Abstract
Modulation of cyclooxygenase-2 (COX-2) mRNA stability plays an important role in the regulation of its expression by oncogenic Ras. Here, we evaluate COX-2 mRNA stability in response to treatment with two known endogenous promoters of gastrointestinal cancer, the bile acid (chenodeoxycholate; CD) and ceramide. Treatment with CD and ceramide resulted in a 10-fold increase in the level of COX-2 protein and a four-fold lengthening of the half-life of COX-2 mRNA. COX-2 mRNA stability was assessed by Northern blot analysis and by evaluating the AU-rich element located in the COX-2 3′-UTR. A known inhibitor of mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase (MEK), PD98059, reversed the effects of CD or ceramide to stabilize COX-2 mRNA. Overexpression of a dominant-negative ERK-1 or ERK-2 protein also led to destabilization of COX-2 mRNA. Treatment with a p38 MAPK inhibitor, PD169316, or transfection with a dominant-negative p38 MAPK construct reversed the effect of CD or ceramide to stabilize COX-2 mRNA. Expression of a dominant-negative c-Jun N-terminal kinase (JNK) had no effect on COX-2 mRNA stability in cells treated with CD or ceramide. We conclude that posttranscriptional mechanisms play an important role in the regulation of COX-2 expression during carcinogenesis.
Keywords: cyclooxygenase, gene regulation, stabilization, MAPK
Introduction
Prostaglandin endoperoxide synthases (Ptgs), commonly referred to as cyclooxygenases (COX), are key enzymes required for prostaglandin synthesis. They convert arachidonic acid to prostaglandin H2, the precursor for prostaglandins and thromboxanes [1]. Two isoforms of cyclooxygenase have been described, COX-1 and COX-2. COX-1 is expressed constitutively in many circumstances [2]; whereas, COX-2 is an immediate-early growth-response gene that is induced by a number of mitogenic and inflammatory stimuli [3–5]. A growing body of evidence suggests that cyclooxygenase activity and prostaglandin synthesis may be involved in intestinal carcinogenesis. COX-2 can regulate tumor angiogenesis [6], metastatic potential [7], and programmed cell death [8]. Lack of the COX-2 gene in mice is associated with decreased intestinal tumor burden [9], and similar effects have been observed in prostaglandin E receptor-1 (EP-1) and cytosolic phospholipase A2 (cPLA2) null mice [10,11].
The involvement of cyclooxygenase in tumor promotion is supported by the observation that inhibitors of enzyme activity such as nonsteroidal anti-inflammatory drugs (NSAIDs) possess anti-neoplastic effects. In population-based studies, a decrease in colorectal carcinoma risk (40% to 50%) is observed in patients who use aspirin regularly [12,13]. Likewise, NSAIDs are effective in reducing tumor burden and growth in mouse models of familial adenomatous polyposis (FAP) [9], azoxy-methane-treated rats [14] and human tumors xenografted in nude mice [15]. At least part of these anti-neoplastic effects have been attributed to inhibition of COX-2, which results in decreased prostaglandin production. Therefore, it is important to identify factors that regulate COX-2 expression in intestinal epithelial cells.
Cytokines, growth factors, oncogenes, and tumor promoters can induce COX-2 expression in several different cell types [16–23]. Most studies evaluating COX-2 regulation have focused on factors involved in transcriptional control. Several consensus cis elements, including NF-κB, NF-IL6, ATF/CRE, and the E-box have been identified in the 5′ region of the COX-2 gene, some of which are important for the transcriptional regulation of COX-2 expression [24–29]. Stimulation of the mitogen-activated protein kinase (MAPK) signaling pathway, including c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and the p38 MAPK cascade has been reported to play a role in the regulation of COX-2 gene expression [16,19,20,23,29–33].
Bile acids serve as endogenous promoters of gastro-intestinal cancer and induce COX-2 expression in epithelial cells [34–37]. Zhang et al. [38] reported that two bile acids, chenodeoxycholate and deoxycholate, induce COX-2 expression in human esophageal adenocarcinoma cells. Bile acid-mediated induction of COX-2 was blocked by inhibition of protein kinase C (PKC) activity. Bile acid treatment also enhanced c-Jun phosphorylation and increased binding of AP-1 to DNA. Recently, Shirvani et al. [39] have shown that COX-2 is regulated ex vivo following exposure to bile salts in Barrett's esophagus and adenocarcinoma. Ceramide, a bioactive lipid, has been linked to inflammation and carcinogenesis [40–42]. Subbaramaiah et al. [23] demonstrated that triggering the ceramide pathway induces COX-2 expression in human mammary epithelial cells and that induction was blocked by the overexpression of dominant-negative MEK, p38 MAPK, and JNK.
Regions of the COX-2 3′-untranslated region (UTR) are highly conserved in many cytokine genes [43] and contain multiple copies of the AUUUA sequence [44,45] that are thought to play a role in mRNA destabilization [46]. Regulation of cytokine and immediate-early gene expression involves, in part, posttranscriptional mechanisms in which mRNA stabilization is involved [47,48]. Recently, posttranscriptional regulation of mRNA stability has been described for COX-2 [49–51]. We have shown that COX-2 levels increase dramatically following activation of Ras and that stabilization of COX-2 mRNA was mainly responsible for increased expression in a rat intestinal epithelial cell line permanently transfected with a Ras cDNA inducible system (RIE-iRas) [16]. Here, in uninduced cells, we examine COX-2 mRNA stability in response to two endogenous promoters of gastrointestinal cancer and evaluate the signaling pathways responsible for this form of posttranscriptional gene regulation.
