Abstract
Telomerase, a cellular reverse transcriptase, adds telomeric repeats to chromosome ends. In normal human somatic cells, telomerase is repressed and telomeres progressively shorten, leading to proliferative senescence. Introduction of the telomerase (hTERT) cDNA is sufficient to produce telomerase activity and immortalize normal human cells, suggesting that the repression of telomerase activity is transcriptional. The telomerase transcript has been shown to have at least six alternate splicing sites (four insertion sites and two deletion sites), and variants containing both or either of the deletion sites are present during development and in a panel of cancer cell lines we surveyed. One deletion (β site) and all four insertions cause premature translation terminations, whereas the other deletion (α site) is 36 bp and lies within reverse transcriptase (RT) motif A, suggesting that this deletion variant may be a candidate as a dominant-negative inhibitor of telomerase. We have cloned three alternately spliced hTERT variants that contain the α, β or both α and β deletion sites. These alternate splicing variants along with empty vector and wild-type hTERT were introduced into normal human fibroblasts and several telomerase-positive immortal and tumor cell lines. Expression of the α site deletion variant (hTERT α-) construct was confirmed by Western blotting. We found that none of the three alternate splicing variants reconstitutes telomerase activity in fibroblasts. However, hTERT α- inhibits telomerase activities in telomerase-positive cells, causes telomere shortening and eventually cell death. This alternately spliced dominant-negative variant may be important in understanding telomerase regulation during development, differentiation and in cancer progression.
Keywords: human telomerase, alternate splicing, dominant-negative inhibitor, telomere, apoptosis
Introduction
Most normal human diploid cells have limited life-span and then undergo a growth arrest state called senescence [1,2]. Telomeres are specialized structures located at the ends of chromosomes. Human telomeric DNA, usually 5 to 20 kb in length, consists of repetitive TTAGGG sequences [3]. Due to the “end replication problem” in linear chromosomes [4,5], telomeric DNA is not completely copied during DNA replication and telomeres progressively shorten. After some telomeres have become sufficiently short, normal human cells enter senescence and stop dividing [6]. Telomerase is a ribonucleoprotein enzyme complex that adds telomeric repeats to the ends of telomeres. The catalytic subunit of telomerase (hTERT in humans), which has reverse transcriptase activity, uses an integral RNA (hTR in humans) that contains a sequence complimentary to the telomeric repeat as a template to elongate telomeres [7–9]. Telomerase is not expressed in most normal human somatic cells [10], whereas it is upregulated or reactivated in most human cancer cells [11]. It has been shown that reactivation of telomerase is a crucial step in tumorigenesis [12–14], even though ectopic expression of telomerase alone does not cause cancer-associated changes [15,16].
Introduction of hTERT cDNA into normal human cells is sufficient to reconstitute telomerase activity and immortalize normal human cells [17,18]. Because normal human diploid cells express the integral hTR but do not contain hTERT transcripts [7,19], it is believed that telomerase is primarily regulated at the level of repression of the telomerase gene. However, telomerase may also be regulated by alternate splicing. A total of six alternate splicing sites (four insertion sites and two deletion sites) have been identified and confirmed within the telomerase mRNA (Genbank accession numbers: AF128893 and AF128894) (Figure 1A) [20,21], and there is evidence that telomerase mRNA is alternately spliced either to abolish telomerase activity before transcription of telomerase becomes fully repressed during embryonic development [22], or when telomerase is reactivated during tumorigenesis [23].
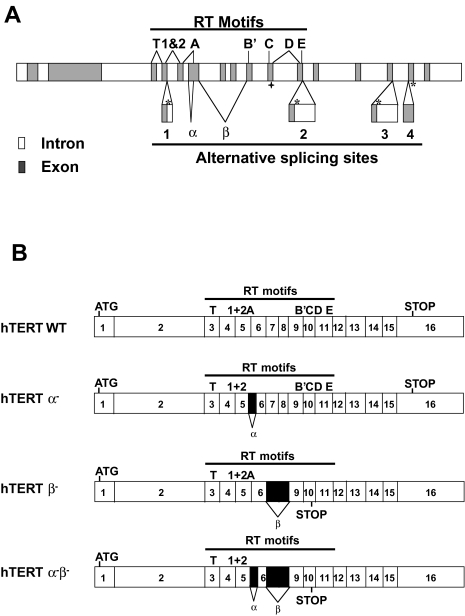
Figure 1.
