Abstract
The recruitment of antigen-specific T lymphocytes to the intestinal mucosa is central to the development of an effective mucosal immune response, yet the mechanism by which this process occurs remains to be fully defined. Here we show that the CC chemokine receptor 9 (CCR9) is selectively and functionally expressed on murine αEβ7+ naive CD8αβ+ lymphocytes and a subset of recently activated CD69+ CD8αβ+ lymphocytes. Using a T cell receptor transgenic transfer model, we demonstrate that CCR9 expression is functionally maintained on CD8αβ+ lymphocytes following activation in mesenteric lymph nodes but rapidly downregulated on CD8αβ+ lymphocytes activated in peripheral lymph nodes. These recently activated CCR9+ CD8αβ+ lymphocytes selectively localized to the small-intestinal mucosa, and in vivo neutralization of the CCR9 ligand, CCL25, reduced the ability of these cells to populate the small-intestinal epithelium. Together these results demonstrate an important role for chemokines in the localization of T lymphocytes to the small-intestinal mucosa and suggest that targeting CCL25 and/or CCR9 may provide a means to selectively modulate small-intestinal immune responses.
Introduction
The intestinal mucosa is continually exposed to a large array of foreign antigens and must respond appropriately to maintain mucosal integrity while at the same time mounting effective immune responses to potential pathogens. The recruitment of T lymphocytes to intestinal effector sites is thought to play a critical role in this process.
Following activation in secondary lymphoid organs, T lymphocytes gain the ability to migrate from the blood to tertiary effector tissues such as the intestine and skin (1). Subsets of previously activated T lymphocytes display selective tissue tropism for these sites, a process that is controlled by specific combinations of cell adhesion molecules (1, 2). Previously activated T lymphocytes homing to the intestine express high levels of α4β7 integrin, whose ligand, MAdCAM-1, is expressed on postcapillary venules in the intestinal lamina propria. Indeed, β7 integrin appears to be critical for the entry of previously activated T lymphocytes into the intestinal lamina propria and epithelium (3, 4).
In addition to cell adhesion molecules, accumulating evidence exists for an involvement of chemokines and their receptors in the recruitment of activated lymphocyte subsets to effector tissues (5). For example, the CC chemokine receptor 4 (CCR4) and the CCR10 ligand, CCL27, were recently shown to contribute to lymphocyte recruitment to inflamed skin (6, 7). The chemokine receptor CCR9 is selectively and functionally expressed on human small-intestinal lymphocytes (8), and its ligand, CCL25, is constitutively expressed by murine and human small-intestinal epithelial cells (9, 10), indicating a potential role for this chemokine receptor/chemokine pair in lymphocyte localization to the small intestine. However, examination of CD8αβ+ lymphocyte numbers in the small-intestinal epithelium of CCR9–/– mice has yielded conflicting results (11, 12). Furthermore, small-intestinal epithelial cells constitutively express a number of chemokines with activity for T lymphocytes, including CXCL12, CCL28, and CX3CL1, indicating a potential role for other chemokines in this process (13–15). Thus the in vivo role of CCL25 and CCR9 in T lymphocyte recruitment to the small intestine remains unclear.
In the current study we have examined expression and regulation of CCR9 on murine CD8αβ+ lymphocytes in vivo and determined the role of CCL25 in the recruitment of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa.
Methods
Mice.
C57BL/6J-Ly5.1 mice were obtained from Charles River Laboratories (Wilmington, Massachusetts, USA). OT-1 mice were kindly provided by A. Mowat (University of Glasgow, United Kingdom). All mice were maintained at the animal facility at the Department of Microbiology, Immunology and Glycobiology, Lund University, and were used between 8 and 14 weeks of age.
Antibodies and reagents.
Anti-CD8β (53-5.8), anti-Ly5.2 (104), anti-CD62L (Mel-14), anti-CD44 (IM7), anti-CD4 (RM4-5), and anti-CD69 (H1.2F3) antibodies and streptavidin-allophycocyanin were from Pharmingen (San Diego, California, USA). Anti-αE (M290) and anti-β7 (FIB-504) antibodies were kindly provided by C. Parker (Dana-Farber Cancer Institute, Boston, Massachusetts, USA). Hybridomas producing anti-CD8α (YTS 169-4), anti-CD4 (GK1.5), anti-B220 (RA3.6B2), anti–FcRII/III (2.4G2), and anti–MHC-II (M5/114) antibodies were from American Type Culture Collection (Rockville, Maryland, USA). Goat anti-rabbit Ig was from Jackson ImmunoResearch Laboratories Inc. (West Grove, Pennsylvania, USA), polyclonal rabbit anti–mouse CCR9 antibody was from G. Márquez (K629; ref. 16), 7-amino-actinomycin D (7AAD) was from Sigma-Aldrich (Steinheim, Germany), and recombinant murine CCL25 was from R&D Systems Europe (Abingdon, United Kingdom). Murine stromal cell-derived–factor 1 was a kind gift from I. Clark-Lewis (University of British Columbia, Vancouver, British Co-lumbia, Canada).
Adoptive transfers.
