Abstract
Using transgenic Nicotiana plumbaginifolia seedlings in which the calcium reporter aequorin is targeted to the chloroplast stroma, we found that darkness stimulates a considerable flux of Ca2+ into the stroma. This Ca2+ flux did not occur immediately after the light-to-dark transition but began ∼5 min after lights off and increased to a peak at ∼20 to 30 min after the onset of darkness. Imaging of aequorin emission confirmed that the dark-stimulated luminescence emanated from chloroplast-containing tissues of the seedling. The magnitude of the Ca2+ flux was proportional to the duration of light exposure (24 to 120 h) before lights off; the longer the duration of light exposure, the larger the dark-stimulated Ca2+ flux. On the other hand, the magnitude of the dark-stimulated Ca2+ flux did not appear to vary as a function of circadian time. When seedlings were maintained on a 24-h light/dark cycle, there was a stromal Ca2+ burst after lights off every day. Moreover, the waveform of the Ca2+ spike was different during long-day versus short-day light/dark cycles. The dark-stimulated Ca2+ flux into the chloroplastidic stroma appeared to affect transient changes in cytosolic Ca2+ levels. DCMU, an inhibitor of photosynthetic electron transport, caused a significant increase in stromal Ca2+ levels in the light but did not affect the magnitude of the dark-stimulated Ca2+ flux. This robust Ca2+ flux likely plays regulatory roles in the sensing of both light/dark transitions and photoperiod.
INTRODUCTION
A remarkable number of physiological stimuli increase cytosolic free Ca2+ levels in plant cells, including light, abscisic acid, gibberellin, touch, osmotic and oxidative stress, fungal elicitors, temperature shocks, and nodulation factors (Bush, 1995; Sanders et al., 1999). One of the most important of these stimuli that relates to the plant's response to its environment is the increase of cytosolic Ca2+ elicited by light/dark signals (Shacklock et al., 1992; Millar et al., 1994). Light-induced Ca2+ fluxes have been implicated in the entrainment of circadian oscillators in plants (Gomez and Simon, 1994), and there are circadian oscillations of cytosolic Ca2+ (and possibly of chloroplastidic Ca2+) in tobacco and Arabidopsis (Johnson et al., 1995). Ca2+ is a regulator of myriad processes in all organisms, including plants, in which a number of Ca2+-modulated proteins have been characterized, including calmodulin and a class of Ca2+-dependent but calmodulin-independent protein kinases called calcium-dependent protein kinases that are found in plants and some protozoa but are absent from animals and fungi (Roberts and Harmon, 1992).
Although much is known about the regulation of cytosolic and organellar Ca2+ in plants, most of the focus of previous research has been on the mechanisms by which cytosolic Ca2+ is controlled. The regulation of Ca2+ in organelles has tended to be relegated to determining the potential role of the organelles in regulating cytosolic Ca2+ (Moore and Akerman, 1984; Bush, 1993, 1995; Sanders et al., 1999). Two particular examples are the role of Ca2+ stores in the endoplasmic reticulum and the vacuole in regulating cytosolic Ca2+, which is mediated by inositol triphosphate, cyclic ADP ribose, and NADP (Sanders et al., 1999; Navazio et al., 2000).
The potential role of chloroplastidic Ca2+ fluxes in regulating processes within the chloroplast and contributing to the regulation of cytosolic Ca2+ has been underappreciated. Isolated chloroplasts take up Ca2+ upon illumination (Muto et al., 1982; Kreimer et al., 1985), a process that probably is mediated by Ca2+ transport across the inner envelope membrane of the chloroplast (Roh et al., 1998). Light-induced Ca2+ uptake into chloroplasts probably occurs in vivo, at least in characean algae, because in Nitellopsis, direct measurement using Ca2+-selective microelectrodes demonstrated that the cytosolic Ca2+ was lower when the plants were illuminated with strong light, and the phenomenon was dependent on photosynthetic electron transport (Miller and Sanders, 1987).
Indirect measurements implied that Ca2+ levels in the chloroplastidic stroma may increase as a result of this light-induced Ca2+ uptake (Kreimer et al., 1988), but direct measurements of stromal Ca2+ under these conditions have not been reported. Based on the Ca2+/H+ antiport into thylakoids (Ettinger et al., 1999), it is possible that the light-induced Ca2+ uptake across the inner envelope is transferred to the thylakoid or to other Ca2+ stores without influencing stromal Ca2+ levels significantly.
Calcium plays both regulatory and structural roles within the chloroplastidic stroma and thylakoids. Within the stroma, Fru-1,6-bisphosphatase (the key Calvin cycle enzyme) is activated by low concentrations and inhibited by high concentrations of Ca2+ (Hertig and Wolosiuk, 1983; Kreimer et al., 1988). Moreover, NAD kinase, which catalyzes the conversion of NAD to NADP and therefore is crucial to providing NADP for photosynthetic reduction to NADPH, is Ca2+ activated, light regulated, and present in the chloroplastidic stroma (Muto et al., 1981; Jarrett et al., 1982). High Ca2+ concentrations within the stroma tend to inhibit photosynthetic CO2 fixation (Portis and Heldt, 1976; Demmig and Gimmler, 1979).
On the other hand, within the thylakoid, functional assembly of photosystem II (PSII) and the oxygen-evolving complex requires proper assembly of polypeptides and cofactors in a process that is light and Ca2+ dependent (Becker et al., 1985; Miller and Brudvig, 1989; Grove and Brudvig, 1998). This Ca2+ dependence is relevant not only to the initial assembly of the PSII/oxygen-evolving complex and to the repair of PSII reaction centers that have been damaged by photoinhibition during a normal day (Mattoo et al., 1989) but also to the mechanism of photosynthetic oxygen evolution (Yocum, 1991). Therefore, Ca2+ is required within the thylakoid lumen for continued proper functioning of PSII during the day.
We set out to measure Ca2+ levels directly in the chloroplast stroma, especially with regard to stromal Ca2+ regulation during the day/night cycle and how it is influenced by prolonged exposure to light. The method we used was that of the Ca2+-selective photoprotein aequorin, which enables continuous noninvasive reporting of Ca2+ in transgenic plant seedlings (Knight et al., 1991). A particular advantage of the aequorin technique is that it can be targeted to specific organelles so that its Ca2+-dependent luminescence is a monitor of a specific organelle's Ca2+ level that is not confounded by signals from other compartments. Previously, we described the development of a transgenic tobacco strain in which apo-aequorin was targeted specifically to the chloroplastidic stroma (Johnson et al., 1995). In that investigation, we found that upon transfer from prolonged light to darkness, there was a large transient increase of Ca2+ that peaked at ∼20 to 25 min after the light-to-dark transition; occasionally, there was a lower level circadian oscillation of Ca2+ in darkness (Johnson et al., 1995).
Many questions remained unanswered after that study. Did these Ca2+ spikes occur after dusk every day on a regular day/night cycle? Was a prolonged light exposure necessary to achieve the Ca2+ spike and/or did a prolonged light exposure affect the magnitude of the Ca2+ spike? Was the Ca2+ spike “gated” by the circadian clock? Did the spectrum of light that was used for the exposure before darkness influence the Ca2+ spike? (If so, this might imply the involvement of a specific signaling photoreceptor.) Finally, what were the subcellular sources of the large Ca2+ flux into the stroma that was evoked by darkness? We address these questions in the current investigation.