Genomic organization of hTERT gene. (A) Solid boxes are exons; shaded boxes are 5′TR or 3′TR; the locations of the RT motifs (T, 1 and 2, A, B′, C, D and E) (8) are indicated on top; and the locations of alternate splicing sites (insertions 1–4 and deletions α and β are marked on the bottom (see Wick et al. [21] for an alternative mapping of the hTERT RT motif boundaries). The locations of termination codons that correspond to the variants containing insertions are marked with *, and ◆ marks the location of the termination codon for the variant containing deletion β; the deletion α does not cause a frameshift. (B) Four possible combinations of hTERT alternate splicing variants containing the deletions.
Six alternate splicing sites produce a very large number of possible combinations, only a few of which have been confirmed [20,21]. The two insertions closest to the 3′ end cause premature terminations after the telomerase RT motifs. One deletion (β site, 182 bp) and the two insertions closest to the 5′ end cause premature translation terminations. Because these deletions and insertions are located before some of the telomerase RT motifs, these alternately spliced variants would not be expected to retain reverse transcriptase activity. The α site deletion site removes 36 bp within RT motif A, and thus should also be inactive. Because the two deletion sites are located close to each other, it is possible to analyze them together (containing no, one or both deletions). In the present study, we have cloned the hTERT variants corresponding to deletion α (hTERT α-), deletion β (hTERT β-) and the combined deletion (hTERT α- β-) (Figure 1B), and examined their effects in normal, immortalized, and cancer cell lines.
Materials and Methods
Cell Culture
Human telomerase-positive nonsmall cell lung cancer cells H1299 (ATCC No.: CRL-5803), SW39 cells (immortal telomerase-positive cells derived from normal human lung fibroblasts IMR90 cells stably transfected with Simian Virus 40 (SV40) large-T antigen), telomerase-negative normal human diploid foreskin fibroblast BJ cells, prostate carcinoma cells DU145 (ATCC No.: HTB-81), and murine packaging cell lines PE501 and PA317 were cultured at 37°C under 5% CO2 in a 4:1 mixture of Dulbecco's MEM: medium 199 supplemented with medium containing 10% fortified bovine calf serum (Hyclone, Logan, UT) and 50 µg/ml of gentamicin (Sigma, St. Louis, MO).
Detection of Alternate Splicing Variants by RT-PCR
Total RNA was isolated and treated with DNase I as described [19]. Reverse transcription was performed using First-strand cDNA synthesis kit from Gibco using gene-specific primer TERT 2565R (5′CGCAAACAGCTTGCAGCTTGTTCTCCATGTC). Subsequent PCR was set up using primers TERT 2109 (5′GCCTGAGCTGTACTTTGTACTTTGTCAA) and TERT 2531R (5′AGGCTGCAGAGTGCAGAGCAGCGTGGAGAGG), of which one was labeled at its 5′-end with 32P. PCR products were separated on a 5% polyacrylamide gel and exposed to a Phosphor-Imager Storage screen. Gel image was analyzed using ImageQuant version 5 software (Molecular Dynamics, CA).
Construction of Retroviral Vectors Containing Alternate Splicing Forms of Telomerase
The retroviral constructs containing alternate splicing forms of hTERT were engineered using the full-length hTERT cDNA by cassette exchange as described below. The EcoRI fragment from pGRN145, containing the hTERT coding sequence [18] with a consensus Kozak sequence was cloned into the EcoRI site of pBluescript SK(+) (Stratagene, CA). H1299 total RNA was isolated using TriPure reagent from Roche Molecular Biochemical (IN) according to the manufacturer's instructions. Reverse transcription was performed using RETROscrip kit from Ambion (Ambion, TX) with random primers supplied with the kit. Subsequent PCR using the above reverse transcription reaction as template was performed with primers TERT2045 (5′CTGGGCGCCTCTGTGCTGGG) and TERT2565R (5′CGCAAACAGCTTGTTCTCCATGTC) using Pfu DNA polymerase (Stratagene). PCR products were digested with EcoRV and BamHI, and fragments corresponding to hTERT α-β- and hTERT β- alternate splicing forms were gelisolated and cloned into pBluescript SK(+) vector. The hTERT α- form was constructed by exchanging the Styl- BamHI fragment from hTERT α-β- with the Styl-BamHI fragment from wild-type sequence. These fragments were then exchanged with the corresponding fragment in the wild-type hTERT coding sequence cloned in pBluescript SK(+). The EcoRI fragments from these constructs and pGRN145, containing the three hTERT alternate splicing variants and wild-type cDNAs, respectively, were then subcloned into the retroviral vector pBabe puro, in which the puromycin resistance gene is under the control of the SV40 promoter [24].
