Abstract
Inflammatory breast cancer (IBC) is a distinct and aggressive form of locally advanced breast cancer. IBC is highly angiogenic, invasive, and metastatic at its inception. Previously, we identified specific genetic alterations of IBC that contribute to this highly invasive phenotype. RhoC GTPase was overexpressed in 90% of archival IBC tumor samples, but not in stage-matched, non-IBC tumors. To study the role of RhoC GTPase in contributing to an IBC-like phenotype, we generated stable transfectants of human mammary epithelial cells overexpressing the RhoC gene, and studied the effect of RhoC GTPase overexpression on the modulation of angiogenesis in IBC. Levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin-6 (IL-6), and interleukin-8 (IL-8) were significantly higher in the conditioned media of the HME-RhoC transfectants than in the untransfected HME and HME-β-galactosidase control media, similar to the SUM149 IBC cell line. Inhibition of RhoC function by introduction of C3 exotransferase decreased production of angiogenic factors by the HME-RhoC transfectants and the SUM149 IBC cell line, but did not affect the control cells. These data support the conclusion that overexpression of RhoC GTPase is specifically and directly implicated in the control of the production of angiogenic factors by IBC cells.
Keywords: inflammatory breast cancer, human mammary epithelial cells, RhoC GTPase, angiogenesis, angiogenic factors
Introduction
Primary inflammatory breast cancer (IBC) accounts for approximately 6% of new breast cancer cases annually in the United States [1]. IBC is characterized by a very rapid course, progressing within 6 months to cause the clinical manifestations of erythmea, skin nodules, peau d'aurange, and nipple retraction due to tumor infiltration of lymphatic and connective tissue [2,3]. IBC tumors are highly angiogenic and at the time of diagnosis nearly all patients have nodal metastasis, whereas approximately 36% have gross distant metastases [2,3]. This number greatly increases 1 year after diagnosis, presumably due to the progression of occult metastases, suggesting that IBC cells acquire metastatic capabilities early in tumor formation. Not surprisingly, even with multimodality treatment, the 5-year disease-free survival is less than 45%, making IBC the most deadly form of locally advanced breast cancer [3].
Until recently, relatively little was known about the genetic mechanisms underlying the development and progression of IBC. In a previous study, our laboratory identified genes that strongly correlate with the aggressive and invasive IBC phenotype [4]. RhoC GTPase is overexpressed in 90% of archival IBC tumors, but not in stage-matched non-IBC tumors. Our laboratory has also demonstrated that RhoC GTPase is a transforming oncogene of human mammary epithelial cell [5]. RhoC is a member of the Ras superfamily of small GTP-binding proteins, which play a crucial role in the control of actin cytoskeletal reorganization and DNA transcription [6–8]. Another oncogene, ras, has been shown to induce the production of angiogenic cytokines such as vascular endothelial growth factor (VEGF) [9,10]. Recent evidence suggests that overexpression of RhoA, a member of the Rho family that is 95% homologous to RhoC, can induce angiogenesis in human prostate cancer as well as interleukin-8 (IL-8) in human endothelial cells [11].
To study the effect of RhoC GTPase overexpression on the modulation of angiogenic factors in human mammary epithelial cells, stable human mammary epithelial (HME)-RhoC transfectants were established as previously described [5]. We found that production of active VEGF, basic fibroblast growth factor (bFGF), IL-6 and IL-8, were significantly increased in the HME-RhoC cells compared with the controls and were comparable to the SUM149 IBC cell line, which also overexpresses RhoC GTPase. The production of these factors was specifically inhibited by the introduction of an inhibitor of Rho activity, C3 exotransferase, into HME-RhoC cells.
Materials and Methods
Cell Lines
Cell lines were maintained under defined culture conditions for optimal growth in each case as previously described [12–14]. Briefly, human mammary epithelial (HME) cells were immortalized with human papilloma virus E6/E7 [15] and grown in 5% FBS (Sigma Chemical Co., St. Louis, MO) supplemented Ham's F-12 medium (JRH BioSciences, Lenexa, KS) containing insulin, hydrocortisone, epidermal growth factor, and cholera toxin (Sigma). HME cells were transfected with either β-galactosidase or RhoC GTPase [5]. Stable HME-β-gal and HME-RhoC cells were maintained in the medium described above supplemented with 100 µg/ml hygromycin (LifeScience Technologies). The SUM149 cell line was developed from a primary IBC tumor and grown in 5% FBS supplemented Ham's F-12 medium containing insulin and hydrocortisone. The HME cells were characterized as being keratin-19 positive, ensuring that they are from the same differentiation lineage as the SUM149 IBC tumor cell line [4].
