Abstract
Neutrophils represent a potential source of genotoxic reactive oxygen and nitrogen species in the tumor microenvironment. Using Mutatect cell lines, which can form subcutaneous tumors in syngeneic C57BL/6 mice, we have previously established that the number of spontaneously infiltrating neutrophils correlates with the number of mutations at the hypoxanthine phosphoribosyltransferase (Hprt) locus. We now describe the properties of four lines that express different levels of the neutrophil chemokine, interleukin-8 (IL-8), from a tetracycline (TET)-responsive promoter. In a series involving 45 animals, IL-8-expressing lines produced tumors with a higher neutrophil content than the control line. Analysis of the 45 tumors revealed that the neutrophil level again strongly correlated with hprt mutant frequency (MF) (P<.0001, r=0.88). Administration of TET was effective in lowering the neutrophil content of low IL-8-expressing tumors, but not high IL-8-expressing tumors. Although the IL-8 transgene was stable in all lines in vitro, high IL-8-expressing lines completely lost the transgene in vivo whereas low IL-8-expressing lines showed no evidence of transgene instability. These results provide further evidence, based on the study of an endogenous gene (hprt) and an IL-8 transgene, that neutrophils may contribute to genetic instability in tumors.
Keywords: disease models, animal, neutrophils, genomic instability, reactive oxygen species
Introduction
Solid tumors are frequently infiltrated with inflammatory cells such as lymphocytes, neutrophils, and/or macrophages, which are sources of cytotoxic and genotoxic reactive nitrogen oxygen species (RNOS). Genetic instability is a common feature of solid tumors and we and others have proposed that mutagenic RNOS generated by these tumor-infiltrating cells are, in some measure, responsible for the accumulation of mutations associated with tumour progression [1–4]. We have established the Mutatect model as an experimental paradigm to study the contribution of RNOS to the induction of mutations in vivo. This model permits the detection of mutations in cells recovered from subcutaneous tumors in syngeneic C57BL/6 mice [2,5]. The seminal observation arising from these studies is a four-fold higher frequency of cells bearing a mutation affecting hypoxanthine phosphoribosyltransferase (Hprt) function in vivo than in vitro [2,6]. This led us to examine the possibility that mutagenic factors are present in the tumor microenvironment. Administration of an antioxidant, vitamin E, to mice bearing Mutatect tumors markedly lowers the mutation frequency [6]. Mutatect tumors are infiltrated with host cells, predominantly neutrophils — a potential source of mutagenic RNOS [7]. Neutrophils stained positively for inducible nitric oxide synthase and there was a correlation between nitric oxide synthase activity and the frequency of Hprt- mutant cells (mutant frequency, MF). Further indication of the presence of RNOS was that the tumor cells stained for nitrotyrosine (an immunohistochemical marker of RNOS) [7], Thus, in the Mutatect model, infiltrating neutrophils appear to be a source of potentially mutagenic RNOS.
Interleukin-8 (IL-8) is a human C-X-C chemokine for neutrophils and an angiogenic factor [8–11]. This proinflammatory cytokine is expressed in many human tumors, such as melanoma [12,13], non-small cell lung tumors [14], bronchioloalveolar carcinoma [15], head and neck squamous carcinoma [16], gastric carcinomas [17], colon carcinoma [18], mesothelioma [19], and various glioblastomas [20,21]. Human IL-8 is also a chemoattractant for mouse neutrophils and other non-human species [9,22,23]. In a previous Mutatect study, we have demonstrated that direct intratumoral injection of IL-8 increases both the number of neutrophils and the MF [7].
In the present report, we describe a series of Mutatect cell lines that express different levels of IL-8 from a tetracycline (TET)-regulatable promoter. Tumors initiated with these cell lines were examined for their neutrophil content, Hprt- MF, and the stability of the transfected IL-8 gene. We report that IL-8 can have pleiotropic effects that are dependent on the level of IL-8 expression.
Materials and Methods
Reagents
Fluorescamine, diaminobenzoic acid, hypoxanthine, aminopterin, thymidine, 6-thioguanine (6-TG), and TET hydrochloride for cell culture were from Sigma-Aldrich (Oakville, Canada). G-418 was from Gibco-BRL (Burlington, Canada) and rabbit anti-myeloperoxidase (MPO) polyclonal antibody was from Lab Vision Corporation (Fremont, CA). TET hydrochloride administered to animals in the drinking water was diluted from a flavoured suspension for oral use (Novo-Tetra, Novopharm, Toronto, Canada).
