Abstract
Immunoglobulin T-cell receptors (IgTCRs) combine the specificity of antibodies with the potency of cellular killing by grafting antibody recognition domains onto TCR signaling chains. IgTCR-modified T cells are thus redirected to kill tumor cells based on their expression of intact antigen on cell surfaces, bypassing the normal mechanism of activation through TCR-peptide-major histocompatibility complex (MHC) recognition. Melanoma is one of the most immunoresponsive of human cancers and has served as a prototype for the development of a number of immunotherapies. The target antigen for this study is the ganglioside GD3, which is highly expressed on metastatic melanoma with only minor immunologic cross-reaction with normal tissues. To determine an optimal configuration for therapy, four combinations of IgTCRs were prepared and studied: sFv-ε, sFv-ζ, Fab-ε, Fab-ζ. These were expressed on the surface of human T cells by retroviral transduction. IgTCR successfully redirected T-cell effectors in an MHC-unrestricted manner, in this case against a non-T-dependent antigen, with specific binding, activation, and cytotoxicity against GD3+ melanoma cells. Soluble GD3 in concentrations up to 100 µg/ml did not interfere with recognition and binding of membrane-bound antigen. Based on the outcomes of these structural and functional tests, the sFv-ζ construct was selected for clinical development. These results demonstrate key features that emphasize the potential of anti-GD3 IgTCR-modified autologous T cells for melanoma therapies.
Keywords: T cell, gene therapy, immunotherapy, signalling
Introduction
The incidence of melanoma has increased faster than any other malignancy in the last decade. There is no standard treatment for advanced melanoma, which remains poorly responsive to chemotherapy and radiotherapy. However, melanoma is one of the most immunoresponsive of human cancers and has served as a prototype for the development of many different immunotherapies. Several studies have shown that melanoma-associated gangliosides are a suitable target for antibody therapies in melanoma patients [1,2]. Gangliosides are sialic-acid-containing glycosphingo-lipids that are located on the surface of animal cells, and are greatly increased in neuroectodermal tumors of humans and other species [3,4].
The capacity of melanoma cells to invade tissues and extracellular matrices is, in part, due to their repertoire of adhesion receptors [5,6]. GD3 has been localized in the plasma membrane and in focal adhesion plaques of human melanoma cells [4], suggesting a potential role for this ganglioside in melanoma cell attachment, an important functional property of metastatic tumor cells [7]. GD3 is expressed heavily on most melanoma cells, with more than 80% of melanomas having high expression of GD3 (106 to 107/cell) [8]. GD3 has low expression on retina pigment cells, central nervous system (CNS), and normal melanocytes. There is minimal expression of GD3 on other normal cells [9]. The fact that high-level expression of GD3 is restricted mainly to melanoma and that the majority of melanoma tumors express it, suggests that this molecule may serve as a useful target antigen for antibody-based immunotherapy.
Many clinical investigations have explored the potential of immune system components for the treatment of malignant disease. This strategy has been two-pronged, exploring the humoral immune system as represented by antibodies [10], and the cellular immune system as represented by lymphokine activated killer cells (LAKs) and tumor infiltrating lymphocytes (TILs) [11]. In general, antibodies have the capability to supply specificity, but often lack potency, whereas cellular effectors display potency but often lack specificity. Apart from select tumor types that have shown responses, neither approach separately has yet had an important general impact on the treatment of malignancy.
Recent studies have investigated the possibility of combining the specificity of antibodies with the homing, tissue penetration, and target-cell destruction of T cells. This is accomplished by grafting T cells with chimeric receptors consisting of the antigen recognition domains of an antibody joined to the signaling portion of the T-cell receptor (TCR) [12,13]. The latter mediates activation of T cells, whereas T-cell specificity is determined by the grafted antibody. The coupling of antibody with the TCR to redirect T cells was first demonstrated by Kuwana et al. in 1987 [14]. Since this time, many applications have evolved from this demonstration [15,16].
The TCR is composed of a disulfide-linked heterodimer (αβ, “Ti”) in association with a set of transmembrane proteins, collectively designated the CD3 complex [17]. The CD3 complex consists of the γ, δ, and ε chains and is associated with a homodimer of two ζ chains. Whereas the function of the Ti heterodimer is to recognize ligand (peptide/MHC), the function of the associated CD3 and ζ subunits is to couple the TCR to intracellular signal transduction mechanisms [18].
In the present study, we describe the construction of four anti-GD3 immunoglobulin TCR (IgTCR) genes and their functional expression in T cells. The four constructs differ in which antibody fragment is used (fragment antigen-binding antibody, Fab, versus single-chain fragment variable of antibody, sFv), and which signaling chain of the TCR is used (ζ or ε). To date, TCR-ζ has been used as standard linkage for the chimerization, and few studies examined the role of the ε-chain for the chimerization. The functions of the cytoplasmic domains of the CD3 chains appear to be similar, and the CD3 chains can induce signal transduction events independently of ζ [19]. The TCR ε-chain has potential advantages over ε-chain in that the ε-chain has a single Ig-like domain, providing a natural spacer/adaptor for the linkage. In contrast, the ζ chain optimally requires a “membrane spacer,” such as CD8α hinge, for the sFv attachment [20,21]. Moreover, ε has a long history as the principal target for T-cell activation through monoclonal antibodies (mAbs, e.g., OKT3) and bifunctional antibodies.
Fab ensures the preservation of affinity for antigen that may frequently be lost in the sFv, and Fab may be directly coupled without a spacer to either ζ or ε. Further, prior studies often modified only the CD8 effector cells [22,23], and anti-tumor efficacy was dependent on co-administration of interleukin 2 (IL2). We employ CD4+ as well as CD8+ T cells to engage the collaborative functions between these two effector arms of the T-cell system. To accomplish efficient and stable gene delivery to T cells, retroviral transduction systems were employed. These anti-GD3 IgTCR-modified T cells recognized antigen and bound specifically to tumor cells in a non-MHC-restricted manner through antibody-type specificity, and transmitted transmembrane signals for T-cell activation and cytotoxicity. The present report extends our prior results with a carcinoem-bryonic antigen (CEA)-specific IgTCR [24], adapting this technology to a new and clinically important non-T-dependent antigen.