We injected 2 × 106 to 3 × 106 CD8+ OT-1 cells intravenously into C57BL/6J-Ly5.1 mice. Two to three days later, we injected mice intraperitoneally with 5 mg ovalbumin (OVA, grade VI; Sigma-Aldrich) with or without 100 μg LPS (Escherichia coli, serotype 055:B5; Sigma-Aldrich). Mice were sacrificed 2–3 days after OVA/LPS injection, and tissues were collected for further analysis. For antibody treatment experiments, mice received anti-CCL25 antibody (89818; R&D Systems Inc.) or rat IgG2b isotype control antibody (A95-1; Pharmingen) intraperitoneally, 6 hours prior to (100 μg) and 1 day (75 μg) and 2 days (50 μg) after OVA administration. For 5- and 6-carboxy-fluorescein diacetate succinimidyl ester (CFSE) staining, cells (107 cells/ml) were incu-bated with 1 μM CFSE (Molecu-lar Probes Inc., Eugene, Oregon, USA) for 8 minutes at room temp-erature in PBS.
Lymphocyte isolation.
Small-intestinal and colonic intraepithelial lymphocytes (IELs) were isolated as previously described (17) or from the cell suspensions obtained following EDTA treatment of intestinal tissue (see below). Small-intestinal lamina propria lymphocytes (LPLs) were isolated essentially as previously described (18). Briefly, intestinal pieces were incubated for 4 × 20 to 6 × 20 minutes in calcium- and magnesium-free (CMF) HBSS supplemented with 10% FCS and 10 mM HEPES (GIBCO BRL; Life Technologies, Paisley, Scotland, United Kingdom) (HBSS-10), containing 5 mM EDTA at 37°C on an orbital shaker (125 rpm). For isolation of colonic IEL, 1mM DTT was included with the EDTA. The remaining tissue pieces were washed free of EDTA and digested with collagenase type VIII (100 U/ml; Sigma-Aldrich) in RPMI supplemented with 10% FCS and 10 mM HEPES (RPMI-10) at 37°C for 1 hour, twice. For isolation of lymphocytes from inflamed skin, ears were split into dorsal and ventral sheets, treated for 20 minutes with EDTA twice, and then digested with collagenase for 1 hour three times as above. For isolation of liver and lung lymphocytes, organs were perfused with 5 ml of cold PBS containing 75 U/ml heparin prior to removal. Tissues were then minced, and the resulting cell suspension was filtered through a 70-μm cell strainer (Costar, Corning Inc., Corning, New York, USA). All tissue lymphocytes were enriched on 40/70 Percoll gradients (Amersham Pharmacis Biotech, Uppsala, Sweden). Isolation of lymphocytes from lymph nodes (LNs) and spleen was performed according to standard techniques.
Flow cytometry.
After incubation with blocking buffer (PBS, anti–FcRII/III antibody [10 μg/ml], 2% FCS, and 0.05% azide) for 20 minutes at 4°C, lymphocytes were incubated with fluorochrome-conjugated or biotin-conjugated antibody at saturating concentrations for 20 minutes at 4°C. For biotinylated antibodies, stained cells were washed and fluorochrome-conjugated streptavidin was added to the cells for 20 minutes at 4°C. For CCR9 staining, 10% normal goat serum was included in the initial blocking buffer. Cells were then incubated with polyclonal rabbit anti–mouse CCR9 antibody for 45 minutes at 4°C, washed, and incubated with biotinylated goat anti-rabbit antibody for 20 minutes at 4°C. Finally, cells were incubated with fluorochrome-conjugated streptavidin for 20 minutes at 4°C. To check staining specificity, anti-CCR9 antibody was preincubated with the immunizing peptide used for generation of the CCR9 antibody (PTELTSLIPGMFDDFSYDST; 5 μg/ml) for 15 minutes at room temperature prior to use. Samples were analyzed on a flow cytometer (FACSCalibur; BD Biosciences Europe, Erembodegem, Belgium) using CellQuest software (BD Biosciences).
Chemotaxis.
Chemotaxis assays and analysis of lymphocyte subset migration were performed as previously described (19). The analysis of lymphocyte subset migration was performed as previously described (8). To determine OT-1 cell chemotaxis, we depleted CD4+, B220+, and MHC-II+ cells from LN cells by magnetic cell sorting beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer’s instructions prior to adding the cells to chemotaxic wells.
Real-time PCR.
RNA was extracted from tissues using the Absolutely RNA kit (Stratagene, La Jolla, California, USA) according to the manufacturer’s instructions, and cDNA was prepared from RNA using Superscript RT II (GIBCO BRL; Life Technologies). Quantitative detection of CCL25 was performed using a LightCycler (Roche Diagnostics, Basel, Switzerland). Briefly, using LightCycler DNA Master Hybridization Probes kit (Roche Diagnostics), 500 nM forward and reverse primers, 200 nM fluorescently labeled probes, 2 μl cDNA product, and RNase- and DNase-free water were added to the master mix to a final volume of 20 μl. The following primers and probes were used: murine CCL25 primers: forward, 5′-CCGGCATGCTAGGAATTATCA-3′, and reverse, 5′-GGC-ACTCCTCACGC-TTGTA-CT-3′; murine GAPDH primers: forward, 5′-CCTGCACCACCAACTGCTTA-3′, and reverse 5′-ATGACCTTGCCCACAGCCT-3′; murine CCL25 probes: 3′ fluorescein-labeled, 5′-GGCGGAAGTAGAATC-TCACAGCACGTA-3′, and 5′ LC Red 640–labeled, 5′-TTGCAGCTTCCACTCACTTCCTGC-3′; GAPDH probes: 3′ fluorescein-labeled, 5′-ATGACCACA-GTCCATGCCATCACTGCC-3′, and 5′ LC Red 640–labeled, 5′-CCCAGAAGA-CTGTCGATGGCCCCT-3′. All primers and probes were designed in cooperation with O. Landt (TIB MOLBIOL, Berlin, Germany). The amount of CCL25 and GAPDH was calculated from a standard curve of dilutions of linearized plasmid encoding CCL25 and GAPDH cDNA.