Cytokine ELISA Assays
Levels of soluble cytokines were determined from cell-conditioned media. Cells were incubated in normal growth medium for 4 days. The cell-conditioned media was harvested, centrifuged for 5 minutes at 2500 rpm, and divided into 1-ml aliquots. The Quantikine hVEGF and human bFbF immunoassays (R&D Systems, Minneapolis, MN) were used to measure protein levels of the 165 amino acid species of VEGF and of bFGF. ELISA was performed per the manufacture recommendations. ELISAs for IL-6, IL-8, and IL-12 were performed by the University of Maryland Cytokine Core Laboratory (www.cytokinelab.com).
Quantitative RT-PCR of Cytokines
Levels of cytokine mRNA was determined by quantitative RT-PCR. Briefly, total RNA was harvested from cells using Trizol reagent (Life Technologies, Gaithersburg, MD) per the recommendations of the manufacturer. One microgram of total RNA was reverse transcribed to cDNA using the AMV reverse transcription kit (Promega, Madison, WI). Aliquots of cDNA totaling 50 ng were amplified by PCR using primers specific for either IL-6, IL-8, bFGF, or VEGF (10 ng/µl final concentration) and β-actin (1 ng/µl final concentration). PCR products were separated on a 1.2% agarose gel and imaged on an Alpha Image 950 documentation system (Alpha Innotech, San Leandro, CA). Densitometry of images was performed using NIH Image (version 1.62).
Rat Aortic Ring Assay
The rat aortic ring assay was used to measure the functional potency of angiogenic factors [16]. Briefly, the aorta was removed from a freshly sacrificed Sprague-Dawley rat and rinsed in ice-cold Hank's buffered saline solution (HBSS) containing penicillin and streptomycin (Life Technologies). Segmental rings, approximately 1 mm in width, were cut from the aorta and embedded in a 50 µl aliquot of 10 mg/ml Matrigel in six-well plates. The rat aortic rings were incubated overnight at 37°C in 2 ml of serum-free medium. The serum-free medium was then exchanged for 2 ml of the same cell-conditioned medium that was used for cytokine determination. Rat aortic ring segments were incubated for 4 days at 37°C, and then analyzed by phase-contrast microscopy for microvessel growth.
C3 Exotransferase Inhibition of RhoC GTPase Activity
The HME-β-gal, HME-RhoC, and SUM149 cells were transiently transfected with pEF-myc C3 transferase using FuGene 6 transfection reagent (Roche-Boehringer Mannheim, Mannheim, Germany). Cells were incubated for 2 days at 37°C, at which time cell-conditioned media and protein were harvested. Expression of C3 transferase was confirmed by Western blot analysis using an antibody to the c-myc-epitope tag. Control cells were transfected with pFLAG-β-galactosidase or FuGene 6 alone.
Active C3 exoenzyme was introduced into cells using a method based on liposome encapsulation and membrane fusion, which we have termed lipoporation. Briefly, cells were grown in six-well plates until reaching a confluence of 40% to 50% and the medium replaced with fresh medium. Three micrograms of human recombinant C3 exotransferase (Cytoskeleton Inc., Denver, CO) was combined with FuGene 6 transfection reagent (Roche-Boehringer Mannheim) and added to the cultures. As controls either an equal quantity of human recombinant tubulin or FuGene 6 alone were added to cell cultures. The cells were incubated for 2 days at 37°C, at which time cell-conditioned medium was harvested, for cytokine assays. Presence of the intracellular C3 exoenzyme was confirmed by visualizing the rhodamine-tagged protein using fluorescent microscopy. The efficiency and activity of both the transfected and lipoporated C3 exoenzyme were confirmed by a quantitative ADP-ribosylation assay [17].
ADP-Ribosylation Assay
The efficiency of in vivo ADP-ribosylation of RhoC GTPase by C3 exotransferase was determined as previously described [17]. Active C3 exotransferase was efficiently introduced into HME-β-gal, HME-RhoC, and SUM149, as described above. Cells were collected 48 hours later, washed in medium, and pelleted. The cells were lysed in 20 mM Hepes pH 8.0 (Sigma) by three repeated freeze/thaw cycles. Cell lysates (10 µg) were combined with 50 ng/ml C3 exotransferase and 5x106 cpm [32P]NAD (Amersham) in ADP-ribosylation buffer (20 mM Hepes, pH 8.0, 1 mM MgCl2, 1 mM AMP, and thymidine, Sigma) and incubated for 30 minutes at 37°C. TCA-precipitable material was then recovered and radioactivity was counted on a Packard scintillation counter.