Mutated Cell Lines
The derivation of the Mutatect model has been described earlier [5]. Conditions for cell culture, detection of Hprt- mutants, and removal of pre-existing mutant cells prior to formation of subcutaneous tumours have been reported elsewhere [2,7]. Mutatect cell lines used in this report include MC-TGS17-51 (referred to as MC17-51), MT-6, and IL-8-expressing TM-3, TM-7, TM-28, TM-34. Development of MT-6 and IL-8 expressing cell lines is described in the Results section. Unless otherwise stated, all cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal calf serum (FCS, Gibco-BRL) in a humidified atmosphere of 5% CO2/95% O2 at 37°C.
Construction of pTRE-IL-8 Plasmid
A human IL-8 cDNA that is able to express biologically active IL-8 protein in Mutatect cells was used to prepare the pTRE-IL-8 plasmid. An 833-bp fragment, containing the entire coding region of IL-8, was isolated by digestion of pcDNA3-IL-8 construct (clone L25-13) (Haqqani, Sandhu, and Birnboim, submitted for publication) with BamHI. A mammalian expression vector, pTRE (Clontech, Palo Alto, CA), was also digested with BamHI. The fragment and the vector were ligated and transformed into competent SURE Escherichia coli (Stratagene Cloning Systems, La Jolla, CA), and ampicillin-resistant clones were selected. pTRE-IL-8 (clone L1-2) was isolated. It contained the IL-8 coding region in a sense orientation (confirmed by restriction endonuclease analysis) under a response element (tTA response element, TRE) for a TET-responsive transcriptional activator (tTA) (Clontech).
Construction of Regulatable IL-8-Expressing Mutatect Cell Lines
The Tet-Off gene expression system (Clontech) was used to construct TET-regulatable IL-8 expressing Mutatect cell lines. In the first step, a pTet-Off plasmid containing genes for the tTA and neomycin (neo) was linearized with ScaI and transfected into MC17-51 cells using DODAC:DOPE LuV liposomes (a gift from INEX Pharmaceuticals Corp., Vancouver, Canada). After 48 hours in culture, 0.5 mg/mL G-418 was added and stable G-418-resistant (MT) clones were selected. MT-6 clone, containing tTA activity that was completely suppressible by TET, was identified as described in the Results section. In the second step, MT-6 cells were co-transfected with the pTRE-IL-8 construct (linearized with ScaI) and pTK-Hyg plasmid (Clontech); cells were maintained in 10 µg/mL TET hydrochloride throughout the transfection. After 48 hours in culture, 0.3 mg/mL of hygromycin (Roche Diagnostics, Laval, Canada) was added, and hygromycin-resistant (TM) stable clones were selected. As described in the Results section, several IL-8-expressing TM clones whose expression could be regulated by TET were identified.
Competitive ELISA for Detection of IL-8 in Culture Medium
Analysis of IL-8 in culture medium was carried out using a competitive ELISA with rabbit anti-human IL-8 polyclonal antibody (Endogen, Woburn, MA), as described elsewhere (Haqqani, Sandhu, and Birnboim, submitted for publication). The assay was essentially linear in the range 0.02 to 1.50 ng of IL-8 per milliliter. Each assay was performed in triplicate.
Mutatect Tumor Formation, Size Measurements, and Tumor Fractionation
Subcutaneous tumors of MT-6, TM-3, TM-7, TM-28, and TM-34 cells were initiated by injection of 5x105 cells in 0.1 mL of PBS into syngeneic, 8 to 10 week old C57BL/6 female mice. For in vitro controls, the same cell types were grown in culture for the entire period of in vivo tumor growth, with subculturing twice weekly. Where indicated, 0.4 mM TET was added to the drinking water, which was protected from light and changed every 2 to 3 days. Once tumors reached a size where they could be measured with calipers (about 1 week after injection), three dimensions of each tumor were measured every 2 to 3 days. Tumor volumes were approximated by the following equation of a hemi-ellipsoid:
where V is the volume of the tumor in cubic millimeters. Once the largest diameter of the control MT-6 tumors was about 1 cm (about 3 weeks), all tumor-bearing mice were euthanized. About one fourth of each tumor was used for histological analysis and isolation of DNA for polymerase chain reaction (PCR) analysis of the IL-8 transgene. The remainder in 5 mL of DMEM plus 10% FCS was minced lightly, mechanically dispersed by repeated passage through a 3-mL syringe, then allowed to settle in a 15-mL tube at 0°C for 5 minutes. The supernatant (cellular fraction) was separated from the particulate (stromal fraction) and analyzed for MPO activity (a neutrophil-specific marker), frequency of Hprt- mutations (MF), and protein. A fluorometric method employing diaminobenzoic acid was used for quantification of DNA [24]. Ex vivo cultures from the cellular fraction were also analyzed for IL-8 protein in the culture supernatant by ELISA. All experiments using animals were carried out at the Animal Care and Veterinary Service of the University of Ottawa in accordance with guidelines of the Canadian Council on Animal Care.