Materials and Methods
Chemicals
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), Opti-MEM, and Cellfectin were obtained from Gibco (Gaithersburg, MD). C6-Ceramide (N-hexanoylsphingosine) was purchased from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA). CD (chenodeoxycholic acid) and DRB (5,6-dichloroben-zimidazole riboside) were obtained from Sigma Chemical Co. (St. Louis, MO). The MEK inhibitor PD98059 (2′-amino-3′-methoxyflavone) and a p38 MAPK inhibitor PD169316 [4-(4-fluorophenyl)-2-(4-nitrophenyl)-5-(4-pyridyl)-1H-imidazole] were purchased from Cal Biochem-Novabiochem Co. (San Diego, CA). The anti-COX-2 antibody, C-20, was purchased from Santa Cruz Biotechnology.
Plasmids and Cultured Cells
The luciferase reporter construct, pcDNA3.1/Zeo(+)-Luc+3′UTR containing the COX-2 3′-UTR cloned downstream of the luciferase reporter gene was provided by Dan Dixon (University of Utah, Salt Lake City, UT). The ERK-1, ERK-2, and p38 MAPK dominant-negative expression vectors, pCEP4-ERK-1, pCEP4-ERK-2 and pCMV5-p38 were gifts from Dr Melanie Cobb (University of Texas, Southwestern). The JNK-1 kinase-defective expression plasmid, pCMV5-JNK-1, was a gift from Dr Harvey Herschman (UCLA, Los Angeles, CA).
The RIE-iRas cell line was prepared from RIE cells stably transfected with a Ha-RasVal12 cDNA inducible system. In this report, we carried out studies evaluating uninduced cells. RIE-iRas/Luc+3′UTR, RIE-iRas/Luc+ARE, and RIE-iRas/Luc+ΔARE represent three RIE-iRas cell lines stably transfected with different luciferase reporter constructs, pLuc+3′UTR, pLuc+ARE, and pLuc+ΔARE, respectively. The pLuc+3′UTR contained 1.5 kilobases of the COX-2 3′-UTR downstream of the luciferase gene. In the pLuc+ARE, the AU-rich elements (ARE) of the COX-2 3′-UTR (415 bp) are linked downstream of the reporter gene. The pLuc+ΔARE was derived from pLuc+3′UTR by removal of the ARE from the full-length COX-2 3′-UTR.
Cell Transfection
The amounts of DNA used for each transfection is specified in the text or figure legend that describes each experiment. Following a 4-hour incubation period, cells were washed and cultured for 24 hours. The agents were added at the indicated amounts 12 hours before harvesting the cells. Luciferase activity was measured as previously described [16]. Triplicate wells were used for all transfections and all experiments were repeated at least twice.
RNA Extraction and Northern Blot Analysis
Total cellular RNA was extracted by using Tri-Reagent (Molecular Research Center, Inc. Cincinnati, OH) according to the manufacturer's protocol. RNA samples (20 µg per lane) were separated on 1.0% formaldehyde agarose gels and blotted onto nylon membranes. The blots were hybridized with cDNA probes labeled with α-[32P]dCTP by random primer extension (Stratagene, La Jolla, CA). After hybridization and washes, the blots were subjected to autoradiography. 18S rRNA signals were used as controls to confirm the integrity of RNA and equality of the loading. For determination of mRNA stability, cells were treated with each agent for 12 hours, then the transcription was stopped by addition of 100 µM of DRB. RNA samples were isolated at 0, 30, 60, 90, 120, and 180 minutes following DRB treatment and analyzed for COX-2 levels by Northern blot analysis.
Western Blot Analysis
Western blotting was performed as previously described [16]. Cells were lysed for 30 minutes in radio-immunoprecipitation assay buffer (RIPA, 1xPBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mg/ml phenylmethyl-sulfonyl fluoride, 10 µg/ml aprotinin, 1 mM orthovanadate), then clarified cell lysates were denatured and fractionated by SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes. The filters were then probed with indicated antibodies, developed using the enhanced chemiluminescence system (ECL, Amersham, Arlington Heights, IL), and exposed to XAR-5 film (Eastman Kodak, Rochester, NY). The anti-COX-2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Results
Ceramide and CD Induce COX-2 Expression in RIE-iRas Cells
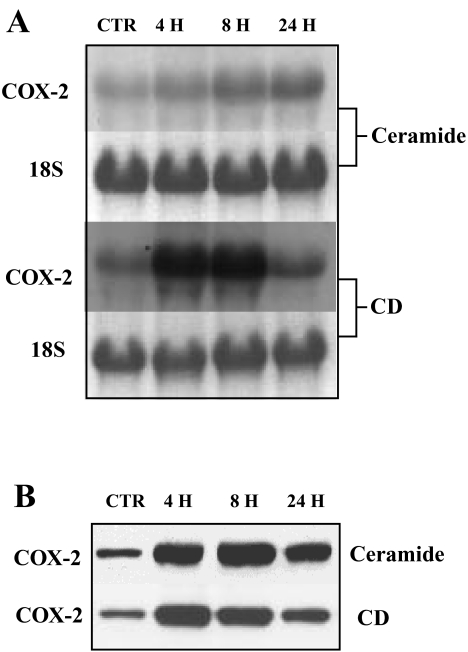
We previously reported that activated Ras and TGF-β1 treatment induces COX-2 in RIE-iRas cells [16]. Recent reports by others indicate that ceramide and CD induce COX-2 expression in human mammary epithelial and esophageal adenocarcinoma cells [23,38]. Northern and Western analyses were carried out to determine whether these agents also induce COX-2 mRNA and protein in RIE-iRas cells. As shown in Figure 1, treatment with ceramide or CD leads to prolonged induction of COX-2 in these cells (at least for 24 hours). COX-2 mRNA and protein levels increased significantly after 4 hours of treatment and by 8 hours both agents led to a four- to eight-fold increase in mRNA levels and a 10-fold induction of COX-2 protein expression.