RESULTS
Patterns of Chloroplastidic Ca2+ under Prolonged Illumination and after Transfer to Darkness
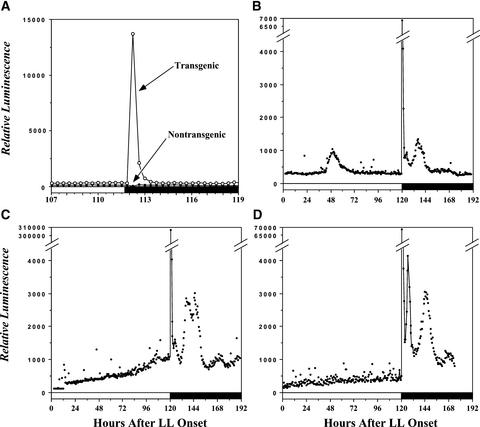
Figure 1A shows that transgenic seedlings (MAQ 6.3) expressing apo-aequorin which is targeted to the chloroplastidic stroma and incubated in coelenterazine exhibited a large burst of luminescence soon after the transition from constant white light (LL) to constant darkness (DD). This burst was not observed in nontransgenic wild-type seedlings that were incubated in coelenterazine (Figure 1A), thereby showing that delayed chlorophyll fluorescence from light excitation during the day could not account for the luminescence profiles we observed in transgenic plants (Jursinic, 1986). To illustrate the variable versus reproducible features of this dark-stimulated luminescence burst that indicates a Ca2+ flux, Figures 1B to 1D depict the patterns of stromal Ca2+ in LL for 5 days followed by DD for 3 days. In LL, there was no circadian variation in stromal Ca2+, unlike the case for cytosolic Ca2+ (Johnson et al., 1995). Sometimes there were brief episodes of fluctuations in stromal Ca2+ in LL (Figure 1B), but these were not consistent.
Figure 1.
Luminescence from Reconstituted Aequorin in Nicotiana plumbaginifolia Seedlings Expressing Aequorin That Has Been Targeted to the Chloroplast Stroma (MAQ 6.3).
Seedlings were in LL (22 μE·m−2·s−1) until the time of transfer to DD (white bars along abscissa indicate LL, and black bars indicate DD).
(A) Comparison of dark-stimulated luminescence from MAQ 6.3 seedlings (open circles) and nontransgenic wild-type seedlings (closed circles). Both MAQ 6.3 and wild-type seedlings were incubated in coelenterazine; transition to DD was at 111.7 h after the beginning of LL. There were 10 seedlings per vial, and seedlings were 10 days old.
(B) to (D) Three independent sets of MAQ 6.3 seedlings transferred from LL to DD at 120 h. There were five seedlings per vial, and seedlings were 22 days old. (C) and (D) are redrawn from Johnson et al. (1995).
Previously, we showed that the total amount of apo-aequorin expression driven by the 35S promoter of Cauliflower mosaic virus is relatively constitutive, with a possible increase over time in LL (Johnson et al., 1995). The slight increase of total apo-aequorin over time in LL may be responsible for the increase of the luminescence readings in LL (Figure 1C), or this might be attributable to continued uptake of Ca2+ into the stroma under illumination. Previously, we estimated the stromal Ca2+ level to be ∼150 to 200 nM in LL (Johnson et al., 1995), but this value might increase over time, especially in the seedlings reported in Figure 1C.
Upon transfer to DD, we reproducibly observed a large transient increase in stromal Ca2+ that began at ∼5 to 10 min and peaked at ∼20 to 25 min after the light-to-dark transition (Johnson et al., 1995; see also the expanded time scale in Figure 4A). We estimated that the peak concentration of stromal Ca2+ reached 5 to 10 μM (Johnson et al., 1995). The luminescence levels decayed from these peak values over the next several hours in DD, but often they displayed damped oscillations that appeared to have a circadian period (Figures 1C and 1D). The fact that these circadian oscillations of luminescence were damped should not be taken as proof that the stromal Ca2+ oscillations were necessarily damped in DD; the large stromal Ca2+ spike that followed the LL-to-DD transition might have discharged most of the aequorin in the stroma, so this damping might be a reflection of the depletion of the reporter. The 5- to 10-min “lag phase” could be reversed by light: if the lights were turned on before the dark-induced Ca2+ spike began, the spike was inhibited, and if the lights were turned on after the Ca2+ spike began, the spike was attenuated (data not shown).
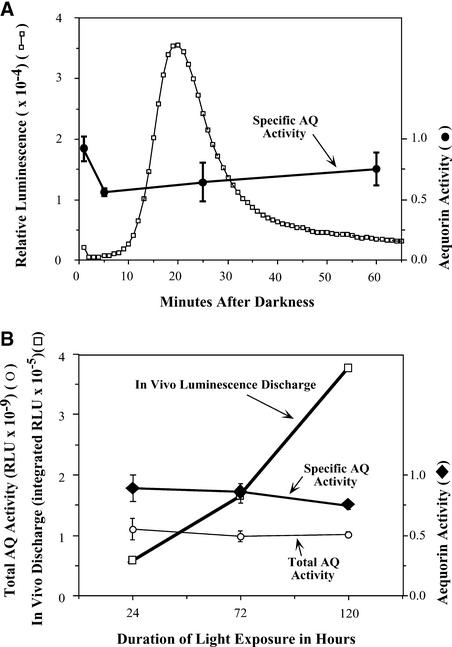
Figure 4.
Comparison of Luminescence of MAQ 6.3 Seedlings with Total Apo-Aequorin Activity at Different Times.
(A) Relative apo-aequorin activity during the first hour after the transfer from LL to DD. Seedlings had been in LL for 5 days (22 μE·m−2·s−1) before transfer to darkness. Left ordinate and open squares show in vivo luminescence of seedlings (in relative light units [RLU]); the abscissa shows time after the LL-to-DD transition in minutes.
(B) Apo-aequorin activity of seedlings that had been exposed to different durations of previous light exposure (22 μE·m−2·s−1). Left ordinate and open squares show in vivo luminescence of seedlings integrated for every minute for 90 min; left ordinate and open circles show total apo-aequorin activity of each sample extracted and assayed as for specific apo-aequorin activity (total activity, not normalized to protein concentration); the abscissa shows the duration of light exposure before the LL-to-DD transition; right ordinates and closed symbols show specific activity of apo-aequorin (normalized to protein concentration) extracted at the times indicated from seedlings under the same conditions as in the samples used for luminescence recordings.
Extracted samples (both total and specific) were prepared in triplicate, and apo-aequorin activity was measured in extracts as described in Methods. Error bars indicate standard error of mean. AQ, apo-aequorin; RLU, relative light units.