Analysis of Hprt- Mutants
Details of this analysis have been described elsewhere [2]. In brief, the frequency of Hprt- mutants was measured in Mutatect cells growing ex vivo and in vitro. Cells from the cellular fraction (1x105 viable cells) of each tumor were plated in triplicate in 10-cm dishes in medium containing 50 µM 6-TG. After 12 days of incubation, the number of 6-TG-resistant colonies (representing Hprt- mutants) was counted and corrected for plating efficiency. MF is expressed as the number of Hprt- mutants per 1x105 clonable tumor cells.
Detection of IL-8 gene loss by PCR
Genomic DNA was isolated from cultured cells or from total Mutatect tumors as described elsewhere [25]. A fragment from the integrated IL-8 transgene was amplified by PCR analysis (forward primer: GAGGCCTAT ATAAGCAGAGC; reverse primer: AGAGCTGCAGAAAT CAGGAA). The 241-bp PCR product corresponded to 137 bp of the pTRE promoter and 104 bp of the 5′ end of IL-8 cDNA (i.e., the entire 5′ untranslated region and the first 5′ nucleotides of the coding region). Failure to detect the specific 241 bp PCR, while detecting a non-specific 670 bp fragment product on an ethidium bromide-stained agarose gel, was taken as evidence of IL-8 gene loss.
Tumor MPO
The cellular fraction of excised tumors (described above) was suspended in PBS, homogenized, and MPO was solubilized with 0.2% hexadecyltrimethylammonium bromide (Sigma-Aldrich). Protein was quantified using fluorescamine [26,27]. MPO activity was measured by a specific assay based on bromide-dependent chemiluminescence of luminol at pH 5 [28]. Each sample was assayed in triplicate.
Statistical Analysis
Nonparametric statistical tests were used, unless otherwise indicated. The Mann-Whitney U test was used to compare two unpaired groups. Spearman rank correlation was used to determine a correlation between two measured variables. A value of P < .05 was considered to be statistically significant. All P values shown are two-tailed.
Results
Construction of Regulatable IL-8-Expressing Mutatect TM Cell Lines
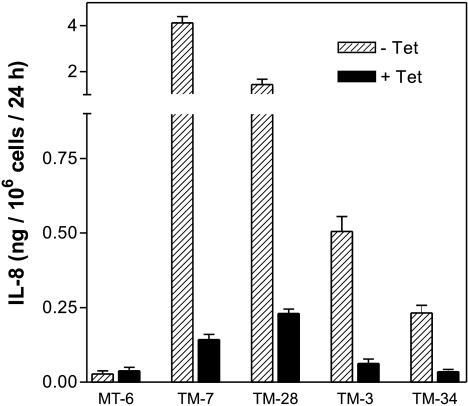
The preparation of TET-regulatable IL-8-expressing cell lines was accomplished in two steps. First, a stable cell line expressing tTA was isolated on the basis of a linked neo gene. Mutatect MC17-51 cells were transfected with tTA/neo and 15 stable G-418-resistant (MT) clones were selected. tTA is a transcriptional activator that can bind to a TRE on an expression plasmid and activate transcription of downstream inserted genes. TET prevents tTA-mediated activation. To screen G418-resistant clones for tTA activity, MT clones were transiently transfected with the pTRE-IL-8 construct and expression of IL-8 was measured by ELISA. Four clones had detectable IL-8 in the culture medium, indicating tTA activity. This was repeated in the presence of TET, which should inhibit tTA activity and IL-8 expression. Of the four clones tested, MT-6 responded best to TET and was chosen for the next step. MT-6 cells were co-transfected with pTRE-IL-8 and a plasmid containing a hygromycin resistance gene. Stable hygromycin-resistant clones (TM series) were isolated and grown in the absence or presence of TET and their culture media were analyzed for IL-8. Media from 13 of 40 clones had detectable IL-8 (data not shown), but only four clones exhibited stable IL-8 expression and effective TET-mediated suppression of IL-8 (Figure 1). Although TET was routinely added to culture medium at 1 µM, IL-8 expression could be inhibited completely by 80 nM (data not shown). This series of TET-regulatable TM clones was used for further experiments and the parental MT-6 clone was used as a control, non-IL-8-expressing cell line.
Figure 1.
TET-responsive IL-8-expressing Mutated TM cell lines. Cells were grown in the absence (hatched bars) or presence of 1 µM TET (filled bars) and IL-8 was detected in the culture media by ELISA. MT-6 is a control, non-IL-8-expressing cell line. Error bars represent mean±SEM of triplicate values.
