Abstract
Mutants of a new gene, TRANSPARENT TESTA GLABRA2 (TTG2), show disruptions to trichome development and to tannin and mucilage production in the seed coat. The gene was tagged by the endogenous transposon Tag1 and shown to encode a WRKY transcription factor. It is the first member of this large, plant-specific family known to control morphogenesis. The functions of all other WRKY genes revealed to date involve responses to pathogen attack, mechanical stress, and senescence. TTG2 is strongly expressed in trichomes throughout their development, in the endothelium of developing seeds (in which tannin is later generated) and subsequently in other layers of the seed coat, and in the atrichoblasts of developing roots. TTG2 acts downstream of the trichome initiation genes TTG1 and GLABROUS1, although trichome expression of TTG2 continues to occur if they are inactivated. Later, TTG2 shares functions with GLABRA2 in controlling trichome outgrowth. In the seed coat, TTG2 expression requires TTG1 function in the production of tannin. Finally, TTG2 also may be involved in specifying atrichoblasts in roots redundantly with other gene(s) but independently of TTG1 and GLABRA2.
INTRODUCTION
In many species, plant hairs (trichomes) are believed to provide physical protection from attack by predators. In Arabidopsis, trichomes occur as large single cells that develop on the upper surface of rosette leaves, the lower surface of cauline leaves, the edges of leaves, stems, and sometimes sepals (Hülskamp et al., 1994). Trichome development on the leaf surface is first seen as the enlargement of a single epidermal cell. Such cells are spaced relatively evenly, approximately three or four cells apart at this stage. As the trichome increases in size and grows outward from the leaf surface, its nucleus endoreduplicates several times, and three branches arise in a stereotypic manner. The surface of the trichome is glassy until it reaches the final stages of differentiation, when it develops surface papillae. At maturity, the trichome is surrounded by a ring of supporting epidermal cells.
Genes involved in controlling the initiation and early stages of morphogenesis of trichomes are now being discovered in Arabidopsis through molecular genetic studies (Hülskamp et al., 1999; Szymanski et al., 2000) (Table 1). The first to be studied in detail was GLABROUS1 (GL1), mutants of which lack hairs on the surface of their leaves (Koornneef et al., 1982; Hülskamp et al., 1994). GL1 encodes a MYB family transcription factor that is expressed in trichomes from their inception and during their early development (Larkin et al., 1993). A second gene, TRANSPARENT TESTA GLABRA1 (TTG1), also results in the loss of leaf surface trichomes when its function is impaired (Koornneef et al., 1982). This gene encodes a protein containing WD40 repeats (Walker et al., 1999), a domain generally believed to promote interaction with other proteins. Genetic studies have indicated that GL1 and TTG1 control the same process in trichome initiation (Larkin et al., 1994, 1999).
Table 1.
Pleiotropic Effects of Trichome Mutants on the Production of Mucilage and Tannins in the Seed Coat, the Anthocyanin in Vegetative Regions, and on Root Hair Development
| Mutant
|
|||||
|---|---|---|---|---|---|
| Plant Property | ttg1 | gl1 | gl2 | gl3 | ttg2 |
| Trichomes | − | − | ↓ | ↓ | ↓ |
| Seed coat mucilage | − | wt | − | wt | − |
| Seed coat tannins | − | wt | wt | wt | − |
| Anthocyanins | − | wt | wt | wt | wt |
| Root hairs | ↑ | wt | ↑ | wt | wt |
| Encoded protein | WD40 | MYB | HD-Zip | bHLH | WRKY |
wt, wild type; −, absent; ↓, reduced morphogenesis; ↑, present on all epidermal cell files.
Recently, evidence for a direct link between GL1 and TTG1 function has come from characterization of the GL3 gene and its function (Koornneef et al., 1982; Payne et al., 2000). Trichome morphogenesis is disrupted in gl3 mutants such that most trichomes now have two instead of three branches and they show one fewer round of endoreduplication on average (Hülskamp et al., 1994). In plants homozygous for gl3-2 (a likely null mutant), trichome number is not reduced significantly, but even so, overexpression of GL3 results in the production of additional leaf trichomes, suggesting that GL3, like GL1 and TTG1, also plays a role in their initiation (Payne et al., 2000). Consistent with this finding, the GL3 protein is capable of interacting physically with both the GL1 and TTG1 proteins in yeast cells, although the latter do not interact (Payne et al., 2000).
GL3 encodes a transcription factor of the large bHLH family (Payne et al., 2000). Significantly, a heterologous bHLH protein encoded by the R gene of maize also can stimulate an increase in the number of leaf trichomes in Arabidopsis when overexpressed in either wild-type or ttg1 mutant plants (Lloyd et al., 1992). Because GL3 is a bHLH gene, it is a candidate ortholog of the maize R gene. They are closely related in sequence, and genetic and molecular evidence indicates that GL3 and R proteins can interact with GL1 (Larkin et al., 1994; Szymanski et al., 1998; Schnittger et al., 1999; Payne et al., 2000). However, overexpression of GL3 is much less effective than overexpression of R in boosting the number of trichomes in a ttg1 mutant background, either alone or combined with the overexpression of GL1, so their functions are not mutually interchangeable (Payne et al., 2000).
A fourth gene known to be involved in early trichome development is GL2. Trichomes arise in gl2 mutants, but their outgrowth is reduced and most are unbranched (Koornneef et al., 1982; Hülskamp et al., 1994). GL2 encodes a transcription factor that combines homeodomain and Leu zipper motifs (HD-ZIP), and it is expressed in trichome cells throughout their development (Rerie et al., 1994). Pair-wise epistatic interactions between gl2 and gl1, ttg1, or gl3 suggest that GL2 trichome function lies downstream of the GL1 and TTG1 genes and that it shares functions with GL3 (Hülskamp et al., 1994), conclusions supported by the fact that the expression of GL2 is reduced in each of the mutants (Di Cristina et al., 1996; Szymanski et al., 1998).
All four of these genes regulate early trichome development, but two of them, TTG1 and GL2, also are involved in the development of other tissues (Table 1). In the wild type, root hairs arise only from trichoblasts, which are files of epidermal cells that overlie junctions between cortical cells (Schiefelbein, 2000). The two epidermal cell files lying between them (atrichoblasts) usually do not generate root hairs. In both ttg1 and gl2 mutants, however, root hairs arise from both cell types (Galway et al., 1994; Di Cristina et al., 1996; Masucci et al., 1996). Gene interaction and expression studies indicate that the function of GL2 is to inhibit root hair development from atrichoblasts, whereas TTG1 apparently acts earlier to control GL2 function, probably through the product of the ortholog of the maize R bHLH gene (Galway et al., 1994; Di Cristina et al., 1996; Hung et al., 1998). In roots, a partner of such a bHLH protein is likely to be the product of the MYB gene WEREWOLF (WER) (Lee and Schiefelbein, 1999), a situation that parallels the proposed interaction between a predicted R-like bHLH protein and the GL1 MYB protein in trichomes discussed above. Furthermore, it was shown recently that GL1 and WER are functionally interchangeable (Lee and Schiefelbein, 2001).
TTG1 and GL2 also are involved in the generation of mucilage in the outer layer of the seed coat (Western et al., 2000; Windsor et al., 2000) (Table 1). Mucilage is absent in both ttg1 and gl2 mutants (Koornneef et al., 1982). GL2 normally is expressed specifically in this layer during development, and its expression is dependent on TTG1 function, although it may be restored in ttg1 mutants by overexpressing the maize R gene (Szymanski et al., 1998). In fact, all disruptions seen in ttg1 mutants are complemented by overexpression of the maize R gene (Lloyd et al., 1992).
Finally, TTG1 also is involved in the regulation of flavonoid production (although GL2 is not; Table 1). In ttg1 mutants, the anthocyanins that give a reddish color to parts of seedlings, stems, and leaves, particularly under stress conditions, are absent (Shirley et al., 1995). Also absent is the dense brown tannin produced by the inner layer of the inner integument of the seed coat (Debeaujon et al., 2000). As a consequence, the testa of seeds appears transparent in ttg1 mutants. The TTG1 protein positively regulates synthesis of the enzyme dihydroflavonol reductase, which generates leucoanthocyanidins, which are precursors of both anthocyanins and tannins (Nesi et al., 2000). In addition, TTG1 also regulates a downstream step in each of the subsequent anthocyanin and tannin biosynthetic pathways, the latter likely encoded by the BANYULS (BAN) gene (Devic et al., 1999). Interestingly, TTG1 is joined in the regulation of two of these steps (control of dihydroflavonol reductase synthesis and of the first step in the tannin pathway) by bHLH and MYB proteins encoded by TRANSPARENT TESTA8 (TT8) (Nesi et al., 2000) and TT2 (Nesi et al., 2001), respectively, and expression of each of these genes is dependent on TTG1 function.
In summary, it seems likely that several developmental processes in Arabidopsis are regulated by the combinatorial action of MYB and bHLH transcription factors in association with the WD40 accessory protein TTG1. Later stages of several of the same processes are controlled by the HD-ZIP transcription factor GL2. Although much is known, a full understanding of the complex regulatory interplay involved in trichome, seed coat, and root hair development requires the identification of additional components.
In this study, we have identified a new gene, TTG2, that also controls the early development of trichomes and the production of mucilage and tannin in seed coats. Based on epistatic interactions, the trichome function of TTG2 is likely to lie downstream from that of TTG1 and GL1 but to overlap that of GL2. The TTG2 gene encodes a member of the plant-specific WRKY transcription factor family whose only previously known functions are associated with their upregulation after pathogen attack, mechanical damage, and senescence (Eulgem et al., 2000). The expression pattern of TTG2 indicates that it plays a role not only in trichome and seed coat development but redundantly in the specification of atrichoblasts in the root epidermis.