Results
Expression of CCR9 by murine T lymphocyte subsets.
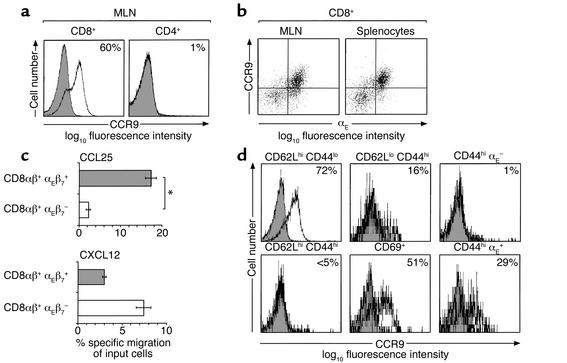
Using an affinity-purified polyclonal antibody (K629) raised against a peptide encoding amino acids 3–22 of the N-terminal portion of murine CCR9, Carramolino and colleagues recently demonstrated low-level expression of CCR9 on CD8+ but not on CD4+ lymphocytes isolated from murine LNs (16). In order to gain further insights into the expression of CCR9 on lymphocyte subsets, we used K629 in combination with a more sensitive staining procedure (see Methods). Under these conditions, about 60% of mesenteric lymph node (MLN) CD8αβ+ lymphocytes (mean 62.0%, SD 9.5, n = 19) and about 55% of peripheral lymph node (PLN) (mean 53.0%, SD 9.7, n = 14) and splenic (mean 55.3%, SD 10.5, n = 8) CD8αβ+ lymphocytes expressed CCR9 (Figure 1a, and data not shown). In contrast, 3% of CD4+ lymphocytes in MLNs and PLNs expressed CCR9 (Figure 1a, and data not shown). The staining for CCR9 was specific, since preincubation with the immunizing peptide reduced staining to levels observed when no primary antibody was used (Figure 1a). Since CCR9 is expressed on αEβ7+ CD8αβ+ human peripheral blood lymphocytes (8), we examined expression of CCR9 on murine αEβ7+ CD8αβ+ lymphocytes. Almost all CCR9+ CD8αβ+ lymphocytes in the spleen, MLNs, and PLNs coexpressed αEβ7 (Figure 1b, and data not shown). To determine whether expression of CCR9 correlated with responsiveness to CCL25, the ability of splenic αEβ7+ and αEβ7– CD8αβ+ lymphocytes to migrate to CCL25 was determined in chemotaxis assays (Figure 1c). Results from this analysis demonstrated that αEβ7+ but not αEβ7– CD8αβ+ lymphocytes migrated efficiently to CCL25. In contrast, αEβ7– CD8αβ+ lymphocytes migrated more efficiently to CXCL12 (Figure 1c). Thus, CCR9 is preferentially and functionally expressed on murine αEβ7+ CD8αβ+ lymphocytes.
Figure 1.
CCR9 expression on murine lymphocytes from normal mice. (a) CD8+ and CD4+ lymphocytes from MLNs were stained with anti-CCR9 antibody (open) or anti-CCR9 antibody that had been preincubated with the CCR9-immunizing peptide (shaded). Numbers represent percentage of CCR9+ cells with background staining removed. Background staining was consistently less than 2%. (b) CCR9+ CD8αβ+ lymphocytes coexpress αEβ7 integrin. (c) CD8αβ+ αEβ7+, but not CD8αβ+ αEβ7–, lymphocytes migrate to CCL25. The ability of CD8αβ+ αEβ7+ and CD8αβ+ αEβ7– lymphocytes to migrate to CCL25 (250 nM) or CXCL12 (100 nM) was determined in chemotaxis assays (see Methods). Results are mean ± SEM of quadruplicate wells from one experiment of two performed. *P < 0.0001. (d) CCR9 expression on CD8αβ+ lymphocyte subsets in MLNs. Numbers represent percentage of CCR9+ cells with background staining removed.
Further phenotypic analysis of CD8αβ+ lymphocyte subsets in MLNs and PLNs demonstrated that CCR9 was expressed on the majority of naive (CD62Lhi CD44lo) CD8αβ+ lymphocytes (Figure 1d, and data not shown), while CD62Lhi CD44hi CD8αβ+ lymphocytes were CCR9– (Figure 1d). Furthermore, a small population of CD62Llo CD44hi (previously activated) and about 50% of CD69+ (recently activated) CD8αβ+ lymphocytes were CCR9+ (Figure 1d). The CD44hi CCR9+ CD8αβ+ lymphocytes also expressed αEβ7, although some CD44hi αEβ7+ CD8αβ+ lymphocytes were CCR9– (Figure 1d). Expression of CCR9 on a subset of recently activated CD8αβ+ lymphocytes indicated that CCR9 may be differentially regulated on CD8αβ+ lymphocytes following their activation in vivo and that CCR9 may function in the localization of a subset of recently activated CD8αβ+ lymphocytes.
CCR9 is selectively maintained on antigen-specific CD8αβ+ lymphocytes following activation in MLNs.