Tumorigenicity of TM Cell Lines
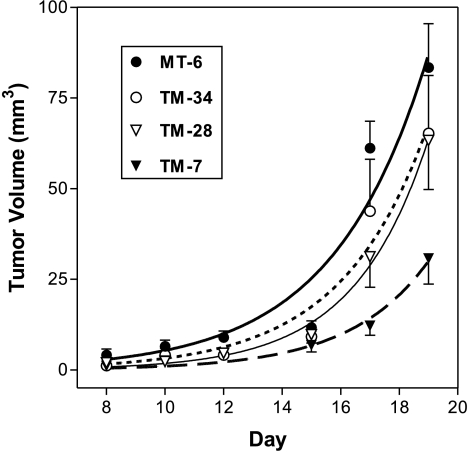
Mutatect cells grow to 1 cm subcutaneous tumors in syngeneic C57BL/6 mice within 2 to 3 weeks. To study the effect of IL-8 expression on tumorigenicity of Mutatect cells, the growth rate of the four TET-regulatable, IL-8-expressing TM clones was compared to the parental MT-6 clone. Cells from each line were injected into groups of eight mice and the volume of tumors was monitored throughout their development. We have recently shown that constitutive high-level IL-8 expression results in very poor subcutaneous growth of Mutatect tumors (Haqqani, Sandhu, and Birnboim, submitted for publication). In an attempt to improve growth during the early phase of tumor development, all mice were administered TET in their drinking water starting from the time of tumor cell injection to suppress IL-8 expression. After 7 days, one half of the animals in each group was given TET-free drinking water and the remainder was maintained on TET. No statistically significant difference in the tumor growth was seen as a function of TET for any clone (data not shown). The average tumor volume for each clone is presented in Figure 2. TM-7 cells produced smaller tumors on day 19 than control MT-6 cells (P=.03), with growth kinetics indicating a lag phase at least 1 week longer than MT-6 tumors (Figure 2). The in vitro growth rates of TM-7 and MT-6 cells were very similar, with doubling times of 14 to 16 hours (data not shown). Tumors initiated with all other TM clones (including TM-3, not shown in the figure) did not differ significantly from MT-6 tumors in their growth characteristics (Figure 2). Thus, cells expressing very high levels of IL-8 seem to be less tumorigenic than cells expressing lower levels.
Figure 2.
Comparison of tumor volumes at the indicated time following subcutaneous injection of Mutatect MT-6, TM-34, TM-28, or TM-7 cells in syngeneic C57BL/6 mice. Tumor volume was calculated as described in the Materials and Methods section. Each point represents mean±SEM of eight animals.
MPO Levels in TM Tumors
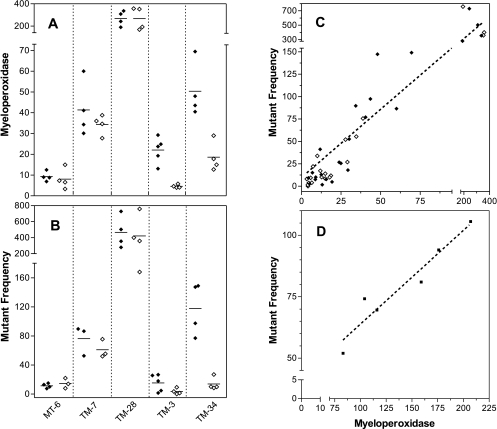
Human IL-8 is a potent chemoattractant for mouse neutrophils and other non-human species [22,23]. To examine the effect of IL-8 expression on neutrophil infiltration in the TM tumors, MPO (a neutrophil-specific enzyme) was measured in the cellular fraction. All four groups of TM tumors had a statistically significantly higher level of MPO than non-expressing MT-6 tumors (Figure 3A; P<.05 for each). This experiment demonstrated that IL-8 expression could increase neutrophil infiltration into tumors. MPO was also measured in mice maintained on TET throughout tumor development to lower IL-8 expression. TM-7, which had the highest in vitro production of IL-8 (Figure 1), had relatively low levels of MPO in tumors and there was no effect of TET in the drinking water. TM-28, which had the next highest in vitro production of IL-8, had the highest level of MPO in tumors and also showed no effect of TET. TM-3 and TM-34, which had the lowest in vitro production of IL-8, had moderate levels of MPO, but showed a clear response to TET in the drinking water. These results indicate that IL-8 expression can increase the number of neutrophil-induced mutations in Mutatect tumors and that this can be regulated in vivo in the case of low IL-8-expressing, TET-responsive lines.
Figure 3.