RESULTS
ttg2 Mutant Phenotype
A new recessive trichome and seed coat mutant was obtained during a transposon tagging screen. Genetic complementation tests revealed that it was not allelic with ttg1. The new mutant was named transparent testa glabra2-1 (ttg2-1). Mutants homozygous for a second independent allele, ttg2-2, kindly provided by Alan Lloyd, showed the same defects to trichome development, seed coat color, and mucilage production to the same degree, indicating that all phenotypic changes arise from a single genetic defect in each case.
A major effect of ttg2-1 on trichome development is to reduce or eliminate branching (Figures 1A and 1B). Most leaf surface trichomes in ttg2-1 mutants were unbranched (>95%), whereas in the wild type, almost all showed three branches (Figures 1D and 1E). The number of trichomes formed per leaf also was reduced by approximately half. By comparison, in ttg1-1 mutants, there were no trichomes on the surfaces of leaves, although a few unbranched trichomes arose around their edges (Figure 1F). Trichome spacing also was disrupted slightly in ttg2-1 mutant plants. In two separate segregating F2 families, 10.1 and 13.2% of trichomes arose closely adjacent to another trichome on the surfaces of the first four leaves of ttg2-1 mutants, compared with 0 and 1.3%, respectively, in wild-type controls.
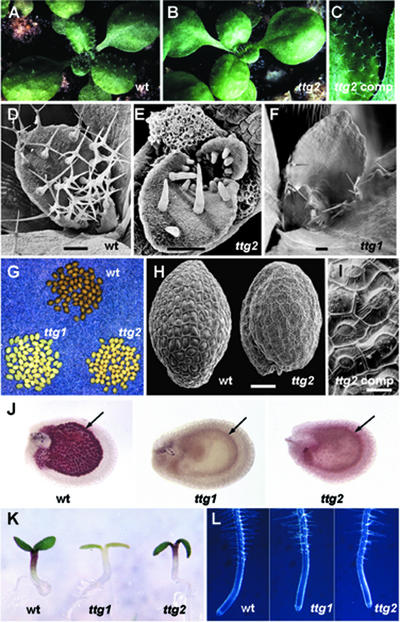
Figure 1.
Phenotype of ttg2-1 Mutant Plants.
(A) to (C) Characteristic appearance of trichomes on rosette leaves. On wild-type leaves, they are densely spaced and mostly three branched (A), whereas on ttg2 leaves, they are sparser and mostly unbranched (B). Trichomes in a complemented ttg2 mutant plant are relatively dense, and three branches are restored (C).
(D) to (F) Scanning electron microscopy of trichomes on the developing third and fourth leaves of wild-type (D), ttg2-1 (E), and ttg1-1 (F) plants. In ttg1 leaves, only a few unbranched trichomes arise on the edges.
(G) Seed coat color of the wild type (top, fully pigmented), ttg1 (bottom left, no pigment), and ttg2 (bottom right, weak pigmentation).
(H) Scanning electron microscopy of seed coats. Raised domes (columellae) covered with dry mucilage occur in the center of each cell of the wild type but not in the ttg2 mutant.
(I) Mucilage and columellae are partially restored in complemented ttg2 seeds.
(J) Seeds at the heart-shaped embryo stage treated with acidic vanillin. The endothelium (arrows) of wild-type seeds stains dark red as a result of the presence of tannin precursors in vacuoles. There is no staining in ttg1 mutants, but in ttg2 mutants, the endothelium and outer layers of the seed coat are palely stained.
(K) Four-day-old seedlings of the wild type, ttg1, and ttg2 showing the lack of anthocyanin pigment in the hypocotyls of ttg1 but not ttg2 plants. (The ttg1 plant also is mutant for the chlorophyll mutation yellow inflorescence that does not affect anthocyanin production.)
(L) Primary roots of 7-day-old seedlings of the wild type, ttg1, and ttg2 showing that root hairs arise only from nonadjacent cell files in the wild type and the ttg2 mutant but from all cell files in ttg1.
wt, wild type. Bars = 100 μm in (D) to (F) and (H) and 20 μm in (I).
The trichomes of ttg2-1 plants also showed a range of other developmental defects (Figures 2B to 2D) compared with those of the wild type (Figure 2A). ttg2-1 mutant trichomes were variable in size, with relatively few reaching the same height as wild-type trichomes. Larger mutant trichomes had the normal raised socket of surrounding support cells, whereas shorter trichomes had correspondingly reduced support cells (Figure 2B). Short rudimentary trichomes lacking surface papillae also were observed. These invariably appeared glassy rather than crystalline under normal light, and in some cases, even larger trichomes appeared glassy (Figure 2C). Finally, some ttg2-1 trichomes showed a range of distorted growth patterns, including forms that were bent or twisted (Figures 2C and 2D) and sometimes blistered.
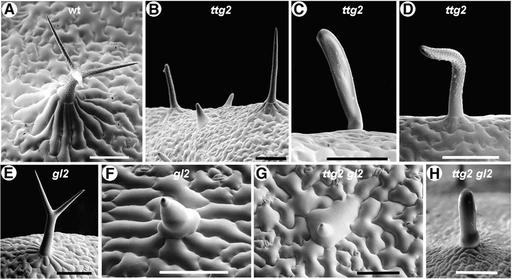
Figure 2.
Scanning Electron Microscopy Showing Trichome Structure in Wild-Type, ttg2-1 Single Mutant, gl2-1 Single Mutant, and ttg2-1 gl2-1 Double Mutant Plants.
(A) Wild-type (wt) trichome showing the three-branched structure with well-developed surface papillae surrounded by a socket of raised epidermal cells.
(B) Trichomes on the surface of a ttg2 mutant leaf, ranging in structure from rudimentary outgrowths to well-developed (although unbranched) trichomes with surface papillae and peripheral epidermal cells.
(C) and (D) Higher magnification of other forms of ttg2 mutant trichomes showing the lack of surface papillae (glassy in visible light) (C) and distorted growth (D).
(E) A gl2 mutant trichome with two branches, surface papillae, and peripheral epidermal cells.
(F) A rudimentary gl2 trichome that is glassy and lacks raised epidermal support cells.
(G) and (H) Examples of ttg2 gl2-1 double mutant trichomes showing much reduced development. Some resemble an epidermal pavement cell with a small raised point (G), and others may be larger, although they are always glassy and may have slight bulges, especially at their base or tip (H).
Bars = 100 μm.
The mature seed coats of wild-type Arabidopsis were brown (Figure 1G), resulting from the presence of tannins in the endothelium, the inner layer of the inner integument. In ttg2 mutants, pigmentation was reduced to a pale fawn, whereas the seeds of ttg1 mutants were even lighter and appeared pale lemon yellow as a result of the cotyledons being visible through the transparent testa (Figure 1G). Tannins are derived from the polymerization and oxidation of flavanols late in seed development. Flavanols turn vanillin dark red (Debeaujon et al., 2000), and the colorless flavanol precursors could be seen earlier in the endothelial layer of developing wild-type seeds after staining with acidic vanillin (Figure 2J). No red staining was visible in the endothelium in developing ttg1-1 mutant seeds, but faint staining was apparent in ttg2-1 seeds, not only in the endothelium but also in all other layers of the seed coat except the epidermis (Figure 2J).
The wild-type seed coat produces mucilage from the epidermal layer of the outer integument. This was associated with raised domes (columellae) within the center of the large tessellated cells that cover the seed's surface (Figure 1H). Such tessellated cells also covered ttg2-1 seeds, but the internal domes of columellae and overlying mucilage were absent (Figure 1H). When wetted, ttg2-1 seeds did not exude any mucilage, although their capacity for germination apparently was unaffected.
Anthocyanins are produced normally in several parts of Arabidopsis plants, including the base of the stem and the hypocotyl. In ttg1-1 mutants, this pigmentation was absent, but it was clearly present in ttg2-1 plants at levels comparable to those in the wild type (Figure 1K).
In the wild-type root, hairs developed from individual files of epidermal cells that usually alternated around the root's surface with pairs of cell files that did not develop hairs (Figure 1L). In ttg1-1 mutants, hairs arose from every cell file. When ttg2-1 roots were examined, however, no differences in root hair growth patterns were seen in comparison with the wild type (Figure 1L). Also, the surface of those cells without hairs often was covered with a waxy deposit in ttg2-1 mutant roots as in the wild type (data not shown).
Thus, of the disruptions seen in ttg1 mutants, ttg2 mutants shared reduced trichome development, pale seed coat color, and the lack of mucilage production, but they did not demonstrate loss of anthocyanin pigmentation or increased root hair formation.
Interactions between TTG2 and TTG1, GL1, and GL2
To determine if the functions of TTG2 overlap or interact with those of other genes, double mutants were generated. In ttg1-1 ttg2-1 double mutants, ttg1 was epistatic to ttg2 in all shared phenotypic properties. There were no trichomes on the surface of leaves, although the few trichomes that arose from the leaf edges in ttg1 single mutants were still present in the double mutants. Seed coat mucilage was absent. The other properties of the ttg1-1 mutant (absence of anthocyanin and increased numbers of root hairs) also were retained. It seems likely that TTG1 function precedes TTG2 function in trichome and seed coat tannin development and that TTG2 does not share functions with TTG1 in anthocyanin or root hair development.
Another trichome mutant, gl1-1, also resulted in no trichomes being produced on leaf surfaces. This, too, was epistatic to ttg2-1 in double mutant plants, although scattered trichomes arose on the edges of leaves in gl1-1 single mutants and gl1-1 ttg2-1 double mutant plants.
