AGPase activation state (Figure 9E) and a marked decrease in the intensity of the 50-kD immunosignal relative to the 100-kD signal in nonreductive SDS-PAGE (Figure 9F).
Figure 9.
Redox Activation State and Dimerization Degree of AGPase in Transgenic Tubers Expressing a Heterologous Suc Phosphorylase in the Cytosol.
Tubers of 8-week-old wild-type plants and three independent transgenic lines were analyzed for Suc content (A), starch (B), hexose phosphates (C), overall AGPase activity when DTT was included in the assay (Vred) (D), redox activation state of AGPase (Vsel/Vred) (E), and dimerization of AGPB protein in nonreductive SDS-PAGE using the maize brittle-2 antibody (F). Each lane contains the proteins originating from 1 mg fresh weight. Results are means ± se; n = 3 tubers from different plants, except for (F), which documents a representative example. FW, fresh weight.
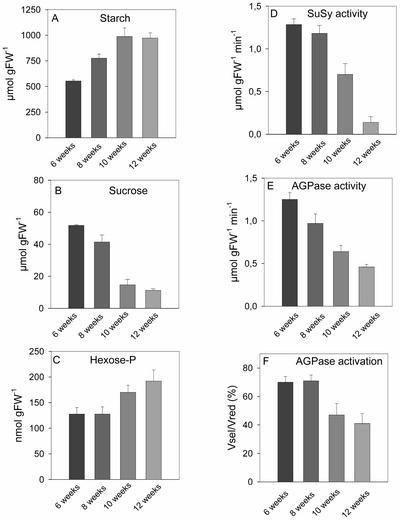
Redox Inactivation of AGPase Correlates with the Decrease in Suc and the Inhibition of Starch Accumulation as Potato Plants Age
The experiments shown in Figures 2 to 9 used tubers from 8-week-old plants, which contain high levels of Suc. A third approach investigated changes in wild-type tubers as the mother plant ages. Tuber starch levels increased on a fresh weight basis between 6 and 10 weeks but did not increase further between 10 and 12 weeks (Figure 10A). Suc levels decreased slightly between 6 and 8 weeks and decreased markedly at 10 and 12 weeks (Figure 10B) (Mares and Marschner, 1980; Merlo et al., 1993). Hexose phosphate levels increased between 10 and 12 weeks, revealing that starch synthesis is inhibited more strongly than Suc breakdown (Figure 10C). As shown previously (Mares and Marschner, 1980; Merlo et al., 1993), SuSy activity decreased sharply (Figure 10D) and overall AGPase activity decreased markedly (assayed with DTT; Figure 10E) as the plant aged. There also was a marked decrease of AGPase activation (Figure 10F).
Figure 10.
Redox Activation State of AGPase in Tubers from Wild-Type Plants of Different Developmental Stages.
Tubers were harvested from 6-, 8-, 10-, and 12-week-old plants and analyzed for starch levels (A), Suc levels (B), hexose phosphate levels (C), SuSy activity (D), AGPase activity (Vred) (E), and redox activation state of AGPase (Vsel/Vred) (F). Results are means ± se; n = 3. FW, fresh weight.
DISCUSSION
A Novel Mechanism Involving Reductive Post-Translational Regulation of AGPase Inhibits Starch Synthesis after Detachment of Tubers from the Mother Plant
The starting point for the experiments presented here was the observation (Geigenberger et al., 1994) that removal of growing potato tubers from the mother plant leads within 1 day to an inhibition of starch synthesis that cannot be explained easily by allosteric regulation or by changes in the expression of AGPase. This led us to suspect that there might be a major gap in our understanding of the regulation of starch synthesis.
The first step in identifying the missing regulatory mechanism was to determine the step(s) at which flux was being inhibited. Substitution of higher plant AGPase with a heterologous bacterial AGPase provided genetic evidence that the inhibitory mechanism required the presence of native potato AGPase (Figure 2). To provide biochemical evidence that AGPase is the unique site at which flux is regulated, we performed a systematic investigation of the subcellular levels of every metabolite in the pathway between Suc and starch. This allowed the mass-action ratio (the product/substrate ratio) to be estimated for every enzyme and transport step between Suc in the cytosol and starch synthesis in the plastid. AGPase was the only step at which this ratio decreased (by 2 orders of magnitude) after detachment of the tubers (Table 2). These genetic and biochemical experiments demonstrate (1) that the regulatory mechanism acts at AGPase and (2) that AGPase is the sole step involved in the inhibition of starch synthesis after tuber detachment.
The second step was to exclude the possibility that known mechanisms are responsible for the inhibition of AGPase activity. Measurements of 3PGA and Pi levels in the plastid showed that the 3PGA/Pi ratio increased after detachment of tubers. Although there was a marked decrease in the transcript levels for AGPB and AGPS1, there were no changes of AGPB protein or AGPase activity. AGPase activity also changes much more slowly than transcript levels during the photoperiod in Arabidopsis leaves (Sokolov et al., 1998) and potato tubers (Geigenberger and Stitt, 2000). Furthermore, studies with antisense AGP transformants have shown that large changes in the levels of AGP transcripts are required to produce a significant change in AGPase activity (Müller-Röber et al., 1992) and that quite large changes of AGPase activity are required to produce a significant inhibition of starch synthesis (Müller-Röber et al., 1992; Geigenberger et al., 1999a).
The last step was to identify the missing mechanism. Since Sowokinos (1981) found that potato AGPase activity is stimulated by DTT, it has been included routinely in the extraction and assay media. An important clue was provided by in vitro experiments by Preiss and co-workers in which they found that when the higher plant heterotetrameric AGPase is overexpressed heterologously in E. coli, it is inactivated partly by an intermolecular disulfide bond that forms between Cys residues in the N-terminal regions of the AGPB subunits (Iglesias et al., 1993; Ballicora et al., 1995; Fu et al., 1998). Following up this clue, we investigated the biochemical and kinetic properties of AGPase extracted from tubers in the absence of DTT using methods adapted to allow rapid extraction in the absence of oxygen.
We found that AGPB exists as a mixture of monomers and dimers in growing tubers and is converted almost completely to dimers in detached tubers. Furthermore, dimerization led to inactivation, as a result of a strong decrease of the substrate affinity, an increased requirement for and decreased sensitivity to activation by 3PGA, and an increased sensitivity to inhibition by Pi. The change in the kinetic properties was especially dramatic when AGPase activity was assayed in the forward reaction. Dimerization and inactivation both were reversed by incubating extracts with DTT.