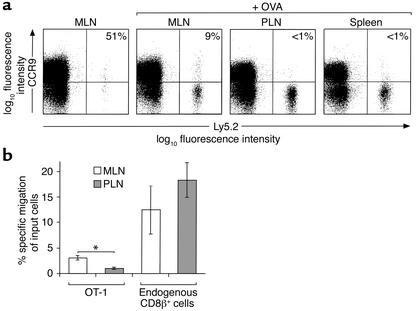
To further understand the mechanisms regulating CCR9 expression on CD8αβ+ lymphocytes following activation in vivo, we used a T cell receptor (TCR) transgenic transfer model in which OVA-specific CD8αβ+ TCR transgenic OT-1 cells were transferred to C57BL/6J-Ly5.1 mice (20). Initial studies confirmed that OT-1 cells showed a pattern of CCR9 expression similar to that of CD8αβ+ lymphocytes from wild-type mice (data not shown). To examine the effect of activation on CCR9 expression, mice were injected with OVA intraperitoneally, and the expression of CCR9 on OT-1 cells in MLNs, PLNs, and spleen was determined. As expected, administration of OVA led to a large increase in the total number of OT-1 cells in LNs and spleen (data not shown). Six to twenty percent (mean 11.5%, SEM 1.7, n = 9) of OT-1 cells expressed CCR9 in the MLNs 3 days after OVA administration (Figure 2a). This staining was specific, since preincubation of the anti-CCR9 antibody with the immunizing peptide against which it was raised reduced staining to background levels (data not shown). In marked contrast, OT-1 cells in PLNs and spleen were CCR9– (Figure 2a). The differential regulation of CCR9 in MLNs and PLNs was selective, since CXCR3, whose ligands are upregulated at sites of inflammation, was induced on OT-1 cells in both MLNs and PLNs (data not shown). To determine whether CCR9 expression on recently activated OT-1 cells in MLNs was functional, the ability of OT-1 cells from MLNs and PLNs to respond to CCL25 was determined in chemotaxis assays (Figure 2b). OT-1 cells from MLNs but not from PLNs of OVA-treated mice migrated to CCL25 in chemotaxis assays. In contrast, endogenous CD8αβ+ lymphocytes from both MLNs and PLNs migrated to CCL25 (Figure 2b).
Figure 2.
Regulation of CCR9 expression on antigen-specific CD8αβ+ lymphocytes following activation in vivo. (a) OT-1 (Ly5.2+) cells were injected into C57BL/6J-Ly5.1 mice, and their expression of CCR9 was determined by flow cytometry 3 days after intraperitoneal OVA administration. Numbers represent the percentage of OT-1 cells that are CCR9+ with background staining removed. Results are from one representative experiment of nine (MLN), seven (PLN), and two (spleen) experiments performed. (b) OT-1 cells from the MLNs of OVA-treated mice respond to CCL25. MLN and PLN lymphocytes were isolated 3 days after intraperitoneal administration of OVA, and the ability of OT-1 cells and endogenous CD8αβ+ lymphocytes to migrate to CCL25 (250 nM) was determined. Results are the mean ± SEM of four experiments with three mice per experiment. *P < 0.005.
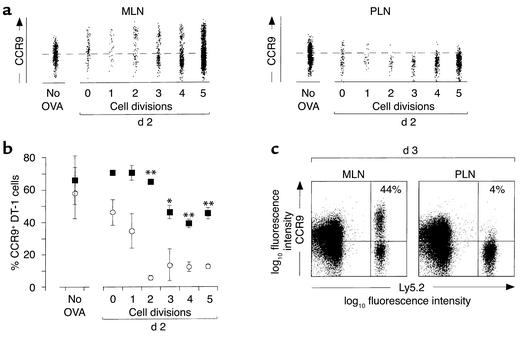
To determine the kinetics of CCR9 expression during the initial stages of antigen-driven CD8αβ+ lymphocyte proliferation, OT-1 cells were labeled with CFSE and injected into recipient mice, and expression of CCR9 on CFSE+ cells was determined (Figure 3). Since adjuvants are commonly used to boost the immune responses to soluble antigens, OVA was coinjected with LPS to increase the number of OT-1 cells available for analysis. Furthermore, to exclude the possibility that the CCR9+ OT-1 cells in the MLNs 3 days after stimulation derived from another site, CCR9 expression was examined on OT-1 cells 2 days after OVA injection, before these cells had started to recirculate. In the absence of activation, the percentage of CCR9+ OT-1 cells within MLNs and PLNs did not differ significantly (Figure 3, a and b). Following activation, CCR9 expression was selectively maintained on a subset of OT-1 cells in MLNs for at least five cell divisions (Figure 3, a and b). Furthermore, occasionally the percentage of CCR9+ OT-1 cells at early divisions was higher than seen in unstimulated OT-1 cells (Figure 3a), and the levels of CCR9 on CCR9+ OT-1 cells were higher after activation (Figure 3a). Together these results indicate that some de novo induction of CCR9 expression may also occur on these cells during their activation in MLNs. In marked contrast, CCR9 expression was rapidly lost on OT-1 cells following activation in PLNs (Figure 3, a and b). Analysis of CCR9 expression on OT-1 cells 3 days after stimulation, at a time point when the large majority of cells had undergone more than five divisions (data not shown), demonstrated that CCR9 continued to be expressed on a large subset of OT-1 cells activated in MLNs but was absent on cells activated in PLNs (Figure 3c). Of note, the percentage of OT-1 cells expressing CCR9 in MLNs was greater following stimulation with OVA plus LPS than with OVA alone (for comparison see Figures 2a and 3c), indicating that a large population of activated CCR9+ CD8αβ+ lymphocytes may be generated in gut-associated lymphoid tissue (GALT) during a productive immune response. Together these results demonstrate that CCR9 expression is maintained on CD8αβ+ lymphocytes following activation in MLNs but downregulated on CD8αβ+ lymphocytes in PLNs.