Retroviral Infection and Cloning of Infected Cells
Recombinant viruses of the empty pBabe puro vector [24], the wild type, and three alternate splicing variants of hTERT were generated by first transfecting the ecotropic packaging cell line PE501 cells using Fugene (Roche Molecular Biochemicals) and selecting with 4 µg/ml puromycin. Viral supernatants derived from these cells were used to infect the amphotrophic PA317 packaging cell line [25] to generate clones containing unrearranged proviral copies of pBabe puro, pBabe puro hTERT, pBabe puro hTERT α-, pBabe puro hTERT β-, and pBabe puro hTERT α-β-. Medium containing released viruses produced from confluent dishes of selected populations of PA317 clones were filtered (0.45 µm) and used to infect BJ cells, SW39 cells, H1299 cells, and DU145 cells. After selection in 750 ng/ml puromycin, cells were plated at 100 to 300 cells per 10-cm dish. Clones were picked and expanded till they became confluent in 15-cm dishes. The clones were then trypsinized and counted using a Coulter ZM cell counter, and the number of population doublings of each cone was calculated by assuming each clone was expanded from a single cell. Each clone was then culture by replating 0.5 to 1x106 cells in a 15-cm plate when it reached confluency.
Western Blotting, Telomerase Activity Assay, and Telomere Measurements
Western blotting analysis was performed with a rat anti-hTERT antibody obtained from Geron Corporation (Menlo Park, CA). Telomerase activities of all cells were performed using TRAP-eze kit from Intergene (Intergene, MA) with [32P]-labeled TS primer as described elsewhere [11,26]. Telomere lengths were determined by digesting genomic DNA with Hinfl and analyzed on 0.7% agarose gels with size markers. Telomere DNA (TRF) was visualized by probing with 5′ [32P]-labeled (T2AG3)4 probe and quantitated as described [6].
Results
Alternate Splicing Forms Are Detected in Cancer Cells
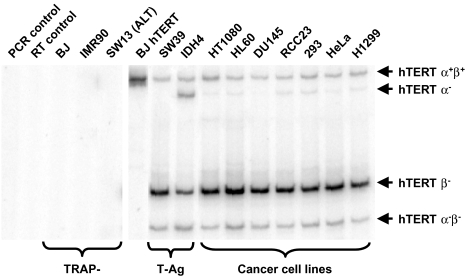
We have surveyed a number of telomerase-positive cell lines to determine the abundance of alternate splicing forms of hTERT transcript by RT-PCR (Figure 1). All four alternate splicing forms of hTERT transcript have also been observed in cultured human cancer cells and SV40 large T-antigen immortalized cells, whereas telomerase-negative normal human fibroblast BJ and IMR90 cells and telomerase-negative ALT pathway cell line SW13 cells do not contain any form of hTERT mRNA (Figure 2).
Figure 2.
