Abstract
The cAMP analogue 8-Cl-cAMP induces apoptosis and inhibits proliferation of a wide variety of malignancies in vitro and in vivo with relatively little toxicity. The antitumor effects of this compound are thought to involve its ability to modulate type I protein kinase A (PKAI). However, a nontoxic metabolite of 8-Cl-cAMP, 8-Cl-adenosine, with no known activity against PKAI, exerts growth inhibitory effects in breast, ovary, pancreas, and colorectal cancer cells in vitro and accumulates in xenografted tumors after 8-Cl-cAMP treatment in vivo. To characterize further the antitumor effects of 8-Cl-adenosine in colorectal cancer, we examined its effects on cell growth in vitro (cell number, 3H-thymidine incorporation, and soft agar colony formation) using the isogenically matched colorectal cancer cell lines HCT116, HCT116-E6 (p53-depleted), and 80S14 (p21WAF1/Cip1-null). 8-Cl-adenosine inhibited cell growth by 89%, 74%, and 79%, respectively in HCT116, HCT116-E6, and 80S14 cells after a 72-hour exposure. Growth inhibition coincided with DNA endoreduplication and subsequent apoptosis. Furthermore, nontoxic doses of 8-Cl-adenosine administered i.p. twice weekly for 4 weeks to athymic mice suppressed growth of HCT116-derived xenografts by 50%. These results show that 8-Cl-adenosine exerts antitumor activity against colorectal cancer independent of p53 and p21WAF1/Cip1.
Keywords: 8-Cl-adenosine, colorectal cancer, cell cycle, endoreduplication, xenograft
Introduction
Colorectal cancer remains the most common fatal cancer among nonsmokers. There is a societal mandate and enormous scientific opportunity to impact on the toll of suffering and mortality through the development of improved approaches to screening, prevention, and treatment. For the last 45 years, the mainstay therapy for advanced colorectal cancer has relied on regimens using the nucleoside analogue 5-fluorouracil (5-FU) [1]. However, even with chemomodulating agents designed to enhance the efficacy and decrease toxicity of 5-FU, response rates remain poor [2]. Recently, a variety of other nucleosides have shown promise in preclinical studies, including 8-chloroadenosine 3′,5′-cyclic monophosphate (8-Cl-cAMP).
8-Cl-cAMP is a site-selective cyclic adenosine 3′,5′-monophosphate (cAMP) analogue that previously has been shown to inhibit growth in a variety of cancer cell lines [3]. This compound undergoes metabolism/degradation to nucleotides (8-Cl-AMP; 8-Cl-IMP), nucleosides (8-Cl-adenosine; 8-Cl-inosine) and bases (8-Cl-adenine; 8-Cl-xanthine and 8-Cl-hypoxanthine). It has been suggested that growth inhibition by 8-Cl-cAMP does not require metabolism and is related to selective effects on PKA accompanied by a reduction in p21ras protein levels [4], However, we and others have shown that 8-Cl-cAMP inhibits tumor cell growth through its major metabolite, 8-Cl-adenosine (Ref. [5]; our unpublished observations). Indeed, 8-Cl-adenosine inhibits growth in a variety of cancer cell lines, including breast, ovary, pancreas, and colon through a cAMP-independent mechanism. In addition, this nucleoside has been presumed to exert its antiproliferative effects only on transformed cells. However, we recently have shown that 8-Cl-adenosine inhibits primary epidermal keratinocytes but this inhibition is completely reversible (D.T. Dransfield and W.B. Bollag, unpublished observations), whereas the growth inhibitory effect induced by 8-Cl-adenosine in transformed cells is completely irreversible even after removal of the agonist (Ref. [4]; our unpublished observations).
Although extensive investigations have focused on characterizing the growth inhibitory effects of 8-Cl-cAMP, relatively little attention has been devoted to 8-Cl-adenosine, independent from the effects of its parental cAMP analogue. We therefore examined the inhibitory properties of this nucleoside against colorectal cancer growth in vitro and in vivo.
