Abstract
The effects of a ligand of the aromatic hydrocarbon receptor (AhR), benzo[a]pyrene (B[a]P), and its metabolite, BPDE (7r,8t-dihydroxy-9t,10t-epoxy-7,8,9,10-tetrahydro-benzo[a]pyrene), on BRCA-1 levels and cell cycle kinetics were determined in MCF-7 breast cancer cells. Exposure of asynchronous MCF-7 cells for 72 hours to a non-cytotoxic dose of 0.5 µM B[a]P triggered a three-fold reduction in BRCA-1 protein. In MCF-7 cells resistant (20% to 30%) to genotoxic concentrations of B[a]P (1 to 5 µM), the loss of BRCA-1 protein was coupled with pausing in S-phase and G2/M, and accumulation of p53, mdm2 and p21. Treatment of MCF-7 cells synchronized in S-phase (72%) with B[a]P prolonged the arrest in S-phase, although this checkpoint was transient since cells resumed to G2/M after 12 hours with reduced levels of BRCA-1. In these cells, levels of p53 were increased, whereas the cellular content of p21 remained unaltered. In contrast, the co-treatment with the AhR antagonist, α-naphthoflavone (ANF), abrogated the deleterious effects of B[a]P on BRCA-1 expression, while preventing the accumulation of p53 and disruption of cell cycle profile. These findings suggest that the AhR mediated the inverse expression patterns of BRCA-1 and p53 upon exposure to B[a]P. The treatment with BPDE induced S-phase arrest and reduced BRCA-1 mRNA levels. The negative effects of BPDE on BRCA-1 expression were not transient since removal of BPDE did not allow complete reversal of the repression. These cumulative data suggest that the B[a]P metabolite, BPDE, may play a key role in disruption of BRCA-1 expression and cell cycle kinetics in breast epithelial cells.
Keywords: benzo[a]pyrene, BPDE, BRCA-1, p53, cell cycle kinetics, sporadic breast cancer
Introduction
The BRCA-1 protein participates in transcription-coupled repair of oxidative damage [1], co-localizes with Rad51 [2], BRCA-2 [3], and the hRad50-hMre11-p95 complex [4]. In addition, an important role in embryogenesis has been attributed to BRCA-1 since nullizygous embryos die early in development, possibly because of accumulation of DNA damage [5,6]. The latter affects subnuclear location and ATM-dependent phosphorylation of BRCA-1 [7–9].
It has been proposed that BRCA-1 may be involved in cell cycle control since the cellular levels of BRCA-1 protein peak in S- and M-phases [10]. This inference is supported by the fact that BRCA-1 contributes to p53-dependent gene expression [11] and the trans-activation of p21 [12,13]. Moreover, the overexpression of mutant BRCA-1 encoding for COOH-terminal residues decreases the doubling time of 184A1 human breast epithelial cells, while inducing the loss of G2/M block by colchicine [14]. These cumulative data are consistent with a role for BRCA-1 in S- and G2/M-phases control [15].
One of the cellular responses to DNA damage is the activation of G1/S and G2/M checkpoints. Stabilization of p53 at the G1/S boundary elicits the trans-activation of a number of genes, including mdm2 and the cyclin-dependent kinase inhibitor p21, which, in concert, controls entry into the S-phase [16]. While upregulation of p21 can occur through a p53-independent pathway, both p53 and p21 are essential for maintaining the G2 checkpoint after DNA damage [17]. Nevertheless, the disruption of these cell cycle regulators by drugs and chemical agents may compromise the fidelity of DNA replication and allow progression of cells harboring genomic aberrations. Alternatively, severe DNA damage may destine cells to lethality [18].
One of the puzzles in breast carcinogenesis is that BRCA-1 is not mutated in sporadic breast tumors [3]. We hypothesized that epigenetic effectors capable of inducing genotoxic damage may cause a concomitant loss of BRCA-1, thus preventing its participation in DNA repair functions. An outcome may be the propagation of mutations or chromosomal aberrations that contribute to drug resistance and cancer development.
Although recent studies have investigated the regulation of BRCA-1 expression by DNA-damaging agents [19], knowledge concerning the contribution of polycyclic aromatic hydrocarbons (PAHs) to the regulation of BRCA-1 is limited. PAHs are known to induce a number of biological responses including G1 arrest [20] and genotoxic stress [21]. Recently [22], we reported that benzo[a]pyrene (B[a]P), a prototype PAH and ligand of the aromatic hydrocarbon receptor (AhR), inhibited the expression of BRCA-1 in estrogen receptor-positive breast (MCF-7) and ovarian (BG-1) cancer cells. Here, we present evidence that in MCF-7 cells, the expression of BRCA-1 and p53 is inversely regulated by B[a]P, which contributes to transition from S- to G2/M-phases in the presence of reduced levels of BRCA-1. We document that disruption of BRCA-1 expression and cell cycle kinetics by B[a]P are mediated, at least in part, by the reactive metabolite, 7r,8t-dihydroxy-9t,10t-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE).
Methods
Cell Culture
Effects of B[a]P on cell proliferation were investigated as described previously [23] in MCF-7 cells obtained from the American Type Culture Collection (Manassas, VA). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM)/F12 medium (Sigma, St. Louis, MO) supplemented with 10% fetal calf serum (FCS; Hyclone Laboratories Inc., Logan, UT). B[a]P, α-naphthoflavone (ANF), and colchicine were obtained from Sigma. Aphidicolin was purchased from Calbiochem Co. (La Jolla, CA). BPDE was obtained from Midwest Research Institute (Kansas City, MO). For cell viability studies, cells were plated at a density of 2x106 cells/100 mm tissue culture dish and maintained overnight in DMEM/F12 plus 10% FCS. Three dishes were assigned to each experimental treatment. At the end of the incubation periods, cell viability was assessed in triplicate (n = 9) by trypan blue exclusion.
Flow Cytometry
Flow cytometry was performed as previously described [19]. Briefly, cells were harvested with trypsin and washed in PBS. Then cells were treated with RNAse and stained with propidium iodide (50 µg/ml in PBS). Cell cycle distribution profiles were recorded with a FACscan (Becton-Dickinson, Franklin Lakes, NJ), using a CELLQuest program. Data were analyzed with the MODFIT.2 software at the Flow Cytometry Laboratory of the Arizona Cancer Center.