MPO and hprt mutant frequency in Mutatect tumors at day 19. TET (0.4 mM) was added to the drinking water of mice either throughout tumor development (open symbols) or only for the first week (filled symbols). (A) MPO (units per microgram of protein) was measured in the cellular fraction of tumors. (B) Mutant frequency (Hprt- mutants per 1x105 viable G418R cells) was measured in cells taken from the cellular fraction of tumors. For both panels A and B, each point represents a single tumor and the horizontal bar represents the mean. (C) Correlation between MPO activity (panel A) and Hprt- MF (panel B). Spearman rank correlation: n=45, P<.0001, r=0.88. (D) Results from a separate experiment using TM-28 tumors in animals receiving no TET. Spearman rank correlation: n=6, P=.O17, r=0.94.
Hprt- MF in TM Tumors
To investigate the effect of IL-8 expression on the MF, cells recovered from TM tumors were challenged with 6-TG to detect Hprt- mutants. The MF (Figure 3B) closely paralleled the measurements of MPO (Figure 3A). TM tumours in animals not maintained on TET had a significantly higher MF than MT-6 (P<.05 for each), with the exception of TM-3 tumors. Of the animals maintained on TET, only TM-34 tumors showed a statistically significant reduction in MF (P=.03). The reduction seen with TM-3 tumors was not statistically significant.
Correlation Between MPO and MF
The number of Mutatect tumor-infiltrating neutrophils has recently been shown to correlate strongly with the MF [6,7]. To determine whether a similar correlation exists for other IL-8-secreting cell lines, the 45 tumors shown in Figure 3A and B were analyzed. This group included animals that received TET either for 1 week (controls) or for the entire tumor growth period (treatment group). A strong statistical correlation between MPO and MF was seen (P<.0001, r=0.88) (Figure 3C). A separate experiment using TM-28 tumors grown in animals that received no TET is shown in Figure 3D. A similar strong correlation between MPO and MF was seen (P=.017, r=0.94).
In Vivo IL-8 Transgene Instability in TM Tumors
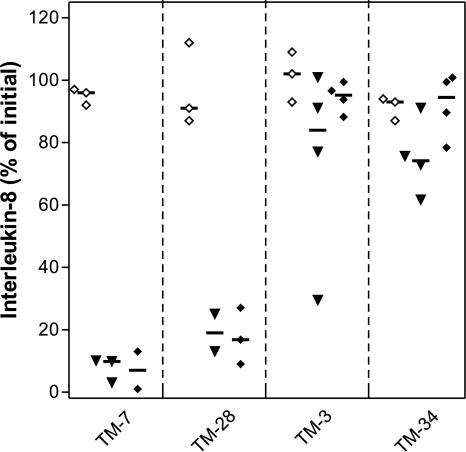
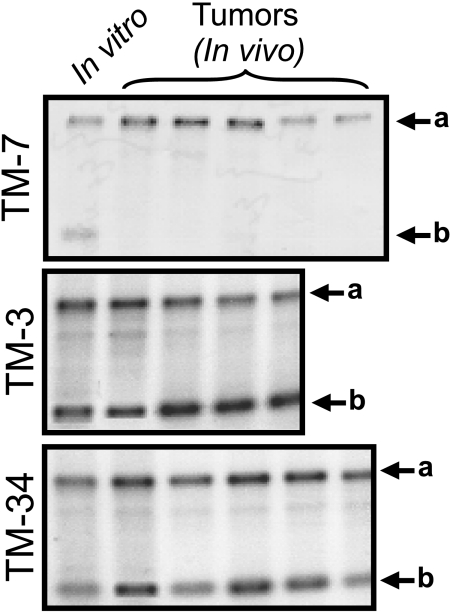
The stability of the IL-8 transgene was studied in TM tumors in animals with or without TET. IL-8 protein expression was measured by ELISA in the supernatants of ex vivo cultures of TM tumor cells and in vitro cultures of the same cells that had never been passaged through an animal (Figure 4). The presence of TET in the drinking water had no discernable effect on the level of IL-8 in ex vivo cultures from any of the four tumor types (Figure 4; filled diamonds versus filled triangles). There was no significant difference in IL-8 expression between ex vivo and in vitro cultures of TM-3 and TM -34 cells. By contrast, IL-8 expression was very low in ex vivo cultures of TM-7 and TM-28 tumors compared to their in vitro controls (P<.0001 and P=.0002, respectively; two-tailed t test). Loss of the pTRE-IL-8 transgene as a possible cause for the low IL-8 expression was studied by PCR analysis of a 241-bp fragment corresponding to the 5′ end of the IL-8 cDNA. DNA from total tumors was isolated. In agreement with measurements of IL-8 protein expression, this IL-8-specific sequence was absent in DNA from TM-7 tumors (Figure 5) or TM-28 tumors (data not shown), whereas DNA from TM-3 and TM-34 tumors retained this sequence (Figure 5). Thus, TM-7 and TM-28 cell lines, which initially expressed high levels of IL-8 (Figure 1), lost their IL-8 transgene in vivo, whereas TM-3 and TM-34 cells, which initially expressed lower levels of IL-8, retained the transgene.