Materials and Methods
Materials
All cell culture reagents were obtained from Media-Tech/Cellgro (Herndon, VA), and Fisher Scientific (Pittsburgh, PA). HCT116 cells were purchased from ATCC (Bethesda, MD). HCT116-E6 and 80S14 cells were generous gifts from Dr. Wafik S. El-Deiry (University of Pennsylvania) and Dr. Bert Vogelstein (Johns Hopkins University), respectively. 3H-Thymidine was purchased from Dupont/NEN (Boston, MA). Propidium iodide was purchased from Molecular Probes (Eugene, OR). Balb/c nu/nu athymic mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN). All other reagents were obtained from standard suppliers and were of the highest grade available.
Cell Culture
HCT116, HCT116-E6, and 80S14 cells were cultured in DMEM supplemented with 2 mM l-glutamine, 50 I.U. penicillin, 50 µg/ml streptomycin, nonessential amino acids, and 10% heat-inactivated FBS. All cell lines were cultured at 37°C in 5% CO2 and media was changed every 48 hours.
Cell Growth Assay
Cells were plated at a density of 2.5x105 cells/100 mm dish and incubated for 16 to 24 hours at 37°C before the addition of control vehicle (H2O) or 8-Cl-adenosine in fresh serum-containing media. At the indicated times, cells were washed twice with 1xPBS, trypsinized, and counted using a hemocytometer.
3H-Thymidine Incorporation
Cells were plated at a density of 13,325 cells/well in six-well plates. After 16 to 24 hours at 37°C, cells were treated with fresh serum-containing media with control vehicle (H2O) or 8-Cl-adenosine for the indicated times. Samples were “pulsed” for 1 hour with 1 µCi 3H thymidine and washed twice with 1xPBS and extracted with 5% (vol/vol) TCA at 4°C. Samples were solubilized with 0.3 N NaOH for 10 minutes at 50°C with gentle shaking, and incorporated 3H-thymidine was determined by liquid scintillation counting.
Flow Cytometric Analysis
Unsynchronized cells were plated at a density of 2.5x105 cells/100 mm dish and incubated for 16 to 24 hours at 37°C prior to the addition of fresh serum-containing media supplemented with control vehicle (H2O) or 8-Cl-adenosine. At the indicated times, attached and floating cells were harvested and pooled. The combined cell pellet was washed once with ice-cold 1xPBS, gently resuspended in ice-cold 70% EtOH and stored at 4°C until processed. DNA content of the nuclei was determined by staining nuclear DNA with propidium iodide (50 µg/ml) for 30 minutes and measuring the relative DNA content of nuclei using a Becton Dickinson FACSCalibur fluorescence-activated cell sorter. MODFIT DNA and CELLQUEST analysis software were used to determine the proportion of nuclei in each phase of the cell cycle.
Colony Formation in Soft Agar
To determine the effect of 8-Cl-adenosine on anchorage-independent cell growth, HCT116, HCT116-E6, and 80S14 cell lines were plated at 1.6x105 cells/35mm dish in media-supplemented with 10% FBS, 0.4% (wt/vol) Sea-Plaque agar (FMC BioProducts, Rockland, ME), and test agent (H2O or 8-Cl-adenosine). Colony number and size were quantified after 5 days using light microscopy with colonies containing greater than five cells counting positive.
Antitumor Effects of 8-Cl-adenosine in a Murine Xenograft Model
Nude athymic mice were injected subdermally with HCT116 cells (1x106). Once tumors had reached an approximate volume of 150 mm3, the mice were randomized into equal groups and injected i.p. twice weekly with either 50 mg/kg 8-Cl-adenosine or 1xPBS vehicle for 4 weeks. Measurements of tumor volume were obtained weekly using a caliper and volume computed with the formula 4/3πab2 with “a” being the radius of the long axis and “b” the radius of the short axis as described by Kooby et al. [16]. Animals were sacrificed on developing signs of lethargy, failure to eat, altered behavior, excessive tumor growth, or tumor ulceration.