Western Blotting
Western blotting was performed as previously described [24]. Cell extracts were normalized to protein content and separated by 4% to 12% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis. Immunoblotting was carried out with antibodies raised against BRCA-1 (Ab-2), p53 (Ab-2), p21 (Ab-1), and mdm2 (Ab-1) obtained from Oncogene Research Products (Cambridge, MA) and p27 (C-19) obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Normalization of Western blots was confirmed by incubating immunoblots with β-actin antibody-1 (Oncogene Research Products). The immunocomplexes were detected by enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL).
Semi-Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Details concerning the experimental conditions for semi-quantitative RT-PCR analysis of BRCA-1 mRNA are described elsewhere [22]. Briefly, total RNA (400 ng) was incubated with random hexamer primers, Moloney murine leukemia virus RT, RNase inhibitor (Life Technologies/Gibco BRL, Gaithersburg, MD), and RT buffer (Ambion Inc., Austin, TX) at 42°C for 1 hours. cDNA was amplified using the forward 5′-AGCTCGCTGAGACTTCCTGGA-3′ and reverse 5′-CAATTCAATGTAGACAGACGT-3′ primers, which produced fragments spanning exon-1A to exon-8. The amplification products were of the expected size (712 bp), and their authenticity to the BRCA-1 sequence (GenBank accession no. U1460) was confirmed by direct sequencing. The primers for the internal standard 18S ribosomal RNA (488 bp) were from Ambion Inc. The expression levels of BRCA-1 were quantified by Alpha Imager (Alpha Innotech Inc., San Diego, CA) analysis and corrected for the expression of the control mRNA (BRCA-1/18S).
Results
Dose-Dependent Effects of B[a]P on Cell Viability and BRCA-1
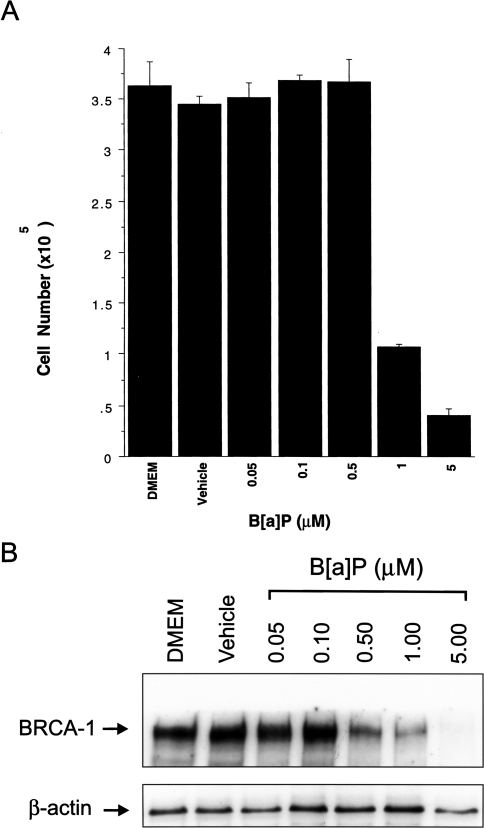
The data depicted in Figure 1 demonstrate a strong correlation between cell number (Figure 1A) and levels of BRCA-1 protein (Figure 1B) in cells treated with varying concentrations of B[a]P. MCF-7 cells treated with 0.05 to 0.5 µM B[a]P proliferated at the same rate as control cells, even though BRCA-1 protein levels were reduced by three-fold at concentrations of 0.5 µM B[a]P (Figure 1B). This observation indicates that non-cytotoxic concentrations of B[a]P might abrogate the expression of BRCA-1 in circumstances of chronic exposure. However, acute doses of 1 and 5 µM B[a]P reduced cell viability 3.5- and 10-fold, respectively, after 72 hours. BRCA-1 protein was reduced six-fold in the presence of 1 µM B[a]P, whereas at 5 µM B[a]P, BRCA-1 immunocomplexes were nearly undetectable. These data lend support to our previous findings [22] that non-lethal doses of B[a]P may reduce BRCA-1 protein, whereas at concentrations higher than 0.5 µM, the loss of BRCA-1 is paralleled by decreased cell viability. The finding that 20% to 30% of cells did not succumb to treatment with cytotoxic levels of B[a]P, but contained little or no BRCA-1, triggered our interest in examining the dynamic changes in BRCA-1 expression and cell cycle kinetics in this sub-cell population.
Figure 1.
Dose-response effects of B[a]P on cell viability and BRCA-1. (A) MCF-7 cells were cultured in the presence of increasing concentrations of B[a]P for 72 hours. Bars represent average cell number±SD from three independent wells counted in triplicate (n = 9). (B) Western blotting of BRCA-1 protein. Cell extracts were obtained from MCF-7 cells cultured for 72 hours in control DMEM/F12 containing 10% FCS, basal medium plus vehicle (DMSO), or increasing concentrations of B[a]P. The bands in panel B are immunocomplexes visualized by incubating Western blots with BRCA-1 (Ab-2) or β-actin (Ab-1) antibodies.