We interpret these results as evidence that the redox changes identified by Preiss and co-workers in heterologously overexpressed AGPase operate as a regulatory mechanism in planta. However, biochemical analyses of the position of the Cys bridges in AGPase holoenzyme isolated from plants is needed to confirm this and to exclude the possibility that other intermolecular or intramolecular Cys bridges also form in planta. Final genetic proof that this mechanism is responsible for the inhibition of starch synthesis after detachment of tubers would require the production of transformants in which the native AGPB is replaced by a modified form of AGPB in which Cys-82 is modified to prevent the formation of the intermolecular bridge. This experiment would require a mutant line in which transcription of the native AGPB is blocked to allow effective overexpression without cosuppression of the modified construct. At present, this is not possible in potato.
Redox Modulation of AGPase Allows Starch Synthesis to Be Increased, Whereas the Levels of Phosphorylated Metabolites Decrease
The dramatic changes in the kinetic properties of AGPase produced by redox modulation explain why starch synthesis is inhibited after tuber detachment even though there are changes in the levels of substrates and allosteric effectors that would otherwise strongly stimulate starch synthesis. To provide independent biochemical evidence that redox activation allows the rate of starch synthesis to be increased in planta in the face of diametrically opposed changes in the levels of substrates and metabolite effectors, discs were prepared from tubers and incubated with DTT to achieve an artificial activation of AGPase. This led to a strong stimulation of starch synthesis and a marked decrease in the levels of phosphorylated metabolites.
Post-Translational Redox Modulation of AGPase Also Makes an Important Contribution to the Regulation of Starch Synthesis in Tubers in Other Conditions
Other situations have been reported in which the rate of starch synthesis in potato tubers changes independently of overall AGPase activity and reciprocally to the changes in the levels of phosphorylated intermediates. When Suc is supplied to potato tuber slices (Geiger et al., 1998), starch synthesis is stimulated even though phosphorylated intermediates, including 3PGA, decrease. When Suc phosphorylase is overexpressed in growing tubers (Trethewey et al., 2001), starch synthesis is inhibited even though phosphorylated intermediates increase and AGPase activity decreases only slightly. The inhibition of starch synthesis in tubers as the mother plant ages is not linked to a general decrease of phosphorylated metabolites (Merlo et al., 1993).
These results can be explained by post-translational redox modulation of AGPase. Feeding Suc to discs led to increased activation of AGPase and stimulated the rate of starch synthesis (Figure 8, Table 3), the heterologous expression of Suc phosphorylase led to redox inactivation of AGPase and a lower tuber starch content (Figure 9), and the gradual changes in the sink-source balance as the mother plant aged led to a decrease of AGPase activation. In the latter two cases, there also was a 30 to 40% decrease of overall AGPase activity, but this alone (see above) is not large enough to explain the increase in hexose phosphates and the decrease in the rate of starch synthesis.
Post-Translational Redox Modulation of AGPase Represents a Component in a Novel Regulatory Pathway That May Link the Rate of Starch Synthesis to Suc Supply
Although the evidence from the individual experiments is circumstantial, together, our findings indicate that post-translational redox regulation of AGPase is part of a novel regulatory loop that links the rate of starch synthesis to changes in the Suc supply. Detachment of tubers led to a sudden interruption of phloem import, and aging of the mother plant led to a gradual change in sink-source relations. In both cases, the decreased rate of Suc import led to a decrease in the tuber Suc content (Figures 2D, 3I, and 10B) (Merlo et al., 1993), which correlates with the redox inactivation of AGPase. In these two treatments, of course, the import of other compounds as well as Suc was interrupted.
When tuber discs were incubated in the absence of Suc, the internal Suc level of the discs decreased and there was a decrease of AGPase activation that could be reversed by the addition of Suc (Figure 9, Table 3). Overexpression of Suc phosphorylase to alter the Suc level and the precise way in which Suc was metabolized in the tuber also led to redox inactivation of AGPase (Trethewey et al., 2001) (Figure 10). Furthermore, in an antisense AGPB transformant line in which AGPase activity was reduced by 40 to 50%, there was an increase of the tuber Suc level (Geigenberger et al., 2000) and a decrease in the dimerization and an increase of the activation state of AGPase (data not shown).
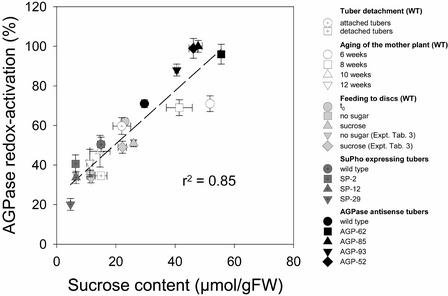
Figure 11 summarizes the data on tuber Suc content and AGPase activation from these different experiments, which were performed over a period of 2 years with many separate batches of plants grown under different conditions. By comparing the two parameters, a correlation coefficient of 0.85 was found. These results provide very strong correlative evidence that post-translational redox modulation of AGPase responds to changes in Suc availability, although it is not possible at this time to determine whether a specific influx, a particular Suc pool, or changes in related metabolites provide the immediate stimulus for the transduction pathway.
Figure 11.
Correlation between Tuber Suc Level and AGPase Redox Activation.
Data were taken from Figures 8B and 8C and Table 3 (feeding to tuber discs), Figures 9A and 9D (Suc phosphorylase–expressing tubers), and Figures 10B and 10F (aging of mother plant). Data from another tuber detachment experiment with wild-type plants and from transgenic tubers with decreased expression of AGPase are included (means ± se; n = 4). FW, fresh weight.
Candidate Components of Signal Transduction That Lead to the Redox Modulation of AGPase Have Yet to Be Identified and Confirmed
The light-dependent redox modulation of Calvin cycle enzymes and enzymes involved in ATP synthesis and NADPH export in chloroplasts is mediated by thioredoxin f and thioredoxin m, respectively (Schümann and Jacquot, 2000). Photosynthetic electron transport leads to a reduction of ferredoxin, and reducing groups then are transferred by ferredoxin:thioredoxin reductase to the thioredoxins, which react with their targets. Oxidation is thought to occur primarily by a reversal of this process. The reaction between the thioredoxins and their individual targets is modulated by changes in pH, Mg2+, and the levels of their substrates or products, which alter the mid-redox potential of the Cys groups on enzymes (Scheibe, 1991; Schümann and Jacquot, 2000).
Ballicora et al. (2000) showed in in vitro experiments with heterologously overexpressed AGPase that DTT can be replaced by thioredoxin f isolated from spinach. More studies are needed to identify which thioredoxin(s) interacts with AGPase in planta and to determine how the flow of electrons to AGPase is regulated. Suc may lead to a large increase in the redox state of the plastid or, alternatively, may act in some way to modulate the transfer of electrons from NADPH over thioredoxin to AGPase.