RT-PCR survey results of telomerase-negative (TRAP-) and telomerase-positive cell types (from BJ hTERT to H1299) as described in the Materials and Methods section. PCR products corresponding to four alternate splicing variants of hTERT containing the deletions are of different sizes and they are indicated on the
Deletion Variants of hTERT Are Inactive
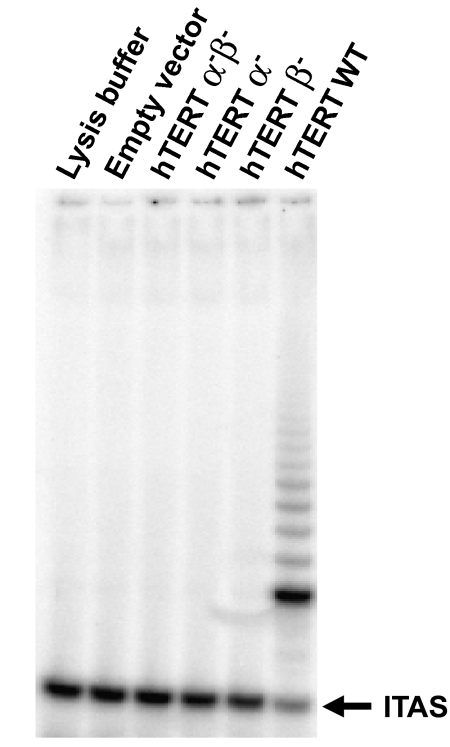
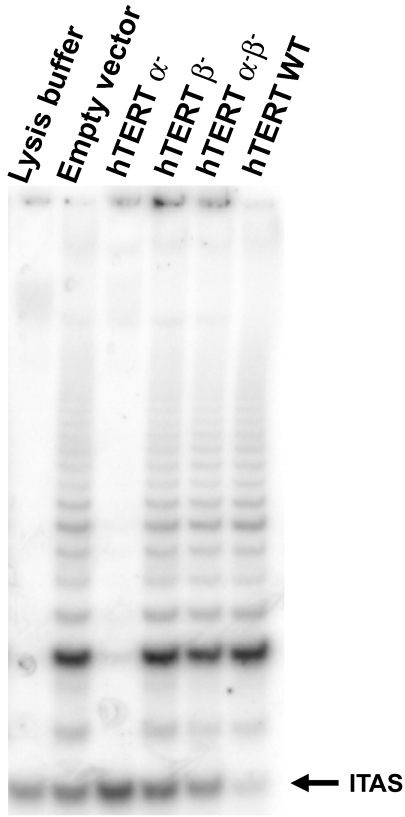
We introduced retroviral vectors containing wild-type hTERT (hTERT WT), hTERT α-, hTERT β-, hTERT α-β-(hTERT α-, hTERT β-, hTERT α-β- were engineered from full-length hTERT cDNA, see Materials and Methods section; Figure 1B) or a control vector expressing only the puromycin resistance marker into human foreskin fibroblast BJ cells. After puromycin selection, the successfully infected cells were assayed for telomerase activity using the PCR-based TRAP assay [11,26]. Although cells expressing hTERT WT exhibited telomerase activity, cells expressing hTERT α-, hTERT β-, hTERT α-β- lacked detectable telomerase activity (Figure 3).
Figure 3.
TRAP assay of telomerase-negative human fibroblast BJ cells infected with pBabe puro vector, pBabe puro hTERT α- β-, pBabe puro hTERT α-, pBabe puro hTERT β- and pBabe puro hTERT WT. Only pBabe puro hTERT WT produces telomerase activity in BJ cells.
Effect of hTERT Variants on Cell Growth, Telomerase Activity, Telomere Dynamics of Telomerase-Positive Cells
To investigate whether these hTERT variants might function as dominant negative inhibitors, we introduced them into three different telomerase-positive cell lines: SW39 (SV40 large-T antigen immortalized human IMR90 lung fibroblasts), DU145 (prostate carcinoma) and H1299 (nonsmall cell lung carcinoma). In each case, after puromycin selection, infected cells were cloned, expanded and continuously cultured. These clones were sampled for hTERT protein expression, telomerase activity, and telomere length (TRF) at various intervals.
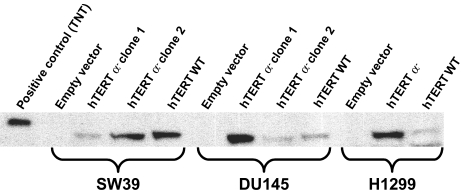
Using an antibody against the C-terminus of hTERT, Western blot analysis confirmed the expression of hTERT wild type and hTERT α- in the clones (Figure 4). The expression of hTERT β- and hTERT α-β- could not be confirmed by Western blot because the premature terminations removed the antibody binding site and there is not an available antibody against the N-terminal half of hTERT.