Figure 3.
CCR9 is maintained on a subset of OT-1 cells following activation in MLNs. (a) CCR9 expression on CFSE+ CD8αβ+ OT-1 (Ly5.2+) lymphocytes. OT-1 cells were labeled with CFSE and injected into C57BL/6J-Ly5.1 mice. MLNs and PLNs were removed 2 days after intraperitoneal administration with OVA plus LPS, and the expression of CCR9 on CFSE+ OT-1 cells was determined by flow cytometry. Results are from one representative experiment of four performed with pooled cells from three to five mice. (b) Combined results of CCR9 expression on OT-1 cells from MLNs (filled symbols) and PLNs (open symbols) 2 days after stimulation with OVA plus LPS. Each symbol represents the mean ± SEM of three to four experiments with cells pooled from three to five mice per experiment. The data from cells that had undergone no divisions and one division are from two experiments, since there were too few cells for analysis in the remaining two experiments. *P < 0.02, **P < 0.002. (c) CCR9 expression on OT-1 (Ly5.2+, CD8αβ+) cells 3 days after stimulation with OVA plus LPS. Results are from the same experiment as in a and are representative of three performed.
CCR9 is selectively expressed on OT-1 lymphocytes localizing to the small-intestinal epithelium.
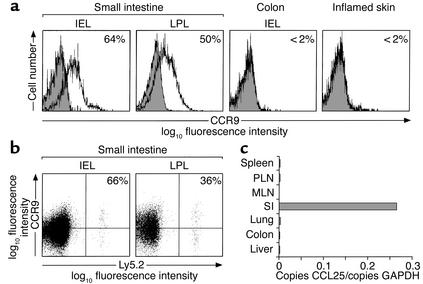
In humans, CCR9 is selectively expressed on all small-intestinal lymphocytes and about 30% of colonic lymphocytes, but not on lymphocytes isolated from other mucosal effector sites or sites of inflammation (10). We therefore determined expression of CCR9 on CD8αβ+ lymphocytes present in murine effector sites (Figure 4a). About 40% of CD8αβ+ small-intestinal IELs (mean 39.6%, SD 14.7, n = 9) and about 50% of CD8αβ+ small-intestinal LPLs (mean 52.3%, SD 2.6, n = 3) expressed CCR9 (Figure 4a). In contrast, CCR9 was not expressed on colonic CD8αβ+ IELs or CD8αβ+ lymphocytes isolated from inflamed delayed-type hypersensitivity skin (Figure 4a). Thus, CCR9 is selectively expressed on previously activated CD8αβ+ lymphocytes in the murine small intestine. To determine whether recently activated CCR9+ OT-1 cells selectively localize to the small intestine, OT-1 cells were transferred to recipient mice, and the expression of CCR9 on OT-1 cells localizing to the small intestine, liver, and lung following OVA stimulation was examined. In unimmunized mice, OT-1 cells in the small intestine were barely detectable (<0.2% of CD8β+ cells; see Figure 5e), consistent with the requirement for activation for entry of these cells to effector sites. After administration of OVA, 57% (SD 6.6, n = 5) of OT-1 cells isolated from the small-intestinal epithelium and 36% (n = 1) of OT-1 cells in the small-intestinal lamina propria expressed CCR9 (Figure 4b). In contrast, less than 2% of OT-1 cells in the lung and liver expressed CCR9 (data not shown).
Figure 4.
Activated CCR9+ OT-1 cells selectively localize to the small intestine. (a) CCR9 expression on CD8αβ+ lymphocytes isolated from tissue effector sites. Cells were stained with anti-CCR9 antibody (open) or anti-CCR9 antibody that had been preincubated with the CCR9-immunizing peptide (shaded), and analyzed by flow cytometry. Results are representative stainings from six mice for small-intestinal and colonic IELs and three mice for small-intestinal LPLs. Skin CD8αβ+ lymphocytes were obtained from the ears of mice with 2,4-dinitro-1-fluorobenzene–induced DTH and are from two stainings using ears pooled from a total of 40 mice. Numbers represent percentage of CD8αβ+ lymphocytes that are CCR9+ with background staining removed. (b) OT-1 cells (Ly5.2+) were injected into C57BL/6J-Ly5.1 mice. Three days after intraperitoneal administration of OVA, lymphocytes were isolated from the small intestine, and the expression of CCR9 on OT-1 lymphocytes was determined by flow cytometry. Results are from five (IEL) and one (LPL) separate stainings using a total of eleven and three mice respectively. Numbers represent the percentage of OT-1 cells expressing CCR9 with background staining removed. (c) CCL25 is selectively expressed in the murine small intestine (SI). Quantitative analysis of CCL25 mRNA expression was determined by real-time RT-PCR.
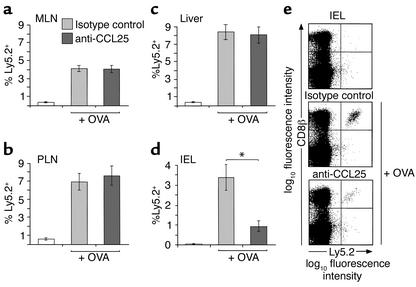
Figure 5.