Dark-Stimulated Luminescence Emanates from Plant Tissues That Have Chloroplasts
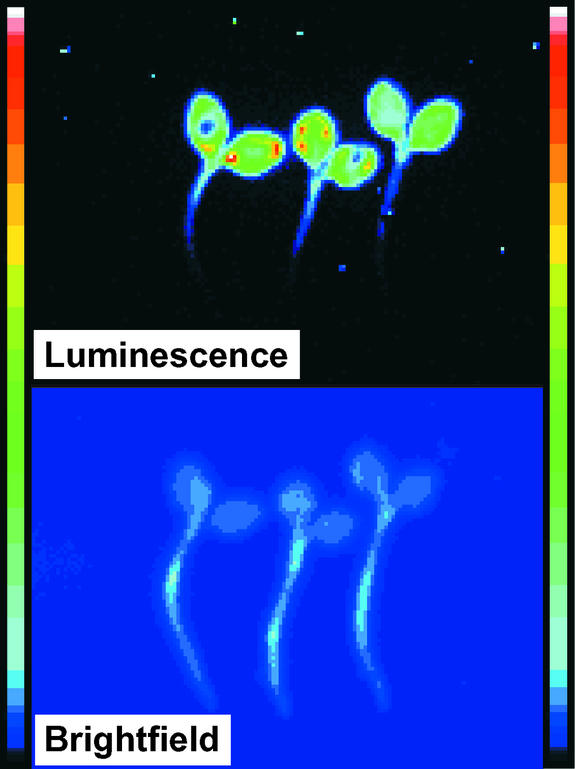
Figure 2 shows the integrated spatial pattern for the luminescence of three MAQ 6.3 seedlings during the first 30 min after the LL-to-DD transition. The relative intensity of luminescence was strongest from the cotyledons, less from the hypocotyl, and almost nonexistent from the rootlets. This spatial distribution corresponds closely to that of chloroplasts in the seedlings and confirms the correct targeting of the apo-aequorin that would be expected from the targeting sequence (rbcS; see Methods) and that was measured previously by Percoll gradient centrifugation of extracts (Johnson et al., 1995).
Figure 2.
Imaging of Luminescence from Three MAQ 6.3 Seedlings.
The top shows luminescence emission from seedlings after transfer from LL (5 days at 22 μE·m−2·s−1) to DD. The image shown represents an integration of the light emission during the first 30 min after the LL-to-DD transition. Integrations from 30 to 60 min and from 60 to 90 min showed the same distribution, but the intensity of luminescence was lower. The bottom shows images of the same seedlings in a bright field (i.e., under room lighting) to indicate the full size of the seedlings. The resolution of the bright-field images is not optimal; for example, the relative widths of the hypocotyls and rootlets are larger in the image than in reality. The color bars on both sides are the pseudocolor scales, with dark blue being dimmest and white being brightest.
Do the Duration and Spectrum of the Light Exposure Affect the Magnitude of the Ca2+ Spike?
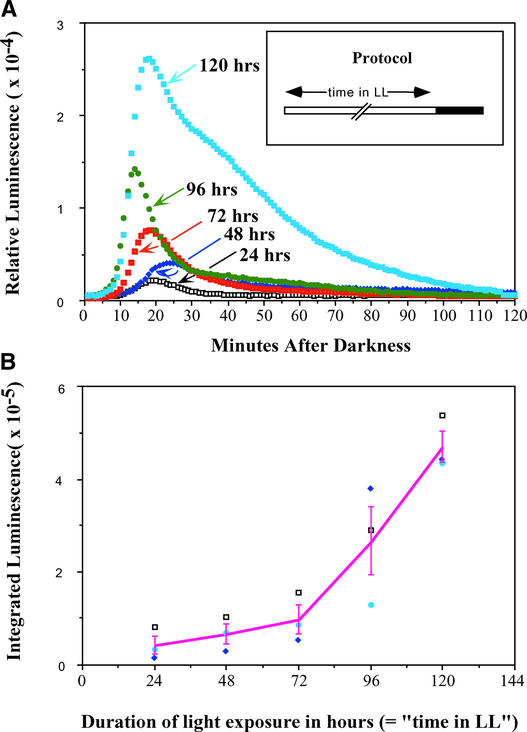
The data described above were obtained by exposing MAQ 6.3 seedlings to LL for 120 h and then transferring them to darkness. To determine whether the duration of LL exposure influenced the magnitude and/or kinetics of the dark-stimulated stromal Ca2+ spike, seedlings were placed in LL for different durations from 24 to 120 h and then transferred to darkness to measure luminescence. We found that both the peak height and the integrated luminescence were directly proportional to the duration of the previous light exposure before they were transferred to dark (Figure 3). The integrated luminescence data (Figure 3B) showed a significant difference among the groups by analysis of variance (ANOVA) (F = 19.62, P < 0.001).
Figure 3.
The Magnitude of the Ca2+ Spike into Chloroplasts Is Proportional to the Duration of Light Exposure before the LL-to-DD Transition.
(A) Relative luminescence immediately after the LL-to-DD transition as a function of the duration of light exposure from 24 to 120 h (time 0 is the time of the transition). The protocol is shown in the inset. Light intensity was 22 μE·m−2·s−1.
(B) Integration of the luminescence from 0 to 90 min after the LL-to-DD transition as a function of the duration of previous LL treatments for three separate experiments, including the one shown in (A). Integrated luminescence was calculated as the cumulative light emitted during 90 min as measured in 1-min bins and added together. The solid line represents the mean from the three individual experiments, and error bars indicate standard error of mean.
Student's t test further indicated significant increases from the exposure of 72 to 96 h and from 96 to 120 h. Although there were no statistical differences among the first three durations of exposure (24, 48, and 72 h) by the t test, the trend of the increase is clear (Figure 3B). These differences in peak height and integrated luminescence were reproducible and significant. On the other hand, the small differences in the kinetics (latency) of the Ca2+ increase (Figure 3A) were not reproducible. The dependence of the magnitude of the Ca2+ spike on the duration of the previous light exposure suggests that light progressively charges a process that the transfer to darkness subsequently discharges, like that of a capacitor being charged by electrical current.
We also wanted to determine if the spectrum of light was important in allowing/preventing the dark-stimulated stromal Ca2+ spike. If specific wavelengths of light inhibited the stromal Ca2+ spike, this might imply the involvement of a particular photoreceptor system. To test this hypothesis, MAQ 6.3 seedlings that had been in LL for at least 16 h were transferred to 1 h of relatively dim light of different colors: blue, green, red, and cool white (all at 1.3 μE·m−2·s−1). Then, each group of seedlings was transferred to DD to determine if the stromal Ca2+ spike had occurred. All four colors of light prevented the Ca2+ flux until the transfer to DD, indicating that low intensities of blue, green, red, or white light are capable of inhibiting the stromal Ca2+ spike until the seedlings are transferred to DD (data not shown). This result indicates that the perception of the LL-to-DD transition that results in the dark-stimulated stromal Ca2+ flux is not accomplished by a single photopigment with a restricted spectral sensitivity.
Total Apo-Aequorin Content Does Not Change after the LL-to-DD Transition
As mentioned above, we found the expression of apo-aequorin from the 35S promoter of Cauliflower mosaic virus to be constant in LL. However, we wanted to confirm that there were no significant changes in apo-aequorin content during the large dark-stimulated Ca2+ spike. On an expanded time scale, the profile of dark-stimulated MAQ 6.3 seedling luminescence confirmed that the signal started to increase between 5 and 10 min after the LL-to-DD transition, peaked at ∼20 to 25 min, and then decayed gradually to the original level (Figure 4A).
From the same batch of seedlings from which this luminescence was recorded, total apo-aequorin activities were measured from the seedling homogenates at four different times after the LL-to-DD transition: (1) immediately after the LL-to-DD transition; (2) at 5 min after the transition; (3) at 25 min after the transition (just after the peak); and (4) at 60 min after the transition. Although there appeared to be slightly higher apo-aequorin activity at the first time point, there were no significant differences among the four groups by one-way ANOVA (F = 1.67, P = 0.249). Also, analysis by Student's t test showed no significant differences among the values at each time point. Therefore, our conclusion is that the large luminescence peak in MAQ 6.3 seedlings after the LL-to-DD transition is not caused by a change in aequorin activity but is an accurate reflection of a large Ca2+ spike within the chloroplast stroma.