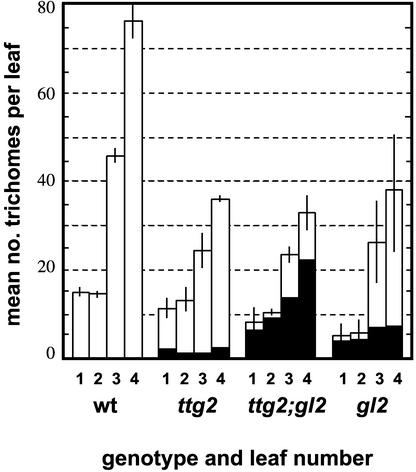
A third mutant with defects in trichome development, gl2-1, resembled ttg2-1 closely in that trichome development was reduced but not abolished. The mean number of trichomes on the first four leaves of gl2-1 single mutants was similar to that seen in ttg2-1, approximately half the wild-type number (Figure 3). This suggests that neither TTG2 nor GL2 plays an essential role in the initiation of trichomes, although each supports it. In the double mutant, the number was not reduced further to a significant degree (Figure 3). Thus, the reductions seen in each single mutant may be the consequence of modification of a common initiation process.
Figure 3.
Mean Number of Trichomes per Leaf on the First Four Leaves of Wild-Type, ttg2, gl2, and ttg2 gl2 Mutant Plants.
Means and standard error values are shown for each genotype grown together under very similar conditions. The number of rudimentary trichomes also is shown (solid areas). wt, wild type.
On the other hand, there is evidence that TTG2 and GL2 play overlapping roles in promoting trichome outgrowth. In the gl2-1 mutant, as in ttg2-1, trichomes reached a wide range of end points in their development. Some were almost as large as wild-type trichomes, with a covering of papillae and a base of supporting cells (Figure 2E). Their branching was reduced, although not as much as in ttg2-1, and branches often occurred at abnormal positions. Many smaller, apparently immature trichomes were present, the smallest of which may have characteristic bulges, especially where they join the leaf epidermis (Figure 2F). All of the smaller trichomes, and some of the larger ones, lacked surface papillae and thus appeared glassy. The proportion of rudimentary trichomes in gl2-1 mutants was somewhat higher than that in ttg2-1 (Figure 3), although they still accounted for <20% of trichomes on later arising leaves.
Strikingly, when gl2-1 and ttg2-1 were combined, there was a marked increase in the severity of defects in trichome development. The number of rudimentary trichomes was increased greatly such that three-quarters of all trichomes were rudimentary (Figure 3). Some were detectable only as small pointed outgrowths on epidermal pavement cells (Figure 2G). The largest were relatively short and always were glassy and unbranched (Figure 2H), and they frequently had a bulge at their base or tip. This synergistic effect indicates that TTG2 and GL2 share functions in controlling trichome outgrowth that are exposed only when both are in mutant form.
Mutants of both gl2-1 and ttg2-1 resulted in the absence of seed coat mucilage, and, as expected, this also was the case in the double mutant. The ttg2-1 mutant alone affected seed coat color, and this was disrupted similarly in the double mutant. Root hairs arose from atrichoblasts as well as trichoblasts in gl2-1 mutants, and the same change was seen in gl2-1 ttg2-1 double mutants. Thus, overlaps in seed coat functions, or in root hair functions, were not detected between these two genes.
In conclusion, double mutant studies indicate that the trichome initiation functions of TTG1 and GL1 are necessary for TTG2 function in trichomes of the leaf surfaces, whereas the function of GL2 overlaps that of TTG2 in the control of trichome outgrowth. In seed coat pigmentation, TTG1 function also is a prerequisite for TTG2 function. The functions of TTG1, TTG2, and GL2 are required individually for mucilage production. Finally, there is no genetic evidence that TTG2 has any function in controlling root hair development, either alone or redundantly with TTG1 or GL2.
TTG2 Encodes a WRKY Transcription Factor
The ttg2 mutant phenotype cosegregated with a new insertion of the endogenous transposon Tag1 (Tsay et al., 1993) among descendants of plants regenerated from transformed root tissue. Sequences adjacent to the new insert were isolated by inverse PCR, and a 20.7-kb cosmid clone that carried corresponding wild-type DNA was isolated from a genomic library (Figure 4A). To determine if this cosmid could complement the ttg2-1 mutant phenotype, it was used to transform ttg2-1 mutant plants. Full restoration of the wild-type phenotype for trichome development (Figure 1C) and of seed coat color and partial restoration of mucilage production (Figure 1I) were observed. These restorations were dominant and cosegregated with transgenic inserts in subsequent generations. Thus, a functional TTG2 gene is present within this 20.7-kb cosmid. Confirmation that the gene disrupted by Tag1 in ttg2-1 mutants is TTG2 comes from the observed disruption of the same gene in a second independently isolated mutant allele, ttg2-2 (see below).
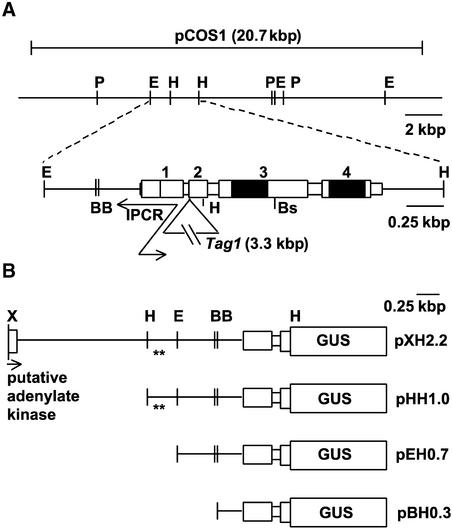
Figure 4.
Structure of the TTG2 Gene.
(A) Cloning of TTG2. The map at top shows the genomic region covered by cosmid pCOS1 (Columbia ecotype; only P and E sites are shown). The map at bottom shows the location of the TTG2 gene within a 2.7-kb EcoRI-HindIII fragment of this cosmid. The isolation of cDNA pRRi1 allowed the limits of four exons (numbered) to be deduced, assuming that the cDNA sequence ends within exon 1 (vertical line). The locations of two WRKY repeats are shown in black. The site of insertion of the Tag1 transposon in the ttg2-1 mutant is indicated, and the extent of the inverse PCR fragment derived from Tag1 is shown with arrows.
(B) TTG2 promoter analysis. A translational fusion of TTG2 and the GUS gene was made using the HindIII site within TTG2. The construct with the longest promoter (pXH2.2) extended 2.2 kb 5′ from the start of translation into a XhoI site within the next deduced gene. Three successive 5′ deletions were made (pHH1.0, pEH0.7, and pBH0.3). Asterisks indicate the sites of a 15-bp tandem repeat that contains a putative regulatory element that may control root expression (ATATT).
B, BamHI; Bs, BspDI; E, EcoRI; H, HindIII; P, PstI; X, XhoI.
A cDNA clone corresponding to TTG2 was recovered from a cDNA library made from Arabidopsis roots cultured in vitro. This clone contained an insert of 1315 bp. Comparison with the cosmid genomic sequence indicated that the 5′ end of this cDNA lies within the first exon and 131 bp downstream of the likely translation start site (Figure 4A). The TTG2 gene contains four exons, and the Tag1 element is inserted in the second exon at codon 74. Genomic sequence comparisons of ttg2-1 and the wild type revealed that an 8-bp duplication (GGCTGAAG) occurred upon Tag1 insertion. No evidence of further movement of the transposon was seen, although the sequences of its termini apparently are intact.
The TTG2 locus was mapped to chromosome 2 between DNA markers g17288 and um579B using recombinant inbred lines (Lister and Dean, 1993). It was not separated by recombination from DNA marker m323 among the 28 lines tested. Subsequent to this study, the full DNA sequence of this genomic region was reported (Arabidopsis Genome Initiative, 2000). TTG2 corresponds to gene At2 g37260, or gene 5 within BAC F3G5. The cDNA sequence obtained here reveals that the gene has an additional exon upstream of those predicted in the database.
The inferred polypeptide product of the TTG2 gene contains a duplicated sequence of 65 or 66 amino acids (Figure 5, shaded) that is identical at 29 positions. This sequence closely resembles a domain present in the WRKY family of plant DNA binding proteins. It has two highly conserved sequence motifs, the WRKYGQK amino acid sequence near the N-terminal region (Rushton et al., 1996), and a conserved C-X4-5-C-X22-23-H-X1-H sequence that resembles zinc finger motifs (de Pater et al., 1996; Rushton et al., 1996). Together, these motifs are referred to as the WRKY domain (Eulgem et al., 2000). In family members with two such domains, the C-terminal domain has been shown to bind to specific sequences of DNA (Ishiguro and Nakamura, 1994; de Pater et al., 1996; Eulgem et al., 1999) in a zinc-dependent manner (Rushton et al., 1995; de Pater et al., 1996; Hara et al., 2000). Other regions of TTG2 share little if any sequence relatedness to known WRKY proteins (Eulgem et al., 2000).