Figure 1.
Time course for induction of COX-2 following treatment with ceramide and CD. RIE-iRas cells were treated for the times indicated with control (0.1% DMSO), ceramide (20 µM) or CD (300 µM). (A) Total cellular RNA samples (20 µg per lane) were separated on 1.0% formaldehyde agarose gels and blotted onto nylon membranes. The blots were hybridized with rat COX-2 cDNA probes labeled with α-[32P]dCTP. After hybridization and washes, the blots were subjected to autoradiography. 18S rRNA signals were used as controls to confirm the integrity of RNA and equality of the loading. (B) Cell lysates (30 µg per lane) were loaded onto 7.5% SDS-polyacrylamide gel, electrophoresed, and transferred onto nitrocellulose filters. The blots were probed with anti-COX-2 antibody C-20.
Stabilization of COX-2 mRNA by Ceramide and CD Treatment
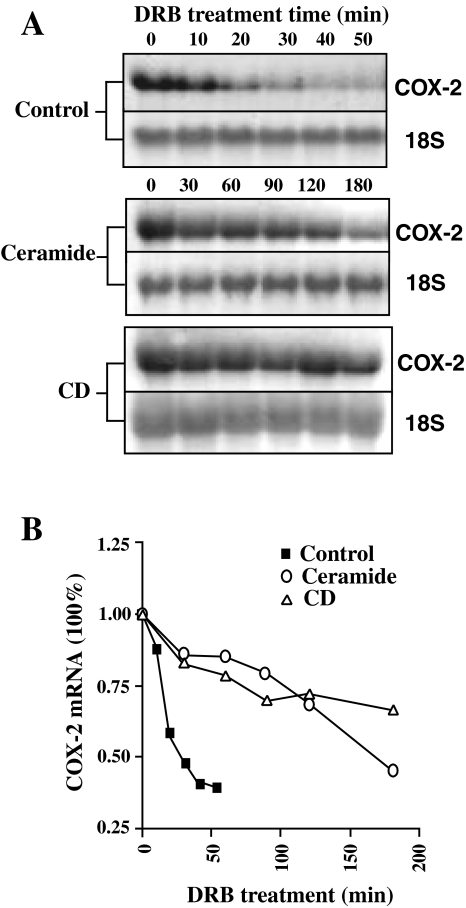
The transcriptional regulation of COX-2 by ceramide and CD has been reported [23,38]. To evaluate potential mechanisms for posttranscriptional regulation, we determined the stability of COX-2 mRNA in cells following treatment with ceramide or CD. Cells were treated with ceramide or CD for 12 hours, then further transcription was inhibited by addition of DRB. Total RNA was isolated at different times following addition of DRB and analyzed for COX-2 levels by Northern blotting (Figure 2A and B). COX-2 mRNA was rapidly degraded in control cells with a half-life of around 30 minutes. Treatment with both ceramide and CD significantly stabilized COX-2 mRNA and extended the half-life to greater than 2 hours.
Figure 2.
Regulation of COX-2 mRNA stability. (A) RIE-iRas cells were treated with control (0.1% DMSO), ceramide (20 µM) or CD (300 µM) for 12 hours. Then transcription was stopped by addition of 100 µM of DRB and total RNA samples were isolated at the indicated time points and analyzed for COX-2 mRNA levels by Northern blot analysis. (B) Degradation curves of COX-2 mRNA. The results from (A) were analyzed by densitometry scanning using the NIH image program and normalized to the loading controls.
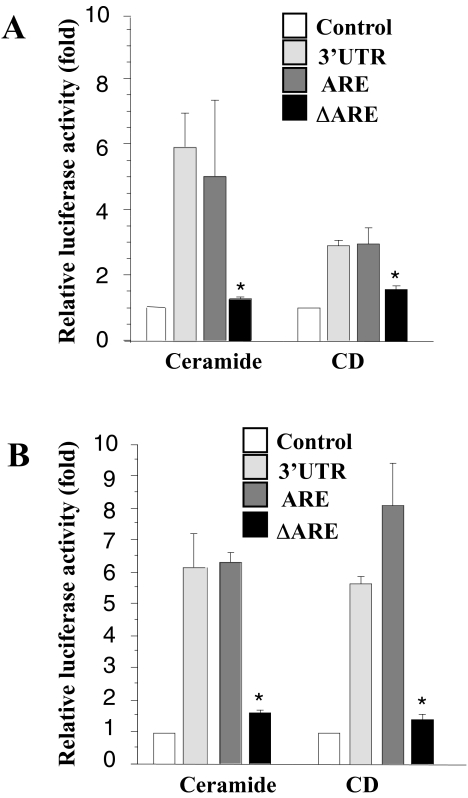
We previously reported that the 3′-UTR of COX-2, especially the AU-rich region, contributes to COX-2 mRNA stability following Ras activation and TGF-β1 treatment [16]. Here, to evaluate the role of the 3′-UTR in mRNA stability, we examined three cell lines, RIE-iRas/Luc+3′UTR, RIE-iRas/Luc+ARE, and RIE-iRas/Luc+ΔARE (see Materials and methods section). These cells were treated with ceramide or CD for 8 and 24 hours, after which the luciferase activities in cell extracts were determined. As shown in Figure 3, both agents increased luciferase activity by 3- to 10-fold in the RIE-iRas/Luc+3′UTR and RIE-iRas/Luc+ARE cells. However, in the RIE-iRas/Luc+ΔARE cells, which did not contain the AU-rich region, the increase was not significant. Therefore, regions of the COX-2 3′-UTR are important in regulating COX-2 levels following treatment with CD or ceramide.