Materials and Methods
Cell Lines and Antibodies
Human melanoma cell lines M21 (GD3+) and M24 (GD3-) were provided by Dr. G. Gammon (John Wayne Cancer Institute, Santa Monica, CA). The human leukemic T-cell line, Jurkat, was purchased from ATCC. These cell lines were grown in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 2 mM l-glutamine, penicillin (100 units/ml), streptomycin (100 µg/ml), and 10% fetal calf serum (FCS; Gibco) and maintained at 37°C in 5% CO2. The amphotropic packaging cell lines Bing, Phoenix, and 293-GPG were provided by Dr. W. S. Pear (MIT), Dr. G. P. Nolan (Stanford University Medical Center), and Dr. R. Mulligan (Children's Hospital, Boston, MA), respectively. All packaging cell lines were grown in DMEM (Gibco) supplemented with 2 mM l-glutamine, penicillin (100 units/ml), streptomycin (100 µg/ml), and 10% FCS and maintained at 37°C in 5% CO2.
Chimeric MB3.6 is an anti-GD3 mAb, and was provided by Dr. R. A. Reisfeld (Scripps Research Institute). Murine anti-human ζ chain antibody was a gift from Dr. S. Schlossman (Dana Farber Cancer Institute). Rabbit anti-ε antibody was purchased from Dako, Glostrup, Denmark.
Anti-Idiotype Antibody (Anti-Id) to MB.3.6
Anti-Id rat mAb to MB3.6 (V66) was initially provided by Dr. S. Ferrone (NYMC, Valhalla), but this antibody subsequently became unavailable to this project. In the following, we describe the preparation of new anti-Id antibodies to replace V66.
Preparation of immunogen The anti-Id needed to recognize both sFv and Fab forms of MB3.6. Thus, we immunized with sFv and screened and/or purified with Fab. Although the sFv is of murine origin, we postulated that coupling of the mouse antibody to a potent bacterial immunogen might stimulate a murine anti-mouse idiotype response. We previously prepared an MB3.6 sFv-PE40 immunotoxin (Yun et al., in preparation), but it would be lethal to animals at appropriate immunizing doses. Instead, we obtained a mutated PE40 (PE40mut; R. Kreitman, NCI/NIH) that lacked the enzymatic activity of the toxin, and prepared and expressed MB3.6 sFv-PE40mut protein. This was our immunogen.
Production of anti-Id antibody against MB3.6 in rabbit Rabbits received five injections of immunogen (100 µg/injection) at intervals of at least 3 weeks over the course of 3 months, with a phased immunization and adjuvant scheme. Titer was assayed by ELISA (below). Anti-Id was purified from serum by affinity chromatography against intact MB3.6 antibody immobilized on an Affigel (Biorad), and reactivity reconfirmed by ELISA (below).
Production of mouse anti-Id against MB3.6 Mice were immunized with immunogen in a standard scheme, with spleen harvest, SP2/0 fusion and HAT selection, and colonies screened for anti-Id reactivity by ELISA. Positive clones were identified by ELISA, expanded, purified, and tested against immobilized MB3.6 sFv or intact antibody, and then against MB3.6 IgTCR transduced cells. Clone “B10” was selected for development and preparation of quantities of anti-Id. Expressing, binding, and activation assays using B10 were equivalent to the original V66 anti-Id antibody (S. Ferrone), and IgTCR studies were then resumed.
ELISA assay A human-mouse chimeric version of MB3.6 (chMB3.6) was used for plate coating, and detection of bound rabbit or mouse anti-Id was obtained by goat anti-(rabbit Fc) or goat anti-(mouse Fc) conjugated with alkaline phosphatase, and developed with p-nitrophenyl phosphate substrate by standard methods.
Construction of Anti-GD3 IgTCRs
Human ζ chain, ε chain, and CD8α hinge were previously cloned in p2.1 mammalian expression vector [24]. For construction of the sFv, the variable heavy chain (VH) and variable light chain (VL) immunoglobulin cDNA sequences of the mAb MB3.6 were amplified by RT-PCR by following immunoglobulin-specific primers (restriction sites are underlined). VH forward: 5′-GGCCCTGCAGGCCGGCTCTGGTGGCTCAGGATCGGAAGTGGTGGTGGTGGAGTC-3′ incorporates PsfI and NaeI sites, VL forward; 5′-CCGCTCTAGACTCGACCATGGTTTTCACACCTCAGA-3′ incorporates an XbaI site, VH reverse: 5′-CGCCCAAGCTTTAGTACTGGAGACGGTGACCG-3′ incorporates HindIII and ScaI sites. VL reverse: 5′-CGCGAGCGCTTCCACCCGATCCTGAGCCACCTTTGATCTCCAGCTTTGTCC-3′ incorporates a Eco47III site. The single-chain linker (a 15 amino acid long bridge) was designed to be (GGSGS)3, and assembled by PCR. VH was first amplified using VH forward and reverse primers, and subsequently cloned into pUC19 using PstI and HindIII (pUC-VH). VL was amplified using VL forward and reverse primers, and subcloned into pUC-VH using XbaI and NaeI/Eco47III, resulting in pUC-sFv. The correct structure was verified by sequencing. The sFv (750 bp) was excised by the XbaI and ScaI restriction sites, respectively, and ligated into the XbaI-SmaI and XbaI-Eco47III sites of p2.1-ε and p2.1-CD8αhinge-ζ, thereby generating completed sFv-ε and sFv-ζ constructs.
For construction of the Fab-TCR, separate plasmids were generated for the light chain (L) and the heavy chain (H)-TCR. For the H-TCR, the Cγ1 hinge fragment with mutated Cys 230 and Cys 239 to Ala, was first subcloned to p2.1-ε/ζ construct after annealing the following complementary synthetic DNA fragments, incorporating a BamH1 site. 5′-CTAGAAAAGGATCCCAAATCTTGTGACAAAACTCACACAGCCCCACCGGCCCCA-3′ and 5′-TGGGGCCGGTGGGGCTGTGTGAGTTTTGTCACAAGATTTGGGATCCTTTT-3′ were annealed and ligated into the p2.1-ε and p2.1-ζ by the XbaI-SmaI sites and XbaI and Klenow filled BamHI, to get p2.1-Cγhinge-ε and p2.1-Cγhinge-ζ, respectively. A DNA fragment encoding VHCγ was amplified by RT-PCR from chMB3.6 transfectoma cells using the following primers, VH forward (see above) and Cγ1 reverse, 5′-CCCCCCGTTAACTCTCTTGTCCACCTTG-3′, incorporating HpaI site. The PCR product was then ligated into the XbaI and HpaI/XhoI sites of p2.1-Cγhinge-ε and p2.1-Cγhinge-ζ vectors, respectively, in frame to the DNA encoding the MB3.6-VHCγ.