Results
8-Cl-adenosine Inhibits Colorectal Cancer Growth In Vitro
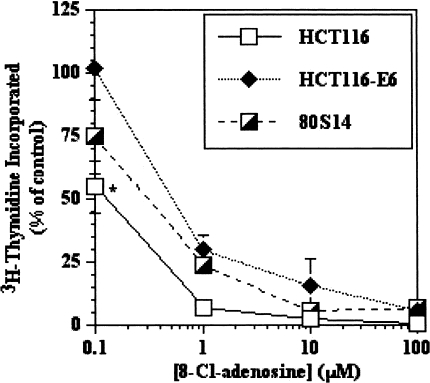
Growth suppressive effects of 8-Cl-adenosine in wild-type p53-bearing HCT116 cells have been reported [5]. In an attempt to determine the p53 dependence of this effect, we used parental HCT116, HCT116-E6 (stably transfected with HPV-E6 and thus depleted of functional p53) and 80S14 cells (HCT116 p21-/-), which are null for p21WAF1/CiP1 through homologous recombination [10]. The use of isogenically matched cell lines for these studies eliminates ambiguity that might arise using genetically distinct cell lines. We initially sought to establish 8-Cl-adenosine dose-response curves for the respective cell lines (HCT116, HCT116-E6 and 80S14). All three cell lines exhibited similar dose-response curves after 8-Cl-adenosine treatment for 72 hours (Figure 1), with the only statistically significant difference observed at the lowest concentration tested (0.1 µM) comparing HCT116 to HCT116-E6 cells. More important though, was the observation that the lowest concentration that yielded a complete inhibition of growth in all three cell lines was ∼5 µM, therefore this concentration was used in all subsequent experiments unless otherwise noted.
Figure 1.
8-Cl-adenosine inhibits growth in colorectal cancer cells in a dose-dependent manner. Selected colorectal cancer cells were treated for 72 hours with various concentrations of 8-Cl-adenosine. The amount of incorporated 3H-thymidine was determined as described in Materials and Methods. Data represent the average±SEM, n=3. *p<0.05, comparing HCT116 to HCT116-E6 at 0.1 µM.
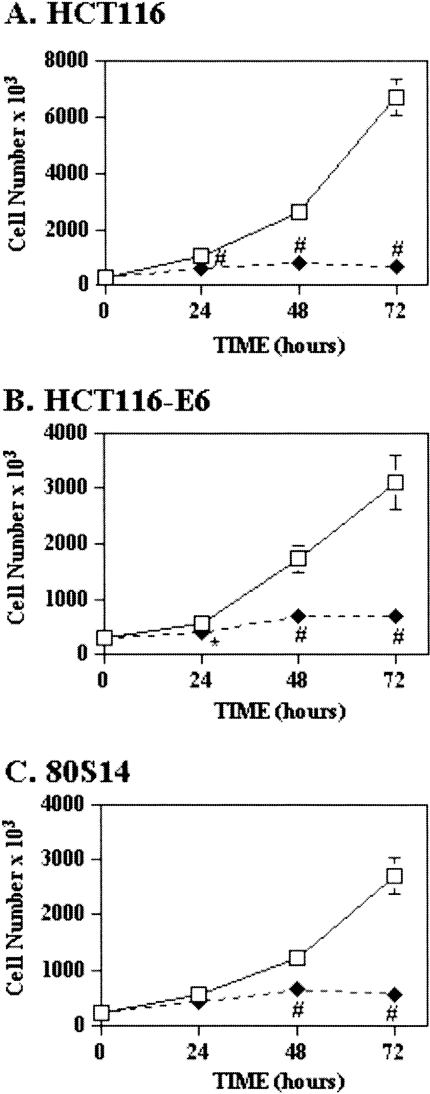
8-Cl-adenosine also inhibited growth in all three cell lines in a time-dependent manner (Figure 2). Growth of HCT116 and HCT116-E6 cells was inhibited significantly by 24 hours, with inhibitions of 89% and 74% observed at 72 hours, respectively (Figure 2A and B). In 80S14 cells, significant growth inhibition was observed at 48 (42%) and 72 hours (79%) after 8-Cl-adenosine treatment (Figure 2C).
Figure 2.