Figure 3.
Luciferase assay for COX-2 mRNA stability after treatment of ceramide or CD. Three cell lines, RIE-iRas/Luc+3′UTR, RIE-iRas/Luc+ARE, and RIE-iRas/Luc+ΔARE, were stably transfected with three luciferase reporter expression vectors (see MaterialS and methods section) respectively, and treated with control (0.1% DMSO), ceramide (20 µM) or CD (300 µM) for 8 hours (A) and 24 hours. (B) Three wells were used for each condition. Luciferase activities were measured and the relative increase with each treatment was plotted as means±S.E. *P<.05 versus 3′-UTR or ARE.
The MAPK Signaling Pathway Is Involved in COX-2 mRNA Stability
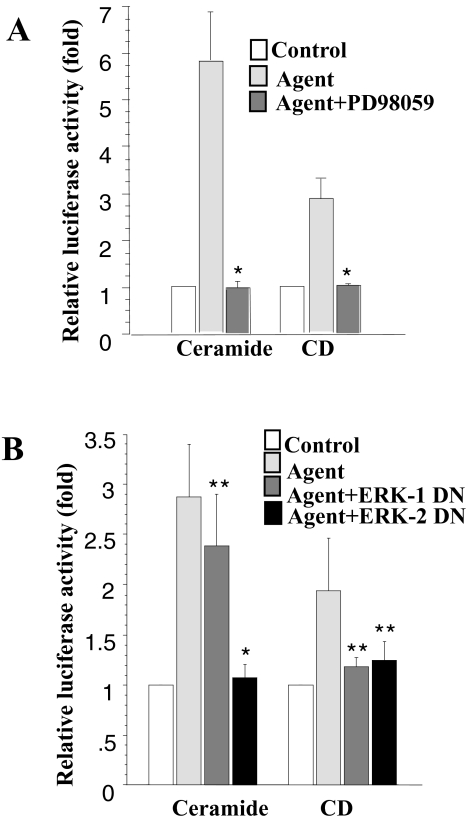
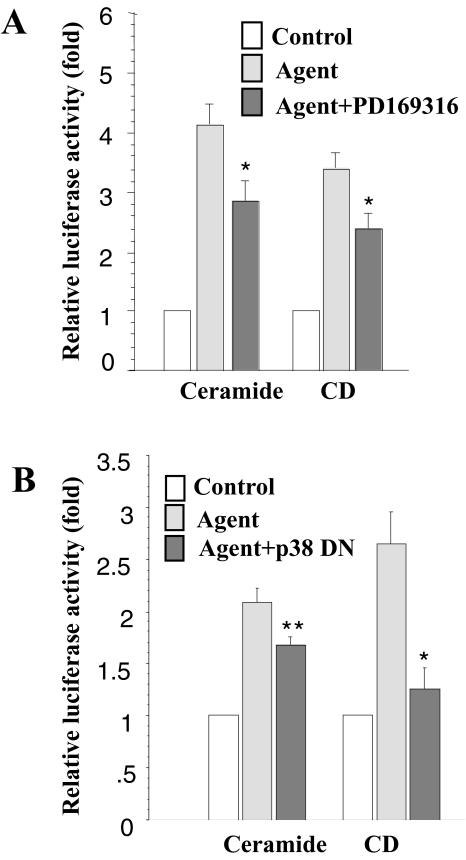
Recent studies by several groups have demonstrated that the Raf1/MAPKK/ERK cascade plays an important role in the regulation of COX-2 expression [19,23]. Treatment with the MEK inhibitor, PD98059, or transfection with a dominant-negative ERK construct can block induction. To evaluate whether the ERK cascade also regulates COX-2 mRNA stability, we treated the cells with PD98059 1 hour before addition of ceramide or CD. As shown in Figure 4A, this inhibitor blocks the increase in luciferase activity induced by ceramide or CD treatment.
Figure 4.
Effect of ERK on COX-2 mRNA stability following treatment with ceramide or CD. (A) RIE-iRas/pLuc+3′UTR cells (see Materials and methods section) were treated with control or the indicated agent plus and minus PD98059 (75 µM). Cells were harvested 12 hours after treatment and assayed for luciferase activity and total protein content. (B) RIE-iRas cells were cotransfected with pcDNA3.1/Zeo (+) Luc+3′UTR (0.4 µg) and either an expression plasmid for dominant-negative ERK-1 protein (0.4 µg) or an expression plasmid for dominant-negative ERK-2 protein (0.4 µg) or 0.4 µg of an empty expression vector. Transfected cells were then treated with DMSO, ceramide or CD. Cells were harvested 12 hours after treatment, assayed for luciferase activity and total protein content. Three wells were used for each condition. Data are expressed as means±SE. *P<.05 versus ceramide or CD treatment, **P>.05 versus ceramide or CD treatment.