Furthermore, we wanted to ascertain if the increased magnitude of dark-stimulated luminescence after increasing light exposures (Figure 3) was the result of increased Ca2+ flux or increased aequorin content. For example, if the long-duration light exposure stimulated the synthesis of aequorin (perhaps merely as a result of growth or developmental changes), it is possible that the progressively increasing luminescence was caused by a larger pool of aequorin and not by changes in the magnitude of the Ca2+ flux. Figure 4B shows that this explanation is not valid. After light exposures of 24, 72, and 120 h, there was a 6.7-fold increase in dark-stimulated luminescence. But there was no significant increase in specific apo-aequorin activity or in total apo-aequorin activity in seedlings during this interval (Figure 4B). These data show that the burst of dark-induced luminescence after progressively longer durations of previous light exposure was attributable to a progressive increase in the magnitude of the Ca2+ flux and not to changes in the apo-aequorin content of the seedlings.
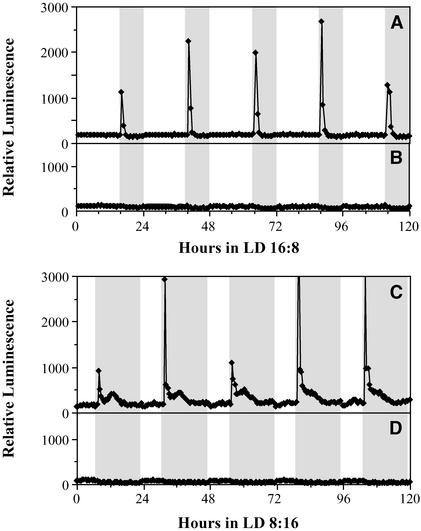
Do Stromal Ca2+ Spikes Occur after Dusk Every Day on a 24-h Day/Night Cycle?
The observation that the duration of LL has a large effect on the magnitude of the stromal Ca2+ flux raised the possibility that prolonged light exposure is necessary to achieve the Ca2+ spike and that such fluxes are not a component of plant cell physiology on a normal 24-h light/dark cycle. Therefore, we tested the Ca2+ profiles of MAQ 6.3 seedlings on 24-h light/dark cycles, both long-day cycles (LD 16:8; 16 h of light/8 h of dark) and short-day cycles (LD 8:16). As shown in Figure 5, under both regimes, Ca2+ spikes occurred every day soon after lights off. In LD 16:8, these spikes lasted for ∼1 h and then returned to the basal level (Figure 5A). In short days (LD 8:16), the profile of Ca2+ fluxes included a second smaller peak later in the night that was not observed in the long-day photoperiod (Figure 5C).
Figure 5.
Stromal Ca2+ Spikes Occur after Lights Off in 24-h Light/Dark Cycles.
(A) and (B) Long-day cycles (LD 16:8).
(C) and (D) Short-day cycles (LD 8:16).
Gray areas indicate dark intervals, and white areas indicate illuminated intervals. Light Intensity was 22 μE·m−2·s−1. MAQ 6.3 seedlings were monitored in (A) and (C), whereas nontransgenic wild-type seedlings were monitored in (B) and (D). Both the transgenic and nontransgenic seedlings were treated with coelenterazine. These data are representative traces for two separate experiments for each photoperiod with four to five replicates for each condition.
The height of the dark-stimulated Ca2+ spike appeared to be variable from cycle to cycle, but this effect almost certainly was attributable to a sampling artifact. Because the 30-channel apparatus we used collected data from each channel approximately every 25 min, the variation of the peak heights was likely the result of the fact that the apparatus will not consistently pick the same phase on the brief Ca2+ spike profile in every cycle. Although it is impossible at present to conclude that the magnitude of the Ca2+ flux is the same from cycle to cycle, it is clear that it does occur after every dusk on 24-h light/dark cycles. Control nontransgenic seedlings (Figures 5B and 5D) did not show any significant changes in their luminescence profiles over the 24-h cycle, again indicating that delayed chlorophyll fluorescence from light excitation during the day could not account for the luminescence profiles we observed in transgenic plants.
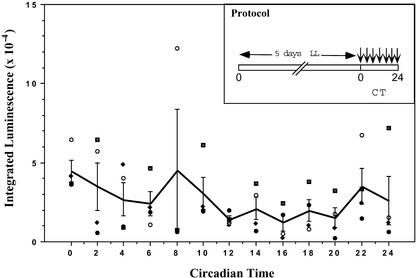
Is the Ca2+ Spike Gated by the Circadian Clock?
The damped circadian oscillation of Ca2+ in DD depicted in Figure 1 (especially [C] and [D]) and the daily dark-stimulated Ca2+ spikes observed in LD cycles (Figure 5) implied that a circadian clock could be involved in the regulation of stromal Ca2+ levels. One way that a clock might be involved is by gating the Ca2+ flux so that at some phases of the circadian cycle the gate is opened wider, allowing a larger Ca2+ flux, than at other phases. To determine if the magnitude of the Ca2+ flux is a function of the circadian time of transfer to darkness, MAQ 6.3 seedlings were germinated and grown in LD 16:8 for 10 days, placed in LL for 5 days, and transferred to darkness at different circadian times. As shown in Figure 6, there was no obvious circadian modulation of the magnitude of the Ca2+ spike. ANOVA and Student's t test pattern analyses showed no statistically significant differences among the different time points. Therefore, even though there was circadian control of basal stromal Ca2+ levels (Figure 1) (Johnson et al., 1995), the dark-stimulated Ca2+ spike appears not to be gated by a circadian timekeeper.
Figure 6.
Ca2+ Flux into Chloroplast Stroma as a Function of Circadian Time.
Seedlings were germinated and grown in LD 16:8 until the time of treatment with coelenterazine, as described in Methods. After the 8-h treatment with coelenterazine in DD, the seedlings were transferred to LL. The protocol from the onset of LL was as shown in the inset: MAQ 6.3 seedlings were maintained in LL for 5 days (22 μE·m−2·s−1), after which they were transferred to DD at different phases of the circadian cycle. The ordinate plots the integrated luminescence (0 to 90 min after the LL-to-DD transition) for four separate experiments, and the abscissa shows the circadian time (CT) of the transfer to darkness (time 0 is subjective dawn, which is the time of transfer in the experiments depicted in Figures 1 to 4). The solid line connects the averages, and the error bars indicate standard error of mean. The large variation at circadian time 8 was caused by one outlying point.
Corresponding Dark-Stimulated Changes in Ca2+ Levels between the Cytosol and the Stroma
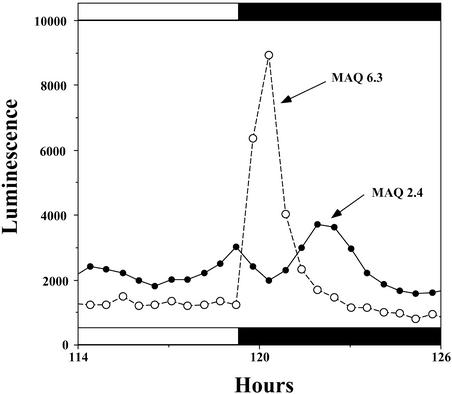
In an attempt to ascertain Ca2+ movements between the cytosol and the chloroplast stroma during the dark-stimulated Ca2+ flux, we compared aequorin luminescence profiles between strains in which the aequorin is targeted to the stroma (MAQ 6.3) and the cytosol (MAQ 2.4). Figure 7 shows the familiar Ca2+ spike from the stroma elicited by LL-to-DD transfer (MAQ 6.3 trace). In the cytosol, Ca2+ decreased slightly during the increasing phase of the stromal Ca2+ spike and increased significantly as the stromal Ca2+ spike decayed (Figure 7). The second phase (cytosolic Ca2+ increase) was reproducible in MAQ 2.4 traces (it occurred in all eight of the matched recordings), but the first phase (cytosolic Ca2+ decrease) appeared in half of the matched MAQ 2.4/MAQ 6.3 comparisons (in four of the eight matched recordings).