8-Cl-adenosine inhibits growth in colorectal cancer cells in a time-dependent manner. Selected colorectal cancer cell lines were treated with (◆) or without (□) 8-Cl-adenosine (5 µM) for the indicated times. Cells were harvested and counted using a hemocytometer. Data represent the average±SEM, n=3. *p<0.05; †p<0.005; #p<0.001 when compared to control-treated cells.
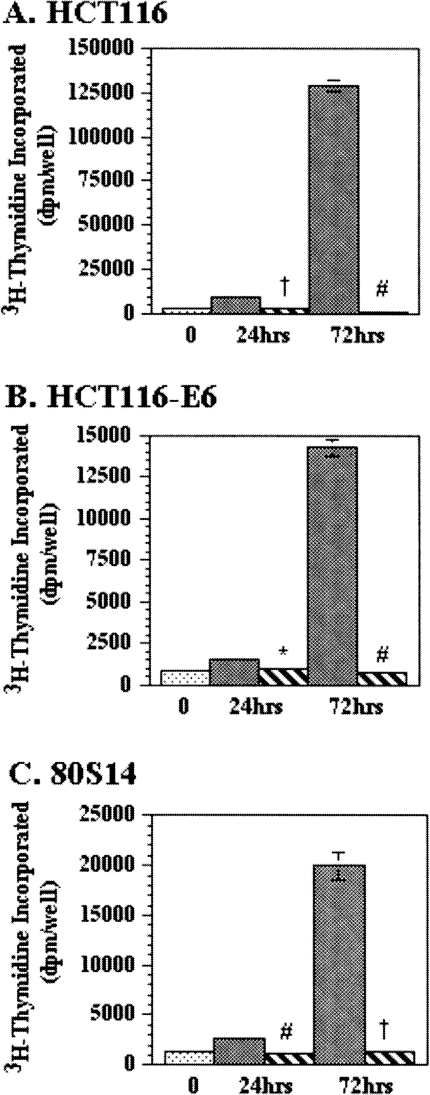
Similar growth inhibitory effects of 8-Cl-adenosine were found when 3H-thymidine incorporation was measured in HCT116, HCT116-E6, and 80S14 cells. 8-Cl-adenosine induced a 30%, 25%, and 50% decrease in 3H-thymidine incorporation compared to control-treated cells at 24 hours with a more drastic 99%, 97%, and 92% decrease observed after 72 hours of 8-Cl-adenosine treatment (Figure 3). 3H-Thymidine incorporation experiments conducted out to 96 and 168 hours demonstrated a greater than 98% inhibition of growth in all three cell lines (data not shown).
Figure 3.
8-Cl-adenosine inhibits 3H-thymidine incorporation in colorectal cancer cells. Selected colorectal cancer cell lines were treated with (hatched bars) or without (solid bars) 8-Cl-adenosine (5 µM) for the indicated times. The amount of incorporated 3H-thymidine was determined as described in Materials and Methods. Data represent the average ± SEM, n = 3–5. *p<0.05; †p<0.005; #p<0.001 when compared to control-treated cells.
8-Cl-adenosine Inhibits Anchorage-Independent Growth
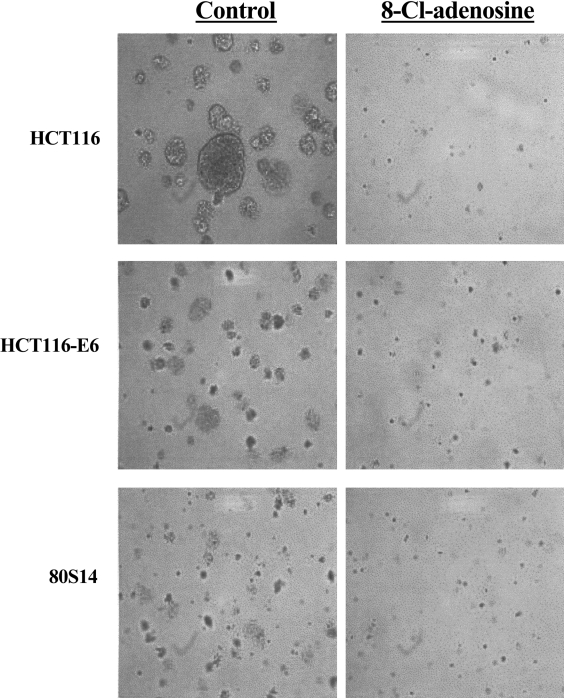
Finally, using an anchorage-independent growth assay, 8-Cl-adenosine significantly reduced the size and total number of colonies formed in soft agar at concentrations of 1 µM (HCT116 and 80S14) and 2.5 µM (HCT116-E6) with the greatest effects seen at ∼5 µM in all three cell lines (Figure 4, Table 1).