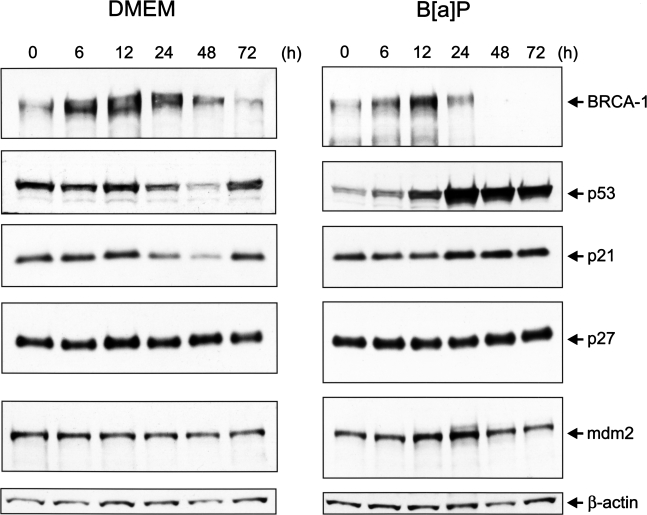
We performed Western blot analysis of cell extracts obtained from asynchronous MCF-7 cells cultured in control medium or medium supplemented with 5 µM B[a]P for various periods of time (Figure 2). In both control and B[a]P-treated cells, BRCA-1 protein peaked at 12 hours. This induction was attributed to stimulated expression of BRCA-1, which is characteristic of rapidly proliferating cells [24]. Whereas BRCA-1 resumed to basal levels in control cells at 24 hours, the presence of B[a]P drastically reduced BRCA-1 levels at both 48 and 72 hours. Between 6 and 12 hours, we detected fluctuations in the levels of p53 in cells cultured in basal medium. Treatment with B[a]P for 12 hours induced a significant increase in the cellular content of p53, which was further augmented at 24 hours, and remained significantly higher than the amount detected in control cells. The accumulation of p53 in B[a]P-treated cells was accompanied by an increase in the level of cyclin-dependent kinase inhibitor p21. In contrast, there were no detectable changes in the cellular content of p27, in the presence or absence of B[a]P. Cellular levels of the p53 regulator, mdm2, remained unchanged throughout the experiment in MCF-7 cells cultured in DMEM/F12, whereas the mdm2 protein accumulated between 12 and 24 hours after treatment with B[a]P. Thus, when comparing the temporal profiles of expression of BRCA-1 and p53, the accumulation of p53 at 12 hours in B[a]P-treated cells preceded by approximately 12 hours the loss of BRCA-1. At 48 and 72 hours after treatment with B[a]P, BRCA-1 was not detectable, whereas the cellular levels of p53 and p21 remained elevated.
Figure 2.
Time-dependent effects of B[a]P on BRCA-1 and cell cycle checkpoints. Asynchronous MCF-7 cells were cultured for various periods of time in basal DMEM/F12 plus 10% FCS or basal medium containing 5 µM B[a]P. At the end of the incubation periods, cell extracts were analyzed for their content in BRCA-1, p53, p21, and mdm2 protein. The control bands are β-actin immunocomplexes.
B[a]P Alters Cell Cycle Kinetics
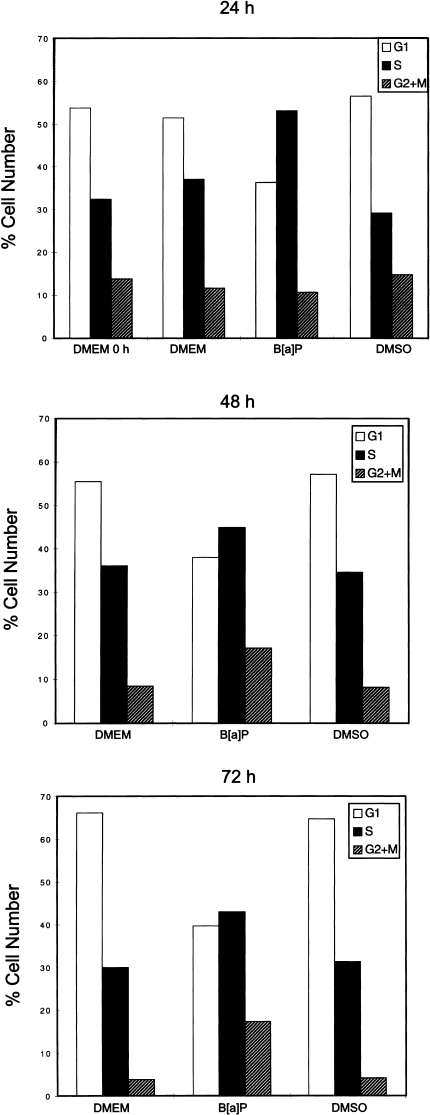
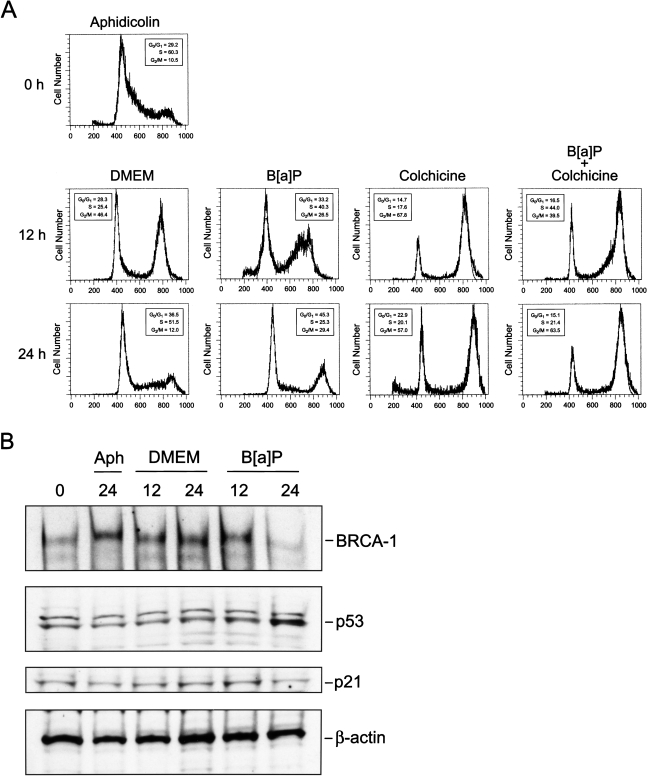
The concomitant loss of BRCA-1, along with the changes in p53 and p21 proteins, prompted us to examine the effects of B[a]P on cell cycle progression. Cell cycle distribution was determined by flow cytometric analysis of propidium iodide stained cells. Treatment of asynchronous MCF-7 cells with B[a]P, when 55% of cells were in G0/G1, induced within a period of 24 hours a significant enrichment in S-phase compared with control cells (53.1% vs. 32.0%). The accumulation in S-phase was paralleled by a reduction in the fraction of cells in G0/G1 (36.3% vs. 54.0%) (Figure 3) and a 2.5- and 4.0-fold increase in the percentage of cells positioned in G2/M at 48 and 72 hours, respectively. Thus, loss of BRCA-1 and stabilization of p53 and p21 correlated with pausing of cells in S-phase and subsequent arrest in G2/M.
Figure 3.
B[a]P induces S-phase and G2/M accumulation. Asynchronous MCF-7 cells were cultured for 24, 48, and 72 hours in basal DMEM/F12 plus 10% FCS, basal medium containing the vehicle (DMSO) or vehicle plus 5 µM B[a]P. Cell cycle profiles were analyzed by flow cytometry as described in Materials and Methods section. Bars represent percentages of cells in G0/G1, S, and G2/M representative of three separate experiments with standard deviations lower than 3%.