Figure 4.
Western blotting of SW39, DU145 and H1299 clones infected with the empty pBabe puro vector, pBabe puro hTERT α- and pBabe puro hTERT WT. In vitro translated wild-type hTERT protein (TNT lane) is larger because it has a (His)6-Anti-Xpress epitope (pcDNA3.1HisC vector from Invitrogen, CA). Cell lysates of 250,000 cells are loaded in each lane, except for the positive control. Under these conditions, the endogenous levels of hTERT protein expressed by SW39, DU145 and H1299 are not detected.
All clones infected with hTERT α- or hTERT α-β- maintained the same level of telomerase activities compared to clones infected with the pBabe puro vector control, within the range of telomerase activity fluctuation (Figure 5). All clones infected with hTERT wild-type cDNA showed increased telomerase activity, whereas the clones infected with hTERT α- showed decreased activity (Figure 5). Although there was some clonal variation compared to empty vector infection controls, the expression of hTERT α- in all three cell lines in general caused an 85% to 95% reduction in telomerase activity, which remained constant during the time of culturing.
Figure 5.
Sample TRAP assays of H1299 clones infected with pBabe puro vector, pBabe puro hTERT α-, pBabe puro hTERT β-, pBabe puro hTERT α-β-, and pBabe puro hTERT WT during the course of cell culturing (250 cells assayed per lane). Telomerase activity levels in all clones have remained constant during the course of cell culturing.
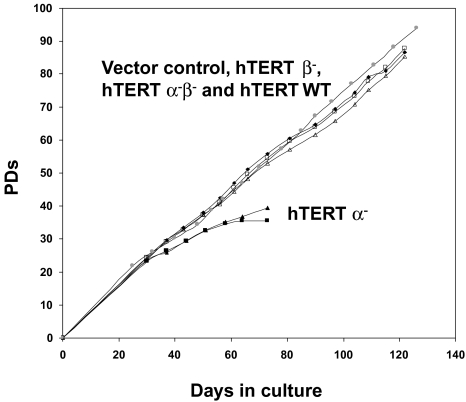
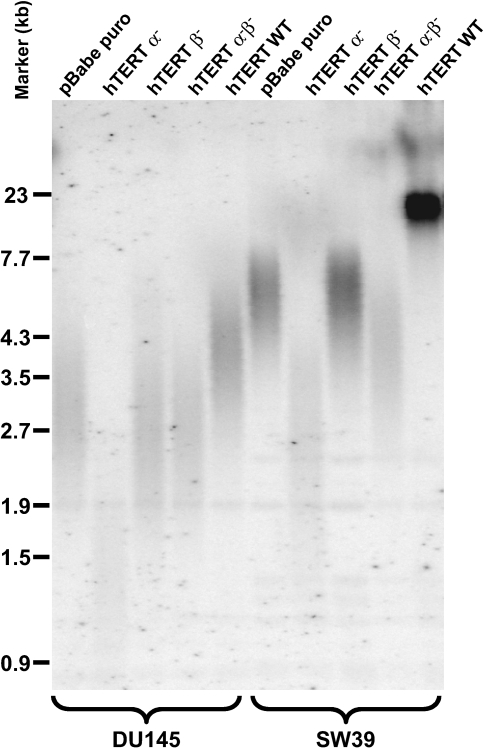
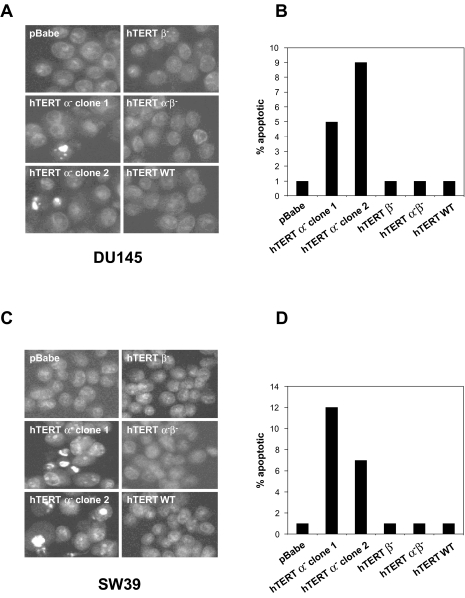
The SW39 and DU145 clones expressing the hTERT α- variant with reduced telomerase activity initially grew at the same rate as all other clones (Figure 6). RNase treatment of all TRAP samples abolished telomerase activity (data not shown). These clones showed progressive telomere shortening (Figure 7) and, after approximately 40 population doublings growth-arrested and underwent apoptosis with very few survivors as determined by 4′6-diamidino-2-phenylindole (DAPI) staining (Figure 8). Clones expressing the control vector, the hTERT β-, hTERT α-β- and hTERT WT showed no sign of apoptosis and continued to proliferate. Due to the extremely long starting telomere size of these cells, the H1299 hTERT α- clone did not die during the time course of the experiment. However, telomere length shortened by about 4 kb.