Figure 4.
8-Cl-adenosine inhibits anchorage-independent growth in colorectal cancer cells. Selected colorectal cancer cell lines were cultured in soft agar for five days in the absence or presence of 8-Cl-adenosine (2.5 µM). Images are representative of three separate experiments yielding similar results.
Table 1.
Colony Formation in Soft Agar.
| 8-Cl-adenosine (µM) | ||||||
| Control | 0.05 | 0.5 | 1 | 2.5 | 5 | |
| HCT116 | 16.4±4.9 | 12.3±1.2 | 12.2±1.6 | 6.8±0.4* | 5.4 ±1.0* | 6.8±0.4* |
| HCT116E6 | 20.8±0.9 | 15.8±0.3 | 17.3±0.9 | 16.0 ±1.4 | 12.9±0.6† | 9.3±2.9† |
| 80S14 | 17.8±1.7 | 16.4±1.5 | 13.8±1.3 | 12.2±1.9* | 6.4±0.9† | 2.8±0.4† |
Each colorectal cancer cell line was treated with 8-Cl-adenosine at the indicated concentrations in soft agar. Colonies were counted using light microscopy as described in Materials and Methods. Data represent the average±SEM of three experiments done in duplicate.
p<0.05;
p<0.005 when compared to control-treated cells.
8- Cl-adenosine Induces Cell Cycle Arrest and Apoptosis In Vitro
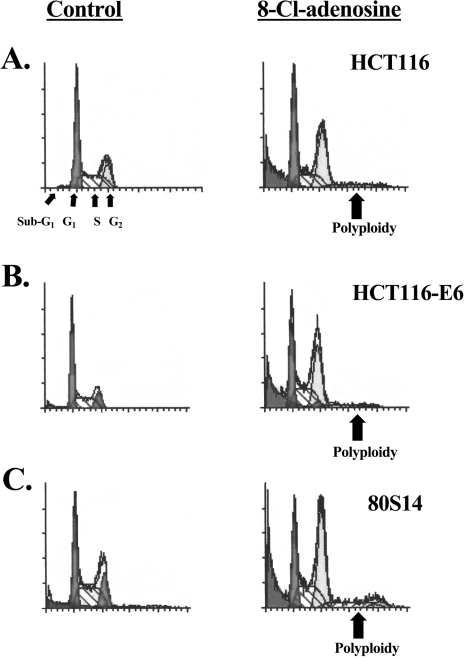
To characterize the effects of 8-Cl-adenosine on cell cycle events, HCT116, HCT116-E6, and 80S14 cells were treated with 8-Cl-adenosine and subjected to flow cytometric analysis. In HCT116 cells, 8-Cl-adenosine-induced a transient G1 cell cycle accumulation by 48 hours of treatment (Table 2) accompanied by the appearance of sub-G1 and polyploid cell populations (Figure 5). In addition, standard Hoescht staining experiments revealed an increase in positively stained HCT116, HCT116-E6, and 80S14 cells in response to a 72-hour treatment with 8-Cl-adenosine suggesting that apoptosis was induced by this nucleoside (data not shown). Sub-G1 and polyploid cell populations also developed in HCT116-E6 and 80S14 cells after 8-Cl-adenosine treatment, but did not show a G1 accumulation. In addition, 8-Cl-adenosine-treated HCT116-E6 and 80S14 cells exhibited an earlier onset and a greater degree of polyploidism than parental HCT116 cells (Table 2).
Table 2.