For the L-TCR construct, the expression vector pBSpGKneo was constructed by subcloning a 1.6-kb Xho/ClaI Klenow filled-in DNA fragment encoding the pgk promoter and neoR gene from pEFPGKneo (gift of Dr. S. Orkin) to the EcoRV site of pBlueScript SK+ (Stratagene, La Jolla, CA). The DNA fragment encoding chMB3.6VLCκ was amplified using VL forward (see above), and Cκ reverse; 5′-GGGGGGCTCGAGCTAACACTCTCCCCTGTTG-3′, incorporating an XhoI site. The amplified VLCκ product was then subcloned into p2.1 vector using XbaI-XhoI sites, making a p2.1VLCκ construct. The resulting SalI cassette of SRαVLCκ was then excised from p2.1VLCκ, filled using Klenow fragment, and subsequently subcloned into the HincII site of PBSpGKneo, generating a pBSMBLpGKneo vector. The sequences of all PCR-amplified DNA fragments were verified by sequence analysis (Sequenase, Amersham, Arlington Heights, IL).
Generation of Jurkat Cells Stably Expressing L-chain
For the Fab-IgTCR constructs, the H- and L-chains were co-expressed in the transfectant. First, DNA (50–100 µg) encoding the light chain, pBSMBLpGKneo, was transfected into 2x107 Jurkat cells by electroporation (one pulse, 275 V, 9400 µF) using a gene pulse electroporator (Bio-Rad, Munich, Germany). After 48 hours, cells were selected with G418 (2 mg/ml) for 7 days, and then expanded to cell lines in 1 mg/ml of G418. L-chain expression was confirmed by Western blot of culture supernatants using anti-human κ light chain antibody. Stable L-chain-expressing transfectants were then used for H-chain transfer (H-ε/ζ) by using retroviral transduction to make a Fab-IgTCR-expressing Jurkat cell line.
Retroviral Transduction
For gene transfer into Jurkat CD4+ human T cells, the SalI-XhoI fragment from SRα-H-TCR cassette and SRαsFv-TCR cassette were subsequently subcloned into the SalI site of the retroviral vector pBabepuro (gift of Dr. W. Pear), generating a pBabepuro-H-TCR and a pBabepurosFv. Vector DNA was transfected into a helper-free, amphotropic packaging cell lines, Bing, Phoenix, and 293-GPG using Ca2+-phosphate transfection method in the presence of 25 µM chloroquine. Culture supernatants from these cells were used for Jurkat cell transduction using standard methods [25]. After transduction, IgTCR-expressing Jurkat cells were selected in puromycin (0.8 µg/ml), and the viability and number of transduced Jurkat cells were determined by trypan blue exclusion. For transduction of normal human T cells, the sFv-ζ cassette was subcloned into the MFG retroviral vector without SRα, employing only the MFG LTR for promoter activity.
Flow Cytometry
Expression of IgTCR on the surface of transduced cells was evaluated by immunofluorescence staining using either an anti-κ antibody (Caltag, Burlingame, CA) against the light chain of human Ig for Fab-TCR or anti-Id against MB3.6 (all constructs). Secondary antibodies used were fluorescein isothiocyanate (FITC) and phycoerythrin (PE)-labeled anti-mouse/rabbit antibody or anti-rabbit/goat antibody (Caltag). Antibody staining was quantified using an EPICS II Coulter flow cytometer.
Conjugation Assay
GD3+ (M21) and GD3- (M24) melanoma target cells and transduced and non-transduced Jurkat effector cells were incubated together and binding was assayed using two-color fluorescence microsopy [24,26]. Melanoma cells stained with hydroethidine and Jurkat cells stained with rhodamin 123 were co-incubated for 1 hour at 37°C. Unbound Jurkat cells were gently washed away, and the remaining cells observed under optical or UV fluorescence microscope. In some experiments, GD3 (Calbiochem, La Jolla, CA) was added to 100 µg/ml to target cells before adding Jurkat effector cells.
IL2 Assay
Transduced, or non-transduced Jurkat cells were stimulated at 1x105 cells/ml in microtiter wells with various target cells and antigens for 24 hours and tested for IL2 as previously described [24].
Cytotoxicity Assay
Cytotoxicity assays were performed as previously described [24,27]. M21 and M24 melanoma cells were seeded separately in six-well culture plates (1x105 per well), and then allowed 24 hours to attach and begin proliferating. Human peripheral blood mononuclear cells were isolated from healthy donors by Ficoll density gradient, and transduced with the anti-GD3 IgTCR retroviral vector and then added at different effector cytotoxic T lymphocyte (CTL) to target (E:T) ratios, ranging from 0.2:1 to 2:1. Every 24 hours, wells were harvested and counted for viable tumor cells by trypan blue exclusion. Both M21 and M24 melanoma cell lines are approximately five-fold larger than human T cells and thus readily distinguished from remaining T cells.
Results
Structure of Anti-GD3 IgTCRs: Fab-ε/ζ and sFv-ε/ζ
To endow T cells with antibody-type recognition, we constructed GD3-specific IgTCRs by fusing the antigen binding domain of the MB3.6 antibody (sFv and Fab) to either the ε or ζ signaling chains of the human TCR. Most IgTCR constructs have employed antibody sFv fragments, but the sFv form may often lose affinity. All antibodies to gangliosides, which are non-T-dependent antigens [28,29], are of low affinity, and any further reduction of affinity could destroy the ability of the IgTCR-modified T cells to recognize and bind to the melanoma cells. Therefore, we also generated Fab constructs to ensure preservation of at least the native affinity to GD3. To minimize antigenicity from murine constant (C) regions, we employed a Fab construct with human C domains. Four IgTCR variants were designed, constructed and tested (Figure 1). For all constructs, the entire TCRε and TCRζ cytoplasmic, transmembrane and extracellular domains (ECD) were preserved in the fusion products, except for a shortening of the ECD of ζ from 9 to 3 amino acids.
Figure 1.
Schematic representation of antibody (Ig), TCR complex, and IgTCR structures. The TCR complex is comprised of six proteins. The structure of the αβ receptor chains is analogous to the Fab portion of an antibody with variable (V) and constant (C) domains. TCR chains γ, δ and ε also have immunoglobulin-like domains. sFv-ζ, sFv-ε, Fab-ζ, and Fab-ε are shown within the complete IgTCR complex. Boxes in the cytoplasmic domains represent signaling motifs of all chains except α and β, which lack them.
To express Fab-TCR chimeras, two separate vectors were constructed, one containing the VH-CH1-TCR (HTCR), and another the VL-CL. To engineer the H-TCR, a natural human IgG heavy chain γ1 hinge region (14 amino acids) was inserted to join the CH1 domain of the Fab heavy chain to ζ chain, resulting in an arrangement of H-γ1 hinge-ζ, in which the two inter-heavy chain cysteines were removed, retaining only the one cysteine involved in L chain bonding [24]. For the expression of Fab-ε/ζ IgTCRs, HTCR and L chain encoding vectors were co-expressed.