Finally, our results raise the question of whether the post-translational regulation of AGPase contributes to the regulation of starch synthesis in other organs. Two lines of evidence indicate that this may be the case. First, anomalies similar to those that prompted our investigations on tubers have been reported for other tissues. Starch synthesis changed independently of overall AGPase activity and the levels of phosphorylated intermediates after phloem transport was inhibited by detaching cotyledons of germinating Ricinus seedlings (Geigenberger and Stitt, 1991), when spinach leaves were cold-girdled to decrease export (Krapp and Stitt, 1995), when sugars were supplied to detached spinach leaves (Krapp et al., 1991) or heterotrophic Chenopodium rubrum suspension cells (Hatzfeld et al., 1990), and in leaves of transgenic tobacco plants when phloem transport was inhibited by the phloem-specific expression of E. coli pyrophosphatase (Geigenberger et al., 1996). Intriguingly, these all involve manipulations that were shown to or are likely to alter sugar export or sugar levels.
Second, the QTCL motif is conserved in the N-terminal region of almost all sequenced AGPB genes of dicots and in some of the sequences available for monocots. Interestingly, the QTCL motif is absent in the major AGPB transcript in cereal endosperm (Thorbjornsen et al., 1996; Hannah et al., 2001), which presumably encodes the cytosolic enzyme. This finding indicates that other mechanisms may be required to link starch synthesis to the Suc supply during the late stages of cereal grain filling.
The QTCL motif is absent in E. coli AGPase (glgC) and in cyanobacterial AGPase and also is absent in the small subunit of the heterotetrameric Chlamydomonas reinhardtii AGPase (STA6) (Zabawinski et al., 2001), indicating that this post-translational regulatory mechanism was developed during the evolution of higher plants, possibly in parallel with the evolution of the use of Suc as a transport metabolite and of thioredoxin function in photosynthetic eukaryotes.
This comparison also implies that it may be necessary to reevaluate the interpretation of experiments in which the overexpression of glgC16 in higher plants was shown to lead to a strong stimulation of starch synthesis (Stark et al., 1992). This stimulation has been taken to date as evidence for the crucial role of the allosteric properties of AGPase in the regulation of starch synthesis. However, it is likely that this genetic switch also interrupts the post-translational redox inactivation of AGPase. The tuber detachment experiments with the double-transformed AF1 lines expressing an AGPase but lacking the QTCL motif and lacking redox modulation suggest this possibility.
Regulation of AGPase at the Level of Expression, Post-Translational Regulation, and Allosteric Regulation
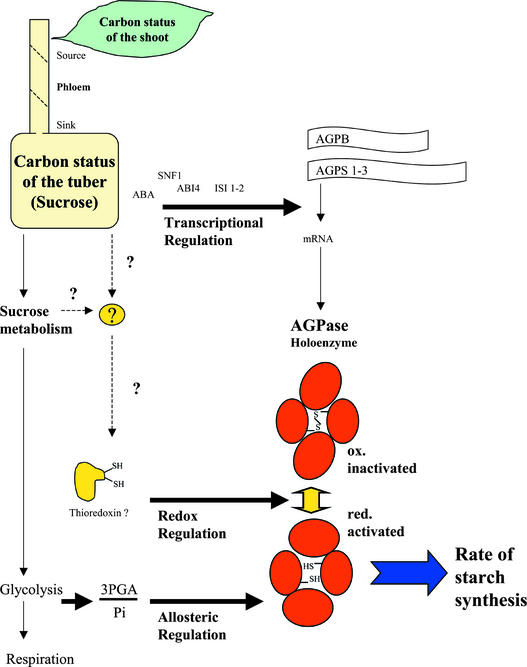
In conclusion, our results show that the redox modulation of AGPase provides a powerful mechanism to regulate starch synthesis. It was overlooked until now because it is reversed rapidly under the standard methods used to extract and analyze AGPase protein and activity. Figure 12 presents a model showing how this novel mechanism combines with known mechanisms to coordinate AGPase activity, forming a network that allows starch synthesis in potato tubers to respond across a range of time scales to a variety of physiological and environmental stimuli.
Figure 12.
AGPase Is a Key Enzyme for the Regulation of Starch Synthesis in Potato Tubers.
Redox modulation of AGPase provides a novel mechanism that combines with known mechanisms to coordinate AGPase activity in a network that allows starch synthesis to respond across a range of time scales to a variety of physiological and environmental stimuli. Allosteric control by 3PGA and Pi operates in a time frame of seconds to adjust the rate of starch synthesis to the balance between Suc breakdown and respiration. Post-translational redox modulation leads to changes in AGPase activity in a time frame of ∼30 to 60 min. Activation occurs in response to factors directly or indirectly related to increased Suc availability and leads to stimulation of starch synthesis and decreased glycolytic metabolite levels. The signaling components leading to redox modulation of AGPase are unknown and may involve thioredoxins as well as putative sugar sensors. Transcriptional regulation in response to changes of Suc allows more gradual changes in AGPase activity, which can require days to develop. ABA, abscisic acid; ABI, abscisic acid insensitive; ISI, insensitive for Suc induction; SNF1, Suc non-fermenting1.
Allosteric control by 3PGA and Pi operates in a time frame of seconds to stimulate starch synthesis when phosphorylated intermediates increase and to inhibit it when they decrease. Existing physiological data indicate that allosteric control does not play a major role in coordinating the rate of starch synthesis with the Suc supply. Instead, it adjusts the rate of starch synthesis to the balance between Suc breakdown and carbon use. Conditions in which changes in the 3PGA/Pi ratio correlate with starch synthesis include high temperature, during which an increasing rate of respiration leads to a depletion of phosphorylated intermediates and an inhibition of starch synthesis (Geigenberger et al., 1998a), and mild water stress, during which post-transcriptional activation of Suc phosphate synthase stimulates the resynthesis of Suc and leads to a decrease of phosphorylated intermediates and an inhibition of starch synthesis (Geigenberger et al., 1997, 1999b).
Post-translational redox modulation leads to changes of activity in a time frame of ∼30 to 60 min. Activation occurs in responses to factors related directly or indirectly to an increase in Suc availability and leads to a stimulation of starch synthesis and a decrease in the levels of phosphorylated intermediates when Suc increases. This could have two important consequences.
First, lower levels of phosphorylated intermediates will favor Suc breakdown via the reversible reactions catalyzed by SuSy and UGPase (Geigenberger and Stitt, 1993), will relieve feedback inhibition of fructokinase by Fru-6-P (Renz and Stitt, 1993), and will decrease Suc resynthesis by inhibiting Suc phosphate synthase (Geigenberger et al., 1999b). Second, stimulation of starch synthesis via a mechanism that simultaneously decreases glycolytic intermediate levels may channel Suc toward starch without this leading to a stimulation of glycolysis and respiration. This is important because internal oxygen concentrations decrease to very low levels during tuber bulking (Geigenberger et al., 2000), and it is important to increase the flow of carbon from Suc to starch without a large increase in oxygen consumption.