Figure 4.
IL-8 in the medium of Mutatect cells either taken from 19-day-old tumors and cultured ex vivo for 7 days (solid symbols) or cells grown in vitro for the equivalent period of time (empty symbols). TET (0.4 mM) was added to the drinking water of mice either throughout tumor development (solid diamonds) or only for the week (solid triangles). The ordinate represents the percent of the initial amount of IL-8 detected in culture in various cells lines prior to injection into animals. IL-8 was detected in the culture media by ELISA as described in the Materials and Methods section.
Figure 5.
Detection of the IL-8 transgene by PCR in 19-day-old tumors. Genomic DNA was extracted from unfractionated TM-7, TM-3, or TM-34 tumors and from same cell types growing in culture for the equivalent period of time (in vitro). The lower bands (arrow b) correspond to an IL-8-specific 241-bp fragment, whereas the upper bands (arrow a) correspond to a non-specific 670-bp fragment used as an internal control for the PCR reaction. Other details as in the Materials and Methods section.
Discussion
IL-8 is a proinflammatory cytokine that is frequently found in human tumors. Its presence is associated with extensive neutrophil infiltration, increased vascularization, and poor prognosis [15–17]. Neutrophils and macrophages are a source of RNOS, known to be genotoxic and cytotoxic. Earlier, we proposed that RNOS generated by these tumor-infiltrating cells might be mutagenic and thereby contribute to the accumulation of mutations in tumors [2]. The Mutatect experimental tumor model was established to test this hypothesis and it has provided the first direct evidence that factors in a tumor microenvironment could be mutagenic [2]. Recently, we demonstrated a strong positive correlation between the number of tumor-infiltrating neutrophils and the MF of tumor cells [6,7]. In the first study, a single intratumoral injection of IL-8 was shown to increase both the number of neutrophils and the MF. To provide a more biologically meaningful source of intratumoral IL-8, we constructed TET-regulatable IL-8-expressing cell lines in which the level of expression could be downregulated in vivo by TET in the drinking water.
Four clones that stably expressed IL-8 in vitro in a TET-regulatable fashion were studied. Clone TM-7, which expressed the highest level of IL-8 in vitro, grew very slowly as a subcutaneous tumor. The other three clones, which expressed lower levels of IL-8, grew at an intermediate rate compared to the parental line. In vivo administration of TET produced no change in MPO or MF levels in TM-7 and TM-28 tumors but lowered the levels in TM-3 and TM-34 tumors, although in vitro all four could be regulated by TET. In TM-3 and TM-34, it reduced MPO (indicative of a reduced number of infiltrating neutrophils), a consequence of which was a reduction in MF. Both TM-7 and TM-28 produced appreciably higher levels of IL-8 in vitro than TM-3 and TM-34. When the level of IL-8 in the culture supernatant was measured in cells recovered from all four tumor types, it was found that cells from TM-7 and TM-28 had largely lost their ability to produce IL-8 while cells from TM-3 and TM-34 recovered from tumors continued to express IL-8. Loss of the IL-8 transgene in TM-7 and TM-28 tumors appeared to be responsible for the loss of IL-8 expression.
The loss of the IL-8 transgene may be the result of complex interactions between high-level IL-8-producing cells and tumor-infiltrating neutrophils. It does not seem to be due to different sites of integration of the transgene in different cell lines since in vitro expression of IL-8 by all lines was stable. Rather, we postulate that chemoattraction of a large number of neutrophils early in the development of tumors by a high level of IL-8 is responsible. Neutrophils can be both cytotoxic and genotoxic. Frequency of loss of IL-8 in vivo was extremely high, approaching 100% in TM-7 and TM-28 tumors, which contrasts with the frequency of mutations at the Hprt locus, where it may approach 1% at its highest. We therefore postulate that selective cytotoxicity toward IL-8-producing cells must be occurring. Because IL-8 can delay apoptosis in neutrophils [29], IL-8-expressing tumor cells may attract neutrophils, while at the same time prolonging their life and extending their cytotoxic capability. The combination of RNOS-mediated genotoxicity (which fosters loss of the transgene) and selective cytotoxicity toward the remaining IL-8-producing cells may explain why cells lacking the transgene eventually dominate the tumor. To explain why TET had an effect in only some tumors, we postulate that TET could not reduce IL-8 sufficiently in high-expressing TM-7 and TM-28 tumors to levels below the chemoattractant threshold. The apparent paradox that the highest IL-8 producer, TM-7, showed relatively low MPO and an intermediate IL-8 producer, TM-28, showed much higher MPO may be explained by the fact that MPO is measured after 3 weeks of tumor growth. TM-7 may have attracted a large number of neutrophils at an early stage in tumorigenesis, causing early loss of the IL-8 transgene. TM-28 may have attracted fewer neutrophils at an early stage, leading to a delay in loss of the transgene. Since the MF correlates well with neutrophil number determined at the time tumors are harvested, this suggests that the process of mutagenesis occurs throughout tumour development.