Labeling of C3 Exotoxin
Cellular internalization of C3 exotoxin was visualized by fluorescence microscopy. C3 exotoxin was labeled using the FluorReporter Rhodamine Red-X Protein Labeling Kit (Molecular Probes Inc., Eugene, OR). HME-β-gal, HME-RhoC, and SUM149 (100,000 cells/chamber) were plated into the chambers of Lab-Tek slides (Nalgene Nunc International, Naperville, IL). C3 exotoxin was introduced into cells as described above and cells were visualized 48 hours later using an Olympus fluorescent microscope equipped with a 573 nm filter.
Results
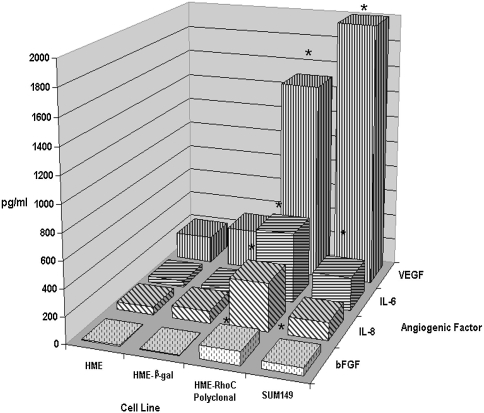
IBC is highly angiogenic, giving rise to profusely vascularized tumors at the primary and metastatic sites, including the skin overlying the breast (reviewed in Ref. [18]). Previous observations by our laboratory have demonstrated that IBC tumors and IBC cell lines produce high levels of angiogenic factors such as VEGF and bFGF (unpublished results). To determine if overexpression of RhoC GTPase could lead to increased production of angiogenic cytokines in mammary epithelial cells, we performed ELISAs on cell-conditioned media from the untransfected HME, HME-β-galactosidase control transfectants, HME-RhoC transfectants, and the wild-type SUM149 IBC cell lines. Cell-conditioned media were tested for the presence of the pro-angiogenic cytokines VEGF, bFGF, IL-6, IL-8, and the anti-angiogenic cytokine IL-12. Results were similar for all of the individual HME-RhoC clones tested (data not shown); thus, for brevity, only data from the polyclonal population is represented in this manuscript. The HME-RhoC polyclonal transfectants produced 10-fold more VEGF and bFGF, and five-fold more IL-6 and IL-8 than the untransfected HME and HME-β-gal control transfected cell lines. Compared with the SUM149 IBC cells, the HME-RhoC cells produced approximately 90% of the level of VEGF, twice the levels of IL-6 and IL-8, and equivalent levels of bFGF (Figure 1). IL-12 production was minimal in all cell lines tested (data not shown), suggesting that IL-12 is not an important angiogenic inhibitor in this system. To determine if the increased production of angiogenic factors correlated with an increase in cytokine message, we performed quantitative RT-PCR on mRNA from the untransfected HME, HME-β-gal control transfectants, HME-RhoC transfectants, and the wild-type SUM149 IBC cell lines. As determined by quantitative RT-PCR, the mRNA levels of IL-6, IL-8, bFGF, and VEGF was 2- to 10-fold higher in the RhoC transfectants and SUM149 IBC cell line compared with the control cell lines (data not shown). These data suggest that the increased cytokine levels were due in part to increased transcription of these genes.
Figure 1.
Comparison of levels of angiogenic factors by HME-RhoC transfectants, SUM149 IBC cell line and the HME-β-gal control cell line as determined by ELISA. The HME-RhoC cells produced significantly higher levels of angiogenic factors compared with the HME-β-gal control cell line, nearly recapitulating the levels produced by the SUM149 IBC cell line. Significant differences (p<0.001) between the control cells and the HME-RhoC cells are denoted by an asterisk (*) with standard deviations within 10% of the reported values.
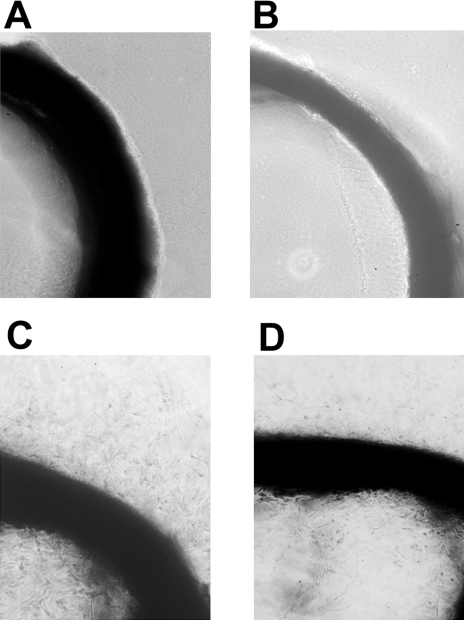
The functional activity of the angiogenic cytokines was determined using the rat aortic ring assay (Figure 2). A fresh 1 mm segment of rat aorta was embedded in Matrigel and incubated with cell-conditioned medium for 4 days. Negligible microvessel growth was observed from the rings incubated with conditioned media from the untransfected HME (panel A) or HME-β-gal control (panel B). In contrast, prominent microvessel growth was observed for cell-conditioned medium from the HME-RhoC (panel C) and the SUM149 IBC (panel D) cell lines. The control samples incubated in growth medium alone did not stimulate microvessel growth in this system (data not shown).