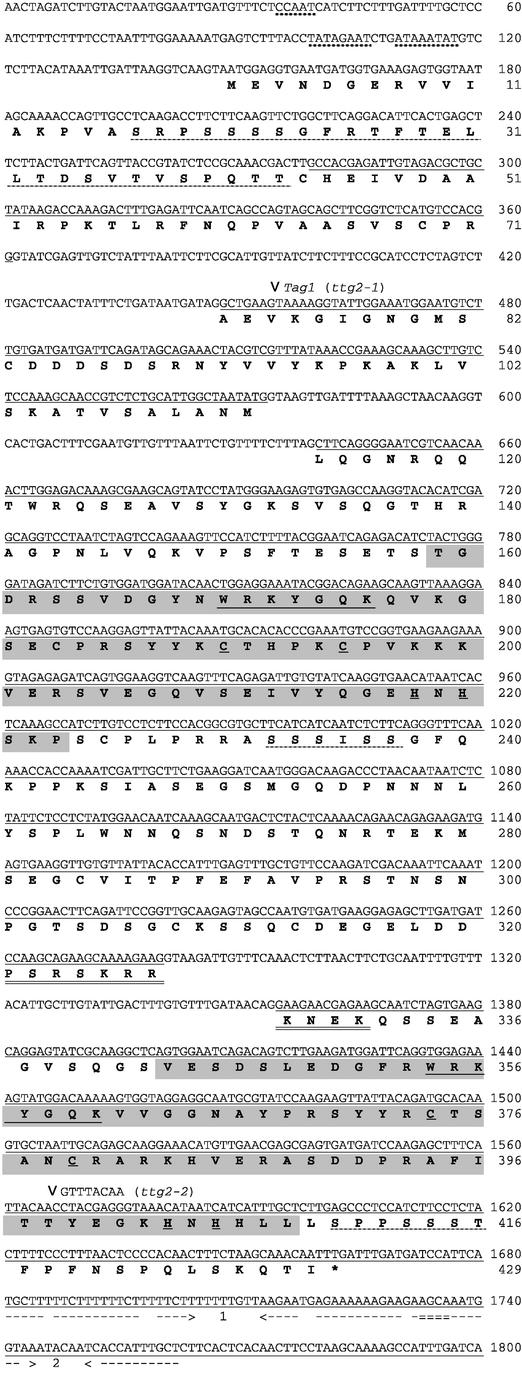
Figure 5.
Sequence of the TTG2 Gene.
The genomic DNA sequence is shown (Columbia ecotype), with regions corresponding to the cDNA recovered in pRRi1 underlined. The deduced amino acid sequence of the TTG2 protein is indicated, commencing at the first Met upstream of the 5′ end of the cDNA. The two WRKY domains are shaded, with the conserved sequence WRKYGQK and the CCHH zinc finger motif underlined in each case. A possible nuclear localization signal (double underline) and putative Ser/Thr-rich activation domains (dotted underline) are highlighted. Also shown are a possible CAAT box and TATA boxes (wavy underline) within the putative promoter and two reverse repeats (1 and 2) located in the 3′ untranslated region (dashed underline). Arrowheads show the sites of insertion of Tag1 in exon 2 in the ttg2-1 mutant and of eight additional nucleotides in the C-terminal WRKY domain in the ttg2-2 mutant. (The ttg2-2 mutant [in Wassilewskija background] also differs from the Columbia genomic sequence at nucleotide 387 in the first intron, in which a C is replaced by a G.)
The ttg2-1 mutant seems likely to result in the loss of functional TTG2 product because the 3.3-kb Tag1 insert is present upstream of the N-terminal WRKY domain and is predicted to result in the early termination of translation (Figure 5). The ttg2-2 mutant allele is associated with the insertion of eight nucleotides in the C-terminal WRKY domain (Figure 5). This frameshift would result in the loss of the two conserved His residues of the putative zinc finger and the generation of a stop codon just downstream of the WRKY domain. Thus, it seems likely that ttg2-2 also produces a nonfunctional product.
The TTG2 protein contains several Ser- and Thr-rich regions (Figure 5, dotted underline), including one near the N terminus (13 of 27 residues), that are characteristic of activation domains in transcription factors. A potential nuclear localization sequence of the SV40 type is present at codons 321 to 331 (Figure 5, double underline). Finally, in the 3′ untranslated region, there are two large reverse repeats that could form stem-loop structures (Figure 5, dashed underlines). Their role is unclear, but they may be involved in mRNA stabilization or in defining the site of polyadenylation.
TTG2 Is Expressed in Trichomes, Seed Coats, and Root Tips
To locate TTG2 mRNA in wild-type plants, tissue sections were probed with labeled antisense RNA generated from the cDNA insert in plasmid pRRi1. In addition, reporter gene constructs were made in which the 5′ promoter and 383 bp of the putative translated region of TTG2 were fused in frame to the β-glucuronidase (GUS) reporter gene (Figure 4B). The longest promoter region used contained 2247 bp upstream of the first ATG (pXH2.2), including the entire 5′ region up to and including some of the C-terminal region of the next deduced gene. Expression patterns consistently present in plants transformed with this promoter, and with the shorter promoter construct pHH1.0, closely matched those detected by in situ hybridization.
TTG2 mRNA was present in developing trichomes when they were first seen as small outgrowths on the adaxial surface of leaves (Figure 6A). As trichome growth and expansion continued, label was detected throughout. Once they were close to maturity, TTG2 mRNA apparently was more abundant in the central region of the trichome, near the branch points (Figure 6C). GUS staining patterns closely matched these expression results (Figures 6B and 6D). Thus, TTG2 transcripts apparently are present in trichomes throughout their growth.
Figure 6.
TTG2 Expression Patterns.
Expression was localized by in situ hybridization of 35S-labeled antisense TTG2 RNA to mRNA ([A], [C], and [G]) or by expression of the GUS reporter gene fused translationally to TTG2 and either 1.0 or 2.2 kb of upstream genomic sequence ([B], [D] to [F], and [H] to [M]). All plants shown are wild type except for those in (L) and (M).
(A) In situ hybridization of antisense TTG2 to transverse sections of developing third, fourth, and fifth leaves. Newly arising trichomes on the adaxial surface of leaves 4 and 5 are heavily labeled (arrows).
(B) GUS staining of a developing leaf (whole mount). Emerging trichomes are strongly stained, and there is patchy internal staining.
(C) Longitudinal section of a vegetative shoot apex of a 7-day-old seedling showing heavy labeling in almost fully mature trichomes (arrows).
(D) Developing leaf (whole mount) showing GUS staining of maturing trichomes in apical regions, newly arising trichomes in basal regions, and internal staining in the pedicel and midrib regions.
(E) Developing silique of a flower soon after fertilization (whole mount) showing heavy GUS staining in developing seeds.
(F) Two developing seeds (whole mount, same scale) with heavy GUS staining seen in the endothelium soon after fertilization (top seed) and later in the other layers of the inner and outer integuments (bottom seed).
(G) Longitudinal section of a developing seed soon after fertilization showing heavy labeling of the endothelium (the innermost layer of the inner integument [arrow]).
(H) Longitudinal section of a developing seed with the embryo at the octant stage showing heavy GUS staining (pink under dark-field conditions) in the endothelium and weaker staining in the embryo and in the endosperm below it.
(I) Longitudinal section of a developing seed at a later stage showing weaker staining in all layers of the seed coat (arrow) except for the now-refringent endothelium.
(J) Root tip (whole mount) showing strong GUS staining in paired files of atrichoblasts from their first appearance and through the cell division and cell elongation zones. Weak staining is still visible in the cell differentiation zone, where root hairs are arising on the intervening files of trichoblasts.
(K) Ten-day-old wild type (wt) seedling (whole mount) showing strong GUS staining in trichomes and in basal regions of younger leaves.
(L) Ten-day-old ttg1 mutant seedling (whole mount) from same family as that shown in (K) showing no GUS staining in leaves. The inset shows GUS staining of the few edge trichomes that have arisen from leaf 5 of the same plant (arrow).
(M) Developing gl2 mutant leaf (whole mount) showing heavy GUS staining in developing trichomes and in the basal regions of the leaf.
Bars = 100 μm.
TTG2 transcription was detected first within ovules immediately after their fertilization (Figure 6E). Unfertilized ovules showed no expression (data not shown). Initially, heavy labeling was seen in the endothelium surrounding the fertilized egg (Figures 6F to 6H). This was strongest at the micropylar end and at the base of the embryo sac at the chalazal end, where the cenocytic cyst occurs (Brown et al., 1999). Expression in the endothelium ceased as soon as densely staining material accumulated in these cells. Weaker expression became detectable somewhat later in all other cell layers of the seed coat (Figures 6F and 6I). Expression continued in the two inner integument layers outside of the endothelium until they were crushed. In the two outer integument layers, expression faded so that none was seen when the outer layer accumulated mucilage.
In addition to trichomes and seed coats, TTG2 was expressed in other tissues. High levels of mRNA detected in situ, and of GUS reporter gene product, were observed in the cell division and cell elongation zones of developing roots (Figure 6J). Specifically, the double files of epidermal cells that do not develop root hairs (atrichoblasts) rapidly accumulated GUS product. Staining levels decreased as the adjacent cell files began to develop root hairs in the cell differentiation zone. In 5-day-old seedlings, very weak staining also was seen in files of hypocotyl epidermis cells that are related by lineage to root atrichoblasts.
Other vegetative tissues also showed significant TTG2 expression. Leaf primordia were labeled with antisense TTG2 RNA and showed uniform and intense GUS expression. As they developed, GUS staining became progressively weaker, at first showing a patchy distribution within the developing mesophyll, and remaining last near the base of the lamina and in the petiole (especially the abaxial epidermis) and in the main vascular bundle (Figure 6D). The shoot apical meristem itself was unstained, but cortical cells below it often were stained at first, as was the developing vasculature. Similar staining was seen in inflorescence shoots. In some transformants, developing sepals were stained in medial abaxial regions. Finally, weak, uniformly distributed expression was seen by in situ hybridization and GUS accumulation in the developing embryo from at least the octant stage to the walking stick stage. The developing endosperm was labeled to a similar degree through the same stages (Figure 6H).
A preliminary investigation of subregions of the TTG2 promoter was performed by generating three 5′ deletions of the GUS reporter construct pXH2.2 (Figure 4B). No elements upstream of 1003 bp from the putative start of translation were detected; GUS patterns in the pHH1.0 construct closely matched those of the longer pXH2.2 construct in intensity and localization. One or more cis-acting elements required to drive intense expression in the root epidermal atrichoblasts was detected within the region 703 to 1003 bp upstream. The construct pEH0.7 generated only very weak staining in these cells even after 24 h of staining. Other staining patterns were unaffected. It is of interest that two direct repeats of 15 bp (separated by 2 bp) occur within the −703 to −1003 region, and these contain the sequence ATATT. This motif also occurs as a tandem duplication in a region of the rolD promoter of the Agrobacterium rhizogenes Ri plasmid that controls root expression (Elmayan and Tepfer, 1995).