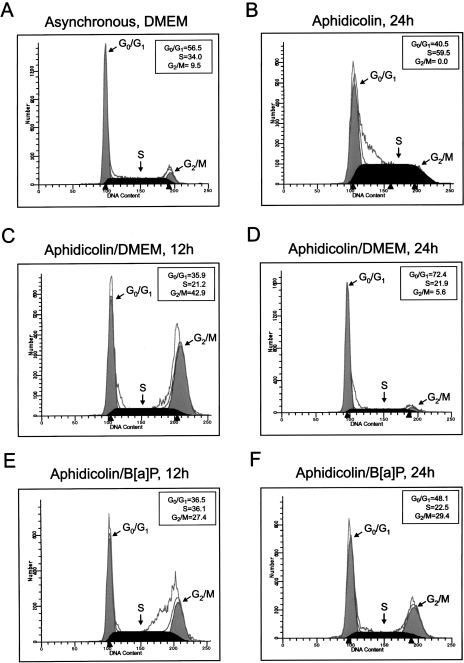
Because levels of BRCA-1 normally peak in S-phase [10], we wished to characterize the effects of B[a]P on levels of BRCA-1 protein and cell cycle progression in MCF-7 cells synchronized in S-phase. After synchronization with 1 µg/ml aphidicolin for 24 hours, cells were released from S-phase arrest by replacing the culture medium with fresh DMEM/F12 plus 10% FCS or medium supplemented with 5 µM B[a]P. The data depicted in Figure 4 showed that, at 12 hours after release, the addition of B[a]P induced arrest in S-phase (36.1% vs. 21.2%) (Figure 4E) and reduced the fraction (27.4%) that progressed to G2/M compared with cells (42%) cultured in basal DMEM/F12 medium. However, while a significant percentage of cells released into control medium transitioned to G0/G1 (72.4%) by 24 hours and assumed the characteristic asynchronous distribution (Figure 4A), the treatment with B[a]P sustained significant accumulation in G2/M (29.4% vs. 5.6%) (Figure 4F). Overall, B[a]P appeared to alter normal cell cycling by lengthening the transition through S and inducing subsequent arrest in G2/M. This inference was confirmed in a G2/M trapping experiment in which cells previously synchronized in S-phase with aphidicolin were released into culture media containing colchicine (0.25 µM) to prevent cycling beyond G2/M. These experiments illustrated that, upon treatment with B[a]P, cells resumed to G2/M with an approximate 12 hours delay compared with cells released in control DMEM/F12 (Figure 5A). In fact, in the presence of B[a]P plus colchicine, 44.0% of cells were positioned in S-phase at 12 hours compared with only 17.6% when cells were treated with colchicine alone. Nevertheless, flow cytometry profiles confirmed that by 24 hours after release, a significant percentage of cells treated with B[a]P plus colchicine had escaped S-phase arrest and occupied the G2/M window at levels (63.5%) similar to those elicited by colchicine (57.0%). Therefore, although B[a]P induced S-phase arrest, this checkpoint was relaxed since cells resumed cycling to G2/M within a 24-hour interval.
Figure 4.
B[a]P delays escape from S-phase and extends transit through G2/M. MCF-7 cells were synchronized in S-phase with aphidicolin (1 µg/ml) for 24 hours, after which cells were released into (C and D) basal DMEM/F12 plus 10% FCS (aphidicolin/DMEM) or (E and F) basal medium plus 5 µM B[a]P (aphidicolin/B[a]P). Cells were harvested at 12 and 24 hours after release. Cell cycle distribution was examined by flow cytometry after propidium-iodide staining. Arrowheads below the DNA histograms represent channels with most events for specific phases of the cell cycle. The profiles are representative of three independent experiments.
Figure 5.
B[a]P-dependent accumulation in G2/M coincides with loss of BRCA-1. (A) MCF-7 cells were synchronized in S-phase with aphidicolin (1 µg/ml) for 24 hours, after which cells were released into basal DMEM/F12 plus 10% FCS, or basal medium plus 5 µM B[a]P, colchicine (0.25 µM), or colchicine plus B[a]P. Cells were harvested at 12 and 24 hours after release. Cell cycle distribution was examined by flow cytometry after propidium-iodide staining. The profiles are representative of three independent experiments. (B) Western blotting of BRCA-1, p53 and p21. After synchronization in S-phase with aphidicolin (1 µg/ml) for 24 hours, cells were released into DMEM/F12 plus 10% FCS or basal medium containing 5 µM B[a]P. Cell extracts were collected at 12 and 24 hours after release. The control bands are β-actin immunocomplexes.
The synchronization in S-phase with aphidicolin elicited the accumulation of BRCA-1 protein, as indicated by Western blotting of protein extracts (Figure 5B). The release from S-phase into basal DMEM was accompanied at 12 hours by a slight reduction in BRCA-1, whose levels increased at 24 hours (lane 4) when 51.5% of cells occupied the S-phase. These dynamic changes in BRCA-1 are in keeping with the notion that levels of BRCA-1 fluctuate during cell progression, but that the BRCA-1 protein is most abundant during transit through S-phase [10]. In contrast, BRCA-1 was significantly reduced after treatment with B[a]P for 24 hours, whereas levels of p53 were increased. Interestingly, the accumulation of p53 did not yield a corresponding increase in p21, whose levels remained unchanged at 12 and 24 hours after release from S-phase. These data indicated that cells containing increased levels of p53 paused transiently in S-phase, but subsequent transition to G2/M occurred with reduced cellular levels of BRCA-1.