Figure 2.
Results of a rat aortic ring assay for functional angiogenic factors produced by untransfected HME (panel A), HME-β-gal control transfectants (panel B), HME-RhoC transfectants (panel C) and the SUM149 IBC cell line (panel D). Segments of rat aorta were embedded in Matrigel and cultured in the corresponding cell-conditioned media for 4 days and then observed for microvessel outgrowth. Conditioned media from the control cell lines (panels A and B) did not induce microvessel outgrowth. However, conditioned medium from the HME-RhoC transfectants (panel C) produced similar levels of new vessel growth as the SUM149 IBC cell line (panel D).
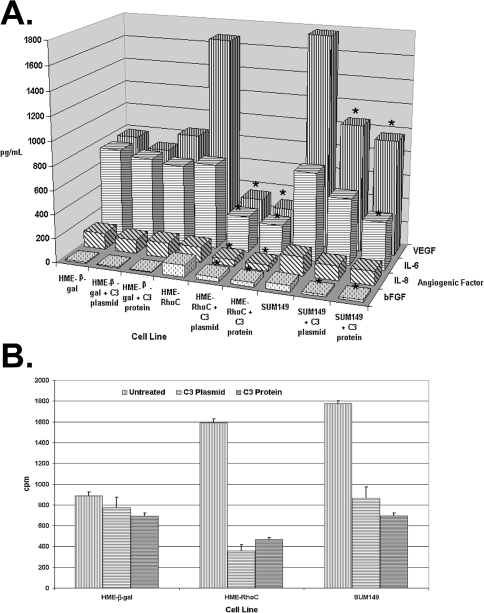
To determine whether the production of angiogenic factors elicited by RhoC GTPase was directly related to its activity, we used C3 exotransferase to inhibit RhoC function. Specific inhibition of RhoC GTPase activity by C3 exotransferase led to decreased production of angiogenic factors. The HME, HME-β-gal control, HME-RhoC, and SUM149 IBC cells were transiently transfected with a c-myc-tagged C3 exotransferase expression construct and assayed 48 hours later for production of VEGF, bFGF, IL-6, and IL-8. As shown in Figure 3A, VEGF production was unchanged in the HME-β-gal controls transiently transfected with C3 exotransferase. In contrast, there was a 4.9- and 1.8-fold decrease in VEGF production by the HME-RhoC and SUM149 cells inhibited with C3, respectively. Expression of C3 exotransferase decreased the production of VEGF by the HME-RhoC and SUM149 cells to a level equivalent to that of the control cells. The levels of bFGF were decreased 37% and 34% in the HME-RhoC and SUM149 cells expressing C3 exotransferase, respectively. The levels of IL-6 production were reduced by 42% and 25% in the HME-RhoC and SUM149 cells, respectively, whereas production of these cytokines in the HME-β-gal control remained unchanged. Production of IL-8 was reduced 2.1- and 1.3-fold in the HME-RhoC and SUM149 cell lines by C3 inhibition.
Figure 3.
Panel A demonstrates the effect on production of angiogenic factors by inhibition of RhoC GTPase with C3 exotransferase. Similar levels of inhibition were accomplished by expressing a C3 exotransferase construct or introducing the active protein directly into the cells. Significant differences (p<0.05) between the untreated and C3 exotransferase treated cells are denoted by an asterisk (*) with standard deviations within 10% of the reported values. Panel B demonstrates the results of an in vitro ADP-ribosylation study to determine the in vivo efficiency of C3 exotransferase inhibition of Rho activity. The assay was performed as outlined in the Materials and Methods section. The potential ADP-ribosylated sites in both the HME-RhoC and SUM149 cell lines were significantly reduced after C3 treatment, thus indicating efficient in vivo inhibition of RhoC GTPase.
The activity of the C3 exotransferase was confirmed by measuring the efficiency of in vivo ADP-ribosylation. As shown in Figure 3B, compared with their non-C3-expressing counterparts, all the C3-expressing cell lines had a significant reduction in the levels of available sites that could be ADP-ribosylated in the in vitro assay. Specifically, the C3-expressing HME-RhoC and SUM149 cells had a two-fold decrease in the number of ADP-ribosylated sites compared to the nontransfected controls. These data indicate that at least half of the RhoC proteins have been ADP-ribosylated in vivo, and therefore inhibited by C3 exotransferase.