Furthermore, we examined the effect of dominant-negative mutants of the ERK1 and ERK2 proteins on luciferase induction by the pcDNA3.1/Zeo(+)Luc-3′UTR reporter gene. Similar to the results shown in Figure 4B, expression of kinase-defective, dominant-negative ERK1 and ERK2 proteins reduced the ceramide- or CD-stimulated increase in luciferase activity.
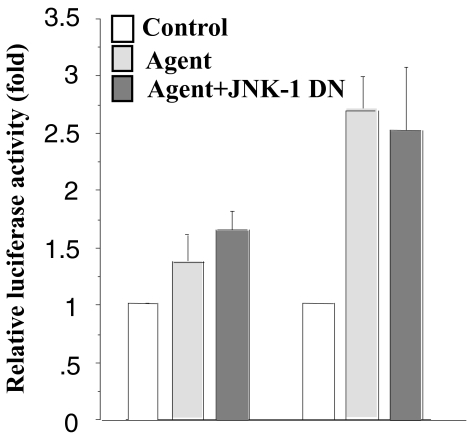
Xie and Herschman [19,20] demonstrated that COX-2 induction also requires activation of the MEKK-1/JNK kinase/JNK/c-Jun cascade. To determine if this pathway also regulates COX-2 mRNA stability, we transfected a dominant-negative JNK-1 with the pcDNA3.1/Zeo(+)Luc-3′UTR reporter construct into RIE-iRas cells. As shown in Figure 5, expression of kinase-defective, dominant-negative JNK-1, which interferes with the phosphorylation of c-Jun, does not affect the luciferase activity driven by the Luc-3′UTR construct. These data suggest that the JNK pathway may only be involved in transcriptional regulation and not the posttranscriptional regulation of COX-2 expression.
Figure 5.
Effect of JNK on COX-2 mRNA stability after induction with ceramide or CD. RIE-iRas cells were cotransfected with pcDNA3.1/Zeo(+)Luc+3′UTR (0.4 µg) and either an expression plasmid encoding a dominant-negative JNK-1 protein (0.4 µg) or the empty vector (0.4 µg). The transfected cells were then treated with control, ceramide or CD, harvested 12 hours after treatment and assayed for luciferase activity and total protein. Three wells were used for each condition. Data are expressed as means ±SE.
In addition to ERK and JNK signaling, p38 MAPK has also been reported to regulate COX-2 expression [31,32,52]. Two p38 MAPK inhibitors, PD169316 and SB203580, have been shown to destabilize COX-2 mRNA [17,52]. Here, we also found that PD169316 treatment reduced the ceramide- and CD-stimulated increase in luciferase activity (Figure 6A). Similar results were obtained with the p38 MAPK dominant-negative transfected cells. As demonstrated in Figure 6B, expression of dominant-negative p38 MAPK protein reduces the ceramide-and CD-stimulated increase in luciferase activity. Therefore, p38 MAPK plays an important role in the regulation of COX-2 mRNA stability.
Figure 6.
Effect of p38 MAPK on COX-2 mRNA stability following treatment with ceramide or CD. (A) RIE-iRas/pLuc+3′UTR cells (see Materials and methods section) were treated with control or the agents indicated plus and minus PD169316 (5 µg/ml) added 1 hour earlier. Cells were harvested 12 hours following treatment and assayed for luciferase activity and total protein. (B) RIE-iRas cells were cotransfected with pcDNA3.1/Zeo(+)Luc+3′UTR (0.4 µg) and either an expression plasmid containing a dominant-negative p38 MAPK protein (0.4 µg) or 0.4 µg of an empty expression vector. The transfected cells were then treated with DMSO, ceramide or CD. Cells were harvested 12 hours after treatment, assayed for luciferase activity and total protein. Three wells were used for each condition. The data are expressed as means±SE. *P<.05 versus ceramide or CD treatment, **P>.05 versus ceramide or CD treatment.
Discussion
Bile acids and ceramide are known to be involved in the promotion of carcinogenesis. Bile acids were shown to affect neoplastic transformation in germ-free and conventional F344 rats [35,53] and in fibroblasts [34]. McMillan et al. recently reported that bile acids reduce the apoptosis-inducing effects of sodium butyrate on human colonic adenoma cells [54]. The precise mechanism(s) by which bile acids promote tumor formation is not clear. Like other tumor promoters, bile acids activate protein kinase C [55,56] and induce AP-1 activity [56,57]. Ceramide is a lipid second messenger that has been linked to pathways involved in apoptosis, inflammation, and carcinogenesis [40–42]. Protein kinase C is activated by ceramide in vitro and in vivo [58,59] and ceramide induces matrix metalloproteinase-1 expression in fibroblasts [42].
Ristimaki et al. [49] and Newton et al. [50] reported that repression of COX-2 is regulated by dexamethasone through destabilization of COX-2 mRNA or shortening of the average length of COX-2 mRNA poly (A) tails. Barrios-Rodiles et al. [51] also demonstrated that COX-2 mRNA transcripts were stabilized following LPS stimulation of macrophages. Two p38 MAPK inhibitors, PD169316 and SB203580, were reported to destabilize COX-2 mRNA in LPS-stimulated human monocytes and TGFα-stimulated human epidermal keratinocytes [17,52]. Previously, we reported that COX-2 mRNA stabilization is responsible for induction of COX-2 following Ras activation or TGF-β1 treatment of RIE-iRas cells [16]. Here we demonstrate that COX-2 expression is regulated by ceramide or CD and that these agents lead to stabilization of COX-2 mRNA, increasing mRNA half-life by greater than four-fold.