No detectable GUS staining was seen in any region when expression was driven using 300 bp upstream of the putative translation start site (pBH0.3). Thus, expression in trichomes, seed coats, and other tissues is controlled by element(s) localized within the −300 to −703 region, whereas root expression elements occur within the −703 to −1003 upstream region.
Generally, TTG2 expression patterns were independent of plant growth conditions. Other known WRKY family genes are expressed relatively rapidly in response to pathogen infection, treatment with salicylate, jasmonic acid, or ethylene, and mechanical wounding (Eulgem et al., 1999; Hara et al., 2000; Robatzek and Somssich, 2001; Yu et al., 2001). To determine if TTG2 expression is induced by salicylate or wounding, plants carrying the GUS reporter construct pXH2.2 were wetted with 2 mM potassium salicylate (pH 6.5) or wounded by compressing leaf tissues with forceps. Compared with controls, no detectable changes in GUS staining were seen anywhere in such plants up to 4 h later (data not shown).
TTG2 Expression Is Supported by TTG1 Function but Is Independent of GL1, GL2, and TTG2 Function
To determine if TTG2 expression requires the function of TTG1, GL1, GL2, or TTG2, representative pTTG2::GUS lines carrying pXH2.2 were crossed to mutants of each gene, and GUS staining patterns were observed in mutant F2 progeny. The most striking effects were seen in ttg1-1 mutants. Staining of leaf primordia was very weak or absent, and no staining was seen in surface regions where trichomes normally develop, although the few trichomes that arose on the edges of leaves were GUS stained (Figures 6K and 6L). Seed coats, too, were very weakly stained in ttg1 mutants. No staining was detected in the young endothelium, where normally it is very strong, although the outer layers were stained during later stages. In roots of ttg1-1 mutants, staining was seen in files of cells in the cell division and cell expansion zones in a pattern similar to that seen in the wild type. Even so, staining was more irregular and patchy, and it was not seen in the cell differentiation zone at the stage when root hairs arose from all cell files. Thus, in general, it seems that TTG2 expression requires TTG1 function in leaf primordia and the endothelium of seed coats, but not in trichomes once they have arisen, in other cells of the seed coat, or in root tip cells.
The other trichome initiation gene, GL1, does not seem to be required for TTG2 expression to occur in leaf primordia, because the levels of GUS staining seen in gl1-1 mutants were close to normal. No trichomes arose on the leaf surface of gl1-1 mutants, and no pTTG2::GUS staining was seen, although the few leaf edge trichomes that arose in gl1-1 mutants were stained just as they were in ttg1 mutants. As expected, TTG2 expression in gl1-1 mutants was normal in both seed coats and root primordia.
The function of the GL2 gene, which genetic evidence suggests shares functions with TTG2 in early trichome outgrowth, is not required for TTG2 expression. In gl2-1 mutants, pTTG2::GUS staining was not detectably different from wild-type staining in leaf primordia or in trichomes, given that the latter do not usually develop to maturity (Figure 6M). pTTG2::GUS staining also apparently was unaffected in gl2-1 seed coats. The pattern in developing gl2-1 roots was similar to that seen in ttg1-1 mutants, revealing that the early localization of TTG2 expression to cells equivalent to atrichoblasts is independent of both GL2 and TTG1 function (although its later expression in the cell differentiation zone is not maintained if GL2 or TTG1 function is compromised).
Finally, we found no evidence that TTG2 regulates its own expression. pTTG2::GUS staining in ttg2-1 mutant plants was indistinguishable from that in the wild type in leaf primordia, trichomes, seed coats, or roots in both pattern and intensity (data not shown).
DISCUSSION
WRKY Genes Have Functions in Morphogenesis as Well as in Stress Response
We have discovered that TTG2 encodes a zinc finger–like transcription factor of the plant-specific WRKY gene family. The best known property of WRKY genes is their rapid expression after pathogen infection (Eulgem et al., 2000). W boxes [(T)(T)TGAC(C/T)] were identified in regulatory regions upstream of genes encoding pathogenesis-related proteins in parsley, and three WRKY proteins were discovered that bound specifically to such regions (Rushton et al., 1996). Later studies showed that expression of these and other WRKY genes is activated rapidly by fungal elicitors or by salicylate and that WRKY proteins interact specifically with W boxes and induce the expression of target genes in vivo (Eulgem et al., 1999; Yu et al., 2001). WRKY gene expression also is associated with the related processes of mechanical wounding (Hara et al., 2000) and senescence (Kim et al., 1997; Eulgem et al., 2000; Robatzek and Somssich, 2001). Historically, WRKY proteins were identified first through their binding to W box–like sequences in promoters of genes encoding the storage protein sporamin and the metabolic enzymes β-amylase in sweet potato (Ishiguro and Nakamura, 1994) and α-amylases in oat (Rushton et al., 1995), but it is not known if this binding has significance in vivo.
TTG2 is the first WRKY gene to be associated with a mutant phenotype. Surprisingly, this phenotype, together with its expression pattern and interaction with other regulatory genes, establishes that TTG2 plays a role unrelated to rapid stress response. Rather, it regulates at least three separate morphogenetic processes in L1-derived cells: trichome development and the production of mucilage and tannin in seed coats. Since the completion of the Arabidopsis genome project, 72 WRKY family members have been identified (Riechmann et al., 2000). TTG2 corresponds to AtWRKY44, a member of group I (Eulgem et al., 2000). This group includes at least 12 other genes that carry two tandemly repeated WRKY domains. Other genes that have only one WRKY domain fall into groups II and III. Proteins involved in stress response occur in all three groups in parsley, tobacco, and Arabidopsis (Eulgem et al., 2000; Robatzek and Somssich, 2001; Yu et al., 2001). Thus, stress response may be an ancestral role of WRKY proteins, and TTG2 may represent a more recent diversification of WRKY function within group I. In this regard, TTG2 does not contain the conserved sequences (regions A and D) found outside the WRKY domains in some other members of group I (Eulgem et al., 2000), although a conserved putative nuclear localization signal (region 2) is present (Robatzek and Somssich, 2001).
TTG2 Functions Downstream of TTG1 and GL1 and Shares Functions with GL2 in Trichome Development
TTG1 and GL1 functions are required for trichomes to be initiated on leaf surfaces, whereas TTG2 function is not (Figure 7). Because surface trichomes do not arise in gl1 or ttg1 mutants, it is not possible to determine from mutant studies whether TTG2 action is controlled directly by these two genes. Once initiated, however, we can say that neither TTG1 nor GL1 function is required to activate TTG2 expression, because the few trichomes that may arise on the edges of ttg1 and gl1 mutant leaves express TTG2 throughout their development. Furthermore, leaf edge trichomes still arise in gl1 ttg2 and ttg1 ttg2 double mutants, suggesting that TTG2 does not share general trichome initiation functions with GL1 or TTG1.
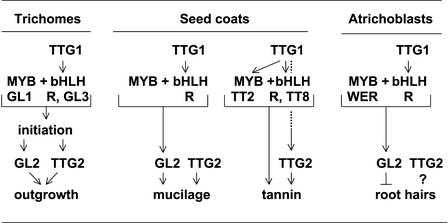
Figure 7.
Proposed Genetic Pathways for the Regulation of Trichome, Seed Coat, and Root Hair Development in Arabidopsis.
Arrows indicate positive regulation, and barred lines indicate negative regulation.
On the other hand, TTG2 shares many trichome properties with the HD-ZIP transcription factor gene GL2 (Figure 7). They are expressed in very similar patterns (in leaf primordia and in trichomes throughout their development), and the consequences of their mutation are similar (a reduction in trichome outgrowth and differentiation). TTG2 does not seem to act directly downstream of GL2, because TTG2 expression is strong in gl2 mutant trichomes. Rather, there is overlap in the processes of trichome development they support, because the extent of trichome outgrowth and differentiation is reduced considerably in gl2 ttg2 double mutants. The number of trichomes seen also is reduced in both ttg2 and gl2 single mutant plants, so each gene may promote trichome initiation without being necessary for it to occur; however, the number is not reduced further in double mutants, so they may promote initiation by the same mechanism. In this regard, it will be informative to examine the consequences of overexpression of TTG2 and GL2 separately and together. A fourth trichome gene, encoding the bHLH transcription factor GL3, also seems to function in promoting trichome initiation (Figure 7) and acts later during trichome morphogenesis, and examination of its genetic and molecular interactions with TTG2 will be of interest.
Clues about the mechanisms of trichome initiation have come from the observation that clusters of trichomes may occur in some genotypes in which initiation is disrupted. This was seen in ttg2-1 mutants, in which ∼10% of trichomes were clustered. Clustering also has been observed in heterozygotes of strong ttg1 mutants and in homozygotes of weaker alleles (Larkin et al., 1994, 1999). Spacing of trichomes is influenced strongly by another gene, TRYPTYCHON (TRY). In try mutants, most trichomes arise in multiple clusters (Hülskamp et al., 1994). There is genetic evidence that the TRY gene plays an inhibitory role in trichome initiation, because the numbers of additional and ectopic trichomes that arise in plants that overexpress either GL1 or R from maize or both are increased further in try mutants (Szymanski et al., 1998; Schnittger et al., 1999). How this TRY-mediated inhibition might occur normally is not known, although it has been proposed that TRY plays a direct role in the lateral inhibition of trichome initiation in neighboring cells (Schnittger et al., 1999) or that the TRY protein is associated directly with TTG1 and GL1 proteins in an inhibitory complex (Szymanski et al., 2000). Once the TRY gene has been cloned, further interpretation will be facilitated.