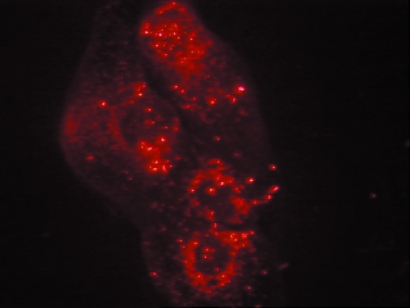
To confirm these results by another approach, RhoC GTPase function was inhibited by direct introduction of C3 exotransferase protein into the cells. Equivalent results were observed when active recombinant C3 exotransferase protein was introduced into the cells using a lipoporation technique. The recombinant C3 protein was rhodamine-labeled and, as demonstrated in Figure 4, the presence of the protein in the cell was confirmed by fluorescent microscopy 48 hours later. VEGF production was significantly reduced in both the HME-RhoC and SUM 149 cells, upon introduction of active C3 exotransferase. As shown in Figure 3A, HME-RhoC cells treated with C3 exoenzyme had a 5.8-fold decrease in VEGF production, whereas the SUM149 cells had a 1.9-fold decrease. A 2.8- and 3.0-fold decrease in bFGF production was observed in the HME-RhoC and SUM149 cells, respectively. Levels of IL-6 production were also moderately affected by C3 treatment. IL-6 levels were decreased by 37% and 53% in the HME-RhoC and SUM 149 cell lines, respectively. Treatment of the HME-β-gal control cells with C3 exoenzyme did not effect the production of VEGF or IL-6. As before, introduction of C3 exotransferase reduced the levels of IL-8 production by 2.4- and 1.3-fold for the HME-RhoC and SUM149 cell lines, respectively. These results were also confirmed by a third method namely, RhoC inhibition using C3 exotoxin purified from Clostridium botulinum (data not shown). As demonstrated in the C3 exotransferase plasmid transfection experiment, there was a two-fold decrease in the number of ADP-ribosylated sites in all the C3-treated cell lines (Figure 3B). Therefore, at least half of the active Rho proteins were inhibited by the introduction of active C3 transferase.
Figure 4.
Rhodamine-labeled C3 exotransferase was introduced into cells using a lipid mediated transfer method (see Materials and Methods section). The presence and efficiency of C3 protein transfer was determined by visualizing cells under a fluorescent microscope.
Taken together these data indicate that RhoC GTPase overexpression can directly lead to the increased production of the pro-angiogenic factors VEGF, bFGF, IL-6 and IL-8. Specific inhibition of RhoC by C3 exotransferase significantly reduced the production of VEGF, bFGF, and IL-8. However, production of IL-6 was only moderately affected by the C3 exoenzyme.
Discussion
Despite recent advances in multimodality treatments, the prognosis for patients with IBC is guarded, leading to poor overall survival and to significant impairment of local control of the disease in the breast and chest wall. This is primarily due to the ability of the tumor to grow quickly and disseminate to distant organs where metastatic cells can establish secondary tumors. Both of these properties are facilitated by and dependent on neovascularization, which provide both nutrients for the primary tumor and a means for metastatic cells to access the circulation [19,20].
Angiogenesis in IBC is dependent on the active production of several potent pro-angiogenic factors and cytokines and the inhibition of anti-angiogenic cytokines [21–23]. Two well-described pro-angiogenic factors that are active in IBC are VEGF and bFGF. These two angiogenic factors can act synergistically to induce angiogenesis [24,25]. Production of VEGF and bFGF has been demonstrated for a variety of tumors such as melanoma, prostate, and lung cancer [26–28]. Previous unpublished studies in our laboratory have demonstrated that IBC cell lines and tumors produce large quantities of VEGF and bFGF. Other laboratories have demonstrated that VEGF expression and production is increased early in preinvasive breast cancers, whereas bFGF is increased in invasive breast tumors in general [29]. The relationship between oncogenic transformation and angiogenesis has been explored in several studies.
Exposure of NIH3T3 cells to TPA, IL-1β, PDGF, or TGF-β can induce expression of VEGF mRNA [30–33]. As these mechanisms use the same signal transduction pathways as ras, other groups have investigated and demonstrated upregulation of VEGF by mutant and/or overexpressed oncogenes such as H- and K-ras, v-raf and v-src [34–37]. It is believed that activation of the MAP kinase pathway leads to the induction of the AP-1 transcription factor, which could bind to four potential AP-1 consensus sites in the human VEGF gene [38,39]. Another hypothesis is that induction of VEGF expression could occur through the phosphatidylinositol 3-kinase pathway, as has been demonstrated in endothelial cell models [40]. An increasing body of evidence suggests that the mode of VEGF induction (i.e., MAPK vs PI3K) by activated H-ras is a cell-type specific process, with cells of epithelial origin signaling more commonly through the MAP kinase pathway whereas those of fibroblastic origin utilizing the PI3K pathway [10].