Figure 6.
Growth curves of clones of SW39 infected with pBabe puro vector, pBabe puro hTERT α-, pBabe puro hTERT β-, pBabe puro hTERT α-β- and pBabe puro hTERT WT All clones grow at the similar rates except at the end of their life-span, when the hTERT α- clones show increased apoptosis.
Figure 7.
Telomere length measurements (TRF) of SW39 clones and DU145 clones infected with pBabe puro vector, pBabe puro hTERT α-, pBabe puro hTERT β-, pBabe puro hTERT α-β- and pBabe puro hTERT WT during the course of cell culture. TRF lengths of all clones stay constant and within the range of clonal variation, except for the TRFs of the clones infected with hTERT α-, which decrease over the time of cell culture.
Figure 8.
The SW39 and DU145 clones that overexpress hTERT α- variant eventually die by apoptosis. Levels of apoptosis are measured by staining with DAPI followed by microscopy. Shown are representative views of stained nuclei of SW39 clones (A) and DU145 clones (C). The apoptosis levels of the SW39 and DU145 clones that overexpressed hTERT α- are 5–12%, whereas that of all other clones are below 1% (B and D).
Discussion
The human telomerase catalytic subunit transcript has at least six alternate splicing sites (two deletion sites and four insertion sites) (Figure 1) [20,21]. The β deletion and all four insertions cause frameshifts and premature terminations, whereas the 36-bp α deletion causes the loss of RT motif A. The hTERT variants containing one or both of the deletions did not reconstitute telomerase activity in human foreskin BJ fibroblasts. When overexpressed in telomerase-positive SW39 (SV40 large-T antigen immortalized IMR90 human lung fibroblast) [27] cells, human cancer cells H1299 (nonsmall lung carcinoma) and DU145 (prostate carcinoma) cells, the hTERT α- variant inhibited telomerase activity, progressively decreased telomere sizes, leading to apoptotic cell death in DU145 and SW39 cells. The hTERT α- alternate splicing variant thus demonstrates dominant negative effects on telomerase activity and telomere maintenance. The other two alternate splicing variants (hTERT β- and hTERT α-β-) did not show any phenotype in these same cells. However, we lacked an antibody that could detect these variants. Because the stability of these variants is unknown, they may not have accumulated to sufficient levels to alter telomerase activity.
A previous study has shown that in fetal heart tissue samples, telomerase activity and telomerase mRNA were coordinately regulated, whereas in fetal kidney samples, hTERT β- mRNA remained at the same level after telomerase activity disappeared [22]. The same study also showed that all four hTERT variants (hTERT α-, hTERT β-, hTERT α-β- and hTERT α+β+) remained at the same levels in fetal liver samples as telomerase activity remained the same [22]. Another study described a tissue sample in which mRNAs of hTERT variants (including hTERT α+β+) were detectable but telomerase activity was not [23]. We have also found that the hTERT transcript is alternatively spliced in cultured cancer cells, and the most prevalent hTERT variant mRNA is hTERT β-, not hTERT α+β+ (wild type at these alternate splicing sites) mRNA. It is possible that alternate splicing plays a role in reducing telomerase activity without reducing hTERT transcription in cells under certain conditions, such as differentiation [28], quiescence [29], during development or tumorigenesis [22,23]. During development, this dominant-negative function of the α- variant of hTERT might be important when certain cells undergo differentiation but hTERT transcription is not yet fully repressed [22]. During tumorigenesis, cancer cells may evolve from differentiated cell types, in which alternate splicing mechanisms may already be in place. Thus, alternate splicing of hTERT in tumor cells may reflect the preexisting alternate splicing mechanisms present in the cells from which they originated.