Transcriptional regulation in response to changes in Suc allows more gradual changes in AGPase activity that may require days to develop. For example, during diurnal rhythms (Geigenberger and Stitt, 2000) and after detachment of tubers (Figure 4), the levels of the AGPS and AGPB transcripts change within 12 to 24 h, but AGPase activity remains high for several days (Geigenberger et al., 1994). Changes in transcription also may be responsible for the gradual decrease of overall AGPase activity on tubers as the mother plants age (Figure 10) and the slightly lower expression of AGPase in tubers that express Suc phosphorylase (Figure 9).
METHODS
Plant Material and Growth Conditions
Wild-type potato plants (Solanum tuberosum cv Desiree) were obtained originally from Saatzucht Fritz Lange (Bad Schwartau, Germany). The generation and characterization of transgenic potato plants expressing antisense ADP-Glc pyrophosphorylase (AGPase), a heterologous gene from a bacterial mutated AGPase enzyme (glgC16 from Escherichia coli), and a bacterial Suc phosphorylase in the cytosol of their tubers were described by Müller-Röber et al. (1992), Lloyd et al. (1999), and Trethewey et al. (2001), respectively.
Potato plants were grown in a growth chamber (350 μmol·m−2·s−1 irradiance at 20°C and 50% RH) under a 14-h-light/10-h-dark regime in 3-L plastic pots in soil supplemented with Hakaphos grün (100 g per 230 L of soil; BASF, Ludwigshafen, Germany) and were watered daily from the top with tap water, or they were grown in a greenhouse during the summer (16-h-light/8-h-dark regime at 20/18°C day/night and 60% RH) with supplementing light (62 400-W AgroSonT lamps per 100 m2) in 20-cm-diameter plastic pots with a 2:1 mixture of soil to sand and were irrigated daily with water (∼200 mL per plant per day) containing nutrients (0.7 g/L Hakaphos rot [8% N, 12% P, 24% K, and 4% Mg]).
Reagents
Enzymes, reagents, and kits for molecular biology were purchased from Roche (Mannheim, Germany), MBI Fermentas (St. Leon-Rot, Germany), Qiagen (Hilden, Germany), and Stratagene (Heidelberg, Germany). Biochemical enzymes were purchased from Roche and Sigma (München, Germany), chemicals were purchased from Roche, Merck (Darmstadt, Germany), and Sigma, and reagents for SDS-PAGE were purchased from Bio-Rad (München). Radiochemicals and x-ray films were purchased from Amersham-Buchler (Braunschweig, Germany). If not stated otherwise, enzymes were grade II and chemicals were of analytical purity.
Tuber Detachment Experiments
Tuber detachment experiments were performed as described by Geigenberger et al. (1994) and were started in the second half of the light period (6 to 8 h into the photoperiod) using 8- to 9-week-old plants. Tubers of these plants contained high Suc synthase activity, which is indicative of rapidly growing tubers.
Sampling of Potato Tuber Tissue
Immediately after harvesting the tuber, a cylinder was cut perpendicular to the stolon-apex axis. Tuber slices that were incubated subsequently with radioactive Glc were cut (8 mm diameter, thickness of 2 mm) and preincubated in 10 mM Mes-KOH, pH 6.5, for 10 to 20 min. Tuber tissues sampled for direct analysis or for analysis after nonaqueous fractionation were sliced immediately into liquid nitrogen and stored at −80°C until use. Metabolic gradients have been reported for potato tubers along the stolon-apex axis (Merlo et al., 1993) and across the tuber (Geigenberger et al., 2000). Therefore, tuber slices were taken from the middle of the tuber, avoiding the outer 3 mm and the tuber skin.
Labeling Experiments with Intact Tubers
Radioactive labeling experiments were performed essentially as described by Geigenberger et al. (1994). To measure carbon fluxes in whole tubers, a 1-mm borehole was made (perpendicular to the stolon-apex axis) into intact or detached tubers using a hypodermic needle. Radioactive Glc (U-14C-Glc; specific activity of 11.5 GBq/mmol) was injected into the borehole, the end was sealed, and after 1 h, a concentric cylinder of 8 mm diameter surrounding the injection hole was cut and frozen in liquid nitrogen. The frozen tissue was weighed before extraction.
Labeling Experiments with Tuber Slices
A cylinder was cut perpendicular to the stolon-apex axis to prepare slices (8-mm diameter, thickness of 2 mm) from intact or detached tubers. Slices were washed briefly in 10 mM Mes-KOH, pH 6.5, blotted with tissue paper, and then incubated for 30 min or 2 h at 22°C in 4 mL of incubation buffer containing 2 mM radioactive U-14C-Glc (18.5 kBq/μmol) or U-14C-Suc (18.5 kBq/μmol) in Erlenmeyer flasks shaken at 90 rpm to maintain aerobic conditions. The incubation times, buffer compositions, and labeled compounds are specified in the figure legends. For each treatment, 10 slices from four different tubers were used (2 slices per replicate sample). After incubation, the discs were washed (three times for 30 s each) in nonlabeled incubation buffer, blotted briefly with paper, and frozen in liquid nitrogen.
Fractionation of 14C-Labeled Potato Tissue
Tuber material was extracted with 80% (v/v) ethanol at 80°C (1 mL/0.1 g fresh weight) and reextracted in two subsequent steps with 50% (v/v) ethanol (1 mL/0.1 g fresh weight at each step). The combined supernatants were dried under an air stream at 40°C, taken up in 1 mL of water, and separated into ionic (phosphate esters, organic acids, and amino acids) and neutral (Suc, Glc, and Fru) components by ion exchange and thin layer chromatography as described by Geigenberger et al. (1997). The residue was analyzed for label in starch as described by Merlo et al. (1993).
Nonaqueous Fractionation of Tuber Tissue
Potato tuber tissue was fractionated as described by Farré et al. (2001), except that (1) five fractions (termed pellet, 0, 1, 2, and 3) were collected from the nonaqueous density gradients and (2) the mass of the dried total aliquot was determined to allow the results to be expressed per gram dry weight. The subcellular distribution of each metabolite was estimated from the distribution of marker enzymes and the metabolite between the gradient fractions, based on equations given by Stitt et al. (1989). The equations were solved to a best fit by the method of least-squares values using an algorithm based on the solver function incorporated into Excel version 5.0 (Microsoft, Redmond, WA). This allowed direct and rapid calculation and evaluation on one spreadsheet using recovery values (percentage of the value found in the total aliquot) of marker enzymes and of metabolites to make the calculations and regressions for subcellular compartmentation. It also allowed more efficient detection of analytic errors than earlier methods.