TTG2 Functions Independently of TTG1 and GL2 in Mucilage Production in the Seed Coat
In Arabidopsis, seed coat mucilage is a pectin-like substance laid down in the epidermis from ∼7 days after pollination (Western et al., 2000). It forms between the plasma membrane and the outer cell wall, compressing the cytoplasm into a column-like shape in the center of each cell (the columella) (Western et al., 2000; Windsor et al., 2000). Another cell wall then is made that separates the mucilage from the plasma membrane. Starch granules appear in both the epidermis and the cell layer under it (the inner layer of the outer integument) soon after fertilization. These disappear as the mucilage and new cell walls arise, perhaps providing components of the new secondary cell wall (Western et al., 2000; Windsor et al., 2000).
Mucilage production is under the control of the trichome genes TTG1, GL2, and TTG2 (Figure 7) among others (Penfield et al., 2001; Western et al., 2001). The pleiotropic trichome mutants lack mucilage in each case, although the two outer cell layers of the developing seed coat are present as normal (Koornneef et al., 1982; this study). GL2 is expressed relatively late and only in the epidermal cell layer (Windsor et al., 2000), and this expression requires TTG1 function (Szymanski et al., 1998). On the other hand, TTG2 expression seems to occur in all four outer cell layers of the developing seed coat from approximately the heart-shaped embryo stage, and this expression is not dependent on either TTG1 or GL2 function. Thus, TTG2 apparently controls a different, perhaps earlier, step in mucilage production than GL2, and it does so independently of TTG1 function.
TTG2 Function Is Dependent on TTG1 Function for Production of Tannin in the Seed Coat
The innermost layer of the seed coat, the endothelium, undergoes specific developmental changes soon after fertilization. Cells become vacuolate, and the vacuoles contain large amounts of the colorless precursors of tannins. Acidic vanillin turns red in the presence of these precursors, specifically flavan-3,4-diol (leucoanthocyanidin) and flavan-3-ol (catechin), which occur as monomers, heterodimers, or as terminal units of their proanthocyanidin polymer (Debeaujon et al., 2000; Nesi et al., 2000, 2001). Ultimately, the proanthocyanidins oxidize to become dark brown tannin as the seeds dry. TTG2 is expressed strongly in the endothelium of ovules immediately after they have been fertilized, revealing that its expression is triggered by this process. It continues until the cells become vacuolate, at which point it declines rapidly.
In ttg2 mutants, the amount of vanillin-stained material present in endothelial cells is reduced greatly. Synthesis of tannins but not anthocyanins is disrupted in ttg2 mutants, so it seems likely that TTG2 regulates the expression of gene(s) involved in the tannin biosynthetic pathway after the leucoanthocyanidin branch point. Consistent with this, the biosynthetic step before leucoanthocyanidin is blocked in ttg1 mutants, and their endothelium is unstained with vanillin. The residual weak vanillin staining seen in ttg2 seed coats may result from the accumulation of free flavanol precursors of tannins, including leucoanthocyanidin. The staining seen in the outer layers may reflect the spreading of excess precursors into these layers.
Candidate tannin genes whose expression may be regulated by TTG2 include BAN, a gene that may control the first step in tannin biosynthesis (the conversion of leucoanthocyanidins to catechins [Devic et al., 1999]), TT12, which seems to be required for the vacuolar transport of tannin precursors within the endothelium (Debeaujon et al., 2000), and the zinc finger gene TT1 (Sagasser et al., 2002). It is known that BAN expression requires TTG1 function (Nesi et al., 2000), together with the bHLH protein TT8 (Nesi et al., 2000) and the MYB protein TT2 (Nesi et al., 2001). Further tests will reveal whether the WRKY protein TTG2 is directly involved as well, although we know that TTG2 expression in the endothelium requires TTG1 function (Figure 7).
TTG2 May Redundantly Control Atrichoblast Development in Roots
Evidence that TTG2 also plays a role in root development comes solely from its strong atrichoblast expression. Loss of TTG2 function is without apparent effect on root development, either alone or in combination with the loss of TTG1 or GL2 function. Furthermore, appropriately localized TTG2 expression still occurs in ttg1 and gl2 mutants, at least in the cell division and cell elongation zones. Thus, neither of these genes limits early TTG2 expression to cell files that normally would lack root hairs (Figure 7), although their function seems to be required later to maintain TTG2 expression in the differentiation zone. In general, it seems that if TTG2 is involved in atrichoblast specification, other unknown genes whose root functions fully encompass that of TTG2 also must be involved. Such genes may include other members of the WRKY family, although TTG2 has no closely similar WRKY relatives. Even the most closely related proteins, WRKY3, -4, -20, -33, and -58, differ from TTG2 in 19 or 20 of the 66 amino acids in the conserved C-terminal DNA binding domain and show little similarity to TTG2 outside of the two WRKY domains (Eulgem et al., 2000).
Overexpression tests of TTG2 may help clarify its possible root functions. It is known that ectopic expression of the maize R bHLH gene blocks root hair development from trichoblasts. This occurs presumably through activation of the GL2 repressor of root hair development, because ectopic expression of GL2 is induced in roots by overexpression of R (Hung et al., 1998; Lee and Schiefelbein, 1999). The MYB protein WER is the likely partner of the Arabidopsis R ortholog in atrichoblasts, and, significantly, GL2 expression in these cells is absolutely dependent on WER function (Lee and Schiefelbein, 1999; Lin and Schiefelbein, 2001) (Figure 7). It will be of interest to determine if TTG2 expression is likewise under direct WER control.
Another MYB gene, CAPRICE (CPC), also is involved in root hair development (Wada et al., 1997). Mutants of cpc lack root hairs, whereas overexpression of CPC results in hairs arising from all cell files. It has been proposed that the CPC protein normally associates with the bHLH R protein in trichoblasts, preventing activation of the root hair repressor gene GL2 (Lee and Schiefelbein, 1999). Interestingly, overexpression of CPC blocks the production of trichomes in leaves (Wada et al., 1997), adding further evidence that regulatory mechanisms are shared between root hairs and trichomes (Lee and Schiefelbein, 2001).
Conclusion
This study has revealed that a new class of transcription factor, the WRKY class, controls trichome, seed coat, and perhaps root hair development in Arabidopsis. It was established previously that the same processes are regulated jointly by MYB and bHLH proteins, a WD40 “accessory protein,” and a HD-ZIP protein (Figure 7). This common involvement suggests these four classes of proteins as possible partners of TTG2. However, at this time, genetic evidence supports only one likely interaction, association of the TTG2 protein with GL2 (an HD-ZIP protein), in controlling trichome outgrowth.
We also determined if TTG1, GL1, or GL2 directly controls TTG2 expression. The answer is no, with the sole exception of TTG1 being necessary for TTG2 expression in leaf primordia and in the endothelium of the seed coat. It is possible that the WD40 protein TTG1 recruits or activates other necessary transcription factors in these tissues. However, it is clear that other genes that jointly regulate these processes remain to be identified, and these may play a direct role in TTG2 regulation. The most important gap in our knowledge is the nature of the proposed Arabidopsis ortholog of the maize R bHLH gene. The GL3 bHLH gene and its close relative 146D23T7 both promote supernumerary trichomes when overexpressed, but neither seems to duplicate fully the effects of R overexpression (Payne et al., 2000). Likewise, additional MYB genes may be involved. It is known that the closest relative of GL1 and WER, AtMYB23, is expressed in developing trichomes, roots, and the seed coat, and its overexpression reveals that its trichome functions overlap with those of GL1 (Kirik et al., 2001).
TTG2 regulates trichome, seed coat, and perhaps root hair development. A fuller understanding of its interactions with other regulatory proteins in the control of these processes may reveal how other members of the large WRKY family interact with partners in their response to stress.
METHODS
Plant Material
Arabidopsis thaliana plants were grown in a 50:50 mix of perlite and seed-raising mixture subirrigated with nutrient solution. They were raised under glasshouse conditions (23 to 25°C, with daylight supplemented by continuous cool-white fluorescent light). Mutant lines of gl1-1, gl2-1, and ttg1-1 were provided originally by the Arabidopsis Information Service (Frankfurt-am-Main, Germany). Double mutant lines were generated by intercrossing two recessive mutants and identifying the weaker of the two mutant phenotypes among the F2 plants. These were allowed to self-fertilize, and the double mutant phenotype was identified among progeny of those F2 plants that were revealed to be heterozygous for the mutant allele of the other gene. J. Brian Windsor and Alan Lloyd (University of Texas at Austin) provided the ttg2-2 mutant line (also homozygous for the erecta mutant) for complementation tests and sequencing. The ttg2-2 mutant allele was identified originally in Wassilewskija plants transformed with T-DNA obtained from the ABRC (Columbus, OH), but it was not T-DNA tagged. Unless stated otherwise, all other plants were of the Landsberg erecta ecotype.
Origin of the ttg2-1 Mutant
The ttg2-1 mutant arose in a transposon tagging experiment set up by inserting the Activation (Ac) element of maize present in the Ti plasmid pBI35SAc11 (Finnegan et al., 1993) into Arabidopsis. T-DNA sequences were transferred into root callus cells by infection with Agrobacterium tumefaciens strain AGL1 using the method of Valvekens et al. (1988). Screening of descendants yielded a recessive trichome and seed coat mutant phenotype, ttg2-1, that did not cosegregate with a new Ac insertion. Segregating plants then were screened for the presence of new bands of the endogenous Tag1 transposon. This was achieved by DNA gel blot hybridization using a probe generated by PCR amplification of the sequence between nucleotides 251 and 1064 of Tag1 (Tsay et al., 1993). The ttg2-1 mutant cosegregated absolutely with a newly arisen Tag1 band in 32 meiotic products from plants heterozygous for both ttg2-1 and the new Tag1 band.