Anti-CCL25 antibody reduces OT-1 cell recruitment to the small-intestinal epithelium. OT-1 (Ly 5.2+) cells were injected into C57BL/6J-Ly5.1 mice, and the percentage of CD8β+ lymphocytes expressing Ly5.2 in the MLNs (a), PLNs (b), liver (c), and small intestine (d) of control or anti-CCL25 antibody–treated mice was determined by flow cytometry 3 days after intraperitoneal challenge with OVA. Results are the mean ± SEM of six (PLN, MLN, liver) and four (small intestine) mice in each group and show one representative experiment of three performed. *P < 0.05. (e) Representative flow cytometry analysis of the small-intestinal IEL populations in each group of mice.
Given that CCR9 is maintained on a subset of CD8αβ+ lymphocytes during activation in MLNs, a possible explanation for the expression of CCR9 on small-intestinal CD8αβ+ lymphocytes is that the CCR9 ligand, CCL25, is involved in the selective localization of recently activated CCR9+ CD8αβ+ lymphocytes to the small-intestinal mucosa. Indeed, previous data have demonstrated that CCL25 is selectively and constitutively expressed in the murine small intestine (9, 12, 21). Since quantitative analysis of CCL25 expression in murine PLNs and MLNs had not been previously performed, we examined expression of CCL25 in secondary lymphoid organs and intestinal and extra-intestinal tissues by real-time RT-PCR. Results from this analysis demonstrated that CCL25 is constitutively and selectively ex-pressed in the small intestine but not in the spleen, MLN, PLN, lung, liver, or colon (Figure 4c). Together these results indicate that CCL25 is involved in the selective localization of CCR9+ CD8αβ+ lymphocytes to the small intestine following their activation in MLNs.
CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa.
To provide proof of concept, we determined the role of CCL25 in CD8αβ+ lymphocyte localization, using neutralizing anti-CCL25 antibody to block CCL25 function in vivo. Following OT-1 cell transfer, recipient mice were injected intraperitoneally with neutralizing anti-CCL25 or control antibody before and after receiving OVA intraperitoneally, and the percentage of OT-1 cells in individual organs was determined 3 days later. The percentage of OT-1 cells isolated from the PLNs, spleen, and MLNs after OVA administration was similar in mice receiving anti-CCL25 or control antibody (Figure 5, a and b, and data not shown). Thus, CCL25 does not play a critical role in the activation and subsequent proliferation of CD8αβ+ lymphocytes in secondary lymphoid organs. In addition, the percentage of OT-1 cells in the liver of mice receiving anti-CCL25 or control antibody did not differ (Figure 5c). In marked contrast, anti-CCL25 antibody treatment led to a significant reduction in the number of OT-1 cells localizing to the small-intestinal epithelium (Figure 5, d and e). Together these results demonstrate an important role for CCL25 in the selective localization of antigen-specific CD8αβ+ lymphocytes to the small-intestinal epithelium.
Discussion
Previous studies, based largely on expression data in humans (8, 10, 22), have provided indirect evidence for a role of CCR9 and CCL25 in small-intestinal immunity. In the present study we demonstrate that CCR9 is preferentially and functionally ex-pressed on murine αEβ7+ CD8αβ+ lymphocytes and is selectively maintained on a subset of these cells following activation in MLNs. These recently activated CCR9+ CD8αβ+ lymphocytes specifically localized to the small-intestinal mucosa, and in vivo neutralization of the CCR9 ligand, CCL25, selectively inhibited localization of these cells to the small-intestinal epithelium. Thus, CCL25 plays an important and selective role in the localization of CD8αβ+ lymphocytes to the small-intest-inal mucosa.
Analysis of CCR9 expression on murine lymphocytes demonstrated that the majority of CD8αβ+ lymphocytes expressed CCR9 and that, as in humans, these cells coexpressed the integrin αEβ7 (8). In contrast to human CD8αβ+ lymphocytes (8, 23), CCR9 and αEβ7 were expressed on the majority of naive murine CD8αβ+ lymphocytes, indicating differential expression of these proteins on naive CD8αβ+ lymphocytes in humans and mice. Further phenotypic analysis of murine CD8αβ+ lymphocytes demonstrated that CD62Lhi CD44hi CD8αβ+ lymphocytes were CCR9–. This pop-ulation of cells has recently been described as containing long-lived memory cells that share key features of “central” memory cells and can migrate to secondary lymphoid organs and sites of inflammation (24–26). Interestingly, the majority of CD62Lhi CD44hi CD8αβ+ lymphocytes were also β7lo (data not shown), indicating that they were unable to enter intestinal effector sites. Finally, a subset of previously activated CD8αβ+ lymphocytes and recently activated CD8αβ+ lymphocytes expressed CCR9. These cells also expressed αEβ7 and likely correspond to the CCR9+ αEβ7+ CD8αβ+ lymphocyte population, previously described in the human peripheral blood, that expresses a previously activated phenotype (8).