By using a higher number of fractions (five) than the number of analyzed compartments (three), the equation system is overdetermined, making the results for subcellular distribution more reliable and robust. Even when the data for one fraction is deleted, the regressions can predict a reliable value (data not shown). Estimates for the subcellular volumes of growing potato tuber tissue derived from planimetry (Farré et al., 2001) were used to calculate the metabolite concentrations in each compartment (vacuole, 3.384 mL/g dry weight; plastid, 0.747 mL/g dry weight; cytosol, 0.655 mL/g dry weight). There were no significant changes in subcellular volumes after detachment (data not shown).
For each gradient, an aliquot of the initial homogenate was taken to determine the recovery values of enzymes applied to the gradient and to determine the dry weight of the potato tissue. The recoveries of enzymes were 72, 82, 89, 78, and 81% for mannosidase, AGPase, UDP-Glc pyrophosphorylase, pyrophosphate:Fru-6-P1 phosphotransferase, and citrate synthase, respectively. Analysis of starch levels in the different fractions revealed that starch is 100% located in the plastids. As has been documented for leaves (Stitt et al., 1989), the distribution of enzymes tracks the distribution of metabolites that are known to be restricted to a particular compartment.
Metabolite Analysis
Tuber material was extracted either directly or after subcellular fractionation (see above) in trichloroacetic acid as described by Weiner et al. (1987) and Jellito et al. (1992); the reliability of the method has been documented by Jellito et al. (1992). Nucleotides were measured by HPLC according to Geigenberger et al. (1997) using a Partisil-10-SAX ion exchange column (4.6 mm × 250 mm; Whatman) and detection at 254 and 230 nm. The nucleotides were identified by comparison of the elution times of authentic standards and the 254/230-nm ratio of absorbance. The metabolite amount was calculated from the peak area using Datasystem 450 MT2 software (Kontron, Munich, Germany).
The levels of Glc-1-P, Glc-6-P, Fru-6-P, UDP-Glc, glycerate-3-phosphate (3PGA), Pi, Suc, and starch were measured as described by Geigenberger et al. (1998b). Inorganic pyrophosphate (PPi) and ADP-Glc were measured in the 96-well format by enzymatic cycling assays (Gibon et al., 2002), except that arsenolysis was not used in the case of PPi determination. Instead, aliquots of the samples were preincubated for 30 min in the presence of glycerokinase, glycerol-3-phosphate oxidase, and glycerol and then heated at 95°C for 20 min before determination of PPi, to decrease the amount of interfering metabolites.
Analysis of Overall Enzyme Activities in Potato Tubers
The activities of Suc synthase and AGPase were measured in desalted tuber extracts as described by Merlo et al. (1993). As detailed below, AGPase also was measured using a newly developed protocol.
Analysis of AGPase Activity and Kinetic Properties
A special extraction and assay protocol was developed to reveal the in planta AGPase activity and redox activation state. The extraction buffer (50 mM Hepes-KOH, pH 7.8, and 5 mM MgCl2) was degassed by bubbling with nitrogen. Tuber discs were prepared rapidly (see above) and frozen in liquid nitrogen. The discs (∼100 mg fresh weight) were homogenized to a powder under liquid nitrogen, extracted with 1 mL of extraction buffer, and centrifuged for 30 s at 10,000g at 4°C; 10 μL of the supernatant was used for the AGPase assay. The entire procedure lasted <2 min. AGPase activity was followed on line and was linear for up to 30 min. The 96-well format allowed us to perform several comparative measurements of different samples at the same time.
AGPase assay in the pyrophosphorolysis direction was performed at 30°C in a total volume of 200 μL containing 50 mM Hepes-KOH, pH 7.8, 5 mM MgCl2, 10 μM Glc-1,6-bisP, 0.6 mM NADP+, 2.5 mM Na-PPi, 1 unit/mL phosphoglucomutase (from rabbit muscle), 2.5 units/mL Glc-6-P dehydrogenase (from yeast), and varying concentrations of ADP-Glc (0 to 1 mM) with or without 5 mM DTT. The assay lacking DTT was termed Vsel, and the assay containing 5 mM DTT was termed Vred.
The reaction was started by adding freshly prepared extract. Parallel assays were performed lacking either Na-PPi or ADP-Glc. Blank activity was negligible for at least 40 min and was subtracted when detected. To analyze the kinetic properties of AGPase, the activator 3PGA or the inhibitor Pi was included in the assay mixtures as indicated in the figure legends. To redox activate AGPase, extract was preincubated for 10 min at 4°C by adding 1 mM ADP-Glc and 5 mM DTT before transfer to the Vred assay. The ratio of Vsel to Vred of the phosphorolysis reaction at 600 μM ADP-Glc and without Pi and 3PGA was termed the activation state of AGPase.
AGPase activity in the ADP-Glc–forming direction was analyzed in a stopped assay. The assay contained 50 mM Hepes-KOH, pH 7.8, 5 mM MgCl2, 1.5 mM Glc-1-P, and 0 to 3 mM ATP in a total volume of 200 μL. The assay was started by the addition of 10 μL of extract (see above), incubated at 30°C for 15 min with shaking at 900 rpm, and stopped by incubating at 95°C for 5 min. The allosteric activator 3PGA or the inhibitor Pi was included in the assays as indicated in the figure legends. The stopped assays were kept at 4°C until quantification. ADP-Glc was quantified via HPLC using a short (25-min) program adapted to separate AMP, ADP-Glc, ADP, and ATP (Geigenberger et al., 1994).
RNA Gel Blot Analysis
Total RNA was isolated from different pools of tubers (two samples per condition) according to Logemann et al. (1987), and RNA gel blot analysis was performed according to Sambrook et al. (1989). Equal loading and transfer were confirmed by staining with ethidium bromide (data not shown). Radioactive hybridization probes were prepared with the random priming labeling kit (Boehringer Mannheim) using 32P-dCTP and following the manufacturer's instructions. EcoRI restriction fragments of plasmid B22-1 and plasmid S25-1 (Müller-Röber et al., 1990; La Cognata et al., 1995) were used as hybridization probes for AGPB and AGPS1, respectively.
Protein Gel Blot Analysis
Frozen plant material was extracted directly with 1 × sample buffer (62.5 mM Tris-Cl, pH 6.9, 2% SDS, 10% glycerol, and 0.02% bromphenol blue, supplemented with 1 mM benzamidine, 1 mM ε-amino caproic acid, 1 mM EDTA, and 1 mM EGTA) that had been degassed either by boiling the buffer or on an argon line. For every milligram of plant material, 5 μL of buffer was added. After centrifugation of the sample for 30 s, the supernatant was boiled immediately for 5 to 10 min. To a part of the sample, 4 mM DTT was added (+DTT), whereas the rest was used as prepared (−DTT). Equal amounts of both samples were loaded onto a 10% acrylamide gel containing SDS (Laemmli, 1970) and separated. After transfer of the proteins to polyvinylidene difluoride, the membrane was incubated with a rabbit antibody raised against the brittle-2 protein (AGPB) from maize (Giroux and Hannah, 1994) and a peroxidase-conjugated anti-rabbit antibody (Bio-Rad). Peroxidase activity was detected on x-ray film by enhanced chemiluminescence.