Cloning of TTG2 and Complementation of ttg2-1
Sequences flanking one side of the newly inserted Tag1 band were recovered by inverse PCR using primers within Tag1 from nucleotides 19 to 2 inclusive and from 251 to 270 inclusive (Tsay et al., 1993) (Figure 4A). Genomic DNA of ttg2-1 mutant plants was digested with Sau3AI and circularized, PCR was performed, and the products were digested with BfaI. Control Landsberg erecta plants generated bands of 360 and 460 bp, presumably corresponding to the two Tag1 elements resident in this strain (Tsay et al., 1993). An additional band of 580 bp was present consistently in ttg2-1 mutant plants. This band was isolated from electrophoretic gels and used as a probe to isolate larger genomic clones from a cosmid library of Columbia genomic DNA made using the vector pOCA18 (Olszewski et al., 1988). (The Columbia ecotype does not contain Tag1 elements.) Three overlapping clones of 20 to 23 kb were isolated. One of these, pCOS1, was chosen for further characterization (Figure 4A). It was used to test for complementation of the mutant phenotype in transformants regenerated from roots of the ttg2-1 mutant using the method of Valvekens et al. (1988).
The site of the new Tag1 insert was mapped using Landsberg/Columbia recombinant inbred lines generated by Lister and Dean (1993). Another genomic cosmid, pCOS2 (overlapping pCOS1 by ∼95%), was used to identify a DraI polymorphism between the Landsberg erecta and Columbia ecotypes. Twenty-eight of the recombinant inbred lines known to show high levels of recombination were probed with pCOS2, and their DraI polymorphism patterns were deduced.
Isolation of TTG2 cDNA
The inverse PCR border fragment was localized within a 0.68-kb BamHI-HindIII fragment of cosmid pCOS1. Preliminary sequencing indicated that the Tag1 insert may have been close to the 5′ end of the gene, so a 0.5-kb HindIII-BspDI subclone adjacent to the BamHI-HindIII sequence on the 3′ side was used to probe cDNA libraries. Approximately 1.2 × 106 plaques of a cDNA library made from inflorescence shoots were probed without a positive result. Another cDNA library, generously provided by John Hamill (Monash University) and made from Landsberg erecta roots that had been transformed with Agrobacterium rhizogenes strain LBA9402, also was tested. In this library, cDNA inserts were cloned directionally (EcoRI-XhoI) in the Uni-Zap XR vector (Stratagene, La Jolla, CA). Approximately 1.2 × 106 plaques were probed, and one positive cDNA clone, pRRi1, was obtained.
Sequencing of the 0.58-kb inverse PCR product, the 2.5-kb EcoRI-HindIII genomic region that spans TTG2, the 1.3-kb cDNA insert in pRRi1, the genomic DNA of ttg2-2 mutant plants (from 70 nucleotides upstream of the putative translation start site to 131 nucleotides downstream of the putative stop codon), and the translational fusions to the β-glucuronidase (GUS) reporter gene was performed using the ABI PRISM Dye Terminator Cycle Sequencing kit (Perkin-Elmer, Foster City, CA).
Scanning Electron Microscopy
Scanning electron microscopy was performed using a Hitachi s570 scanning electron microscope (Tokyo, Japan) at an accelerating voltage of 5 to 15 kV. Fresh samples were cryopreserved and coated with gold using an Emscope SP2000 (Dynavac, Ashford, UK) and then observed directly. To examine the general pattern of trichome distribution on leaves, whole seedlings were fixed in 2% glutaraldehyde in 25 mM sodium phosphate buffer, pH 6.8, treated with OsO4 (0.5% in the same buffer at 12.5 mM), dehydrated through an ethanol series, critical point dried in CO2, and coated with gold using an Eiko 1B.5 sputter coater (Eiko, Tokyo, Japan). Dry seeds were coated with gold directly and observed subsequently.
In Situ Hybridization
Plant material was fixed in 3.7% formaldehyde, 5% acetic acid, and 50% ethanol (v/v) for 3 to 4 h at room temperature, dehydrated through an ethanol series, and embedded in paraffin (Paraplast Plus, Oxford, St. Louis, MO). Serial sections of 8 μm were dried onto slides. To prepare the antisense probe, the full-length cDNA clone pRRi1 was linearized, and runoff transcription was performed using T7 RNA polymerase and nucleotides including α-35S-UTP (625 Ci/mmol). Sense probes of TTG2 cDNA were made using T3 RNA polymerase for use as negative controls. The paraffin was dissolved from sections using xylene, and the tissue was rehydrated.
Approximately 1.5 × 107 cpm of probe (in 50% formamide, 300 mM NaCl, 10 mM Tris, pH 7.5, 1 mM EDTA, 1 × Denhardt's solution [1 × Denhardt's solution is 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% BSA], 70 mM DTT, and 10% dextran sulfate) was added to each slide, a cover slip was lowered over the solution, and the slides were incubated at 42°C for ∼12 h. After treatment with RNaseA, slides were washed at low stringency and then at high stringency (15 mM NaCl, 1 mM NaH2PO4, pH 7.4, 0.1 mM EDTA, and 0.1 × SSPE [1 × SSPE is 0.115 M NaCl, 10 mM sodium phosphate, and 1 mM EDTA, pH 7.4] at 57°C) for 30 to 60 min. After drying, the slides were dipped in LM-1 nuclear emulsion (Amersham), exposed for 4 to 6 weeks, and developed using Kodak D19 developer. Sections were stained using 0.1% toluidine blue in 0.1% sodium borate.
To determine if the full-length cDNA antisense probe specifically identified TTG2 sequences, wild-type genomic DNA and cosmid pCOS1 DNA were digested, transferred to nylon filters after size fractionation, and hybridized with labeled antisense probe under low-stringency conditions (5 × SSPE at 47°C). Filters then were washed first with 2 × SSPE at 51°C (low stringency) and subsequently with 0.1 × SSPE at 57°C (high stringency). Bands additional to those resulting from TTG2 sequences, presumably derived from related family members, were visible after the low-stringency wash, but they were not detectable after the high-stringency wash.
Construction and Expression of pTTG2::GUS Translational Fusions
A 2247-bp sequence upstream of the first ATG in the TTG2 cDNA, and 383 bp of the TTG2 coding region, were translationally fused in frame with the uidA GUS reporter gene. This was achieved by inserting pCOS1 genomic sequences into pBluescript II SK+ and transferring the full insert plus some adjacent polylinker sequence into the polylinker of plasmid pBI101.1. This construct was named pXH2.2::GUS. Three shorter promoter constructs also were generated in which the 5′ region was shortened progressively, based on restriction sites in the promoter region. These contained 1003 bp upstream of the first Met (to a HindIII site; pHH1.0::GUS), 703 bp upstream (EcoRI; pEH0.7::GUS), or 300 bp upstream (BamHI; pBH0.3::GUS). Inserts in all constructs were sequenced to confirm their structure. Constructs were electroporated into A. tumefaciens strain AGL1, and plants of Landsberg erecta ecotype (or the Columbia ecotype for some of the pHH1.0::GUS clones) were transformed by floral dip infiltration. Between 13 and 20 independent transformants were obtained for each of the four constructs, and only consistently observed expression patterns are reported.
To stain for GUS, tissues were fixed in 90% (v/v) acetone:water for 20 min, rinsed in 50 mM sodium phosphate buffer, pH 7.2, containing 3 mM potassium ferricyanide and 3 mM potassium ferrocyanide, and then transferred to the same solution containing in addition 2 mM 5-bromo-4-chloro-3-indolyl β-d-glucuronic acid (Guivarc'h et al., 1996). Tissues were vacuum-infiltrated for 3 min and then incubated at 37°C for various times from 2 min to 24 h. Chlorophyll was extracted by passing through increasing concentrations of ethanol. Tissues were examined as whole mounts or as sections. For sections, stained tissues were further fixed, embedded in paraffin, and sectioned as for in situ hybridization. They were mounted in DePeX (BDH, Poole, UK) and viewed using dark-field microscopy. Under these conditions, the blue product of the GUS reaction appears pink.
Accession Number
The accession number for BAC F3G5 is AC005896.2. The accession number for pRRi1 is AF516172.
Acknowledgments
We are grateful to Stan Alvarez, Peter Bundock, Yasmin Bylstra, Jean-Pierre Eid, Bob Elliott, Megan Griffith, Michael Groszmann, Marcus Heisler, Paul Howles, and Brian Kwan for their interest and assistance. We thank Jean Finnegan and Liz Dennis for providing the plasmid pBI35SAc11, Neil Olzewski for the cosmid library, Detlef Weigel and John Hamill for cDNA libraries, Jean-Pierre Eid for generating preliminary GUS constructs, J. Brian Windsor and Alan Lloyd for providing ttg2-2, and Phil Brewer and Teodora Paicu for sequencing it. Gunta Jaudzems provided excellent advice for electron microscopy. C.S.J. was awarded a Phyllis Hillgrove Scholarship from Monash University. This work was supported by a Monash University–Commonwealth Scientific and Industrial Research Organization Collaborative Grant and by the Australian Research Council (Grant A19531283).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.001404.