The sFv constructs followed a 5′-VL-linker-VH-3′ design [30,31]. The VL and VH were joined together by a short artificial linker, (GGSGS)3, modified from the canonical (GGGGS)3 linker for improved hydration and flexibility. Following previous work [15], we inserted a CD8α hinge spacer between the sFv and ζ chain in the sFv-ζ construct (sFv-CD8αh-ζ) to improve projection of the sFv away from the T-cell membrane and improve antigen engagement, and thereby compensate for the short ECD of ζ (above). For sFv-ε, we postulated that the Ig-like ECD of ε (Figure 1) would serve this spacer function without an added hinge, and that the CH1-CL domains would supply this “spacer function” in Fab-ζ of Fab-ε constructs alike.
The optimal context for initiation of translation in the vicinity of the AUG codon has been shown as GCC (A/G) CCATGG, with a purine (preferably A) in position -3 and a G in position +4 having the strongest effects on modulating translation (at least 10-fold) [32]. However, the natural leaders for the MB3.6 VH chain for Fab, and VL for sFv structure did not contain this optimal Kozak sequence, but instead contained sequences of ACCATGA and CATATGG, respectively. Therefore, the sequences of Fab and sFv constructs were re-engineered to incorporate optimized Kozak sequences.
Expression of Anti-GD3 IgTCRs in 293 Cells
The human embryonic kidney cell line 293, was transiently transfected with expression vectors, p2.1-sFv-ε/ζ, or p2.1-H-ε/ζ with p2.1-VLCκ. These vectors contain the SRα promoter inserted 5′ to the immunoglobulin sequences. Expression of the chimeric polypeptide chains was analyzed in whole cell lysates by Western blot analysis using the anti-ε and anti-ζ mAb. All four chimeras were readily detected, and distinguished from endogenous ε and ζ by size (data not shown). Surface expression of IgTCR on the transfected 293 cells was tested by staining cells with anti-Id antibody to the MB3.6 antibody, and assaying by flow cytometry (Figure 2). Expression was detected on Fab/sFv-ζ transfected cells, but not on the Fab/sFv-ε transfectants. The endoplasmic reticulum (ER) retention signal in ε-chain prevents surface transport and expression except in the context of the other chains of the TCR, which are absent in the 293 cells [33–35].
Figure 2.
Surface expression of IgTCR on transiently transduced 293. Cells were assayed by flow cytometry with staining anti-Id antibody against MB3.6 (solid line) or with negative control antibody (dotted line).
Expression of Anti-GD3 IgTCRs in Human T Cells
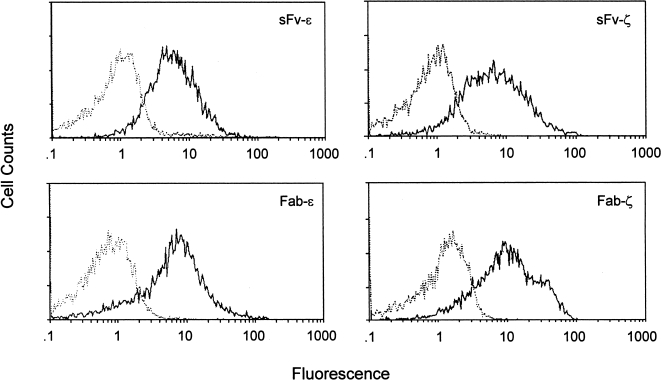
The Jurkat human T-cell line was transduced with Fab-ε/ζ and sFv-ε/ζ. All four IgTCRs were expressed on the surface of the cells, with expression in approximately 70–80% of the transduced cells (Figure 3). In contrast to the negative surface expression of constructs in 293 cells (Figure 2), both Fab/sFv-ε were well expressed on the surface of T cells, as anticipated in the context of all normal TCR chains (Figure 3).
Figure 3.
Surface expression of MB3.6 IgTCR on stably transduced Jurkat cells. Cells were stained as in Figure 2.
IgTCR as Functional Receptors: Redirection of T-Cell Recognition
The specific binding of IgTCR-modified T cells to ligand (antigen or anti-Id antibody) was assayed visually by light microscopy. T cells were incubated on plates coated with either nonspecific antibody (UPC) or anti-Id. IgTCR-modified T cells adhered only to anti-Id coated plates (Figure 4), whereas unmodified cells were nonadherent. Similar results were obtained with the other three constructs (data not shown).
Figure 4.
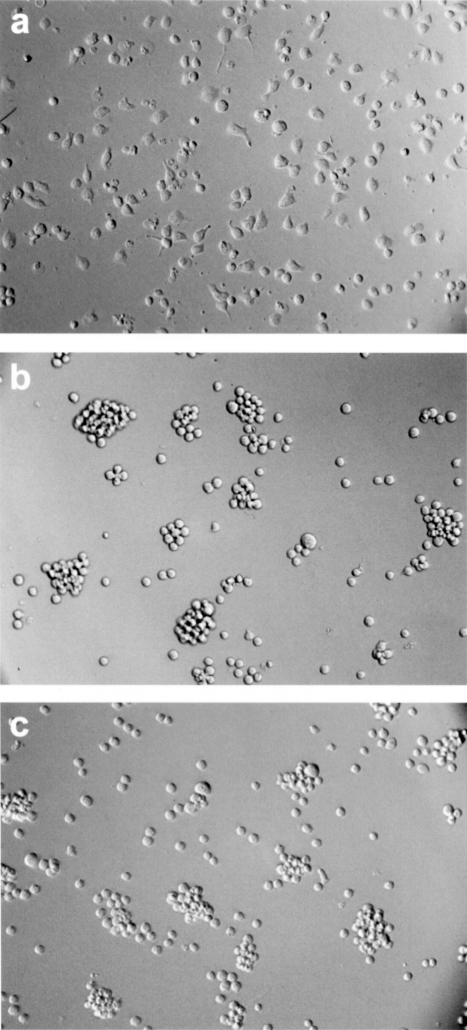
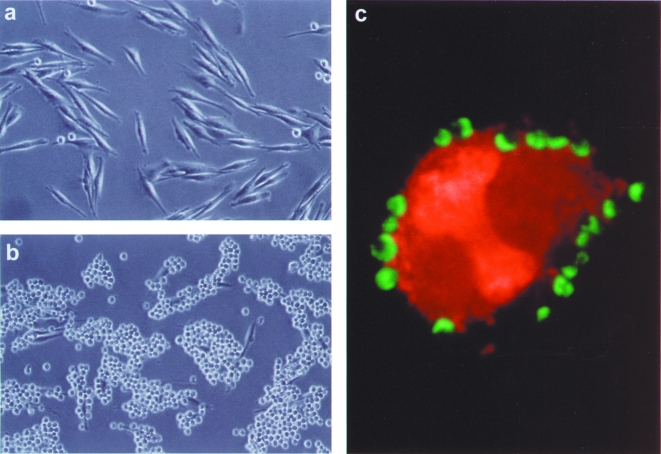
Recognition-mediated adhesion of IgTCR+ T cells. Modified or nonmodified Jurkat T cells were incubated on plates coated with either anti-Id or nonspecific antibody (UPC). Anti-GD3 IgTCR modified T cells bind immobilized anti-Id tightly, inducing cells to flatten on the plate surface (a). On plates coated with UPC, cells did not adhere, and kept their normal round morphology (b). Similarly, no binding occurred with nonmodified Jurkat cells on anti-Id-coated plates (c).