The 3′-UTR of the COX-2 cDNA is extremely AU-rich, and contains 14 copies of the Shaw-Kamens sequence (AUUUA) [44,45]. This motif is present in several immediate-early and cytokine genes and is thought to be involved in the regulation of mRNA degradation [46,60]. Previously we found that a segment of the AU-rich region in the 3′-UTR of the COX-2 gene regulates mRNA instability [16]. Here, we evaluated ceramide and CD for their ability to modulate COX-2 mRNA stability and our results indicate that these agents cause a dramatic increase in mRNA stability.
COX-2 expression is regulated by a variety of MAP kinase signaling pathways that varies depending on the type of inducing agent used [19,20,29,31,32]. Xie and Herschman [19,20] and Reddy et al. [29] reported that COX-2 induction by v-src, serum, and PDGF in NIH-3T3 cells is mediated through both Ras/MEKK1/JNK/c-Jun and Ras/Raf-1/MAPKK/ERK-signaling pathways. IL-1β induction of COX-2 expression in both NIH-3T3 cells and rat mesangial cells involves the activation of both JNK/SAPK and p38 MAP kinase pathways [31,32]. In rat gastric epithelial cells, Jones et al. [18] found that HGF triggers the activation of COX-2 through the ERK2 signaling pathway. Also, ceramide-mediated induction of COX-2 in human mammary epithelial cells requires activation of ERK, JNK, and p38 MAPK pathways [23]. In these previous studies, expression of kinase-defective, dominant-negative mutant kinase proteins, or treatment with kinase inhibitors inhibits the induction of COX-2 protein and mRNA. Therefore, we evaluated if these MAPK pathways also regulate COX-2 mRNA stability.
Activation of ERK signaling stabilizes COX-2 mRNA and PD98059 blocks the increase of luciferase activity in response to treatment with stabilizing agents. To establish that the effects of PD98059 on COX-2 mRNA destabilization were due to the inhibition of ERK, we transfected cells with dominant-negative ERK-1 or ERK-2 expression vectors and a luciferase reporter construct containing the COX-2 3′-UTR region cloned downstream of a luciferase reporter gene. The ERK-1 and-2 mutant proteins blocked the induction of luciferase activity in response to treatment with CD or ceramide. Matsuura et al. [17] reported that PD98059 exhibited only a slight effect on the stability of COX-2 mRNA in TGFα-treated cells. The difference between these results and our findings reported here might be due to differences in cell types and treatment regimens employed.
MAPK inhibitors (SB203580 and PD 169316) have been shown to destabilize COX-2 mRNA [17,52]. Dean et al. [52] demonstrated that following LPS induction of COX-2 in human monocytes, a p38 MAPK inhibitor (SB203580) reduced COX-2 transcription by 60%, which led to a rapid disappearance of COX-2 mRNA. Matsuura et al. [17] reported that a p38 MAPK inhibitor, PD169316, caused a dramatic decrease in the stability of COX-2 mRNA in TGFα-treated human epidermal keratinocytes. Here, we report that PD 169316 and a p38 dominant-negative protein affects COX-2 mRNA stability following treatment with ceramide or CD. From these results, we conclude that activation of the MEK-ERK1/ERK2 pathway and the p38 MAPK pathway both contribute to the stabilization of COX-2 induced by ceramide or CD.
It is clear from our results and work by others that mRNA stability plays an important role in the regulation of COX-2 expression. We provide evidence supporting an additional role of the ERK and p38 MAPK pathways in the regulation of COX-2 mRNA stability. It will be important to determine whether this process occurs in other cell types and for other immediate-early genes. Future efforts will be directed at obtaining a better understanding of the downstream pathways and molecular mechanisms responsible for this type of posttranscriptional regulation during colorectal carcinogenesis.
Acknowledgements
We acknowledge the T.J. Martell Foundation and Katie Couric for their generous support.
Abbreviations
- 3′-UTR
3′-untranslated region
- ARE
AU-rich element
- CD
chenodeoxycholic acid
- COX
cyclooxygenase
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- MAP
mitogen-activated protein
- MAPK
MAP kinase
- MEK
MAP/ERK kinase
- RIE-iRas
rat intestinal epithelial cell that conditionally expresses Ha-RasVal12
Footnotes
This work was supported in part by the United States Public Health Services Grants DK47297 (R.N.D.), P0 CA77839 (R.N.D. and R.D.B.), P30 CA68485 (R.N.D.), P01 CA77839 (R.D.B.), CA69457 (R.D.B.) and DR52334 (R.D.B.). RND is a recipient of a Veterans Affairs Research Merit Grant.