Heterologous Expression of Potato AGPB and AGPS1 in E. coli
The potato sequences of the presumed mature proteins of AGPB (complete SWISS-PROT entry P23509) and AGPS1 (starting at amino acid 41 of SWISS-PROT entry Q00081) were cloned into the vector pQE (Qiagen), causing the encoding of the N-terminal extension MRGSHHHHHHGSVD. The proteins were expressed in E. coli and purified under denaturing conditions on a nickel–nitrilotriacetic acid agarose column. The resulting preparations were >90 and ∼50% pure for AGPB and AGPS1, respectively. The identities of the purified preparations were confirmed by matrix-assisted laser-desorption ionization time-of-flight analysis of peptides, generated by in-gel digestion with trypsin (Walz et al., 2002), before they were used to determine the specificity of the maize brittle-2 and potato AGPB antibodies.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We are grateful to L. Curtis Hannah (University of Florida) for the gift of the maize brittle-2 antibody, to Lothar Willmitzer, Jens Kossmann, and Bernd Müller-Röber for providing transgenic plants, and to Ursula La Cognata for providing the potato AGPB and AGPS cDNAs. We are grateful to Tobias Haas, Katrin Prescha, and Melanie Höhne for excellent technical help and to Joost van Dongen for help in preparing the figures. This work was supported by the Deutsche Forschungsgemeinschaft, Grant Ge 878/1-1 (to A.T., A.B., and P.G.), and the Max Planck Society (support to J.H.M.H., M.S., Y.G., and E.M.F.). The article is dedicated to Hans W. Heldt on the occasion of his retirement.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.003640.
References
- Avigad, G. (1982). Sucrose and other disaccharides. In Encyclopedia of Plant Physiology, T.A. Loewus and W. Tanner, eds (Heidelberg, Germany: Springer), pp. 217–347.
- Ballicora, M.A., Frueauf, J.B., Fu, Y., Schürmann, P., and Preiss, J. (2000). Activation of the potato tuber ADP glucose pyrophosphorylase by thioredoxin. J. Biol. Chem. 275, 1315–1320. [DOI] [PubMed] [Google Scholar]
- Ballicora, M.A., Fu, Y., Frueauf, J.B., and Preiss, J. (1999). Heat stability of the potato ADP-glucose pyrophosphorylase: Role of Cys subunit 12 in the small subunit. Biochem. Biophys. Res. Commun. 257, 782–786. [DOI] [PubMed] [Google Scholar]
- Ballicora, M.A., Laughlin, M.J., Fu, Y., Okita, T.W., Barry, G.F., and Preiss, J. (1995). Adenosine 5′-diphosphate-glucose pyrophosphorylase from potato tuber: Significance of the N terminus of the small subunit for catalytic properties and heat stability. Plant Physiol. 109, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T., and Kende, H. (1979). Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 63, 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert, S., Grosse, H., and Heldt, H.W. (1989). Specific transport of inorganic phosphate, glucose-6-phosphate, dihydroxyacetone-phosphate and 3-phosphoglycerate into amyloplast from pea roots. FEBS Lett. 253, 183–186. [Google Scholar]
- Dancer, J.E., Neuhaus, H.E., and Stitt, M. (1990). The subcellular compartmentation of uridine nucleotides and nucleoside-5-diphosphate kinase in leaves. Plant Physiol. 92, 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré, E.M., Tiessen, A., Roessner, U., Geigenberger, P., Trethewey, R.N., and Willmitzer, L. (2001). Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, amino acids and sugar alcohols in potato tubers using a non-aqueous fractionation method. Plant Physiol. 127, 685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y., Ballicora, M.A., Leykam, J.F., and Preiss, J. (1998). Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J. Biol. Chem. 273, 25045–25052. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., Fernie, A.R., Gibon, Y., Christ, M., and Stitt, M. (2000). Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol. Chem. 381, 723–740. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., Geiger, M., and Stitt, M. (1998. a). High-temperature perturbation of starch synthesis is attributable to inhibition of ADP-glucose pyrophosphorylase by decreased levels of glycerate-3-phosphate in growing potato tubers. Plant Physiol. 117, 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger, P., Hajirezaei, M., Geiger, M., Deiting, U., Sonnewald, U., and Stitt, M. (1998. b). Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205, 428–437. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., Langenberger, S., Wilke, I., Heineke, D., Heldt, H.W., and Stitt, M. (1993). Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta 190, 446–453. [Google Scholar]
- Geigenberger, P., Lerchl, J., Stitt, M., and Sonnewald, U. (1996). Phloem-specific expression of pyrophosphatase inhibits long distance transport of carbohydrates and amino acids in tobacco plants. Plant Cell Environ. 19, 43–55. [Google Scholar]
- Geigenberger, P., Merlo, L., Reimholz, R., and Stitt, M. (1994). When growing potato tubers are detached from their mother plant there is a rapid inhibition of starch synthesis, involving inhibition of ADP-glucose pyrophosphorylase. Planta 193, 486–493. [Google Scholar]
- Geigenberger, P., Müller-Röber, B., and Stitt, M. (1999. a). Contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis is decreased by water stress in growing potato tubers. Planta 209, 338–345. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., Reimholz, R., Deiting, U., Sonnewald, U., and Stitt, M. (1999. b). Decreased expression of sucrose phosphate synthase strongly inhibits the water stress-induced synthesis of sucrose in growing potato tubers. Plant J. 19, 119–129. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., Reimholz, R., Geiger, M., Merlo, L., Canale, V., and Stitt, M. (1997). Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 201, 502–518. [Google Scholar]
- Geigenberger, P., Stamme, C., Tjaden, J., Schulz, A., Quick, P.W., Betsche, T., Kersting, H.J., and Neuhaus, H.E. (2001). Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol. 125, 1667–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger, P., and Stitt, M. (1991). A “futile” cycle of sucrose synthesis and degradation is involved in regulating partitioning between sucrose, starch and respiration in cotyledons of germinating Ricinus communis L. seedlings when phloem transport is inhibited. Planta 185, 81–90. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., and Stitt, M. (1993). Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 189, 329–339. [DOI] [PubMed] [Google Scholar]
- Geigenberger, P., and Stitt, M. (2000). Diurnal changes in sucrose, nucleotides, starch synthesis, and AGPS transcript in growing potato tubers that are suppressed by decreased expression of sucrose phosphate synthase. Plant J. 23, 795–806. [DOI] [PubMed] [Google Scholar]
- Geiger, M., Stitt, M., and Geigenberger, P. (1998). Metabolism in potato tuber slices responds differently after addition of sucrose and glucose. Planta 206, 245–252. [Google Scholar]