Flow Cytometric Analysis of Colorectal Cancer Cell Lines Treated with 8-Cl-adenosine.
| Sub G1 | G1 | S | G2 | Polyploid | |
| HCT116 | |||||
| 0 h | 3.61±1.79 | 38.54±7.00 | 44.55±2.82 | 16.91±5.86 | - |
| 24 h | 5.35±0.79 | 39.60±1.15 | 43.28±4.25 | 17.13±4.40 | - |
| 48 h | 15.50±1.27* | 56.62±4.42 | 19.00±2.62† | 24.38±4.51 | - |
| 72 h | 15.58±5.91 | 41.71±1.92 | 29.12±3.20* | 29.17±5.05 | + |
| HCT116-E6 | |||||
| 0 h | 5.18±1.62 | 24.07±4.26 | 43.43±0.64 | 32.50±4.89 | - |
| 24 h | 7.33±0.54 | 26.29±1.85 | 39.09±3.48 | 34.62±3.95 | - |
| 48 h | 15.33±0.54† | 24.68±1.72 | 27.75±2.48f | 47.57±2.39 | + |
| 72 h | 14.75±2.55* | 18.25±4.66 | 35.16±4.09 | 46.58±2.95 | +++ |
| 80S14 | |||||
| 0 h | 10.48±3.73 | 28.02±3.95 | 40.37±0.62 | 31.61±3.60 | - |
| 24 h | 17.19±4.41 | 39.02±1.51 | 36.20±1.79 | 24.78±1.86 | - |
| 48 h | 22.45±1.79* | 40.64±3.74 | 28.48±1.76 | 30.88±5.49 | + |
| 72 h | 22.73±1.62* | 25.39±3.41 | 28.66±0.92† | 45.96±4.32 | +++ |
Each colorectal cancer cell line was treated with 8-Cl-adenosine (5 µM) for the indicated times. Cells were harvested and subjected to flow cytometric analysis as described under Materials and Methods. Data represent the average±SEM of three experiments.
p<0.05;
p<0.005 when compared to 0-hour sample.
Figure 5.
Cell cycle profiles of colorectal cancer cells treated with 8-Cl-adenosine. Selected colorectal cancer cell lines were treated with or without 8-Cl-adenosine (5 µM) for 72 hours, and subjected to flow cytometric analysis as described in Materials and Methods. The profiles are representative of three experiments yielding similar results. Note, the profiles generated from the 8-Cl-adenosine-treated cells were enlarged to illustrate the cell cycle distributions.
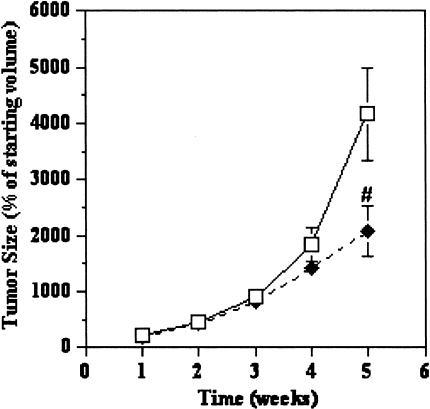
8-Cl-adenosine Slows Tumor Progression in HCT116-derived Xenografts in Nude Athymic Mice
Animals treated with 8-Cl-adenosine (50 mg/kg) injected i.p. twice weekly exhibited a slowed tumor progression (50%) compared to control-treated animals (Figure 6). It is important to note that none of the animals in this study exhibited either a change in body weight or eating habits. Also, both gross and histological examination of mouse hearts, lungs, liver, kidney, stomach, small intestine and colons revealed no significant alterations in tissue morphology.
Figure 6.
8-Cl-adenosine slows tumor progression in HCT116-derived xenografts. Xenografts established in nude athymic mice were treated with (◆) 8-Cl-adenosine (50 mg/kg) or (□) 1xPBS twice/week for a period of 4 weeks and tumor size was measured weekly. Data represent the average±SEM, n=24 for control and 25 for treated. #p<0.001 when compared to control-treated animals.