The Rho genes, which were originally cloned on the basis of their homology to ras, also use the same signal transduction pathways to induce gene transcription (reviewed in Ref. [41]). However, it was not previously known whether the Rho proteins modulate the production of angiogenic factors in cancer cells. In this study, we demonstrate strikingly high levels of VEGF production by HME cells overexpressing RhoC GTPase and not by HME-β-gal controls. The HME-RhoC cells had a five-fold increase in VEGF production over the control transfected cells, nearly recapitulating the VEGF levels produced by the SUM149 IBC cell line. Furthermore, VEGF levels were reduced to a level equivalent to the control cells when the HME-RhoC cells were treated with recombinant human C3 exotransferase, a specific inhibitor of Rho activity (reviewed in Ref. [42]). Similar results were seen for the SUM149 cell line, which also overexpresses RhoC GTPase, when treated with the C3 exoenzyme. In contrast to the RhoC overexpressing cells, the HME-β-gal controls were unaffected by C3 treatment. Although the C3 exotransferase is not a specific inhibitor of RhoC itself, it may therefore be inhibiting more than one Rho molecule or more than one process. However, because both the HME-β-gal and the HME-RhoC transfectants are genotypically identical except for the expression of RhoC GTPase, we can confidently state that inhibition of angiogenic factor production is due to inhibition of RhoC GTPase. Taken together, these results not only demonstrate that the specific GTPase function of RhoC is required for the increased production of VEGF and bFGF, but that RhoC overexpression is specifically responsible for this effect. This latter conclusion derives from the lack of effect of C3 exotransferase on VEGF and bFGF production in HME-β-gal controls that express RhoA, RhoB, and other members of the Rho family, at normal levels.
A recent study has demonstrated that acidic FGF is transcriptionally regulated by ras, rac, and cdc42 [43]. Ras and rac were shown to activate the bFGF promoter, although it is not known whether bFGF transcription was increased. Our data clearly indicate that RhoC GTPase overexpressing cells produce more bFGF than the corresponding controls. Similar to the results obtained for VEGF, production of bFGF by these cells was diminished, although not entirely eliminated, by treatment with C3 exotransferase. This suggests that the basal production of bFGF may be RhoC independent in IBC.
IL-6 is an inflammatory cytokine that has become known as an indirect effector of angiogenesis because it can induce VEGF expression [44]. Whether RhoC overexpression can directly induce IL-6 production has yet to be addressed. Although the data in this study demonstrate that expression levels of IL-6 are higher in both the HME-RhoC and SUM149 IBC cells as compared with normal and HME-β-gal control cells, the increase in expression may not be a direct effect, but due to induction of expression by bFGF. Other studies suggest that bFGF, acting in an autocrine and paracrine fashion, can induce IL-6 expression through the p38-MAP kinase pathway [45]. Levels of IL-6 were moderately reduced when the cells were treated with C3 exotransferase, suggesting that the presence of bFGF could be inducing IL-6 expression independent of RhoC activity. It is therefore unknown at this time whether induction of IL-6 is directly linked to RhoC expression. However, it is clear from these data that VEGF expression is induced by RhoC, at least in part, independently of IL-6.
It has been suggested that Rho proteins and the p38-MAP kinase pathway modulate the expression of IL-8 [11,46]. IL-8 expression has profound biologic consequences: it is a potent angiogenic, mitogenic, and chemotactic factor, as has been shown for several tumor types, particularly melanoma, breast, prostate, bladder, and lung cancers [47–51]. Furthermore, IL-8 can increase the growth rate of both tumor and endothelial cells, and although its role in the establishment of metastases is unclear, it can increase both tumor cell growth rates and metastatic potential in nude mice [52]. In this study, we demonstrate a 10-fold increase in IL-8 expression by the HME-RhoC cells over the control-transfected cells, and a two-fold increase over the SUM149 IBC cell lines. IL-8 production was significantly reduced in the HME-RhoC transfectants by treatment with C3 exotransferase. However, these levels are not significantly decreased by inhibition of RhoC by C3 exotransferase in the SUM149 IBC cell line. IL-8 production in these cells may be driven by another factor that signals to the nucleus in the presence of RhoC overexpression, with bFGF being one possible candidate.
Conclusion
We have demonstrated increased expression of VEGF, bFGF, IL-6, and IL-8 in HME-RhoC stable transfectants compared with HME control transfectants. These levels recapitulated those of the wild-type SUM149 IBC cell line, which also overexpresses RhoC GTPase, thus demonstrating a key role for RhoC overexpression in modulation of angiogenesis by mammary epithelial cells. Further studies are needed to understand in detail the molecular basis for the modulation of production of the individual angiogenic factors by RhoC GTPase.