The expression of CCR9 on a subset of recently activated murine CD8αβ+ lymphocytes indicated that CCR9 may be differentially regulated during activation of these cells in vivo. Indeed, in an OVA-specific TCR transgenic transfer model, CCR9 was found to be functionally maintained on a subset of CD8αβ+ lymphocytes activated in MLNs but was rapidly downregulated on these cells following activation in PLNs. To our knowledge, together, these results provide the first direct in vivo demonstration that chemokine receptor expression on lymphocytes can be differentially regulated depending on the site of initial antigen encounter, and they suggest an important role for GALT in the generation of CCR9+ effector CD8αβ+ lymphocytes. Campbell et al., using a similar transfer model with OVA-specific CD4+ lymphocytes, recently demonstrated that transgenic cells isolated from MLNs but not from PLNs of mice that had received OVA and LPS migrated to CCL25 in chemotaxis assays (27). In the context of the present study, this suggests that CCR9 may be selectively induced on CD4+ lymphocytes following activation in MLNs but not in PLNs. These authors also demonstrated that acquisition of intestinal (α4β7hi) and skin (P-selectin ligand+) tropic phenotypes in CD4+ lymphocytes occurs soon after their activation within GALT and PLNs, respectively. Thus, the microenvironment of secondary lymphoid organs plays a critical role in establishment of tissue tropic T lymphocyte subsets. Our results indicate that selective regulation of chemokine receptors may be part of this process. Finally, the lack of CCR9 expression on a subset of OT-1 cells activated in MLNs may be of relevance, since lymphocytes localizing to the human and the murine large intestine are CCR9– (10, 22) (Figure 4). Thus, activated CCR9– CD8αβ+ lymphocytes in MLNs may be destined for extra-intestinal or intestinal effector sites outside of the small intestine, such as the colon or rectum.
Examination of CCR9 expression on CD8αβ+ lymphocytes resident within tissue effector sites and CCL25 levels in different organs demon-strated that CCR9 and CCL25 are selectively expressed in the murine small intestine. These findings are in agreement with previous studies in humans (10) and indicate a selective role for this chemokine/receptor pair in murine and human small-intestinal immune responses. Interestingly, the levels of CCR9 expressed on murine small-intestinal CD8αβ+ lymphocytes were consistently lower than those found on CCR9+ CD8αβ+ lymphocytes in MLNs, suggesting that partial downregulation of this che-mokine receptor may occur once these cells have taken up residence within the small-intestinal mucosa. In addition, results from the adoptive transfer experiments demonstrated the presence of recently activated CCR9+ CD8αβ+ lymphocytes in the small-intestinal mucosa, indicating a potential role for CCL25 in the initial localization of these cells to this site. Direct evidence for a role of CCL25 in the localization of CD8αβ+ lymphocytes in the small intestine was obtained using anti-CCL25 antibody to neutralize CCL25 activity in vivo. In this set of experiments, neutralization of CCL25 activity led to a selective reduction in the number of CD8αβ+ TCR transgenic lymphocytes localizing to the small-intestinal epithelium. The reduction of CD8αβ+ lymphocyte numbers in the small-intestinal epithelium was not absolute, consistent with the observation that not all recently recruit-ed CD8αβ+ IELs expressed CCR9. Since all human IELs and LPLs express CCR9 (8), it seems plausible that this chemokine/chemokine receptor pair may play an even more dominant role in the localization of lymphocytes to the human small-intestinal mucosa.
Given the well-characterized function of chemokines as chemoattractants, the most likely mechanism by which CCL25 promotes CD8αβ+ lymphocyte localization within the small-intestinal epithelium is the initial recruitment of recently activated CCR9+ CD8αβ+ lymphocytes, either directly from the circulation or from the small-intestinal lamina propria, to the epithelium. However, there are a number of other possible mechanisms, including a role for CCL25 in the short-term retention, survival, or proliferation of CD8αβ+ lymphocytes within the epithelium. Several lines of evidence argue against the involvement of CCL25 in the retention and/or survival of CD8αβ+ lymphocytes in the epithelium. Firstly, endogenous CD8αβ+ IEL numbers would have been equally affected by anti-CCL25 antibody treatment, and as a consequence the percentage of OT-1 cells in this compartment would have remained unchanged. Secondly, administration of anti-CCL25 antibody to wild-type mice, as performed in the present study, has no effect on CD3+ IEL numbers, as determined by quantitative immunohistochemical analysis (28). Finally, while IL-7 or IL-15 promotes the survival of CD8αα+ and CD8αβ+ IELs in vitro, incubation of freshly isolated CD8αα+ or CD8αβ+ IELs with CCL25 alone or in combination with suboptimal doses of these cytokines fails to enhance their survival (ref. 28 and our unpublished observations). Thus, CCL25 is unable to directly prevent CD8αβ+ IELs from undergoing apoptosis. In conclusion, while the reduced OT-1 numbers in the epithelium of anti-CCL25 antibody–treated mice suggest a deficiency in recruitment, only more direct short-term tracking studies will resolve this issue.