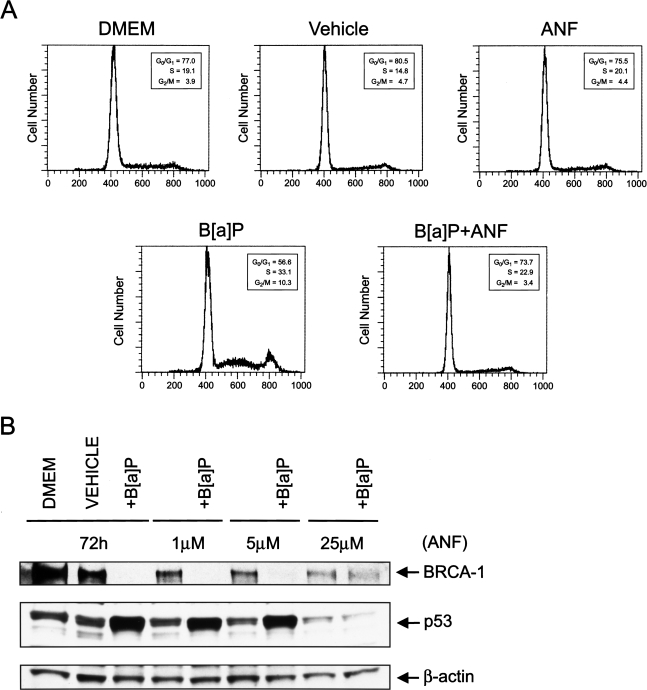
ANF Counteracts the Loss of BRCA-1 Expression and Disruption of Cell Cycle Kinetics
We wished to obtain direct evidence of the involvement of the AhR pathway in the disruption of cell cycle kinetics by B[a]P. Therefore, we tested whether co-treatment with the AhR antagonist, ANF, prevented the perturbations in cell cycle kinetics triggered by B[a]P, and restored BRCA-1 expression. The co-treatment with ANF abrogated the transient arrest of MCF-7 cells in S-phase and the subsequent accumulation in G2/M, suggesting that the AhR pathway mediated the disruptive effects of B[a]P on cell cycle progression. In addition to restoring normal cell cycle distribution (Figure 6A), the co-treatment with ANF counteracted the loss of BRCA-1 and accumulation of p53 (Figure 6B). Taken together, these data confirmed that the changes in cell cycle kinetics as well as the fluctuations in BRCA-1 and p53 involved the participation of the AhR pathway, and attributed to ANF a protective effect against B[a]P. Furthermore, these findings suggest the existence of an inverse relationship between BRCA-1 and p53 status in MCF-7 cells with regard to exposure to B[a]P.
Figure 6.
ANF restores normal cell cycle distribution, BRCA-1, and inhibits the accumulation p53. Asynchronous MCF-7 cells were cultured for 72 hours in basal DMEM/F12, basal medium containing vehicle (DMSO), or 5 µM B[a]P plus increasing concentrations of ANF. Bands represent immunocomplexes of BRCA-1 and p53. The control bands are β-actin immunocomplexes.
BPDE Induces S-Phase Arrest and Reduces the Potential for BRCA-1 Expression
In previous studies [22], we reported that B[a]P, but not 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), reduced the expression of BRCA-1. The fact that TCDD binds with high affinity to the AhR, but is not metabolized, led us to consider the possibility that downregulation of BRCA-1 may be mediated, at least in part, by metabolites of B[a]P. Therefore, we investigated further the effects of the metabolite BPDE on cell cycle progression and expression of BRCA-1.
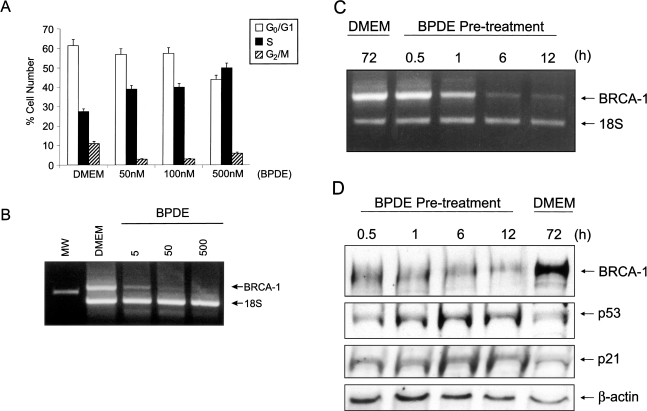
Flow cytometry analysis of MCF-7 cells documented that, similar to B[a]P, BPDE induces a dose-dependent arrest of cells in S-phase. The fraction of cells positioned in S-phase at 24 hours increased from 28% for the DMEM control to 50% for MCF-7 cells treated with 500 nM BPDE (Figure 7A). The BPDE-dependent accumulation in S-phase was paralleled by a reduction in the percentage of cells positioned in G0/G1 (from 60% to 44%) and G2/M (from 10% to 5%). RT-PCR analysis of total RNA revealed that treatment with increasing amounts of BPDE elicited a dose-dependent loss of BRCA-1 transcripts (Figure 7B), which were nearly undetectable at concentrations equal to 500 nM BPDE.
Figure 7.
BPDE induces S-phase arrest and reduces the potential for BRCA-1 expression. (A) Flow cytometry profile of MCF-7 cells cultured for 24 hours in basal DMEM/F12 plus 10% FCS, or basal medium plus increasing concentrations of BPDE. Bars represent average percentage of cells in G0/G1, S, and G2/M from two separate experiments. (B) RT-PCR analysis of BRCA-1 mRNA from MCF-7 cells treated with nanomolar concentrations of BPDE for 24 hours. (C) MCF-7 cells were treated for 72 hours in basal medium, or pre-treated in BPDE (500 nM)-containing medium for 0.5 1, 6 and 12 hours. At the end of the pre-incubation periods, cells were washed and cultured up to 72 hours in fresh DMEM/F12 plus 10% FCS free of BPDE. In (B) and (C), changes in BRCA-1 mRNA were assessed by RT-PCR analysis of total RNA. The PCR products represent BRCA-1 (712 bp) and control ribosomal 18S RNA (488 bp) from input cDNA corresponding to 400 ng of total RNA. Experimental conditions for semi-quantitative RT-PCR analysis of BRCA-1 were those described in Material and Methods section [22]. (D) Western blot analysis of BRCA-1 and p53. MCF-7 cells were pre-treated with BPDE (500 nM) for various periods of time and then cultured up to 72 hours in basal DMEM/F12 plus 10% FCS medium. Bands represent immunocomplexes of BRCA-1, p53 and p21. The control bands are β-actin immunocomplexes.