References
- Arabidopsis Genome Initiative. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Brown, R.C., Lemmon, B.E., Nguyen, H., and Olsen, O.-A. (1999). Development of endosperm in Arabidopsis thaliana. Sex. Plant Reprod. 12, 32–42. [Google Scholar]
- Debeaujon, I., Léon-Kloosterziel, K.M., and Koornneef, M. (2000). Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 122, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater, S., Greco, V., Pham, K., Memelink, J., and Kijme, J. (1996). Characterization of a zinc-dependent transcriptional activator from Arabidopsis. Nucleic Acids Res. 24, 4624–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devic, M., Guilleminot, J., Debeaujon, I., Bechtold, N., Bensaude, E., Koornneef, M., Pelletier, G., and Delseny, M. (1999). The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. Plant J. 19, 387–398. [DOI] [PubMed] [Google Scholar]
- Di Cristina, M., Sessa, G., Dolan, L., Linstead, P., Baima, S., Ruberti, I., and Morelli, G. (1996). The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. Plant J. 10, 393–402. [DOI] [PubMed] [Google Scholar]
- Elmayan, T., and Tepfer, M. (1995). Evaluation in tobacco of the organ specificity and strength of the rolD promoter, domain A of the 35S promoter and the 35S2 promoter. Transgenic Res. 4, 388–396. [DOI] [PubMed] [Google Scholar]
- Eulgem, T., Rushton, P.J., Robatzek, S., and Somssich, I.E. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Eulgem, T., Rushton, P.J., Schmenlzer, E., Hahlbrock, K., and Somssich, I.E. (1999). Early nuclear events in plant defence signalling: Rapid gene activation by WRKY transcription factors. EMBO J. 18, 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan, E.J., Lawrence, G.J., Dennis, E.S., and Ellis, J.G. (1993). Behaviour of modified Ac element in flax callus and regenerated plants. Plant Mol. Biol. 22, 625–633. [DOI] [PubMed] [Google Scholar]
- Galway, M.E., Masucci, J.D., Lloyd, A.M., Walbot, V., Davis, R.W., and Schiefelbein, J.W. (1994). The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev. Biol. 166, 740–754. [DOI] [PubMed] [Google Scholar]
- Guivarc'h, A., Caissard, J.C., Azmi, A., Elmayan, T., Chequi, D., and Tepfer, M. (1996). In situ detection of expression of a GUS reporter gene in transgenic plants: Ten years of blue genes. Transgenic Res. 5, 281–298. [Google Scholar]
- Hara, K., Yagi, M., Kusano, T., and Sano, H. (2000). Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol. Gen. Genet. 263, 30–37. [DOI] [PubMed] [Google Scholar]
- Hülskamp, M., Miséra, S., and Jürgens, G. (1994). Genetic dissection of trichome cell development in Arabidopsis. Cell 76, 555–566. [DOI] [PubMed] [Google Scholar]
- Hülskamp, M., Schnittger, A., and Folkers, U. (1999). Pattern formation and cell differentiation: Trichomes in Arabidopsis as a genetic model system. Int. Rev. Cytol. 186, 147–178. [DOI] [PubMed] [Google Scholar]
- Hung, C.-Y., Zhang, M., Pollock, S., Marks, D.M., and Schiefelbein, J. (1998). A common position-dependent mechanism controls cell-type patterning and GLABRA2 regulation in the root and hypocotyl epidermis of Arabidopsis. Plant Physiol. 117, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, S., and Nakamura, K. (1994). Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. 244, 563–571. [DOI] [PubMed] [Google Scholar]
- Kim, D.-J., Smith, S.M., and Leaver, C.J. (1997). A cDNA encoding a putative SPF1-like DNA binding protein from cucumber. Gene 185, 265–269. [DOI] [PubMed] [Google Scholar]
- Kirik, V., Schnittger, A., Radchuk, V., Adler, K., Hülskamp, M., and Bäumlein, H. (2001). Ectopic expression of the Arabidopsis AtMYB23 gene induces differentiation of trichome cells. Dev. Biol. 235, 366–377. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Dellaert, S.W.M., and van der Veen, J.H. (1982). EMS- and radiation-induced mutation frequencies at individual loci in Arabidopsis thaliana (L.) Heynh. Mutat. Res. 93, 109–123. [DOI] [PubMed] [Google Scholar]
- Larkin, J.C., Oppenheimer, D.G., Lloyd, A.M., Paparozzi, E.T., and Marks, M.D. (1994). Roles of the GLABROUS1 and TRANSPARENT TESTA GLABRA genes in Arabidopsis trichome development. Plant Cell 6, 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, J.C., Oppenheimer, D.G., Pollock, S., and Marks, M.D. (1993). Arabidopsis GLABROUS1 gene requires downstream sequences for function. Plant Cell 5, 1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, J.C., Walker, J.D., Bolognesi-Winfield, A.C., Gray, J.G., and Walker, A.R. (1999). Allele-specific interactions between ttg and gl1 during trichome development in Arabidopsis thaliana. Genetics 151, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.M., and Schiefelbein, J. (1999). WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99, 473–483. [DOI] [PubMed] [Google Scholar]
- Lee, M.M., and Schiefelbein, J. (2001). Developmentally distinct MYB genes encode functionally equivalent proteins in Arabidopsis. Development 128, 1539–1546. [DOI] [PubMed] [Google Scholar]
- Lin, Y., and Schiefelbein, J. (2001). Embryonic control of epidermal cell patterning in the root and hypocotyl of Arabidopsis. Development 128, 3697–3705. [DOI] [PubMed] [Google Scholar]
- Lister, C., and Dean, C. (1993). Recombinant inbred lines for mapping RFLP and phenotypic markers in Arabidopsis thaliana. Plant J. 4, 745–750. [DOI] [PubMed] [Google Scholar]
- Lloyd, A.M., Walbot, V., and Davis, R.W. (1992). Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and C1. Science 258, 1773–1775. [DOI] [PubMed] [Google Scholar]
- Masucci, J.D., Rerie, W.G., Foreman, D.R., Zhang, M., Galway, M.E., Marks, M.D., and Schiefelbein, J. (1996). The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Nesi, N., Debeaujon, I., Jond, C., Lelletier, G., Caboche, M., and Lepiniec, L. (2000). The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 12, 1863–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesi, N., Jond, C., Debeaujon, I., Caboche, M., and Lepiniec, L. (2001). The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13, 2099–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski, N.E., Martin, F.B., and Ausubel, F.M. (1988). Specialized binary vector for plant transformation: Expression of the Arabidopsis thaliana AHAS gene in Nicotiana tabacum. Nucleic Acids Res. 16, 10765–10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, C.T., Zhang, F., and Lloyd, A.M. (2000). GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 156, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield, S., Meissner, R.C., Shoue, A.A., Carpita, N.C., and Bevan, M.W. (2001). MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 13, 2777–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann, J.L., et al. (2000). Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 290, 2105–2110. [DOI] [PubMed] [Google Scholar]
- Rerie, W.G., Feldmann, K.A., and Marks, M.D. (1994). The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev. 8, 1388–1399. [DOI] [PubMed] [Google Scholar]
- Robatzek, S., and Somssich, I.E. (2001). A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 28, 123–133. [DOI] [PubMed] [Google Scholar]
- Rushton, P.J., Macdonald, H., Huttly, A.K., Lazarus, C.M., and Hooley, R. (1995). Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of α-Amy2 genes. Plant Mol. Biol. 29, 691–702. [DOI] [PubMed] [Google Scholar]
- Rushton, P.J., Torres, J.T., Parniske, M., Wernert, P., Hahlbrock, K., and Somssich, I.E. (1996). Interaction of elicitor-inducing DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 15, 5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Sagasser, M., Lu, G.-H., Hahlbrock, K., and Weisshaar, B. (2002). A. thaliana TRANSPARENT TESTA1 is involved in seed coat development and defines the WIP family of plant zinc finger proteins. Genes Dev. 16, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein, J. (2000). Constructing a plant cell: The genetic control of root hair development. Plant Physiol. 124, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger, A., Folkers, U., Schwab, B., Jürgens, G., and Hülskamp, M. (1999). Generation of a spacing pattern: The role of TRYPTYCHON in trichome patterning in Arabidopsis. Plant Cell 11, 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley, B.W., Kubasek, W.L., Storz, G., Bruggemann, E., Koornneef, M., Ausubel, F.M., and Goodman, H.M. (1995). Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 8, 659–671. [DOI] [PubMed] [Google Scholar]
- Szymanski, D.B., Jilk, R.A., Pollock, S.M., and Marks, D. (1998). Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Szymanski, D.B., Lloyd, A.M., and Marks, D. (2000). Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends Plant Sci. 5, 214–219. [DOI] [PubMed] [Google Scholar]
- Tsay, Y.-F., Frank, M.J., Page, T., Dean, C., and Crawford, N.M. (1993). Identification of a mobile endogenous transposon in Arabidopsis thaliana. Science 260, 342–344. [DOI] [PubMed] [Google Scholar]
- Valvekens, D., Van Montagu, M., and Lisjbettens, M.V. (1988). Agrobacterium tumefaciens mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. USA 85, 5536–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, T., Tachibana, T., Shimura, Y., and Okada, K. (1997). Epidermal cell differentiation in Arabidopsis determined by a Myb homolog CPC. Science 277, 1113–1116. [DOI] [PubMed] [Google Scholar]
- Walker, A.R., Davison, P.A., Bolognesi-Winfield, A.C., James, C.M., Srinivasan, N., Blundell, T.L., Esch, J.J., Marks, D.M., and Gray, J.C. (1999). The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11, 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western, T.L., Burn, J., Tan, W.L., Skinner, D.J., Martin-McCaffrey, L., Moffatt, B.A., and Haughn, G.W. (2001). Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol. 127, 998–1011. [PMC free article] [PubMed] [Google Scholar]
- Western, T.L., Skinner, D.J., and Haughn, G.W. (2000). Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol. 122, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor, J.B., Symonds, V.V., Mendenhall, J., and Lloyd, A.M. (2000). Arabidopsis seed coat development: Morphological differentiation of the outer integument. Plant J. 22, 483–493. [DOI] [PubMed] [Google Scholar]
- Yu, D., Chen, C., and Chen, Z. (2001). Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13, 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]