A prerequisite of T-cell-dependent killing is the formation of conjugates between the effector and the target cells, which is mediated by the specificity of the antibody portion of the IgTCR. We examined the ability of IgTCR-transduced T cells to bind specifically to GD3+ and GD3- melanoma cells. Nonmodified T cells showed no target cell binding, whereas IgTCR-modified T cells bound multiply to GD3+ M21 tumor cells (Figure 5). When incubated with GD3- M24 tumor cells, neither IgTCR+ T cells nor unmodified T cells showed binding (data not shown). Similar results were obtained with T cells expressing all four IgTCR constructs.
Figure 5.
Cell conjugation of IgTCR+ T cells with GD3+ melanoma cells. Noncytotoxic Jurkat T cell effectors were used to avoid M21 target cell lysis during the binding assay. After co-incubation of nonadherent T-cell effectors and adherent tumor cell targets on plates, unbound T cells were removed by washing. Nonmodified T cells were nearly completely washed away, showing only the tumor cells left in plate (a). However, IgTCR+ modified T cells were tightly bound to M21 (b). In panel (c), fluorescence staining was employed. A single giant melanoma tumor cell target (orange; apparently in mitosis) is bound by 19 effector T cells (green).
Soluble GD3 exists in plasma at concentrations of 1–10 µg/ml in melanoma patients [36], that could potentially bind to the IgTCR and block tumor cell recognition and binding. The addition of soluble GD3 at 100 µg/ml did not inhibit GD3+ M21 tumor cell binding by IgTCR modified T cells (not shown), providing reassurance that the modified cells will function in vivo.
IgTCR as Functional Receptors: T-Cell Activation
To test whether chimeric IgTCR could function as active signalling receptor molecules, we analyzed the ability of transduced Jurkat cells to undergo antigen-specific stimulation. IL2 secretion by Jurkat is the most commonly used criterion to assess human T-cell activation. In this assay, TCR crosslinking by the target antigen in the presence of phorbal ester leads to IL2 release. TCRε and ζ signalling chains, with their respective immunoreceptor tyrosine-based activation motifs (ITAMs), are each known to function efficiently for T-cell activation [37–39]. Although differences in potency have been noted when ε and ζ are studied as isolated chains, they appear to be equivalent in the context of the full TCR complex on T cells [24].
In preliminary tests, all constructs were able to activate T cells as determined by IL2 secretion. In pairwise comparisons with other constructs, the sFv-ζ chimera was at least as good as other IgTCR in signaling potency and antigen selectivity. Structurally, the sFv design is advantageous over Fab for its simpler expression requirements. Further, the more extended CD8α-hinge-ζ configuration was previously shown to have steric advantages over sFv-ε for cellular antigen binding [24]. Accordingly, we focused on the sFv-ζ version for detailed examination (Figure 6) in anticipation of eventual clinical development.
Figure 6.
IL2 secretion of IgTCR transduced cells after stimulation with immobilized proteins and melanoma cell lines. Unmodified and sFv-ζ modified Jurkat T cells were coincubated with various stimuli, and the supernatants were harvested and tested for IL2 concentration. Stimulators were: UPC, nonspecific antibody; OKT3, anti-CD3 antibody; anti-Id, anti-idiotypic antibody to MB3.6; M21, GD3+ melanoma cell line; M24, GD3- cell line. IL2 levels were standardized to OKT3-stimulated secretion for each group (= 100) to control for variation in cell numbers and cell condition, with a range of 400–1200 IU/ml in different assays. The use of no stimulator or bovine serum albumin (BSA) gave no IL2 secretion (not shown) that was equivalent to UPC. The SE was ±20% in quadruplicate assays.
Control, nontransduced Jurkat T cells secreted IL2 only when treated with ionomycin, an ionophore that induces calcium flux and nonspecific activation, and with immobilized OKT3, which engages the native TCR (Figure 6). Nonspecific antibody, and tumor cells, whether GD3+ or GD3-, had no stimulatory effect. By comparison, the sFv-ζ transduced T cells were similarly activated with OKT3 and ionomycin, but they were additionally activated with immobilized ligand to IgTCR (anti-Id), that had no effect on unmodified Jurkat cells. Most importantly, cellular GD3 antigen on M21 tumor cells induced strong IL2 secretion, equivalent to that obtained with immobilized OKT3. The GD3- M24 cell line did not activate the transduced T cells, demonstrating the specificity of the IgTCR-GD3 interaction for GD3-expressing cells. These results show that activation through IgTCR occurs in a highly selective and antigen-specific manner that depends entirely on GD3 expression on target tumor cells.
IgTCR as Functional Receptors: T-Cell Cytotoxicity
An important functional criterion for the chimeric receptor is that it must redirect CTLs to mediate GD3 specific target cell lysis. For these analyses, we modified normal human T cells, since Jurkat is a CD4+ line without cytotoxic activity. The vector used for these studies was developed for clinical use and does not contain a prokaryotic drug selection marker. To obtain a high frequency of transduced cells, we applied recently developed techniques for high-efficiency transduction without drug selection to introduce IgTCR into T cells (Figure 7) (EJB and RPJ, unpublished results). We previously showed that expression of an IgTCR gene using this vector was stable for at least three months, and that retroviral transduction of IgTCR into human primary T cells infects CD4 and CD8 cells with equal efficiency [24,27].
Figure 7.
IgTCR expression on primary T lymphocytes. T lymphocytes were purified from PBMCs, transduced with the sFv-ζ IgTCR encoding retroviral vector, and analyzed for surface expression with anti-Id (IgTCR+) and negative control antibody, as indicated.