Figure 7.
Comparison of Luminescence Profiles of Stroma-Targeted (MAQ 6.3) versus Cytosol-Targeted (MAQ 2.4) Aequorin.
The ordinate shows relative luminescence, and the abscissa shows time in hours. The LL-to-DD transition occurred between 119 and 120 h, as shown by the black bar. Data are representative traces from three separate experiments in which matched sets of MAQ 2.4 versus MAQ 6.3 seedlings were compared (n = 8 for MAQ 2.4, n = 12 for MAQ 6.3).
It also appeared that the first phase might be dependent on the time during the circadian cycle when the LL-to-DD transition occurred. The first phase suggests that some Ca2+ flows from the cytosol into the stroma, possibly contributing to the increased Ca2+ in the stroma, whereas the second phase suggests that the dissipation of the large dark-stimulated increase of stromal Ca2+ is at least partially attributable to a Ca2+ flux from the stroma to the cytosol. These data suggest that dark-stimulated Ca2+ fluxes occur between these two compartments (discussed below and illustrated in Figure 9).
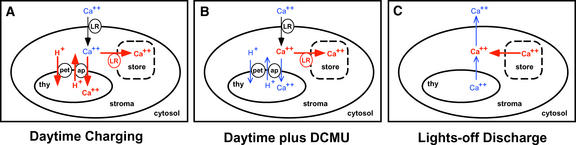
Figure 9.
Model for Ca2+ Fluxes into and out of the Chloroplast Modulated by Light and Dark.
Relatively high concentrations of Ca2+ and strong Ca2+ fluxes are shown with thick red lines, and relatively low concentrations of Ca2+ and weak Ca2+ fluxes are shown with thin blue lines. The source of Ca2+ that is discharged by darkness is a hypothetical calcium store. This calcium store is shown with dashed lines because it is not known (1) whether it is in the stromal lumen or the thylakoid, and (2) whether it is membrane bound (e.g., if it is identical with the thylakoid). Calcium stores in the cytosol and extracellular space are not shown, but they certainly are involved in the regulation of cytosolic calcium. ap, Ca2+/H+ antiporter; LR, light-regulated Ca2+ uptake into chloroplasts; pet, photosynthetic electron transport; thy, thylakoid lumen.
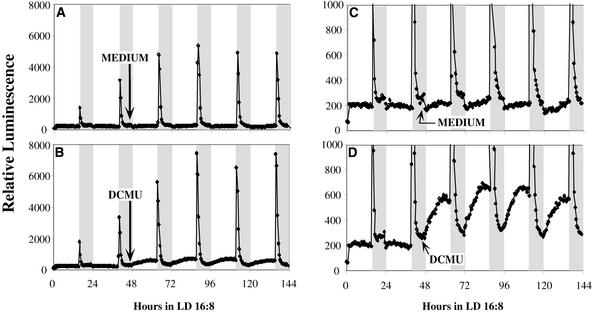
Inhibition of Photosynthetic Electron Transport Promotes a Ca2+ Leak into the Stroma but Does Not Reduce the Dark-Stimulated Ca2+ Flux
The data reported by Ettinger et al. (1999) imply that a Ca2+/H+ antiporter in the thylakoid membrane is involved in the regulation of stromal Ca2+ levels. The activity of this antiporter should depend on the proton electrochemical gradient across the thylakoid membrane; therefore, it should be at least partially inhibited when photosynthetic electron transport is inhibited. If this Ca2+/H+ antiporter is involved in the dark-stimulated Ca2+ flux into the stroma (possibly by charging a thylakoid Ca2+ store in the light), we would expect that inhibition of photosynthetic electron transport would block the dark-stimulated Ca2+ flux or at least promote a leak of Ca2+ into the stroma (presumably a leak from thylakoids to the stroma). Figure 8 shows that an inhibitor of photosynthetic electron transport, DCMU, did not prevent the dark-stimulated Ca2+ flux. However, it did cause a significant increase in stromal Ca2+ levels that might have been caused by leaking of Ca2+ from the thylakoid lumen to the stroma or by a reduced rate of Ca2+ transport from the stroma to the thylakoid.
Figure 8.
DCMU Promotes a Light-Dependent Increase of Ca2+ in the Stroma but Does Not Inhibit the Dark-Stimulated Ca2+ Flux in LD 16:8.
DCMU or MS medium was added to MAQ 6.3 seedlings at h 44 of LD 16:8. A total of 200 μL of MS medium or of 100 μM DCMU (dissolved in MS medium) was added on top of seedlings on 2 mL of agar medium. The final DCMU concentration after diffusion into the agar medium was 10 μM. (C) and (D) show magnified versions of (A) and (B) ([C] is magnified from [A] and [D] is magnified from [B]). Representative traces are shown for measurements from 18 different samples for each treatment (two separate experiments with three DCMU treatments at 2 μM, three DCMU treatments at 10 μM, and three medium treatments in each experiment). To ensure that the signal observed with DCMU was specific for stroma-targeted aequorin (and not for, e.g., delayed fluorescence [Jursinic, 1986]), we also treated nontransgenic seedlings that had been incubated with coelenterazine and treated with DCMU. In those seedlings, there was no increase in basal luminescence levels after DCMU treatment, nor was there a dark-stimulated luminescence burst.
We used two different concentrations of DCMU (2 and 10 μM) with equivalent results, except that the magnitude of the stromal Ca2+ increase in the day was larger at 10 μM (10 μM is the concentration used in the experiment depicted in Figures 8B and 8D). Clearly, the DCMU permeated the seedlings and had an effect, based on the significant increase in daytime stromal Ca2+ levels for several days (cf. Figures 8C and 8D), but there was no significant effect on the magnitude of the dark-stimulated Ca2+ flux (cf. Figures 8A and 8B). This result indicates that the inhibition of photosynthetic electron transport with DCMU affected the regulation of stromal Ca2+ levels (Figure 8D) but did not inhibit the charging of the Ca2+ store that was discharged by lights off.
DISCUSSION
Dark-Stimulated Ca2+ Fluxes
The data shown in Figures 5 and 8 indicate that Ca2+ spikes in the chloroplast stroma are an everyday occurrence after lights off. Note that throughout the day on both LD 16:8 and LD 8:16, Ca2+ levels remained low in the stroma, even though Ca2+ uptake from the cytosol into the chloroplast is expected during the day (Muto et al., 1982; Miller and Sanders, 1987). These daily fluxes have potential regulatory consequences (see below). Lights off discharges the process, and light “charges” the process almost like a capacitor (Figure 3), but the magnitude of the discharge was not affected by either the circadian phase (Figure 6) or the inhibition of photosynthetic electron transport (Figure 8). We also show that there was an exchange of Ca2+ between the cytosol and the stroma at lights off (Figure 7). The dark-stimulated luminescence reported by aequorin was not the result of changes in the levels of apo-aequorin (Figure 4), and the luminescence of aequorin was specific for Ca2+, so it is almost certain that the luminescence we observed was caused by Ca2+ fluxes.