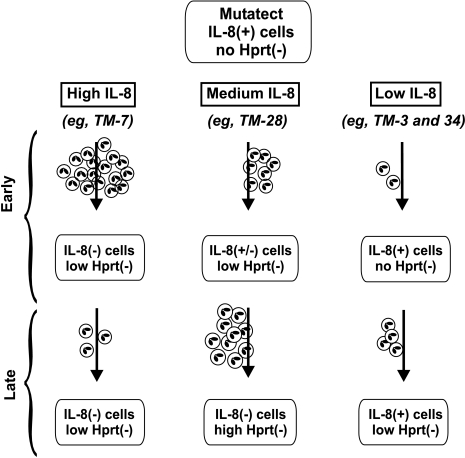
The model presented in Figure 6 summarizes some of the complex biological effects that we have observed in Mutatect tumors secreting different levels of IL-8. It is proposed that the level of IL-8 expression can have a strong influence on tumor biology. At high levels, the predominant effect may be early influx of neutrophils responsible for extensive cytotoxicity and loss of the IL-8 transgene. At lower levels, less cytotoxicity and transgene loss may occur, but there may still be a sufficient neutrophil-generated RNOS to mediate mutagenicity.
Figure 6.
Model of genetic changes occurring in Mutatect tumors expressing different levels of IL-8. High IL-8-expressing lines such as TM-7 attract large number of neutrophils early in tumor development. This produces high cytotoxicity and a lag in tumor growth (not indicated). Neutrophil-derived RNOS produce genotoxicity, leading to loss of the IL-8 transgene and enrichment of these IL-8(-) cells for reasons described in the Discussion section. Hprt (-) mutations are also induced, but there is no enrichment of these mutant cells. Later in tumor development, once IL-8(-) cells dominate the tumor, few neutrophils are attracted. Low IL-8-expressing lines such as TM-3 and TM-34 attract relatively few neutrophils early in tumor development. Cytotoxicity and genotoxicity are relatively low, with no loss of the IL-8 transgene and continued low level attraction of neutrophils both early and late in tumor development. Medium IL-8-expressing lines such as TM-28 attract an intermediate number of neutrophils. This produces intermediate genotoxicity: partial loss of the IL-8 transgene and a low number of hprt (-) mutants. Although the IL-8 transgene continues to be lost, IL-8 levels are sufficient to continue to attract neutrophils. By the end of the experiment when tumors are harvested, neutrophils are still present and a large number of hprt (-) mutant cells have accumulated.
Acknowledgements
We thank Donna Grant and Denise Proulx for their excellent technical assistance. This work was supported by Medical Research Council of Canada grant no. MT-8728 to H.C.B. A.S.H. is a recipient of a Doctoral Research Award from Medical Research Council of Canada. H.C.B. is a Senior Career Scientist of Cancer Care Ontario.
Abbreviations
- 6-TG
6-thioguanine
- Hprt
hypoxanthine phosphoribosyltransferase
- IL-8
interleukin-8
- MF
mutant frequency
- MPO
myeloperoxidase
- neo
neomycin
- RNOS
reactive nitrogen oxygen species
- TET
tetracycline
- TRE
tTA response element
- tTA
TET-responsive transcriptional activator
References
- 1.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res Fundam Mol Mech Mutagen. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson D, Sandhu JK, Breneman JW, Tucker JD, Birnboim HC. Hprt mutants in a transplantable murine tumour arise more frequently in vivo than in vitro. Br J Cancer. 1995;72:1234–1240. doi: 10.1038/bjc.1995.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamir S, Tannenbaum SR. The role of nitric oxide (NO) in the carcinogenic process. Biochim Biophys Ada Rev Cancer. 1996;1288:F31–F36. doi: 10.1016/0304-419x(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 4.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 5.Birnboim HC, Wilkinson D, Sandhu JK, McLean JR, Ross W. Mutatect: a mouse tumour model for detecting radiation-induced mutations in vivo. Mutat Res. 1999;430:275–280. doi: 10.1016/s0027-5107(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 6.Sandhu JK, Haqqani AS, Birnboim HC. Effect of dietary vitamin E on spontaneous or nitric oxide donor-induced mutations in a mouse tumor model. J Natl Cancer Inst. 2000;92:1429–1433. doi: 10.1093/jnci/92.17.1429. [DOI] [PubMed] [Google Scholar]
- 7.Sandhu JK, Privora HF, Wenckebach G, Birnboim HC. Neutrophils, nitric oxide synthase, and mutations in the Mutatect murine tumor model. Am J Pathol. 2000;156:509–518. doi: 10.1016/S0002-9440(10)64755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene intercrine cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 9.Furuta R, Yamagishi J, Kotani H, Sakamoto F, Fukui T, Matsui Y, Sohmura Y, Yamada M, Yoshimura T, Larsen CG. Production and characterization of recombinant human neutrophil chemotactic factor. J Biochem (Tokyo) 1989;106:436–441. doi: 10.1093/oxfordjournals.jbchem.a122870. [DOI] [PubMed] [Google Scholar]