As an in vitro model of human tumor therapies, IgTCR-modified normal human T cells were incubated with GD3+ tumor cell targets at varying CTL-to-target cell ratios and surviving tumor cells were counted daily by trypan blue exclusion (Figure 8). Previous studies [27] have shown that this method of measuring tumor cell killing has a number of advantages over the commonly applied 51Cr-release assay. First, cell counting directly measures the actual number of viable tumor cells, whereas 51Cr-release is only an indirect measure of killing. Second, the statistics inherent to 51Cr assays [40] do not allow assessment of multilog changes in surviving cell numbers as permitted by direct counting. Third, the long time intervals necessary to show T-cell recycling and multiple tumor-cell killing per T cell are incompatible with the high spontaneous release rates of 51CrO4 by these cell lines [40]. With direct cell counting, lower effector-to-target cell ratios and longer times can be employed that are more reflective of perceived in vivo applications, hence providing a more sensitive and appropriate assay for the objectives of this study.
Figure 8.
Cytotoxicity of IgTCR+ CTLs against GD3+ melanoma cells. GD3+ M21 tumor targets were seeded at 2x105 cells per well (120 mm2) in six-well tissue culture plates, and then anti-GD3 IgTCR+ CD8+ CTLs were added at different E:T ratios, ranging from 0.2:1 to 2:1. 0:1 indicates growth of tumor cells without added effector CTLs. Similar results were obtained from three independent experiments.
Transduced CD8+ T cells killed GD3+ M21 cells in a dose-dependent fashion, with specific target lysis at the lowest CTL:target cell ratios tested (0.2:1) (Figure 8). At this lowest ratio, there is a five-fold excess of tumor cells, yet they are >99% eliminated after 4 days, thereby demonstrating recycling and repeated tumor-cell killing per single T cell. (The T-cell numbers are approximately constant over time in this experimental setting [41].) No significant cytolytic activity was observed when sFv-ζ modified T cells were co-incubated with GD3- M24 cells (not shown). Similarly, no lysis of GD3+ M21 cells was observed with unmodified, mock-transduced effector T cells, or by IgTCR-modified T cells specific to CEA, an irrelevant antigen not expressed on M21 (not shown). This cytolytic response was thus highly potent and specific for GD3+ tumor cells. We previously showed that IgTCR-directed cytotoxic potency was stable on serial assays of transduced T cells over at least 2 months in culture, the maximum period after transduction that this test was examined [24,27].
Discussion
In this study we report the construction and expression of four different IgTCRs on human T cells as an antigen-specific receptor. These IgTCRs endowed T cells with antibody-type specificity in an MHC-independent fashion, that permitted GD3-dependent tumor cell recognition and binding, resulting in T-cell activation and target cell lysis in a sensitive and specific manner.
The Selection of Tumor Antigen: GD3
Gangliosides are polymorphic glycolipids found on the surface membranes of most cell types [42] and, as a rule, are weakly immunogenic. The major antigenic determinants of gangliosides are carbohydrates, which typically generate T-cell-independent humoral immune responses. GD3 is a ganglioside that is abundantly expressed on most human melanoma cells and other neuroectodermal tumors, with minor expression on normal tissues [3,43,44]. GD3 has received attention as a potential target for active and passive immunotherapy of human melanoma and other neuroectoderm-derived tumors [4,45]. Antibody-based therapies against this antigen have shown modest clinical efficacy with selective targeting in patients with advanced melanomas without significant toxicity [46–48]. GD3 is thus a suitable antigen for targeting melanoma by IgTCR-modified T cells.
IgTCR as Functional Receptor
All IgTCR showed binding through the anti-GD3 chimeric receptor. However, not all constructs are equally practical from a therapeutic standpoint. The sFv-TCR are easier to apply clinically than the Fab constructs, because they permit expression of only one gene instead of a pair of genes as for Fab-TCR, simplifying both vector construction and the gene-transfer methods required. Our major concern about committing to the sFv in advance of a systematic development and testing was the potential loss of affinity in the sFv [49] that could abrogate IgTCR binding and activation. However, the sFv fragment designed for this study retained adequate binding activity against the natural cell-associated GD3 molecule to confer GD3 specific recognition and tumor-cell binding. This confirmed that the sFv molecule was properly folded and retains its activity when expressed on the membrane surface, and justified our subsequent focus on the sFv-ζ for the remainder of our preclinical evaluation. Further, specific binding was not abrogated by very high levels of soluble GD3, providing assurance that IgTCR-i-T cells will continue to recognize GD3+ cells in the tumor bed that may also be shedding antigen. This parallels results of ourselves [24] and others [50] with analogous CEA-specific IgTCR that was not inhibited by soluble CEA, explained as the affinity enhancement of multivalent intercellular binding that is not effectively overcome by high levels of monovalent soluble ligand [24,51].
To evaluate the signalling and activation-dependent functions of the anti-GD3 IgTCR in human T cells, we examined their ability to trigger IL2 secretion and cytolytic effector functions following successful antigen recognition. When GD3-specific IgTCR was introduced into a human CD4+ T-cell line, cells were activated and secreted IL2 at high levels on contact with GD3+ melanoma cells. When introduced into normal human CD8+ T cells, the IgTCR-bearing cells were efficiently and specifically redirected to kill GD3-expressing cells in vitro. This result demonstrates that primary human T cells can be stably modified to recognize and bind to tumor cells, and mediate target-cell destruction.
The Feasibility and Potential of IgTCR Strategy for Adoptive Immunotherapy
The increasing availability and fine specificity of antibodies to surface markers present on malignant cells enable the development of chimeric molecules with great practical potential for tumor adoptive immunotherapy. These chimeras can be used for many different tumors, such as breast, colon, prostate, brain, liver, and lung cancer, by using appropriate antibodies directed against the targeted tumor-associated antigen. The chimeric IgTCR receptors can confer antibody type specificity on T lymphocytes toward any predefined antigen, homing to their targets, with specific recognition, activation, and execution of cytotoxic functions on encountering the antigen at the tumor target site. The present study extends these concepts to include ganglioside antigens, which cannot normally be presented to T cells.
In this study, we have shown that human T cells expressing IgTCR genes interact specifically with GD3+ target cells and undergo activation as measured by IL2 secretion and antigen-specific tumor cell killing. These data demonstrate the functional expression of a continuous polypeptide that possesses both antigen-binding and signal-transducing properties. These results substantiate and extend our previous studies using chimeric IgTCR receptors against human CEA [24], and continue the advancement of gene modified T-cell strategies in adoptive immunotherapies [52]. The anti-GD3 sFv-ζ IgTCR is currently under development for use in human clinical trials as a new type of anti-melanoma immuno-gene therapy.