- Gerhardt, R., and Heldt, H.W. (1984). Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in non-aqueous media. Plant Physiol. 75, 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon, Y., Vigeolas, H., Tiessen, A., Geigenberger, P., and Stitt, M. (2002). Sensitive and high throughput metabolite assays using a novel enzymic cycling system: Utilisation to investigate the impact of decreased external oxygen concentration on energy and storage metabolism in developing Arabidopsis thaliana seeds. Plant J., 30, 221–235. [DOI] [PubMed] [Google Scholar]
- Giroux, M.J., and Hannah, L.C. (1994). ADP-glucose pyrophosphorylase in shrunken2 and brittle2 mutants of maize. Mol. Gen. Genet. 243, 400–408. [DOI] [PubMed] [Google Scholar]
- Hajirezaei, M., Sonnewald, U., Viola, R., Carlisle, S., Dennis, D., and Stitt, M. (1994). Transgenic potato plants with strongly decreased expression of pyrophosphate:fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta 192, 16–30. [Google Scholar]
- Hannah, L.C., Shaw, J.R., Giroux, M.J., Reyss, A., Prioul, J.-L., Rae, J.-M., and Lee, J.-Y. (2001). Maize genes encoding the small subunit of ADP-glucose pyrophosphorylase. Plant Physiol. 127, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld, W.D., Dancer, J.E., and Stitt, M. (1990). Fructose 2,6-bisphosphate, metabolites and “coarse” control of pyrophosphate:fructose-6-phosphate phosphotransferase during triose phosphate cycling in heterotrophic cell suspension cultures of Chenopodium rubrum. Planta 180, 205–211. [DOI] [PubMed] [Google Scholar]
- Heldt, H.W., Chon, C.J., Maronde, D., Herold, A., Stankovic, Z.S., Walker, D.A., Kraminer, A., Kirk, M.R., and Heber, U. (1977). Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplast. Plant Physiol. 59, 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, A. (1980). Regulation of photosynthesis by sink activity: The missing link. New Phytol. 86, 131–144. [Google Scholar]
- Iglesias, A.A., Barry, G.F., Meyer, C., Bloksberg, L., Nakata, P.A., Greene, T., Laughlin, M.J., Okita, T.W., Kishore, G.M., and Preiss, J. (1993). Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J. Biol. Chem. 268, 1081–1086. [PubMed] [Google Scholar]
- Jellito, T., Sonnewald, U., Willmitzer, L., Hajirezaei, M.R., and Stitt, M. (1992). Inorganic pyrophosphate content and metabolites in leaves and tubers of potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188, 238–244. [DOI] [PubMed] [Google Scholar]
- Jenner, H.L., Winning, B.M., Millar, A.H., Tomlinson, K.L., Leaver, C.J., and Hill, S.A. (2001). NAD malic enzyme and the control of carbohydrate metabolism in potato tubers. Plant Physiol. 126, 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer, B., Fischer, K., Hilpert, B., Schubert, S., Gutensohn, M., Weber, A., and Flugge, U.I. (1998). Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: The glucose 6-phosphate/phosphate antiporter. Plant Cell 10, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W.T., Franceschi, V.R., Okita, T.W., Robinson, N.L., Morell, M., and Preiss, J. (1989). Immunocytochemical localization of ADPglucose pyrophosphorylase in developing potato tuber cells. Plant Physiol. 91, 217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowsky, L. (1994). Glucose activation and metabolism through UDP-glucose pyrophosphorylase in plants. Phytochemistry 37, 1507–1515. [Google Scholar]
- Krapp, A., Quick, W.P., and Stitt, M. (1991). Rubisco, other Calvin cycle enzymes and chlorophyll decrease when glucose is supplied to mature spinach leaves via the transpiration stream. Planta 186, 58–69. [DOI] [PubMed] [Google Scholar]
- Krapp, A., and Stitt, M. (1995). An evaluation of direct and indirect mechanisms for the “sink regulation” of photosynthesis of spinach: Changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold girdling of source leaves. Planta 195, 313–323. [Google Scholar]
- Kruger, N.J. (1997). Carbohydrate synthesis and degradation. In Plant Metabolism, D.T. Dennis, D.H. Turpin, D.D. Lefebvre, and D.B. Layzell, eds (Harlow, UK: Longman), pp. 83–104.
- La Cognata, U., Willmitzer, L., and Müller-Rober, B. (1995). Molecular cloning and characterization of novel isoforms of potato ADP-glucose pyrophosphorylase. Mol. Gen. Genet. 246, 538–548. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Laing, W.A., Stitt, M., and Heldt, H.W. (1981). Control of CO2 fixation: Changes in the activity of ribulose 5-phosphate kinase and fructose- and sedoheptulose-bisphosphatase in chloroplasts. Biochim. Biophys. Acta 637, 348–359. [Google Scholar]
- Lloyd, J.R., Springer, F., Buleon, A., Müller-Röber, B., Willmitzer, L., and Kossmann, J. (1999). The influence of alterations in ADP-glucose pyrophosphorylase activities on starch structure and composition in potato tubers. Planta 209, 230–238. [DOI] [PubMed] [Google Scholar]
- Logemann, J., Schell, J., and Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163, 21–26. [DOI] [PubMed] [Google Scholar]
- MacDonald, F.D., and Preiss, J. (1986). The subcellular location and characteristics of pyrophosphate-fructose-6-phosphate phosphotransferase from suspension-cultured cells of soybean. Planta 167, 240–245. [DOI] [PubMed] [Google Scholar]
- Mares, D.J., and Marschner, H. (1980). Assimilate conversion in potato tubers in relation to starch deposition and cell growth. Ber. Dtsch. Bot. Ges. 95, 299–313. [Google Scholar]
- Martin, C., and Smith, A.M. (1995). Starch biosynthesis. Plant Cell 7, 971–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo, L., Geigenberger, P., Hajirezaei, M.R., and Stitt, M. (1993). Changes of carbohydrates, metabolites and enzyme activities in potato tubers during development, and within a single tuber along a stolon-apex gradient. J. Plant Physiol. 142, 392–402. [Google Scholar]
- Müller-Röber, B.T., Kossmann, J., Hannah, L.C., Willmitzer, L., and Sonnewald, U. (1990). One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol. Gen. Genet. 224, 136–146. [DOI] [PubMed] [Google Scholar]
- Müller-Röber, B.T., Sonnewald, U., and Willmitzer, L. (1992). Inhibition of ADP-glucose pyrophosphorylase leads to sugar storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J. 11, 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus, H., and Stitt, M. (1990). Control analysis of photosynthate partitioning: Impact of reduced activity of ADP-glucose pyrophosphorylase or plastid phosphoglucomutase on the fluxes to starch and sucrose in Arabidopsis thaliana L. Heynh. Planta 182, 445–454. [DOI] [PubMed] [Google Scholar]
- Nielsen, T.H., Krapp, A., Röper-Schwarz, U., and Stitt, M. (1998). The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by nitrogen and phosphate. Plant Cell Environ. 21, 443–455. [Google Scholar]
- Okita, T.W., Nakata, P.A., Anderson, J.M., Sowokinos, J., Morell, M., and Preiss, J. (1990). The subunit structure of potato tuber ADPglucose pyrophosphorylase. Plant Physiol. 93, 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss, J. (1988). Biosynthesis of starch and its regulation. In The Biochemistry of Plants, Vol. 14, J. Preiss, ed (San Diego, CA: Academic Press), pp. 181–254.