- 10.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 11.Schröder JM, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin-1 activity. J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]
- 12.Singh RK, Varney ML, Bucana CD, Johansson SL. Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res. 1999;9:383–387. doi: 10.1097/00008390-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin-8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- 14.Colasante A, Mascetra N, Brunetti M, Lattanzio G, Diodoro M, Caltagirone S, Musiani P, Aiello FB. Transforming growth factor beta-1, interleukin-8 and interleukin-1, in non-small cell lung tumors. Am J Respir Crit Care Med. 1997;156:968–973. doi: 10.1164/ajrccm.156.3.9701122. [DOI] [PubMed] [Google Scholar]
- 15.Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J. Neutrophil alveolitis in bronchioalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 16.Eisma RJ, Spiro JD, Kreutzer DL. Role of angiogenic factors: coexpression of interleukin-8 and vascular endothelial growth factor in patients with head and neck squamous carcinoma. Laryngoscope. 1999;109:687–693. doi: 10.1097/00005537-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ, Tahara E. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93–100. [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto K, Masuda T, Mita S, Ishiko T, Nakashima Y, Arakawa H, Egami H, Harada S, Matsushima K, Ogawa M. Interleukin-8 is constitutively and commonly produced by various human carcinoma cell lines. Int J Clin Lab Res. 1992;22:216–219. doi: 10.1007/BF02591427. [DOI] [PubMed] [Google Scholar]
- 19.Galffy G, Mohammed KA, Dowling PA, Nasreen N, Ward MJ, Antony VB. Interleukin-8: an autocrine growth factor for malignant mesothelioma. Cancer Res. 1999;59:367–371. [PubMed] [Google Scholar]
- 20.Desbaillets I, Diserens AC, Tribolet N, Hamou MF, Van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997;186:1201–1212. doi: 10.1084/jem.186.8.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Meir E, Ceska M, Effenberger F, Walz A, Grouzmann E, Desbaillets I, Frei K, Fontana A, De Tribolet N. Interleukin-8 is produced in neoplastic and infectious diseases of the human central nervous system. Cancer Res. 1992;52:4297–4305. [PubMed] [Google Scholar]
- 22.Rot A. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 1991;3:21–27. doi: 10.1016/1043-4666(91)90006-y. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara T, Miyamoto M, Takayama S, Kato M. Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J Pharmacol Toxicol Methods. 1995;33:91–100. doi: 10.1016/1056-8719(94)00062-9. [DOI] [PubMed] [Google Scholar]
- 24.Vytasek R. A sensitive fluorometric assay for the determination of DNA. Anal Biochem. 1982;120:243–248. doi: 10.1016/0003-2697(82)90342-6. [DOI] [PubMed] [Google Scholar]
- 25.Birnboim HC. Extraction of high molecular weight RNA and DNA from cultured mammalian cells. Methods Enzymol. 1993;216:154–160. doi: 10.1016/0076-6879(92)16018-f. [DOI] [PubMed] [Google Scholar]
- 26.Udenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972;178:871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- 27.De Vouge MW, Yamazaki A, Bennett SAL, Chen J-H, Shwed PS, Couture C, Birnboim HC. Immunoselection of GRP94/endoplasmin from a KNRK cell-specific lambdagt11 library using antibodies directed against a putative heparanase amino-terminal peptide. Int J Cancer. 1994;56:286–294. doi: 10.1002/ijc.2910560224. [DOI] [PubMed] [Google Scholar]
- 28.Haqqani AS, Sandhu JK, Birnboim HC. A myeloperoxidase-specific assay based upon bromide-dependent chemiluminescence of luminol. Anal Biochem. 1999;273:126–132. doi: 10.1006/abio.1999.4206. [DOI] [PubMed] [Google Scholar]
- 29.Kettritz R, Gaido ML, Haller H, Luft FC, Jennette CJ, Falk RJ. Interleukin-8 delays spontaneous and tumor necrosis factor-α-mediated apoptosis of human neutrophils. Kidney Int. 1998;53:84–91. doi: 10.1046/j.1523-1755.1998.00741.x. [DOI] [PubMed] [Google Scholar]