A Dark-Dischargeable Ca2+ Store
The nature of the dark-dischargeable Ca2+ store within the chloroplast is not known. When photosynthetic electron transport is allowed to proceed, Ca2+ that is taken up during the day does not accumulate in the stroma as free Ca2+; rather, it is either transported into other compartments (e.g., the thylakoid) or bound (Kreimer et al., 1987). It has been reported that Ca2+ performs vital functions in the thylakoid (Becker et al., 1985; Miller and Brudvig, 1989; Grove and Brudvig, 1998). A Ca2+/H+ antiporter has been characterized from thylakoids (Ettinger et al., 1999), and it is attractive to consider the possibility that this antiporter transports Ca2+ into the thylakoid, driven by the proton electrochemical gradient generated by photosynthetic electron transport. The data shown in Figure 8D demonstrate that inhibition of photosynthetic electron transport alters Ca2+ regulation within the stroma, indicating that the Ca2+/H+ antiporter may be at least a partial aid in the regulation of stromal basal Ca2+ concentration in the face of light-induced Ca2+ uptake across the inner envelope (Muto et al., 1982; Miller and Sanders, 1987; Roh et al., 1998).
However, inhibiting photosynthetic electron transport with DCMU appears to have little or no effect on the magnitude of the dark-stimulated Ca2+ flux (Figure 8B). This result indicates that the source of the dark-stimulated Ca2+ flux is not a store whose charging is strictly dependent on photosynthetic electron transport. The dark-stimulated Ca2+ flux is unlikely to be primarily from cytoplasm to stroma, because our results indicate that there is no reproducible decrease in cytoplasmic Ca2+ at the time of the stromal Ca2+ flux (Figure 7). Therefore, although there might be some contribution from the cytoplasm, we favor the hypothesis that the Ca2+ flux at lights off comes primarily from within the chloroplast. But from where in the chloroplast?
One alternative is that the large Ca2+ binding capacity of stromal proteins (Kreimer et al., 1987) might be enlisted to bind Ca2+ during the day and release Ca2+ soon after sunset (membranes and/or small molecules in the stroma also might perform this function). Another possibility is that there is light-dependent uptake of Ca2+ into the thylakoid that is not inhibited significantly by treatment with 10 μM DCMU. Because the identity of the relevant Ca2+ store is unknown, it is depicted in Figure 9 with dashed lines and outside of the thylakoid. If subsequent research discovers light-dependent, DCMU-independent uptake of Ca2+ into the thylakoid, then this Ca2+ store might be identical with the thylakoid.
Based on our data and those reported by Ettinger et al. (1999), our hypothesis is that Ca2+ levels are relatively low at the end of the night in the cytosol, stroma, Ca2+ store, and thylakoid lumen (Figure 9). Beginning at dawn, a light-dependent Ca2+ flux into the stroma increases stromal Ca2+, which is transferred rapidly to the Ca2+ store by an unknown mechanism and to the thylakoid by the Ca2+/H+ antiporter (Figure 9A). Photosynthetic electron transport pumps protons into the thylakoid lumen, generating an electrochemical gradient that is used for ATP production but also by the Ca2+/H+ antiporter to exchange Ca2+ for H+ across the thylakoid membrane. As suggested in Figure 9B, DCMU reduces the electrochemical gradient, which results in less efficient reduction of stromal Ca2+ levels (or allows a Ca2+ leak from the thylakoid to the stroma).
Nevertheless, the Ca2+ store charges continuously under illumination in the presence or absence of DCMU. By the end of the day, Ca2+ accumulation in the store has reached a relatively high level. When the illumination is prolonged even further (Figure 3), the Ca2+ level within the store charges to even higher levels. Under these prolonged LL conditions, it is possible that stromal Ca2+ levels begin gradually to drift upward beyond the usual basal levels as a result of saturation of the store (Figures 1C and 1D imply such an effect).
Five to 10 min after lights off, the Ca2+ store (with a possible minor contribution from the cytosol) dumps its load of Ca2+ into the stroma, where it reaches a peak concentration at ∼20 to 25 min after darkness begins (Figure 9C). Subsequent to that peak, the Ca2+ level decreases again to basal levels, probably by transfer of Ca2+ to the cytosol, where it transiently increases cytosolic Ca2+ levels (the “second phase” in the MAQ 2.4 data shown in Figure 7), which then are brought back to basal levels by transport of the Ca2+ to intracellular Ca2+ stores (e.g., vacuole and endoplasmic reticulum) and/or by pumping into the extracellular space. Eventually, the Ca2+ bolus is transported out of the stroma, and Ca2+ concentrations return to basal levels in the cytosol, stroma, and thylakoid lumen.
A potential alternative that should be considered, however, is that the dark-stimulated Ca2+ increase in the stroma might be attributable to the dark-stimulated dissipation of the proton gradient across the thylakoid membrane. This effect could lead to protons leaking back into the stroma, displacing bound Ca2+ (Ca2+ and protons frequently compete for the same binding sites) and increasing the level of free calcium ions in the stroma. If this were true, we would expect a correspondence between the time course of the dissipation of the electrochemical gradient across the thylakoid membrane and the kinetics of the Ca2+ spike within the stroma. However, it has long been known that the proton electrochemical gradient across the thylakoid membrane in isolated chloroplasts is dissipated completely within 30 s of lights off (Schuldiner et al., 1972). The increase of Ca2+ within the stroma does not begin until >5 min after the transfer to darkness (Figures 3A and 4A). (Aequorin responds within milliseconds to changes in Ca2+ levels.) Consequently, there is no correspondence between the H+ and the Ca2+ fluxes.
This conclusion must be tempered by the possibility that proton electrochemical gradients might equilibrate across the thylakoid membrane more slowly in the living plant than in isolated chloroplasts. Nevertheless, the DCMU results shown in Figure 8B also argue against the stromal Ca2+ increase being the result of displacement by protons, because inhibiting photosynthetic electron transport would be expected to reduce the proton electrochemical gradient and thus reduce the proton flux into the stroma at lights off. Therefore, the data available at present suggest that the dark-stimulated burst of Ca2+ within the stroma is not likely to be a direct result of the equilibration of proton gradients across the thylakoid membrane.
Potential Regulatory Consequences of the Ca2+ Burst
One of the most intriguing aspects of the dark-stimulated Ca2+ spike is the 5- to 10-min lag between lights off and the increase of Ca2+ in the stroma. If the lights are turned on before the Ca2+ spike begins, the spike is inhibited. Moreover, if the lights are turned on after the Ca2+ spike has begun, the spike is attenuated. This lag implies a signaling/regulatory function—it is as if some process is waiting for 5 to 10 min to be “sure” that lights off has occurred. Once the waiting period is over, the Ca2+ spike is initiated as an “end-of-day” signal. Possible regulatory roles of the stromal Ca2+ spike are relevant to both the chloroplast and the cytosol. These will be considered in turn.
For the chloroplast, the very large increase of Ca2+ (up to 5 to 10 μM; Johnson et al., 1995) could have significant consequences to the metabolic processes therein. The key Calvin cycle enzyme, Fru-1,6-bisphosphatase, is inhibited by high concentrations of Ca2+ (Hertig and Wolosiuk, 1983; Kreimer et al., 1988). On the other hand, NAD kinase is activated by Ca2+ (Muto et al., 1981; Jarrett et al., 1982). High Ca2+ concentrations within the stroma tend to inhibit photosynthetic CO2 fixation (Portis and Heldt, 1976; Demmig and Gimmler, 1979). Therefore, it is quite likely that the Ca2+ burst in the chloroplast stroma could help to put photosynthetic processes “to bed for the night.” Furthermore, it is likely that the Ca2+ spike phases the subsequent Ca2+ oscillations in the stroma in DD (Johnson et al., 1995); therefore, it may be an entrainment signal to the pacemaker that is regulating the stromal Ca2+ rhythm.