- Renz, A., and Stitt, M. (1993). Substrate specificity and product inhibition of different forms of fructokinase and hexokinases in developing potato tubers. Planta 190, 166–175. [Google Scholar]
- Riens, B., Lohaus, G., Heineke, D., and Heldt, H.W. (1991). Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol. 97, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston, F.S. (1972). A theoretical background to the use of measured intermediates in the study of the control of intermediary metabolism. Curr. Top. Cell. Regul. 5, 47–75. [Google Scholar]
- Salanoubat, M., and Belliard, G. (1989). The steady-state level of potato sucrose synthase mRNA is dependent on wounding, anaerobiosis and sucrose. Gene 84, 181–185. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Scheibe, R. (1991). Redox-modulation of chloroplast enzymes: A common principle for individual control. Plant Physiol. 96, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible, W.R., González-Fontes, A., Lauerer, M., Müller-Röber, B., Caboche, M., and Stitt, M. (1997). Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9, 783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott, K., Borchert, S., Müller-Röber, B., and Heldt, H.W. (1995). Transport of inorganic phosphate, and C3- and C6-sugar phosphates across the envelope membranes of potato tuber amyloplast. Planta 196, 647–652. [Google Scholar]
- Schümann, P., and Jacquot, J.-P. (2000). Plant thioredoxin systems revisited. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 371–400. [DOI] [PubMed] [Google Scholar]
- Silverstein, R., Voet, J., Reed, D., and Abeles, R.H. (1967). Purification and mechanism of action of sucrose phosphorylase. J. Biol. Chem. 242, 1338–1345. [PubMed] [Google Scholar]
- Smith, A.M., Denyer, K., and Martin, C. (1997). The synthesis of the starch granule. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 67–87. [DOI] [PubMed] [Google Scholar]
- Sokolov, L.N., Dejardin, A., and Kleczkowski, L.A. (1998). Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress). Biochem. J. 336, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos, J.R. (1981). Pyrophosphorylases in Solanum tuberosum. II. Catalytic properties and regulation of ADP-glucose and UDP-glucose pyrophosphorylase activities in potatoes. Plant Physiol. 68, 924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos, J.R., and Preiss, J. (1982). Phosphorylases in Solanum tuberosum. III. Purification, physical and catalytical properties of ADP-glucose pyrophosphorylase in potatoes. Plant Physiol. 69, 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, D.M., Timmerman, K.P., Barry, G.F., Preiss, J., and Kishore, G.M. (1992). Regulation of the amount of starch in plant tissues by ADP-glucose pyrophosphorylase. Science 258, 287–292. [DOI] [PubMed] [Google Scholar]
- Stitt, M., Huber, S., and Kerr, P. (1987). Control of photosynthetic sucrose synthesis. In The Biochemistry of Plants, Vol. 10, M.D. Hatch and N.K. Boardman, eds (New York: Academic Press), pp. 327–409.
- Stitt, M., Lilley, R.M., Gerhardt, R., and Heldt, H.W. (1989). Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 174, 518–550. [Google Scholar]
- Stitt, M., Lilley, R.M., and Heldt, H.W. (1982). Adenine nucleotide levels in the cytosol, chloroplast, and mitochondria of wheat leaf protoplasts. Plant Physiol. 70, 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt, M., and Krapp, A. (1999). The interaction between elevated carbon dioxide and nitrogen nutrition: The physiological and molecular background. Plant Cell Environ. 22, 583–621. [Google Scholar]
- Stryer, L.M. (1990). Biochemistry. (Weinheim, Germany: Verlag Chemie).
- Tauberger, E., Fernie, A.R., Emmermann, M., Renz, A., Kossman, J., Willmitzer, L., and Trethewey, R.N. (2000). Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J. 23, 43–53. [DOI] [PubMed] [Google Scholar]
- Thorbjornsen, T., Villand, P., Kleczkowski, L.A., and Olsen, O.-A. (1996). A single gene encodes two different transcripts for the ADP-glucose pyrophosphorylase small subunit from barley (Hordeum vulgare). Biochem. J. 313, 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden, J., Möhlmann, T., Kampfenkel, K., Henrichs, G., and Neuhaus, H.E. (1998). Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of starch. Plant J. 16, 531–540. [Google Scholar]
- Trethewey, R.N., Fernie, A.R., Bachmann, A., Fleischer-Notter, H., Geigenberger, P., and Willmitzer, L. (2001). Expression of a bacterial sucrose phosphorylase in potato tubers results in a glucose-independent induction of glycolysis. Plant Cell Environ. 24, 357–365. [Google Scholar]
- Trethewey, R.N., Geigenberger, P., Hajirezaei, M., Sonnewald, U., Stitt, M., Riesmeier, J., and Willmitzer, L. (1998). Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J. 15, 109–118. [DOI] [PubMed] [Google Scholar]
- Walz, C., Juenger, M., Schad, M., and Kehr, J. (2002). Evidence for the presence and activity of a complete antioxidant defence system in mature sieve tubes. Plant J. 31, 189–197. [DOI] [PubMed] [Google Scholar]
- Weiner, H., Stitt, M., and Heldt, H.W. (1987). Subcellular compartmentation of pyrophosphate and pyrophosphatase in leaves. Biochim. Biophys. Acta 893, 13–21. [Google Scholar]
- Wirtz, W., Stitt, M., and Heldt, H.W. (1982). Light activation of Calvin cycle enzymes as measured in pea leaves. FEBS Lett. 142, 223–226. [Google Scholar]
- Zabawinski, C., van den Koornhuyse, N., D'Hulst, C., Schlichting, R., Giersch, C., Delrue, B., Lacroix, J.-M., Preiss, P., and Ball, S. (2001). Starchless mutants of Chlamydomonas reinhardtii lack the small subunit of a heterotetrameric ADP-glucose pyrophosphorylase. J. Bacteriol. 183, 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]