The ability of neutralizing anti-CCL25 antibody to reduce CD8αβ+ localization within the small-intestinal epithelium is in apparent conflict with data obtained with CCR9–/– mice (11, 12). In one report, CCR9–/– mice were found to have normal numbers of small-intestinal CD8αβ+ TCRαβ+ IELs (11), while a subsequent report examining separately derived CCR9–/– mice concluded that CD8αβ+ TCRαβ+ IEL numbers were reduced in these animals (12). However, this conclusion was based on the observation that CCR9–/– mice showed a reduced percentage of CD8αβ+ TCRαβ+ IELs among TCRαβ+ IELs. Since the percentage of TCRαβ+ cells among CD3+ IELs was significantly increased due to a loss of TCRγδ+ IELs, the total number of CD8αβ+ TCRαβ+ IELs was in fact unaltered. Thus, results from CCR9–/– mice demonstrate that CCR9 is not required for generation of the CD8αβ+ TCRαβ+ IEL compartment. Several explanations for the differences between our results and those obtained with CCR9–/– mice may be proposed. Firstly, CCL25 may be functioning through an alternative receptor to CCR9, such as CCR11, which has been shown to bind to CCL25 (29). However, the observation that CD8αβ+ lymphocytes from CCR9–/– mice fail to migrate to CCL25, indicating that CCR9 is the sole CCL25 receptor on these cells (11, 12), together with the strong correlative data on CCR9 expression presented in the current study, strongly argues against this possibility. Secondly, since CD8αα+ TCRγδ+ IELs are greatly reduced in number in CCR9–/– mice (11, 12), CD8αβ+ IELs may exhibit increased expansion in situ in these mice to fill the IEL compartment. Thirdly, other epithelial-derived chemokines such as CXCL12, CCL28, and CX3CL1 may compensate over time for the CCR9 deficiency, resulting in numbers of CD8αβ+ IELs similar to those in wild-type mice. Indeed, as described above, not all recently activated CD8αβ+ IELs expressed CCR9, and anti-CCL25 antibody failed to completely block lymphocyte entry into the epithelium. Such redundancy in chemokine usage was recently described for the chemokine CCL27 and the chemokine receptor CCR4 in antigen-specific T cell recruitment to the inflamed skin (6).
In conclusion, results from the current study demonstrate a critical role for GALT in the establishment of effector CCR9+ CD8αβ+ lymphocytes and provide the first in vivo demonstration (to our knowledge) of a role of chemokines in lymphocyte trafficking to the intestinal mucosa. Together these results suggest that CCR9 and CCL25 may provide interesting targets for selective modification of small-intestinal immune responses.
Acknowledgments
We would like to thank P. Kearney and A. Runström for kindly providing inflamed ears from mice with 2,4-dinitro-1-fluorobenzene–induced delayed-type hypersensitivity reactions, and F. Ivars for constructive comments during the course of this work. This work was supported by the Swedish Medical Research Council (MFR 13131), the Crafoordska, Österlund, Åke Wiberg, Richard and Ruth Julins, Nanna Svartz, and Kocks Foundations, a Lund Family American Cancer Society grant, the Royal Physiographic Society, and a Crohn’s and Colitis Foundation of America project grant to W.W. Agace. W.W. Agace is an Assistant Professor with the Swedish Science Research Council.
Footnotes
See the related Commentary beginning on page 1079.
Conflict of interest: No conflict of interest has been declared.Nonstandard abbreviations used: CC chemokine receptor (CCR); ovalbumin (OVA); 5- and 6-carboxy-fluorescein diacetate succinimidyl ester (CFSE); intraepithelial lymphocyte (IEL); lamina propria lymphocyte (LPL); lymph node (LN); mesenteric lymph node (MLN); peripheral lymph node (PLN); T cell receptor (TCR); gut-associated lymphoid tissue (GALT).
References
- 1.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 3.Wagner N, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 4.Lefrancois L, et al. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J Exp Med. 1999;189:1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- 6.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homey B, et al. CCL27-CCR10 interactions regulate T cell–mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 8.Zabel BA, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–1256. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurbel MA, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–271. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wurbel MA, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 12.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymph-ocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 13.Agace WW, et al. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr Biol. 2000;10:325–328. doi: 10.1016/s0960-9822(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 14.Pan J, et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–2949. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- 15.Muehlhoefer A, et al. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lympho-cytes in the small intestinal mucosa. J Immunol. 2000;164:3368–3376. doi: 10.4049/jimmunol.164.6.3368. [DOI] [PubMed] [Google Scholar]
- 16.Carramolino L, et al. Expression of CCR9 beta-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8(+) T cells from secondary lymphoid organs. Blood. 2001;97:850–857. doi: 10.1182/blood.v97.4.850. [DOI] [PubMed] [Google Scholar]
- 17.Schon MP, et al. Mucosal T lympho-cyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 18.Harriman GR, Hornqvist E, Lycke NY. Antigen-specific and polyclonal CD4+ lamina propria T-cell lines: phenotypic and functional characterization. Immunology. 1992;75:66–73. [PMC free article] [PubMed] [Google Scholar]
- 19.Agace WW, et al. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur J Immunol. 2000;30:819–826. doi: 10.1002/1521-4141(200003)30:3<819::AID-IMMU819>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Kim SK, et al. Activation and migration of CD8 T cells in the intestinal mucosa. J Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- 21.Vicari AP, et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 22.Papadakis KA, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specializa-tion of the mucosal immune system. J Immunol. 2000;165:5069–5076. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 23.Cerf-Bensussan N, et al. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 25.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manjunath N, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi:10.1172/JCI200113296. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsal, J., et al. 2002. Involvement of CCL25 (TECK) in the generation of murine small intestinal CD8αα+CD3+ intraepithelial lymphocyte compartment. Eur. J. Immunol. In press. [DOI] [PubMed]
- 29.Gosling J, et al. Cutting edge: identification of a novel chemokine receptor that binds dendritic cell- and T cell-active chemokines including ELC, SLC, and TECK. J Immunol. 2000;164:2851–2856. doi: 10.4049/jimmunol.164.6.2851. [DOI] [PubMed] [Google Scholar]