References
- 1.Smith WL, Marnett LJ. Prostaglandin endoperoxide synthase: structure and catalysis. Biochim Biophys Acta. 1991;1083:1–17. doi: 10.1016/0005-2760(91)90119-3. [DOI] [PubMed] [Google Scholar]
- 2.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 3.DuBois RN, Awad J, Morrow J, Roberts LJ, Jr, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-alpha and phorbol ester. J Clin Invest. 1994;93:493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- 5.Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993;268:9049–9054. [PubMed] [Google Scholar]
- 6.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 7.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 9.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Ushikubi F, Narumiya S, Sugimura T, Wakabayashi K. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59:5093–5096. [PubMed] [Google Scholar]
- 11.Takaku K, Sonoshita M, Sasaki N, Uozumi N, Doi Y, Shimizu T, Taketo MM. Suppression of intestinal polyposis in Apc{Delta716} knockout mice by an additional mutation in the cytosolic phospholipase A2 gene. J Biol Chem. 2000 doi: 10.1074/jbc.C000585200. (in Press) [DOI] [PubMed] [Google Scholar]
- 12.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer [see comments] N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women [see comments] N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 14.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 15.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng H, Shao J, Dixon DA, Williams CS, Prescott SM, DuBois RN, Beauchamp RD. Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J Biol Chem. 2000;275:6628–6635. doi: 10.1074/jbc.275.9.6628. [DOI] [PubMed] [Google Scholar]
- 17.Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling TE, Dannenberg AJ, Tanabe T, Inoue H, Arata J, Jetten AM. Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1999;274:29138–29148. doi: 10.1074/jbc.274.41.29138. [DOI] [PubMed] [Google Scholar]
- 18.Jones MK, Sasaki E, Halter F, Pai R, Nakamura T, Arakawa T, Kuroki T, Tarnawski AS. HGF triggers activation of the COX-2 gene in rat gastric epithelial cells: action mediated through the ERK2 signaling pathway. FASEB J. 1999;13:2186–2194. doi: 10.1096/fasebj.13.15.2186. [DOI] [PubMed] [Google Scholar]
- 19.Xie W, Herschman HR. Transcriptional regulation of prostaglandin synthase 2 gene expression by platelet-derived growth factor and serum. J Biol Chem. 1996;271:31742–31748. doi: 10.1074/jbc.271.49.31742. [DOI] [PubMed] [Google Scholar]
- 20.Xie W, Herschman HR. v-src induces prostaglandin synthase 2 gene expression by activation of the c-Jun N-terminal kinase and the c-Jun transcription factor. J Biol Chem. 1995;270:27622–27628. doi: 10.1074/jbc.270.46.27622. [DOI] [PubMed] [Google Scholar]
- 21.Perkins DJ, Kniss DA. Rapid and transient induction of cyclo-oxygenase 2 by epidermal growth factor in human amnion-derived WISH cells. Biochem J. 1997;321:677–681. doi: 10.1042/bj3210677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng H, Williams CS, Shao J, Liang P, DuBois RN, Beauchamp RD. Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:22120–22127. doi: 10.1074/jbc.273.34.22120. [DOI] [PubMed] [Google Scholar]
- 23.Subbaramaiah K, Chung WJ, Dannenberg AJ. Ceramide regulates the transcription of cyclooxygenase-2. Evidence for involvement of extracellular signal-regulated kinase/c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:32943–32949. doi: 10.1074/jbc.273.49.32943. [DOI] [PubMed] [Google Scholar]
- 24.Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Fischer SM. Transcriptional regulation of cyclooxygenase-2 in mouse skin carcinoma cells. Regulatory role of CCAAT/enhancer-binding proteins in the differential expression of cyclooxygenase-2 in normal and neoplastic tissues. J Biol Chem. 1998;273:27686–27694. doi: 10.1074/jbc.273.42.27686. [DOI] [PubMed] [Google Scholar]
- 26.Morris JK, Richards JS. An E-box region within the prostaglandin endoperoxide synthase-2 (PGS-2) promoter is required for transcription in rat ovarian granulosa cells. J Biol Chem. 1996;271:16633–16643. doi: 10.1074/jbc.271.28.16633. [DOI] [PubMed] [Google Scholar]
- 27.Xie W, Fletcher BS, Andersen RD, Herschman HR. v-src induction of the TIS10/PGS2 prostaglandin synthase gene is mediated by an ATF/CRE transcription response element. Mol Cell Biol. 1994;14:6531–6539. doi: 10.1128/mcb.14.10.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 29.Reddy ST, Wadleigh DJ, Herschman HR. Transcriptional regulation of the cyclooxygenase-2 gene in activated mast cells. J Biol Chem. 2000;275:3107–3113. doi: 10.1074/jbc.275.5.3107. [DOI] [PubMed] [Google Scholar]
- 30.Barry OP, Kazanietz MG, Pratico D, FitzGerald GA. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999;274:7545–7556. doi: 10.1074/jbc.274.11.7545. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J Physiol (London) 1999;520(Pt 2):399–406. doi: 10.1111/j.1469-7793.1999.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan Z, Buckman SY, Pentland AP, Templeton DJ, Morrison AR. Induction of cyclooxygenase-2 by the activated MEKK1, SEK1/MKK4, p38 mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:12901–12908. doi: 10.1074/jbc.273.21.12901. [DOI] [PubMed] [Google Scholar]
- 33.Paul A, Cuenda A, Bryant CE, Murray J, Chilvers ER, Cohen P, Gould GW, Plevin R. Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell Signal. 1999;11:491–497. doi: 10.1016/s0898-6568(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 34.Kaibara N, Yurugi E, Koga S. Promoting effect of bile acids on the chemical transformation of C3H/10T1/2 fibroblasts in vitro. Cancer Res. 1984;44:5482–5485. [PubMed] [Google Scholar]