The effects of BPDE on BRCA-1 expression were further examined in a washout experiment in which MCF-7 cells were pre-treated with 500 nM BPDE for 0.5, 1, 6 and 12 hours. Then, after washing out of media containing the BPDE, cells were cultured in fresh DMEM/F12 plus 10% FCS medium up to 72 hours. At the end of the incubation period, we analyzed the levels of BRCA-1 mRNA in total RNA, and protein in cell extracts. While at 72 hours the levels of BRCA-1 mRNA in control cells were similar to those observed in cells harvested at the time of induction (data not shown), BRCA-1 transcripts were reduced five-fold in cells pre-treated with BPDE for 6 to 12 hours (Figure 7C). Similarly, BRCA-1 protein levels were reduced significantly by the pre-treatment for 6 to 12 hours with BPDE (Figure 7D). The loss of BRCA-1 at these time points was paralleled by accumulation of p53 and p21. Because the half-life of BDPE is approximately 5 to 20 minutes [25], we discounted the likelihood that residual BPDE may have been responsible for the reduction in BRCA-1 mRNA and protein. In fact, cells were washed twice and then cultured in fresh medium for at least 60 hours after removal of media containing the BPDE. Based on these considerations, we concluded that the short-term exposure to BPDE exerted a signature effect by reducing the potential for BRCA-1 expression and increasing the cellular levels of p53 and p21.
Discussion
In the absence of a causal relationship between the occurrence of sporadic breast cancer and mutations in the BRCA-1 gene, efforts directed to investigating the contribution of environmental xenobiotics as epigenetic effectors of BRCA-1 are warranted. PAHs, ubiquitous pollutants known to induce mammary tumors in rodents [26], are found in tobacco smoke, industrial pollution and auto exhaust [27]. Among PAHs, B[a]P is a prototype known to act both as a tumor initiator and promoter, and to stimulate the expression of gene products of the cytochrome P450 family, which metabolize B[a]P to the ultimate carcinogen, BPDE [28]. The BPDE damages DNA by forming bulky adducts and apurinic sites that are degraded further to DNA strand breaks [29], thus contributing to inhibition of DNA synthesis [30] and cell cycle arrest [31].
To elucidate the function of BRCA-1, genetic models defective in BRCA-1 and other cell cycle regulators have been developed [11,13–15,32]. In previous work [22], we documented that B[a]P inhibited BRCA-1 expression in estrogen receptor-positive breast MCF-7 and ovarian BG-1 cancer cells in a dose- and time-dependent fashion. In this study, we hypothesized that one modality of PAH-mediated breast oncogenesis may involve the coordinate disruption of BRCA-1 and cell cycle regulation. The loss of BRCA-1 may abrogate normal defense mechanisms, such as cell cycle and growth control functions, thus predisposing to accumulation of DNA damage that may be critical for tumor development. Our findings provide novel evidence that non-cytotoxic doses of B[a]P (0.5 µM) reduce the levels of BRCA-1 protein in MCF-7 cells. The significance of this observation is that BRCA-1 may be a cellular target for repression by chronic exposure to PAHs, which may lower DNA repair functions and predispose to the fixation of mutations.
At cytotoxic concentrations (1 to 5 µM), nearly 20% to 30% of cells survived the treatment with B[a]P, but contained decreased or no BRCA-1 protein. Because cytotoxic damage and multi-drug resistance are hallmarks of cancer, we examined whether cells resistant to B[a]P, having reduced levels of BRCA-1, had acquired altered cell cycle characteristics. Our results indicated that acute exposure to cytotoxic levels of B[a]P lengthened the S-phase interval and led to G2/M accumulation. The stabilization of p53 and p21 and dynamic fluctuations in mdm2 confirmed that surveillance mechanisms were alerted to halt cell cycle progression [16,33]. However, results obtained with MCF-7 cells synchronized with aphidicolin showed that the arrest in S-phase was transient. In the presence of B[a]P, cycling from S to G2/M occurred with an approximate 12-hour delay compared with cells released into control medium. In fact, 24 hours after release from S-phase, a large fraction of cells treated with B[a]P occupied the G2/M window with increased levels of p53, but reduced BRCA-1, whereas levels of p21 were unaffected. The failure of p21, which is required for maintaining the G2 block [17], to increase under these conditions could be attributed, at least in part, to the downregulation of BRCA-1. The latter has been shown to contribute to trans-activation of the p21 promoter [13]. These findings suggest that B[a]P contributes to accumulation in G2/M-phase of cells containing reduced levels of BRCA-1, and complement recent work [15] documenting that embryonic fibroblasts carrying an exon-11 BRCA-1 deletion are defective in G2/M checkpoint. The prolonged arrest in G2 of cells harboring DNA damage is known to cause aberrant mitosis [34]. Because BRCA-1 is a target for phosphorylation by the ATM protein kinase [9] and is required for S-phase [13] and G2/M regulation [15], one could envision that the carcinogenicity of B[a]P may stem, at least in part, from reduction of BRCA-1 expression and repair functions involving the ATM-BRCA-1 cascade.
Previous studies have reported on the inhibition of BRCA-1 by chemical agents, including adriamycin and ultraviolet radiation in breast cancer cells [19]. In this study, we provided evidence that disruption of cell cycle kinetics by B[a]P correlated with inverse expression patterns of BRCA-1 and p53 in MCF-7 cells. In fact, co-treatment with ANF restored normal cell cycle distribution and counteracted the loss of BRCA-1 expression, while preventing the accumulation of p53. Because embryonic death associated with disruption of the BRCA-1 gene was less pronounced in p53-null embryos [6], one could attribute the inverse expression patterns of BRCA-1 and p53 to antagonistic interactions among factors ubiquitous to the regulation of BRCA-1 and p53 [35].
With respect to loss of cell viability induced by B[a]P, one could expect that the coordinate loss of BRCA-1 and accumulation of p53 may lead to p53-dependent apoptosis of severely damaged cells. However, electron microscopy analysis of cells treated with B[a]P did not reveal changes in cellular architecture or morphology characteristic of apoptosis, such as chromatin condensation and membrane blebbing (unpublished data). Considering that approximately 30% of cells did not succumb to the treatment with cytotoxic levels of B[a]P, one could envision that B[a]P-resistant cells expressing lower levels of BRCA-1 may have acquired a cytotoxic drug resistance phenotype [36].