For the cytosol, the possible consequences of an end-of-day signal are relevant to the entrainment of the circadian systems of the plant. The magnitude of the Ca2+ increase in the cytosol after the Ca2+ bolus in the stroma is not large (estimated to be 300 to 400 nM, increasing from a basal level of 150 to 200 nM), but locally (especially in cytosol near the chloroplast envelope), this Ca2+ increase could be significant. There is abundant evidence for a role of Ca2+ fluxes in the entrainment of circadian rhythms in animals (Ding et al., 1994; Geusz and Block, 1994; Colwell, 2000), and there is some evidence in plants as well (Gomez and Simon, 1994; Johnson et al., 1995). In some studies, light has been suggested to stimulate cytosolic Ca2+ increases in plants (Shacklock et al., 1992; Millar et al., 1994), but the mechanism depicted in Figure 9 and suggested by the data shown in Figure 7 indicates that there could be a dark-stimulated cytosolic Ca2+ flux as well. This Ca2+ flux appears to emanate from a Ca2+ store that is charged in the light by a process that depends on the duration of illumination but is not strongly dependent on the wavelength of the light.
A final titillating observation from this study is the difference in the profiles of the stromal Ca2+ spike when plants are in long-day versus short-day photoperiods (Figure 5). A prolonged Ca2+ transient within the stroma in short days could lead to a prolonged end-of-day signal within either the stroma or the cytosol and could be a means by which plants perceive the difference between long days and short days and thereby induce photoperiodic responses such as flowering.
METHODS
Strains and Medium
Two transgenic lines of Nicotiana plumbaginifolia were used in this study. Strain MAQ 2.4 has been transformed genetically with the cDNA for apo-aequorin downstream of the 35S promoter of Cauliflower mosaic virus (Knight et al., 1991); in this strain, the apo-aequorin is targeted to the cytosol. Strain MAQ 6.3 contains the 35S promoter–driven apo-aequorin transgene with the coding sequence of the transit peptide of the small subunit of pea ribulose-1,5-bisphosphate carboxylase (rbcS) fused to the 5′ end of the apo-aequorin cDNA coding sequence in frame. As expected from other studies showing that the fusion of this signal sequence to foreign proteins results in their targeting to the stroma (Van den Broeck et al., 1985), the apo-aequorin is targeted to the chloroplast in MAQ 6.3, as confirmed by Percoll gradient centrifugation of lysed protoplasts (93.8% of total apo-aequorin was in the chloroplast fraction; Johnson et al., 1995).
More recent studies have shown that intact chloroplasts from MAQ 6.3 have an aequorin signal as measured in vitro, but osmotically shocked chloroplasts from MAQ 6.3 in which the inner and outer envelopes are disrupted selectively but the thylakoid membranes are intact have lost the aequorin signal (R. Shingles, personal communication). These results support the conclusion that apo-aequorin is targeted to the stroma in MAQ 6.3 seedlings. In both MAQ 2.4 and MAQ 6.3, apo-aequorin is reconstituted into active aequorin by incubation of seedlings with the luminophore coelenterazine (see below).
For germination, plant seeds were rinsed in 70% ethanol, sterilized in 20% Clorox (5.25% sodium hypochlorite) for 15 min, and washed in sterilized distilled water three times. The sterilized seeds were soaked in 10 μM gibberellic acid overnight to synchronize germination. These seeds were germinated on 0.8% agar containing half-strength Murashige and Skoog (1962) (MS) medium (Sigma) supplemented with 1 × MS vitamins (Sigma) in a light/dark cycle (16 h of light/8 h of dark) at 25°C. No carbon source was added to the medium. The light source for germination and growth was cool-white fluorescent light at 45 μE·m−2·s−1. Seeds of transgenic strains were germinated on the same medium, to which 100 μg/mL kanamycin was added.
Reconstitution of Aequorin in Vivo and Measurement of Luminescence
The luminophore substrate coelenterazine (Biosynth, Naperville, IL) was dissolved in a small amount of ethanol and then diluted with distilled water to a final concentration of 10 μM. Ten-day-old seedlings were floated on a freshly prepared 10 μM coelenterazine solution for 8 h in darkness to reconstitute aequorin. The reconstituted seedlings were placed in groups of 10 seedlings on fresh half-strength MS medium (0.8% agar) in 20-mL scintillation vials, and luminescence emission was monitored with either (1) an automated 30-channel photomultiplier/photon-counting apparatus (Johnson et al., 1995) or (2) a single-channel photon-counting luminometer (Zylux FB12 luminometer, Oak Ridge, TN). The former apparatus was used for the long-term recordings. Recordings from seedlings in light were performed as follows: luminescence was measured every 30 min by placing the seedlings in darkness for 40 s, then their luminescence was measured for 40 s, and then the seedlings were returned to light for 30 min until the next measurement. Our standard illumination conditions are relatively dim (∼22 μE·m−2·s−1), and under these conditions, chlorophyll fluorescence is dim and decays to undetectable levels within 30 s; therefore, our luminescence measurements are not contaminated by photons originating from fluorescence.
Imaging of Luminescence from Whole Seedlings
Luminescence from whole seedlings was imaged through a 50-mm lens with a Princeton Instruments (Trenton, NJ) cooled charge-coupled device camera (TE/CCD512BKS; Kondo et al., 1994). The images shown at top in Figure 2 are 30-min integrations of the luminescence emitted by MAQ 6.3 seedlings in darkness. The images shown at bottom are of seedlings visualized under room lighting. The camera was controlled and images were saved by a program (CCDfocus) written by Takao Kondo (Nagoya University, Nagoya, Japan).
In Vitro Assay of Apo-Aequorin Activity
Ten-day-old seedlings were collected at different time points (10 seedlings at each time point), snap-frozen in liquid nitrogen, and stored at −80°C until all samples were collected. The protocol to reconstitute and discharge aequorin in vitro was modified from Johnson et al. (1995). Seedlings were homogenized in 0.5 mL of buffer containing 0.5 M NaCl, 5 mM EDTA, 0.1% gelatin, 10 mM Tris-Cl, pH 7.4, and 5 mM β-mercaptoethanol. After a brief spin, the supernatants were incubated in 2.5 μM coelenterazine in darkness for 3 h, and the reconstituted aequorin was discharged with 1 mL of 40 mM CaCl2. The luminescence signal was integrated for 10 s and used to calculate the apo-aequorin activity in the extracts. Total apo-aequorin activity was the calculated in vitro luminescence of the entire sample; specific apo-aequorin activity was the in vitro luminescence normalized to the protein concentration in the homogenates. These are expressed in relative light units per milligram of protein (protein concentration was measured by the Bradford assay; Bio-Rad).
Statistical Analyses
Data were analyzed first by one-way analysis of variance to test the differences among the groups and then by Student's t test for between-group differences. All analyses were performed with the program JMP (SAS Institute, Carey, NC).