Discussion
Existing therapies for advanced stages of colorectal cancers are limited by unsatisfactory responses and limiting toxicity. In this study, we characterized the cytostatic and cytotoxic properties of a nontoxic biological agent, 8-Cl-adenosine. 8-Cl-adenosine inhibited growth in wild-type p53, p53-depleted, and p21WAF1/Cip1-null phenotypes of HCT116 colorectal cancer cells. Furthermore, 8-Cl-adenosine alone delayed tumor growth of HCT116-derived xenografts in vivo, justifying efforts to test this compound in human clinical trials.
The antitumor efficacy of DNA-damaging agents, including 5-FU is reduced among colorectal cancers harboring mutations in p53 [8]. Because the majority of colorectal cancers (approximately 80%) contain p53 mutations [9], it is not surprising that clinical response rates to 5-FU hover below 20%. The cytostatic and cytotoxic actions of 5-FU, therefore, appear to be dependent on induction of the p53/p21WAF1/CiP1 pathways for G1 cell cycle arrest and apoptosis [10]. In contrast, we report within that the growth inhibitory effects of 8-Cl-adenosine were equally efficacious among wild-type and p53-depleted HCT116 cells. Furthermore, loss of p21WAF1/CiP1 in HCT116 cells, delayed, but did not abolish the growth inhibitory effects of 8-Cl-adenosine. Flow cytometric analysis revealed that the growth inhibitory actions of 8-Cl-adenosine on parental HCT116 cells involved a G1 cell cycle arrest and induction of apoptosis as judged by a marked shift of cells into a sub-G1 fraction. Although loss of either p53 or p21WAF1/Cip1 prevented 8-Cl-adenosine-induced G1 cell cycle arrest, supporting the role of these proteins in G1-S cell cycle checkpoint regulation [10], the accumulation of sub-diploid cells was fully retained regardless of p53 or p21WAF1/CiP1 status. These effects are not cell-line dependent, because similar effects have been observed in a variety of colorectal cancer cells (SW480, HT-29 and LS1034), irrespective of their p53 status (data not shown). These observations are in stark contrast to those obtained with many of the currently used anticancer agents such as 5-FU, ionizing radiation, etoposide, and adriamycin, which have been shown to a cause a p53-dependent apoptosis [11].
In the absence of p53 or p21WAF1/CiP1, 8-Cl-adenosine treatment induced HCT116 cells to undergo at least one round of DNA endoreduplication (72 hours, Figure 5). A similar finding has been reported in 80S14 cells treated with the DNA-damaging agent adriamycin [12], or the microtubule inhibitor nocodazole [13]. This unhindered entry into mitosis, leading to polyploidy, results from loss of cell-cycle checkpoint(s), most likely secondary to loss of p21WAF1/Cip1 or p53 function [7]. Clearly, the mechanism (s) by which 8-Cl-adenosine inhibits colorectal cancer growth remain to be more fully elucidated.
There is a second pathogenic pathway for tumor development in the colon, unrelated to loss of functional p53, which requires inactivation of the DNA mismatch repair (MMR) system. About 15% of all colorectal cancers have microsatellite instability (MSI), the hallmark of a defective DNA MMR system (reviewed in Ref. [14]). Because these two pathways to colorectal carcinogenesis are separate and independent, it has been suggested that an effective preventative or treatment strategy for one pathway may not work for another [14]. MMR can influence drug cytotoxicity, including 5-FU, in colorectal cancer [15]. In these studies, we used human colorectal cancer cell lines with a defective DNA MMR system. Even in this setting, and irrespective of p53 or p21WAF1/CiP1 status, 8-Cl-adenosine significantly inhibited anchorage-independent cell growth in vitro (Figure 4, Table 1) and slowed tumor progression in vivo (Figure 6). Together, these findings justify efforts to test this compound in human clinical trials. For example, this compound may offer benefit in an adjuvant setting to accompany surgical resection by delaying local recurrence and hindering metastatic spread. Furthermore, our ability to achieve growth inhibitory effects at doses significantly lower than those previously reported to be toxic (1052±52 mg/kg as a single i.p. injection [17]) infer that improved efficacy might be achievable by increasing the dosage of 8-Cl-adenosine without increasing toxicity. The lack of apparent toxicity from 8-Cl-adenosine offers significant advantages over currently used cytotoxic agents, including 5-FU [6].