With regard to the mechanisms through which B[a]P may repress BRCA-1, one could extrapolate that through general inhibition of RNA synthesis, B[a]P may preferentially deplete the cellular levels of proteins with a rapid turnover, such as BRCA-1, as opposed to p53, which is regulated at the posttranscriptional level following DNA damage. This hypothesis is not supported by our earlier data [22], which document that in estrogen receptor-negative HBL-100 and M DA-MB-231 breast cancer cells, neither BRCA-1 nor cell growth was compromised by equimolar (5 µM) concentrations of B[a]P. Moreover, in the same study, we found that the dioxin-like TCDD, which exhibits high affinity for the AhR but is not metabolized, did not repress BRCA-1 mRNA and protein levels [22]. Therefore, we hypothesized that the responsiveness of MCF-7 cells to B[a]P may be a consequence of their ability to metabolize B[a]P to reactive end products. To test this contention, we treated MCF-7 cells with the metabolite BPDE. Consistent with this hypothesis, BPDE induced S-phase arrest and suppressed BRCA-1. These effects were observed at doses 10-fold lower (50 to 500 nM) than those used for B[a]P (0.5 to 5 µM). More importantly, we observed that the short-term exposure to BPDE reduced the potential to express BRCA-1, since removal of BPDE did not allow for complete reversal of the repression. In contrast, levels of p53 and p21 were increased in cells pre-treated with BPDE for at least 6 to 12 hours. We did not attribute the loss of BRCA-1 to residual BPDE since BPDE is hydrolyzed within minutes in cellular systems [25]. In addition, MCF-7 cells were cultured in fresh media after washing out of the pre-treatment medium. Rather, these cumulative observations suggested that the effects of BPDE were not transient. A possible interpretation of these data is that BPDE may repress transcription of BRCA-1 or alter the expression of other factors such as p53, which may contribute to repression of BRCA-1. For example, the functionality of cellular checkpoints may contribute to the deleterious effects of BPDE on BRCA-1 expression. MCF-7 cells have functional p53 and Rb, both of which complex with BRCA-1 [11]. Conversely, in SV40-transformed HBL-100 cells, which are refractory to B[a]P, both p53 and Rb are inactivated [37]. Investigations are currently in progress in our laboratory to clarify whether elevation of p53 is an absolute requirement for BPDE-dependent regulation of BRCA-1.
In summary, there are ample data suggesting that environmental factors can initiate and promote chemical carcinogenesis [38]. While maintenance of genome integrity is the result of balance between production and repair of DNA [39], this work provides novel evidence that B[a]P disrupts normal BRCA-1 expression and cell cycle kinetics. These effects are mediated, at least in part, by the reactive metabolite BPDE, which reduces the potential for BRCA-1 expression. The identification of a possible link between carcinogenicity induced by PAHs and loss of BRCA-1 offers an exciting opportunity to gain insights into the potential role of PAH-gene interactions in the development of sporadic breast cancer.
Acknowledgements
The financial support of Arizona Disease Control Research Commission grants 9722 and 10017 (to D.R.) and Pilot Project P30-ES06694 NIEHS, Southwest Environmental Health Sciences Center (to D.R.) is greatly appreciated. Special thanks are due to T. A. Weinert and G. Tim Bowden for suggestions on cell cycle experiments and useful discussions.
Abbreviations
- PAH
polycyclic aromatic hydrocarbon
- B[a]P
benzo[a]pyrene
- AhR
aromatic hydrocarbon receptor
- BPDE
7r,8t-dihydroxy-9t,10t-epoxy-7,8,9,10-tetrahy-drobenzo[a]pyrene
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- ANF
α-naphthoflavone
- FCS
fetal calf serum
- DMEM
Dulbecco's modified Eagle's medium
References
- 1.Gowen LC, Avrutskaya AV, Latour AM, Koller BH, Leadon SA. BRCA-1 required for transcription-coupled repair of oxidative damage. Science. 1998;286:804–810. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- 2.Scully R, Chen J, Plug A, Xiao Y, Waver D, Feunteun J, Ashley T, Livingston DM. Association of BRCA-1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Tombline G, Weber BL. BRCA-1, BRCA-2 and DNA damage response: collision or collusion. Cell. 1998;92:433–436. doi: 10.1016/s0092-8674(00)80936-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhong Q, Chen C-F, Li S, Chen Y, Wang C-C, Xiao J, Chen P-L, Sharp ZD, Lee W-H. Association of BRCA-1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science. 1999;285:747–750. doi: 10.1126/science.285.5428.747. [DOI] [PubMed] [Google Scholar]
- 5.Hakem R, de la Pompa JL, Elia A, Potter J, Mak TW. Partial rescue of BRCA-1, (5–6) early embryonic lethality by p53 or p21 null mutation. Nat Genet. 1997;16:298–302. doi: 10.1038/ng0797-298. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of BRCA-1, BRCA-2, BRCA-1/BRCA-2, BRCA-1/p53, and BRCA-2/p53 nullizygous embryos. Genes Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 7.Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J, Livingston DM. Dynamic changes of BRCA-1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 8.Thomas JE, Smith M, Tonkinson JL, Rubinfeld B, Polakis P. Induction of phosphorylation on BRCA-1 during the cell cycle and after DNA damage. Cell Growth Differ. 1997;8:801–809. [PubMed] [Google Scholar]
- 9.Cortez D, Wang Y, Qin J, Eleldge SJ. Requirement of ATM-dependent phosphorylation of BRCA-1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1171. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Farmer A, Chen C-F, Jones DC, Chen P-L, Lee W-H. BRCA-1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 1996;56:3168–3172. [PubMed] [Google Scholar]