Abbreviations
- TCR
T-cell receptor
- IgTCR
immunoglobulin TCR
- Fv
fragment variable of antibody
- sFv
single-chain Fv
- Fab
fragment antigen-binding of antibody
- V
variable
- VH and VL
heavy and light variable
- C
constant
- IL2
interleukin 2
- MHC
major histocompatibility complex
- CTL
cytotoxic T lymphocyte
- mAb
monoclonal antibody
- BSA
bovine serum albumin
- ER
endoplasmic reticulum
- ITAM
immunoreceptor tyrosine-based activation motif
- anti-Id
anti-idiotype antibody
References
- 1.Chapman PB, Lonberg M, Houghton AN. Light chain variants of an IgG3 anti-GD3 monoclonal antibody and the relationship among avidity, effector functions, tumor targeting, and antitumor activity. Cancer Res. 1990;50:1503–1509. [PubMed] [Google Scholar]
- 2.Portoukalian J, Carrel S, Dore JF, Rumke P. Humoral immune response in disease-free advanced melanoma patients after vaccination with melanoma-associated gangliosides. EORTC Cooperative Melanoma Group. Int J Cancer. 1991;49:893–899. doi: 10.1002/ijc.2910490616. [DOI] [PubMed] [Google Scholar]
- 3.Pukel CS, Lloyd KO, Travassos LR, Dippold WG, Oettgen HF, Old LJ. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982;155:1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheresh DA, Honsik CJ, Staffileno LK, Jung G, Reisfeld RA. Disialoganglioside GD3 on human melanoma serves as a relevant target antigen for monoclonal antibody-mediated tumor cytolysis. Proc Natl Acad Sci USA. 1985;82:5155–5159. doi: 10.1073/pnas.82.15.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheresh DA. Human melanoma cell attachment involves an Arg-Gly-Asp-directed adhesion receptor and the disialoganglioside GD2. Prog Clin Biol Res. 1989;288:3–24. [PubMed] [Google Scholar]
- 6.Cheresh DA. Structure, function and biological properties of integrin alpha v beta 3 on human melanoma cells. Cancer Metastasis Rev. 1991;10:3–10. doi: 10.1007/BF00046839. [DOI] [PubMed] [Google Scholar]
- 7.Dippold WG, Dienes HP, Knuth A, Meyer zum Buschenfelde KH. Immunohistochemical localization of ganglioside GD3 in human malignant melanoma, epithelial tumors, and normal tissues. Cancer Res. 1985;45:3699–3705. [PubMed] [Google Scholar]
- 8.Ravindranath MH, Tsuchida T, Morton DL, Irie RF. Ganglioside GM3:GD3 ratio as an index for the management of melanoma. Cancer. 1991;67:3029–3035. doi: 10.1002/1097-0142(19910615)67:12<3029::aid-cncr2820671217>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Urmacher C, Cordon-Cardo C, Houghton AN. Tissue distribution of GD3 ganglioside detected by mouse monoclonal antibody R24. Am J Dermatopathol. 1989;11:577–581. doi: 10.1097/00000372-198912000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Junghans R, Sgouros G, Scheinberg D. Antibody-based immunotherapies for cancer. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott, PA: 1996. pp. 655–689. [Google Scholar]
- 11.Rosenberg S. Principles and applications of biologic therapy. In: DeVita TJ, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. V. Lippincott, PA: 1993. pp. 293–324. [Google Scholar]
- 12.Hwu P, Shafer GE, Treisman J, Schindler DG, Gross G, Cowherd R, Rosenberg SA, Eshhar Z. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993;178:361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stancovski I, Schindler DG, Waks T, Yarden Y, Sela M, Eshhar Z. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol. 1993;151:6577–6582. [PubMed] [Google Scholar]
- 14.Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, Nagase F, Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 15.Moritz D, Groner B. A spacer region between the single chain antibody- and the CD3 zeta-chain domain of chimeric T cell receptor components is required for efficient ligand binding and signaling activity. Gene Ther. 1995;2:539–546. [PubMed] [Google Scholar]
- 16.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan AC, Irving BA, Weiss A. New insights into T-cell antigen receptor structure and signal transduction. Curr Opin Immunol. 1992;4:246–251. doi: 10.1016/0952-7915(92)90072-m. [DOI] [PubMed] [Google Scholar]
- 18.Kronenberg M, Siu G, Hood LE, Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- 19.Aoe T, Goto S, Ohno H, Saito T. Different cytoplasmic structure of the CD3 zeta family dimer modulates the activation signal and function of T cells. Int Immunol. 1994;6:1671–1679. doi: 10.1093/intimm/6.11.1671. [DOI] [PubMed] [Google Scholar]
- 20.Newton DL, Xue Y, Olson KA, Fett JW, Rybak SM. Angiogenin single-chain immunofusions: influence of peptide linkers and spacers between fusion protein domains. Biochemistry. 1996;35:545–553. doi: 10.1021/bi951650w. [DOI] [PubMed] [Google Scholar]
- 21.Fitzer-Attas CJ, Schindler DG, Waks T, Eshhar Z. Harnessing Syk family tyrosine kinases as signaling domains for chimeric single chain of the variable domain receptors: optimal design for T cell activation. J Immunol. 1998;160:145–154. [PubMed] [Google Scholar]
- 22.Roberts MR, Qin L, Zhang D, Smith DH, Tran AC, Dull TJ, Groopman JE, Capon DJ, Byrn RA, Finer MH. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood. 1994;84:2878–2889. [PubMed] [Google Scholar]
- 23.Bitton N, Gorochov G, Debre P, Eshhar Z. Gene therapy approaches to HIV-infection: immunological strategies: use of T bodies and universal receptors to redirect cytolytic T-cells. Front Biosci. 1999;4:D386–D393. doi: 10.2741/bitton. [DOI] [PubMed] [Google Scholar]
- 24.Nolan KF, Yun CO, Akamatsu Y, Murphy JC, Leung SO, Beecham EJ, Junghans RP. Bypassing immunization: Optimized design of “designer T cells” against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin Cancer Res. 1999;5:3928–3941. [PubMed] [Google Scholar]
- 25.Kotani H, Newton PB, 3rd, Zhang S, Chiang YL, Otto E, Weaver L, Blaese RM, Anderson WF, McGarrity GJ. Improved methods of retroviral vector transduction and production for gene therapy. Hum Gene Ther. 1994;5:19–28. doi: 10.1089/hum.1994.5.1-19. [DOI] [PubMed] [Google Scholar]
- 26.Chamow S, Zhang DZ, Mhatre SM, Tan XY, Peers DH, Byrn RA, Askenazi A, Junghans RP. A humanized bispecific immunoadhesin-antibody that retargets CD3+ effectors to kill HIV-infected cells. J Immunol. 1994;153:4268–4280. [PubMed] [Google Scholar]