Abbreviations
- IBC
inflammatory breast cancer
- HME
human mammary epithelial cell
- VEGF
vascular endothelial growth factor
- bFGF
basic fibroblast growth factor
- IL
interleukin
Footnotes
This work was supported by National Institutes of Health (NIH) R01 CA 77612-(S.D.M.), and NIH 5T32 CA09537-16, DOD-DAMD17-OD-1-0345 (S.D.M. and K.v.G.), and by Susan G. Komen Foundation Postdoctoral Fellowship (K.V.G.) and grant (S.D.M.).
References
- 1.Levine PH, Steinhorn SC, Ries IG. Inflammatory breast cancer. The experience of the surveillance, epidemiology, and end results (SEER) program. J Natl Cancer Inst. 1985;74:291–297. [PubMed] [Google Scholar]
- 2.Jaiyesimi I, Buzdar A, Hortobagyi G. Inflammatory breast cancer: a review. J Clin Oncol. 1992;10:1014–1024. doi: 10.1200/JCO.1992.10.6.1014. [DOI] [PubMed] [Google Scholar]
- 3.Beahrs O, Henson D, Hutter R. Manual for Staging of Cancer. (3rd ed) 1988:145–150. [Google Scholar]
- 4.van Golen KL, Davies S, Wu ZF, Wang Y, Bucana CD, Root H, Chandrasekharappa S, Strawderman M, Ethier SP, Merajver SD. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511–2519. [PubMed] [Google Scholar]
- 5.van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832–5838. [PubMed] [Google Scholar]
- 6.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 7.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 9.Okada F, Rak JW, St. Croix B, Lieubeau B, Kaya M, Roncari L, Shirasawa S, Sasazuki T, Kerbel RS. Impact of oncogenes in tumor angiogenesis: mutant ki-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3609–3614. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria-Petit A, Filmus J, Mansour SJ, Ahn NG, Kerbel RS. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 2000;60:490–498. [PubMed] [Google Scholar]
- 11.Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krull M, Eichel-Streiber C, Goebeler M, Ludwig S, Suttorp N. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood. 2000;95:3044–3051. [PubMed] [Google Scholar]
- 12.Ethier SP, Kokeny KE, Ridings JW, Dilts CA. erbB family receptor expression and growth regulation in a newly isolated human breast cancer cell line. Cancer Res. 1996;56:899–907. [PubMed] [Google Scholar]
- 13.Ethier SP. Human breast cancer cell lines as models of growth regulation and disease progression. J Mammary Gland Biol Neoplasia. 1996;1:111–121. doi: 10.1007/BF02096306. [DOI] [PubMed] [Google Scholar]
- 14.Sartor C, Dziubinski M, Yu CL, Jove R, Ethier SP. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997;57:978–987. [PubMed] [Google Scholar]
- 15.Band V, Zajchowski D, Kulesa V, Sager R. Human papilloma virus DNAs immortalize normal epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci USA. 1990;87:463–467. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- 17.Stasia MJ, Vignais PV. In: Methods in Enzymology. Abelson JM, Simon MI, editors. Vol. 256. New York: Academic Press; 1995. pp. 324–327. [DOI] [PubMed] [Google Scholar]
- 18.Kleer CG, van Golen KL, Merajver SD. Inflammatory breast cancer: clinical syndrome and molecular determinants. Submitted to Breast Cancer Res. 2000;2:423–429. doi: 10.1186/bcr89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 20.Fidler IJ, Kumar R, Bielenberg DR, Ellis LM. Molecular determinants of angiogenesis in cancer metastasis. Cancer J Sci Am. 1998;4:S58–S66. [PubMed] [Google Scholar]
- 21.Folkman J. Angiogenesis and breast cancer. J Clin Oncol. 1994;12:441–443. doi: 10.1200/JCO.1994.12.3.441. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J. Angiogenesis and its inhibitors. In: DeVita V, Hellman S, Rosenberg S, editors. Important Advances in Oncology. Philadelphia, PA: J.B. Lippincott; 1985. pp. 42–62. [PubMed] [Google Scholar]
- 23.Folkman J. Angiogenesis inhibitors generated by tumors. Mol Med. 1995;1:120–122. [PMC free article] [PubMed] [Google Scholar]
- 24.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:II365–II371. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- 25.Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- 26.Harper ME, Glynne-Jones E, Goddard L, Mathews P, Nicholson RI. Expression of androgen receptor and growth factors in premalignant lesions of the prostate. J Pathol. 1998;186:169–177. doi: 10.1002/(SICI)1096-9896(1998100)186:2<169::AID-PATH164>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Volm M, Koomagi R, Mattern J, Stammler G. Angiogenic growth factors and their receptors in non-small cell lung carcinomas and their relationships to drug response in vitro. Anticancer Res. 1997;17:99–103. [PubMed] [Google Scholar]