- 11.Ouchi T, Monteiro AN, August A, Aaronson SA, Hanafusa H. BRCA-1 regulates p53-dependent gene expression. PNAS. 1998;95:2302–2306. doi: 10.1073/pnas.95.5.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Chen P-L, Subramanian T, Chinnadurai G, Tonlinson G, Osborne CK, Sharp ZD, Lee W-H. Binding of CtIP to the BRCT repeats of BRCA-1 involved in the transcription regulation of p21 is disrupted upon DNA damage. J Biol Chem. 1999;275:11334–11338. doi: 10.1074/jbc.274.16.11334. [DOI] [PubMed] [Google Scholar]
- 13.Somasundaram K, Zhang H, Zeng Y-X, Houvras Y, Peng Y, Zhang H, Wu GS, Licht JD, Weber BL, El-Deiry WS. Arrest of the cell cycle by the tumor suppressor BRCA-1 requires the CDK inhibitor p21 WAF1/CiPI. Nature. 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]
- 14.Larson JS, Tonkinson JL, Lai MT. A BRCA-1 mutant alters G2-M cell cycle control in human mammary epithelial cells. Cancer Res. 1997;57:3351–3355. [PubMed] [Google Scholar]
- 15.Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang X-W, Harris CC, Ried T, Deng C-X. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA-1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 16.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 17.Bunz F, Dutriaux A, Lenauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 18.Brugarolas J, Jacks T. Double indemnity: p53, BRCA and cancer. Nat Med. 1997;3:721–722. doi: 10.1038/nm0797-721. [DOI] [PubMed] [Google Scholar]
- 19.Andres JL, Fan S, Turlek GJ, Wang J-A, Twu N-F, Yuan R-Q, Lamszus K, Goldberg ID, Rosen EM. Regulation of BRCA-1 and BRCA-2 expression in human breast cancer cells by DNA-damaging agents. Oncogene. 1998;16:2229–2241. doi: 10.1038/sj.onc.1201752. [DOI] [PubMed] [Google Scholar]
- 20.Vaziri C, Faller DV. A benzo[a]pyrene-induced cell cycle checkpoint resulting in p53-independent G1 arrest in 3T3 fibroblasts. J Biol Chem. 1997;272:2762–2769. doi: 10.1074/jbc.272.5.2762. [DOI] [PubMed] [Google Scholar]
- 21.Shackelford RE, Kaufmann, Paules RS. Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ Health Perspect. 1999;107:5–24. doi: 10.1289/ehp.99107s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffy BD, Schultz EU, Selmin O, Gudas JM, Bowden GT, Romagnolo D. Inhibition of BRCA-1 expression by benzo[a]-pyrene and its diol epoxide. Mol Carcinogen. 1999;26:100–118. doi: 10.1002/(sici)1098-2744(199910)26:2<100::aid-mc5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Romagnolo D, Annab LA, Lyon TT, Risinger JI, Terry LA, Barrett JC, Afshari CA. Estrogen upregulation of expression of BRCA-1 with no effect on localization. Mol Carcinogen. 1998;22:102–109. doi: 10.1002/(sici)1098-2744(199806)22:2<102::aid-mc5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Gudas JM, Nguyen H, Li T, Cowen KH. Hormone-dependent regulation of BRCA-1 in human breast cancer cells. Cancer Res. 1995;55:4561–4565. [PubMed] [Google Scholar]
- 25.MacLeod MC, Selkirk JK. Physical interactions of isomeric benzo[a]pyrene diol epoxides with DNA. Carcinogenesis. 1982;2:287–292. doi: 10.1093/carcin/3.3.287. [DOI] [PubMed] [Google Scholar]
- 26.Ronai Z, Gradia S, el-Bayoumy K, Amin S, Hecht SS. Contrasting incidence of ras mutations in rat mammary and mouse skin tumors induced by anti-benzo[a]phenan-threne-3,4-diol-1,2-epoxide. Carcinogenesis. 1994;15:2113–2116. doi: 10.1093/carcin/15.10.2113. [DOI] [PubMed] [Google Scholar]
- 27.Batsch H, Hietanen E. The role of individual susceptibility in cancer burden related to environmental exposure. Environ Health Perspect. 1996;104:569–577. doi: 10.1289/ehp.96104s3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nebert DW, Petersen DD, Fornace AJ., Jr Cellular responses to oxidative stress: the Ah gene battery as a paradigm. Environ Health Perspect. 1990;88:13–25. doi: 10.1289/ehp.908813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakravarti D, Pelling JC, Cavalieri EI, Rogan EG. Relating aromatic hydrocarbon-induced DNA adducts and c-H-ras mutations in mouse skin papillomas: the role of apurinic sites. PNAS. 1995;92:10422–10426. doi: 10.1073/pnas.92.22.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanishi DT, Bowden GT, Cress AE. An analysis of DNA replication in synchronized CHO cells treated with benzo[a]pyrene diol epoxide. Biochim Biophys Ada. 1987;910:34–42. doi: 10.1016/0167-4781(87)90092-3. [DOI] [PubMed] [Google Scholar]
- 31.Black KA, McFarland RD, Grisham JW, Smith GJ. S-phase block and cell death in human lymphoblasts exposed to benzo[a]pyrene diol epoxide or N-acetoxy-2-acetylaminofluorene. Toxicol Appl Pharmacol. 1989;97:463–472. doi: 10.1016/0041-008x(89)90251-2. [DOI] [PubMed] [Google Scholar]
- 32.Cressman VL, Backlund DC, Hicks EM, Gowen LC, Godfrey V, Koller BH. Mammary tumor formation in p53- and BRCA-1-deficient mice. Cell Growth Differ. 1999;10:1–10. [PubMed] [Google Scholar]
- 33.Orren DK, Petersen LN, Bohr VA. Persistent DNA damage inhibits S-phase and G2 progression, and results in apoptosis. Mol Biol Cell. 1997;8:1129–1142. doi: 10.1091/mbc.8.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson PA, Clements P, Hudson K, Caldecott KW. A mitotic spindle requirement for DNA damage-induced apoptosis in Chinese hamster ovary cells. Cancer Res. 1999;59:2696–2700. [PubMed] [Google Scholar]
- 35.Koonin EV, Altschul SF, Bork P. BRCA-1 protein products: functional motifs. Nat Genet. 1996;13:266–268. doi: 10.1038/ng0796-266. [DOI] [PubMed] [Google Scholar]
- 36.Moore M, Wang X, Lu Y-F, Wormke M, Craig A, Gerlach JH, Burghardt R, Barhoumi R, Safe S. Benzo[a]pyrene-resistant MCF-7 human breast cancer cells. J Biol Chem. 1994;269:11751–11759. [PubMed] [Google Scholar]
- 37.Aprelikova ON, Fang BS, Meissner EG, Cotter S, Campbell M, Kuthiala A, Bessho M, Jensen RA. BRCA-1-associated growth arrest is RB-dependent. PNAS. 1999;96:11866–11871. doi: 10.1073/pnas.96.21.11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minamoto T, Mai M, Ronai Z. Environmental factors as regulators and effectors of multistep carcinogenesis. Carcinogenesis. 1999;20:519–527. doi: 10.1093/carcin/20.4.519. [DOI] [PubMed] [Google Scholar]
- 39.Kinzler KW, Vogelstein B. Cancer susceptibility genes: gatekeepers and caretakers. Nature. 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]