- 35.Reddy BS, Mangat S, Weisburger JH, Wynder EL. Effect of high-risk diets for colon carcinogenesis on intestinal mucosal and bacterial beta-glucuronidase activity in F344 rats. Cancer Res. 1977;37:3533–3536. [PubMed] [Google Scholar]
- 36.Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N′-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974;53:1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- 37.Reddy BS, Wynder EL. Large-bowel carcinogenesis: fecal constituents of populations with diverse incidence rates of colon cancer. J Natl Cancer Inst. 1973;50:1437–1442. doi: 10.1093/jnci/50.6.1437. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F, Subbaramaiah K, Altorki N, Dannenberg AJ. Dihydroxy bile acids activate the transcription of cyclooxygenase-2. J Biol Chem. 1998;273:2424–2428. doi: 10.1074/jbc.273.4.2424. [DOI] [PubMed] [Google Scholar]
- 39.Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–496. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 40.Ballou LR, Chao CP, Holness MA, Barker SC, Raghow R. Interleukin-1-mediated PGE2 production and sphingomyelin metabolism. Evidence for the regulation of cyclooxygenase gene expression by sphingosine and ceramide. J Biol Chem. 1992;267:20044–20050. [PubMed] [Google Scholar]
- 41.Kirtikara K, Laulederkind SJ, Raghow R, Kanekura T, Ballou LR. An accessory role for ceramide in interleukin-1beta induced prostaglandin synthesis. Mol Cell Biochem. 1998;181:41–48. doi: 10.1023/a:1006824009546. [DOI] [PubMed] [Google Scholar]
- 42.Reunanen N, Westermarck J, Hakkinen L, Holmstrom TH, Elo I, Eriksson JE, Kahari VM. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J Biol Chem. 1998;273:5137–5145. doi: 10.1074/jbc.273.9.5137. [DOI] [PubMed] [Google Scholar]
- 43.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302:723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, Takahashi E, Tanabe T. Characterization of the human gene PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur J Biochem. 1994;221:889–897. doi: 10.1111/j.1432-1033.1994.tb18804.x. [DOI] [PubMed] [Google Scholar]
- 46.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Yang YC. Regulation of interleukin (IL)-11 gene expression in IL-1 induced primate bone marrow stromal cells. J Biol Chem. 1994;269:32732–32739. [PubMed] [Google Scholar]
- 48.Henics T, Sanfridson A, Hamilton BJ, Nagy E, Rigby WF. Enhanced stability of interleukin-2 mRNA in MLA 144 cells. Possible role of cytoplasmic AU-rich sequence-binding proteins. J Biol Chem. 1994;269:5377–5383. [PubMed] [Google Scholar]
- 49.Ristimaki A, Garfinkel S, Wessendorf J, Maciag T, Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J Biol Chem. 1994;269:11769–11775. [PubMed] [Google Scholar]
- 50.Newton R, Seybold J, Kuitert LM, Bergmann M, Barnes PJ. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and posttranscriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem. 1998;273:32312–32321. doi: 10.1074/jbc.273.48.32312. [DOI] [PubMed] [Google Scholar]
- 51.Barrios-Rodiles M, Tiraloche G, Chadee K. Lipopolysaccharide modulates cyclooxygenase-2 transcriptionally and posttranscriptionally in human macrophages independently from endogenous IL-1 beta and TNF-alpha. J Immunol. 1999;163:963–969. [PubMed] [Google Scholar]
- 52.Dean JL, Brook M, Clark AR, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J Biol Chem. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- 53.Reddy BS, Watanabe K, Weisburger JH, Wynder EL. Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats. Cancer Res. 1977;37:3238–3242. [PubMed] [Google Scholar]
- 54.McMillan L, Butcher S, Wallis Y, Neoptolemos JP, Lord JM. Bile acids reduce the apoptosis-inducing effects of sodium butyrate on human colon adenoma (AA/C1) cells: implications for colon carcinogenesis. Biochem Biophys Res Commun. 2000;273:45–49. doi: 10.1006/bbrc.2000.2899. [DOI] [PubMed] [Google Scholar]
- 55.Huang XP, Fan XT, Desjeux JF, Castagna M. Bile acids, non-phorbol-ester-type tumor promoters, stimulate the phosphorylation of protein kinase C substrates in human platelets and colon cell line HT29. Int J Cancer. 1992;52:444–450. doi: 10.1002/ijc.2910520319. [DOI] [PubMed] [Google Scholar]
- 56.Qiao D, Chen W, Stratagoules ED, Martinez JD. Bile acid-induced activation of activator protein-1 requires both extracellular signal-regulated kinase and protein kinase C signaling. J Biol Chem. 2000;275:15090–15098. doi: 10.1074/jbc.M908890199. [DOI] [PubMed] [Google Scholar]
- 57.Hirano F, Tanada H, Makino Y, Okamoto K, Hiramoto M, Handa H, Makino I. Induction of the transcription factor AP-1 in cultured human colon adenocarcinoma cells following exposure to bile acids. Carcinogenesis. 1996;17:427–433. doi: 10.1093/carcin/17.3.427. [DOI] [PubMed] [Google Scholar]
- 58.Lozano J, Berra E, Municio MM, Diaz-Meco MT, Dominguez I, Sanz L, Moscat J. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 59.Sbrissa D, Yamada H, Hajra A, Bitar KN. Bombesin-stimulated ceramide production and MAP kinase activation in rabbit rectosigmoid smooth muscle cells. Am J Physiol. 1997;272:G1615–G1625. doi: 10.1152/ajpgi.1997.272.6.G1615. [DOI] [PubMed] [Google Scholar]
- 60.Newton R, Seybold J, Liu SF, Barnes PJ. Alternate COX-2 transcripts are differentially regulated: implications for post-transcriptional control. Biochem Biophys Res Commun. 1997;234:85–89. doi: 10.1006/bbrc.1997.6586. [DOI] [PubMed] [Google Scholar]