- 27.Beecham EJ, Ortiz-Pujols S, Junghans RP. Dynamics of tumor cell killing by human T lymphocytes armed with an anti-CEA chimeric immunoglobulin-T cell receptor. J Immunother. 1900;23:332–343. doi: 10.1097/00002371-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Marcus DM. Measurement and clinical importance of antibodies to glycosphingolipids. Ann Neurol. 1990;27:S53–S55. doi: 10.1002/ana.410270714. [DOI] [PubMed] [Google Scholar]
- 29.Mizutamari RK, Wiegandt H, Nores GA. Characterization of anti-ganglioside antibodies present in normal human plasma. J Neuroimmunol. 1994;50:215–220. doi: 10.1016/0165-5728(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 30.Luo D, Mah N, Krantz M, Wilde K, Wishart D, Zhang Y, Jacobs F, Martin L. Vl-linker-Vh orientation-dependent expression of single chain Fv-containing an engineered disulfide-stabilized bond in the framework regions. J Biochem (Tokyo) 1995;118:825–831. doi: 10.1093/oxfordjournals.jbchem.a124986. [DOI] [PubMed] [Google Scholar]
- 31.Tsumoto K, Nakaoki Y, Ueda Y, Ogasahara K, Yutani K, Watanabe K, Kumagai I. Effect of the order of antibody variable regions on the expression of the single-chain HyHELIO Fv fragment in E. coli and the thermodynamic analysis of its antigen-binding properties. Biochem Biophys Res Commun. 1994;201:546–551. doi: 10.1006/bbrc.1994.1736. [DOI] [PubMed] [Google Scholar]
- 32.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 33.Mallabiabarrena A, Fresno M, Alarcon B. An endoplasmic reticulum retention signal in the CD3 epsilon chain of the T-cell receptor. Nature. 1992;357:593–596. doi: 10.1038/357593a0. [DOI] [PubMed] [Google Scholar]
- 34.Berkhout B, Alarcon B, Terhorst C. Transfection of genes encoding the T cell receptor-associated CD3 complex into COS cells results in assembly of the macromolecular structure. J Biol Chem. 1988;263:8528–8536. [PubMed] [Google Scholar]
- 35.Carson GR, Kuestner RE, Ahmed A, Pettey CL, Concino MF. Six chains of the human T cell antigen receptor. CD3 complex are necessary and sufficient for processing the receptor heterodimer to the cell surface. J Biol Chem. 1991;266:7883–7887. [PubMed] [Google Scholar]
- 36.Bernhard H, Mayer zum Buschenfelde KH, Dippold Ganglioside GD3 shedding by human malignant melanoma cells. Int J Cancer. 1989;44:155–160. doi: 10.1002/ijc.2910440127. [DOI] [PubMed] [Google Scholar]
- 37.Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 38.Letourneur F, Klausner RD. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992;255:79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- 39.Shinkai Y, Ma A, Cheng HL, Alt FW. CD3 epsilon and CD3 zeta cytoplasmic domains can independently generate signals for T cell development and function. Immunity. 1995;2:401–411. doi: 10.1016/1074-7613(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 40.Junghans RP. A strategy for evaluating lymphokine activation and novel monoclonal antibodies in ADCC and effector cell retargeting assays. Cancer Immunol Immunother. 1990;31:207–212. doi: 10.1007/BF01789170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beecham EJ, Ma QZ, Ripley R, Junghans RP. Coupling of CD28 co-stimulation to IgTCR molecules: dynamics of T cell proliferation and death. J Immunother. 2000 doi: 10.1097/00002371-200011000-00004. in press. [DOI] [PubMed] [Google Scholar]
- 42.Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- 43.Nudelman E, Hakomori S, Kannagi R, Levery S, Yeh MY, Hellstrom KE, Hellstrom I. Characterization of a human melanoma-associated ganglioside antigen defined by a monoclonal antibody, 4.2. J Biol Chem. 1982;257:12752–12756. [PubMed] [Google Scholar]
- 44.Hellstrom I, Brankovan V, Hellstrom KE. Strong antitumor activities of IgG3 antibodies to a human melanoma-associated ganglioside. Proc Natl Acad Sci USA. 1985;82:1499–1502. doi: 10.1073/pnas.82.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravindranath MH, Morton DL. Role of gangliosides in active immunotherapy with melanoma vaccine. Int Rev Immunol. 1991;7:303–329. doi: 10.3109/08830189109114877. [DOI] [PubMed] [Google Scholar]
- 46.Vadhan-Raj S, Cordon-Cardo C, Carswell E, Mintzer D, Dantis L, Duteau C, Templeton MA, Oettgen HF, Old LJ, Houghton AN. Phase I trial of a mouse monoclonal antibody against GD3 ganglioside in patients with melanoma: induction of inflammatory responses at tumor sites. J Clin Oncol. 1988;6:1636–1648. doi: 10.1200/JCO.1988.6.10.1636. [DOI] [PubMed] [Google Scholar]
- 47.Houghton AN, Mintzer D, Cordon-Cardo C, Welt S, Fliegel B, Vadhan S, Carswell E, Melamed MR, Oettgen HF, Old LJ. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci USA. 1985;82:1242–1246. doi: 10.1073/pnas.82.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajorin DF, Chapman PB, Wong GY, Cody BV, Cordon-Cardo C, Dantes L, Templeton MA, Scheinberg D, Oettgen HF, Houghton AN. Treatment with high dose mouse monoclonal (anti-GD3) antibody R24 in patients with metastatic melanoma. Melanoma Res. 1992;2:355–362. doi: 10.1097/00008390-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Brinkmann U, Reiter Y, Jung S-U, Lee B, Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci USA. 1993;90:7538–7542. doi: 10.1073/pnas.90.16.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hombach A, Koch D, Sircar R, Heuser C, Diehl V, Kruis W, Pohl C, Abken H. A chimeric receptor that selectively targets membrane-bound carcinoembryonic antigen (mCEA) in the presence of soluble CEA. Gene Ther. 1999;6:300–304. doi: 10.1038/sj.gt.3300813. [DOI] [PubMed] [Google Scholar]
- 51.Junghans RP. Cruel antibody fictions! Cellular antigen enumeration by “saturation” binding. Immunol Today. 1999;20:401–406. doi: 10.1016/s0167-5699(97)01178-x. [DOI] [PubMed] [Google Scholar]
- 52.Kasid A, Morecki S, Aebersold P, Cornetta K, Culver K, Freeman S, Director E, Lotze MT, Blaese RM, Anderson WF, Rosenberg SA. Human gene transfer: characterization of human tumor-infiltrating lymphocytes as vehicles for retroviral-mediated gene transfer in man. Proc Natl Acad Sci USA. 1990;87:473–477. doi: 10.1073/pnas.87.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]