In summary, 8-Cl-adenosine is a potent antineoplastic agent against colorectal cancer cell growth both in vitro and in vivo, independent of p53 or p21WAF1/CiP1 status. In addition, this compound has potent apoptotic effects in a MMR-deficient colorectal cancer cell line. The mechanism(s) of action of this compound requires further investigation and potentially will lead to a role for 8-Cl-adenosine as adjuvant therapy for colorectal cancer.
Acknowledgements
We thank Dr. William E. Karnes, Jr. (Mayo Clinic) and Dr. Jennifer Pietenpol (Vanderbilt University) for helpful suggestions and discussions. We also would like to thank Dr. Meral Keskintepe (Institute of Molecular Medicine and Genetics FACS Core Facility) for assistance with the flow cytometric analysis. This work was supported by institutional funds from the Institute of Molecular Medicine and Genetics, Medical College of Georgia (D.T.D.).
References
- 1.Piedbois P, Buyse M, Rustum Y, et al. Advanced colorectal cancer meta-analysis project: modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol. 1992;10:896–903. doi: 10.1200/JCO.1992.10.6.896. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB. Therapy for advanced colorectal cancer. Sem Oncol. 1998;25(5):2–11. [PubMed] [Google Scholar]
- 3.Noguchi K, Murata T, Cho-Chung YS. 8-chloroadenosine 3′,5′-monophosphate (8-Cl-cAMP) selectively eliminates protein kinase A type I to induce growth inhibition in c-ras-transformed fibroblasts. Eur J Cancer. 1998;34(8):1260–1267. doi: 10.1016/s0959-8049(98)00051-3. [DOI] [PubMed] [Google Scholar]
- 4.Tagliaferri P, Katsaros D, Clair T, et al. Synergistic inhibition of growth of breast and colon human cancer cell lines by site-selective cyclic AMP analogues. Cancer Res. 1988;48:1642–1650. [PubMed] [Google Scholar]
- 5.Taylor CW, Yeoman LC. Inhibition of colon tumor cell growth by 8-chloro-cAMP is dependent upon its conversion to 8-chloro-adenosine. Anti-Cancer Drugs. 1992;3:485–491. doi: 10.1097/00001813-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Pritchard DM, Potten CS, Hickman JA. The relationship between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine intestinal epithelia. Cancer Res. 1998;58:5453–5465. [PubMed] [Google Scholar]
- 7.Stewart ZA, Mays D, Pietenpol JA. Defective G1-S cell cycle checkpoint function sensitizes cells to microtubule inhibitor-induced apoptosis. Cancer Res. 1999;59:3831–3837. [PubMed] [Google Scholar]
- 8.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104(3):263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 10.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 11.Lowe S, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 12.Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature (London) 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 13.Stewart ZA, Leach SD, Pietenpol JA. p21Waf1/Cip1 inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol. 1999;19:205–215. doi: 10.1128/mcb.19.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boland R, Sinicrope FA, Brenner DE, Carethers JM. Colorectal cancer prevention and treatment. Gastro. 2000;118:S115–S128. doi: 10.1016/s0016-5085(00)70010-2. [DOI] [PubMed] [Google Scholar]
- 15.Carethers JM, Chauhan DP, Fink D, Nebel S, Bresalier RS, Howell SB, Boland CR. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastro. 1999;117:123–131. doi: 10.1016/s0016-5085(99)70558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kooby DA, Carew JF, Halterman MW, Mack JE, Bertino JR, Blumgart LH, Federoff HJ, Fong Y. Oncolytic viral therapy for human colorectal cancer and liver matastases using a multi-mutated herpes simplex virus type-1 (G207) FASEB J. 1999;13(11):1325–1334. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Shi Y, Zhang L. Antitumor activities of 8-Cl-adenosine in vivo and in vitro. Chin J Oncol. 1995;17(1):5–8. [PubMed] [Google Scholar]