- 28.Danielsen T, Rofstad EK. VEGF, BFGF and EGF in the angiogenesis of human melanoma xenografts. Int J Cancer. 1998;76:836–841. doi: 10.1002/(sici)1097-0215(19980610)76:6<836::aid-ijc12>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Heffelfinger SC, Miller MA, Yassin R, Gear R. Angiogenic growth factors in preinvasive breast disease. Clin Cancer Res. 1999;5:2867–2876. [PubMed] [Google Scholar]
- 30.Wang D, Huang HJ, Kazlauskas A, Cavenee WK. Induction of vascular endothelial growth factor expression in endothelial cells by platelet-derived growth factor through the activation of phosphatidylinositol 3-kinase. Cancer Res. 1999;59:1464–1472. [PubMed] [Google Scholar]
- 31.Li J, Perrella MA, Tsai JC, Yet SF, Hsieh CM, Yoshizumi M, Patterson C, Endege WO, Zhou F, Lee ME. Induction of vascular endothelial growth factor gene expression by interleukin-1 beta in rat aortic smooth muscle cells. J Biol Chem. 1995;270:308–312. doi: 10.1074/jbc.270.1.308. [DOI] [PubMed] [Google Scholar]
- 32.Finkenzeller G, Marme D, Weich HA, Hug H. Platelet-derived growth factor-induced transcription of the vascular endothelial growth factor gene is mediated by protein kinase C. Cancer Res. 1992;52:4821–4823. [PubMed] [Google Scholar]
- 33.Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–6274. [PubMed] [Google Scholar]
- 34.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995;270:25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 35.Konishi T, Huang CL, Adachi M, Taki T, Inufusa H, Kodama K, Kohno N, Miyake M. The K-ras gene regulates vascular endothelial growth factor gene expression in non-small cell lung cancers. Int J Oncol. 2000;16:501–511. doi: 10.3892/ijo.16.3.501. [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay D, Tsiokas L, Sukhatme VP. Wild-type p53 and v-Src exert opposing influences on human vascular endothelial growth factor gene expression. Cancer Res. 1995;55:6161–6165. [PubMed] [Google Scholar]
- 37.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 38.Milanini J, Vinals F, Pouyssegur J, Pages G. p42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts. J Biol Chem. 1998;273:18165–18172. doi: 10.1074/jbc.273.29.18165. [DOI] [PubMed] [Google Scholar]
- 39.Damert A, Ikeda E, Risau W. Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327(Pt 2):419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signaling pathways. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- 42.Aktories K. Rho proteins: targets for bacterial toxins. Trends Microbiol. 1997;5:282–288. doi: 10.1016/S0966-842X(97)01067-6. [DOI] [PubMed] [Google Scholar]
- 43.Chotani MA, Touhalisky K, Chiu IM. The small GTPases Ras, Rac and Cdc42 transcriptionally regulate expression of human fibroblast growth factor 1. J Biol Chem. 2000;275:30432–30438. doi: 10.1074/jbc.M003545200. [DOI] [PubMed] [Google Scholar]
- 44.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 45.Kozawa O, Tokuda H, Matsuno H, Uematsu T. Involvement of p38 mitogen-activated protein kinase in basic fibroblast growth factor-induced interleukin-6 synthesis in osteoblasts. J Cell Biochem. 1999;74:479–485. [PubMed] [Google Scholar]
- 46.Warny M, Keates AC, Keates S, Castagliuolo I, Zacks JK, Aboudola S, Qamar A, Pothoulakis C, LaMont JT, Kelly CP. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Invest. 2000;105:1147–1156. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodeck U, Becker D, Herlyn M. Basic fibroblast growth factor in human melanoma. Cancer Cells. 1991;3:308–311. [PubMed] [Google Scholar]
- 48.Speirs V, Atkin SL. Production of VEGF and expression of the VEGF receptors Fit-1 and KDR in primary cultures of epithelial and stromal cells derived from breast tumours. Br J Cancer. 1999;80:898–9030. doi: 10.1038/sj.bjc.6690438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer [in process citation] Clin Cancer Res. 2000;6:2104–2119. [PubMed] [Google Scholar]
- 50.Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998;18:77–81. [PubMed] [Google Scholar]
- 51.Mizuno K, Sone S, Orino E, Mukaida N, Matsushima K, Ogura T. Spontaneous production of interleukin-8 by human lung cancer cells and its augmentation by tumor necrosis factor alpha and interleukin-1 at protein and mRNA levels. Oncology. 1994;51:467–471. doi: 10.1159/000227385. [DOI] [PubMed] [Google Scholar]
- 52.Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12–18. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]