Telomerase reactivation is an important event during tumorigenesis [30]. Inhibition of telomerase should limit the growth potential of tumor cells because their telomeres will not be maintained [13,14]. It has been demonstrated that without telomerase expression, cells expressing SV40 large T-antigen and Ras failed to form tumors in nude mice or grow in soft agar [12], indicating the growth potential brought by telomerase expression might be very important for tumor growth. Our finding that one of the alternate splicing variants of hTERT has dominant-negative effect on telomerase activity and expression of this variant causes cell death of cancer cells opens a new possibility for cancer therapy. Alternate splicing variants of hTERT are observed in cancer cells and premalignant tissues [23]. Alternate splicing variants of hTERT are also observed very early during development [22]. With a better understanding of the factors that regulate alternate splicing variants of hTERT, it may be possible to limit the growth potential of tumor cells by promoting the production of nonfunctional or dominant-negative type of alternate splicing variants of hTERT.
Acknowledgements
BJ neonatal foreskin fibroblasts were kindly provided by James Smith, Baylor University Medical Center, Houston, TX. The hTERT cDNA and rat anti-hTERT antibody were provided by Geron Corporation (Menlo Park, CA). We thank Wendell Wu and Bryan C. Frank for excellent technical assistance.
Abbreviations
- hTERT
human telomerase reverse transcriptase
- TRAP
telomerase repeat amplification protocol
- TRF
terminal restriction fragment
- PD
population doubling
Footnotes
This work was supported by NIA grant AG01228. Jerry W. Shay is an Ellison Medical Foundation senior scholar.
References
- 1.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 3.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MDMJ, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 5.Watson JD. Origin of concatemeric T7 DNA. Nature (London) New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 6.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 9.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 10.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 12.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 13.Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, Beijersbergen RL, Knoll JH, Meyerson M, Weinberg RA. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 14.Herbert B, Pitts AE, Baker SI, Hamilton SE, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 16.Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- 17.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 18.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 19.Yi X, Tesmer VM, Savre-Train I, Shay JW, Wright WE. Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol Cell Biol. 1999;19:3989–3997. doi: 10.1128/mcb.19.6.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilian A, Bowtell DD, Abud HE, Hime GR, Venter DJ, Keese PK, Duncan EL, Reddel RR, Jefferson RA. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011–2019. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 21.Wick M, Zubov D, Hagen G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT) Gene. 1999;232:97–106. doi: 10.1016/s0378-1119(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 22.Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4172. [PubMed] [Google Scholar]
- 23.Ulaner GA, Hu JF, Vu TH, Oruganti H, Giudice LC, Hoffman AR. Regulation of telomerase by alternate splicing of human telomerase reverse transcriptase (hTERT) in normal and neoplastic ovary, endometrium and myometrium [in process citation] Int J Cancer. 2000;85:330–335. [PubMed] [Google Scholar]
- 24.Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AD, Rosman G. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 26.Wright WE, Shay JW, Piatyszek MA. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995;23:3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shay JW, Wright WE. Quantitation of the frequency of immortalization of normal human diploid fibroblasts by SV40 large T-antigen. Exp Cell Res. 1989;184:109–118. doi: 10.1016/0014-4827(89)90369-8. [DOI] [PubMed] [Google Scholar]
- 28.Xu D, Gruber A, Bjorkholm M, Peterson C, Pisa P. Suppression of telomerase reverse transcriptase (hTERT) expression in differentiated HL-60 cells: regulatory mechanisms. Br J Cancer. 1999;80:1156–1161. doi: 10.1038/sj.bjc.6690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holt SE, Wright WE, Shay JW. Regulation of telomerase activity in immortal cell lines. Mol Cell Biol. 1996;16:2932–2939. doi: 10.1128/mcb.16.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]