Acknowledgments
We are grateful to Takao Kondo for the Luminescence Vial Analysis and CCDfocus programs and for extensive advice and assistance in measuring and imaging luminescence. We thank Till Roenneberg and Walter Taylor for the Chrono analysis program and conversion programs. We appreciate the advice of Marc Knight, Anthony Trewavas, and Ann Haley concerning transgenic plants expressing aequorin and for providing the original stocks of MAQ 6.3 and 2.4. Finally, Govindjee, Richard Shingles, and William Ettinger provided helpful suggestions on the manuscript. This research was supported by the National Institute of Mental Health (Grants MH43836 and MH01179 to C.H.J.).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.000653.
References
- Becker, D.W., Callahan, F.E., and Cheniae, G.M. (1985). Photoactivation of NH2OH-treated leaves: Reassembly of released extrinsic PSII polypeptides and religation of Mn into the polynuclear Mn catalyst of water oxidation. FEBS Lett. 192, 209–214. [Google Scholar]
- Bush, D.S. (1993). Regulation of cytosolic calcium in plants. Plant Physiol. 103, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, D.S. (1995). Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 95–122. [Google Scholar]
- Colwell, C.S. (2000). Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur. J. Neurosci. 12, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig, B., and Gimmler, H. (1979). Effect of divalent cations on cation fluxes across the chloroplast envelope and on photosynthesis of intact chloroplasts. Z. Naturforsch. 34c, 233–241. [Google Scholar]
- Ding, J.M., Chen, D., Weber, E.T., Faiman, L.E., Rea, M.A., and Gillette, M.U. (1994). Resetting the biological clocks: Mediation of nocturnal circadian shifts by glutamate and NO. Science 266, 1713–1717. [DOI] [PubMed] [Google Scholar]
- Ettinger, W.F., Clear, A.M., Fanning, K.J., and Peck, M.L. (1999). Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol. 119, 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geusz, M.E., and Block, G.D. (1994). Intracellular calcium in the entrainment pathway of molluscan circadian pacemakers. Neurosci. Biobehav. Rev. 18, 555–561. [DOI] [PubMed] [Google Scholar]
- Gomez, L.A., and Simon, E. (1994). Circadian rhythm of Robina pseudoacacia leaflet movements: Role of calcium and phytochrome. Photochem. Photobiol. 61, 210–215. [Google Scholar]
- Grove, G.N., and Brudvig, G.W. (1998). Calcium binding studies of photosystem II using a calcium-selective electrode. Biochemistry 37, 1532–1539. [DOI] [PubMed] [Google Scholar]
- Hertig, C.M., and Wolosiuk, R.A. (1983). Studies on the hysteretic properties of chloroplast fructose-1,6-bisphosphatase. J. Biol. Chem. 258, 984–989. [PubMed] [Google Scholar]
- Jarrett, H.W., Brown, C.J., Black, C.C., and Cormier, M.J. (1982). Evidence that calmodulin is in the chloroplast of peas and serves a regulatory role in photosynthesis. J. Biol. Chem. 257, 13795–13804. [PubMed] [Google Scholar]
- Johnson, C.H., Knight, M.R., Kondo, T., Masson, P., Sedbrook, J., Haley, A., and Trewavas, A.J. (1995). Circadian oscillations of cytosolic and chloroplastidic free calcium in plants. Science 269, 1863–1865. [DOI] [PubMed] [Google Scholar]
- Jursinic, P.A. (1986). Delayed fluorescence: Current concepts and status. In Light Emission by Plants and Bacteria, Govindjee, J. Amesz, and D.J. Fork, eds (Orlando, FL: Academic Press), pp. 291–328.
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526. [DOI] [PubMed] [Google Scholar]
- Kondo, T., Tsinoremas, N.F., Golden, S.S., Johnson, C.H., Kutsuna, S., and Ishiura, M. (1994). Circadian clock mutants of cyanobacteria. Science 266, 1233–1236. [DOI] [PubMed] [Google Scholar]
- Kreimer, G., Melkonian, M., Holtum, J.A.M., and Latzko, E. (1985). Characterization of calcium fluxes across the envelope of intact spinach chloroplasts. Planta 166, 515–523. [DOI] [PubMed] [Google Scholar]
- Kreimer, G., Melkonian, M., Holtum, J.A.M., and Latzko, E. (1988). Stromal free calcium concentration and light-mediated activation of chloroplast fructose-1,6-bisphosphatase. Plant Physiol. 86, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer, G., Surek, B., Woodrow, I.E., and Latzko, E. (1987). Calcium binding by spinach stromal proteins. Planta 171, 259–265. [DOI] [PubMed] [Google Scholar]
- Mattoo, A.K., Marder, J.B., and Edelman, M. (1989). Dynamics of the photosystem II reaction center. Cell 56, 241–246. [DOI] [PubMed] [Google Scholar]
- Millar, A.J., McGrath, R.B., and Chua, N.-H. (1994). Phytochrome phototransduction pathways. Annu. Rev. Genet. 28, 325–349. [DOI] [PubMed] [Google Scholar]
- Miller, A.F., and Brudvig, G.W. (1989). Manganese and calcium requirements for reconstitution of oxygen-evolution activity in manganese-depleted photosystem II membranes. Biochemistry 28, 8181–8190. [DOI] [PubMed] [Google Scholar]
- Miller, A.J., and Sanders, D. (1987). Depletion of cytosolic free calcium induced by photosynthesis. Nature 326, 397–400. [Google Scholar]
- Moore, A.L., and Akerman, K.E.O. (1984). Calcium and plant organelles. Plant Cell Environ. 7, 423–429. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Muto, S., Izawa, S., and Miyachi, S. (1982). Light-induced Ca2+ uptake by intact chloroplasts. FEBS Lett. 139, 250–254. [Google Scholar]
- Muto, S., Miyachi, S., Usuda, H., Edwards, G.E., and Bassham, J.A. (1981). Light-induced conversion of nicotinamide adenine dinucleotide to nicotinamide adenine dinucleotide phosphate in higher plant leaves. Plant Physiol. 68, 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio, L., Bewell, M.A., Siddiqua, A., Dickinson, G.D., Galione, A., and Sanders, D. (2000). Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc. Natl. Acad. Sci. USA 97, 8693–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis, A.R., Jr., and Heldt, H.W. (1976). Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim. Biophys. Acta 449, 434–446. [DOI] [PubMed] [Google Scholar]
- Roberts, D.M., and Harmon, A.C. (1992). Calcium-modulated proteins: Targets of intracellular calcium signals in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 375–414. [Google Scholar]
- Roh, M.H., Shingles, R., Cleveland, M.J., and McCarty, R.E. (1998). Direct measurement of calcium transport across chloroplast inner-envelope vesicles. Plant Physiol. 118, 1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, D., Brownlee, C., and Harper, J.F. (1999). Communicating with calcium. Plant Cell 11, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner, S., Rottenberg, H., and Avron, M. (1972). Determination of ΔpH in chloroplasts. 2. Fluorescent amines as a probe for the determination of ΔpH in chloroplasts. Eur. J. Biochem. 25, 64–70. [DOI] [PubMed] [Google Scholar]
- Shacklock, P.S., Read, N.D., and Trewavas, A.J. (1992). Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 358, 753–755. [Google Scholar]
- Van den Broeck, G., Timko, M.P., Kausch, A.P., Cashmore, A.R., Montagu, M.V., and Herrera-Estrella, L. (1985). Targeting of foreign protein to chloroplasts by fusion to the transit peptide from the small subunit of ribulose 1,5-bisphosphate carboxylase. Nature 313, 358–363. [DOI] [PubMed] [Google Scholar]
- Yocum, C.F. (1991). Calcium activation of photosynthetic oxygen evolution. Biochim. Biophys. Acta 1059, 1–15. [Google Scholar]