Abstract
The protein kinase A (PKA) is classified as type I or II depending on the association of the catalytic subunit with either the RI or RII regulatory subunits. Alterations in the levels of these regulatory subunits and PKA activity itself appear to affect cellular proliferation and tumorigenesis. We examined colorectal tumor specimens from 45 patients to investigate the potential role of cAMP-related signaling molecules in regulating tumorigenesis. Western blot analysis (PKA subunit protein levels) and in vitro kemptide phosphorylation assays (PKA catalytic subunit activity) were performed on human colorectal tumor tissue homogenates. RIβ protein levels were decreased 200% in ascending and 50% in descending colonic tumors compared to adjacent mucosa. RII protein levels were decreased 77% in descending colonic tumors but no change was observed in ascending colonic tumors compared to adjacent mucosa. PKA activity and the absolute amount of catalytic subunit protein in ascending and descending tumors were unchanged compared to adjacent mucosa. Differences in cAMP-related signaling molecules exist between neoplastic and normal colorectal tissues. These differences may not only serve as potential therapeutic targets for chemotherapeutic agents, but also lead to the identification of novel regulatory mechanisms involved in cellular proliferation and tumorigenesis.
Keywords: colorectal tumors, protein kinase A, cAMP, regulatory subunit, catalytic subunit
Introduction
Colon adenocarcinomas represent one of the leading causes of death in the United States. This year, it is estimated that 56,500 people will die as a result of colorectal cancer, with 132,000 new cases diagnosed. One theory of tumorigenesis proposes that colorectal cancer develops from the stepwise progression from benign adenoma into carcinoma. Support for this model was presented in studies by Vogelstein et al. [1], in which they examined 172 colorectal tumor specimens of different neoplastic degrees. They demonstrated an increased incidence of molecular abnormalities in four genetic markers (ras oncogene mutations and allelic deletions of chromosomes 5, 17, and 18) that coincided with the pathological manifestation of the tumors. Other candidate genetic mutations such as occur in the tumor suppressor p53 and the adenomatous polyposis coli (APC) gene lend credence to the progression of adenoma to carcinoma [2]. Although significant strides have been made regarding the molecular, cellular, and biochemical events associated with the development of adenocarcinomas, the recurrence of disease following surgical resection and the inability to treat advanced stage disease remain major problems often resulting in death.
Abnormalities in proliferative rates are central to the development of epithelial cancers. Neoplastic cells are characterized by unregulated progression through the cell cycle ultimately leading to uncontrolled proliferation. Signal transducing pathways which elevate cAMP levels and activate protein kinase A (PKA) are intricately involved in the proliferative process. For example, in Swiss 3T3 cells DNA synthesis and cell proliferation are stimulated by cAMP increases [3] while in Xenopus, the cAMP concentration and PKA activity fluctuate depending on the phase of the cell cycle [4]. PKA activity also is fundamental for cell cycle progression and growth in yeast [5] and for phosphorylation of cyclin D1, a nuclear protein believed to play a significant role in the transition from G1 to S phase [6]. The PKA activator forskolin increases the amount of hyperphosphorylated retinoblastoma protein in dog thyroid cells, elevating proliferative rates [7].
In addition to their role in cell cycle progression, the different PKA regulatory subunit isoforms (RI and RII) play specific roles in the proliferative or differentiative response to certain agonists. RII is primarily involved in the control of differentiation, whereas RI is implicated in the control of cellular proliferation [8,9]. Overexpression of RI is associated with cellular proliferation and neoplastic transformation [10]. The differential regulation of PKA regulatory subunits and associated changes in the proliferation/differentiation ratio have been observed in a number of carcinoma cell lines, including those of the colon [11].
Although significant strides have been made investigating the role of cAMP/PKA in regulating cellular proliferation in colorectal cells in vitro, very little is known regarding any abnormalities that may exist in the cAMP signaling system in the neoplastic colon in vivo. Therefore, we have examined surgical colorectal tumor specimens for specific alterations in cAMP-related signaling molecules to establish a potential relationship to location and staging.
Materials and Methods
Materials
Nitrocellulose and Kodak BioMax film were purchased from Fisher Scientific (Pittsburgh, PA). Immobilon-P transfer membrane was obtained from Millipore (Bedford, MA). Goat anti-murine or anti-rabbit HRP conjugates and SignaTECT PKA assay kits were purchased from Promega (Madison, WI). SuperSignal Chemiluminescent Kits and BCA protein assay kits were purchased from Pierce (Rockford, IL). Polyclonal antibodies to PKA-RI and PKA-catalytic were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-murine RII was raised using bacterially expressed RIIα as an immunogen [12]. [γ-32P]-ATP was purchased from Dupont/NEN (Boston, MA). All other reagents were of the highest grade obtainable and solutions were made per standard protocol unless otherwise specified.
Preparation of Cellular Extracts from Human Colorectal Biopsy Samples
Consent of all patients was obtained before the procurement of biopsy material in accordance with the Human Assurances Committee Institutional Review Board. Final pathology was confirmed by an attending pathologist at the institution from which the sample was obtained. Human colorectal mucosal samples were obtained from the operative theater and immediately frozen and stored in liquid nitrogen until processing. The mucosal samples were minced and washed three times in ice-cold 1X PBS (phosphate-buffered saline). The minced tissue was resuspended in 1 ml of extraction buffer (25 mM Tris-HCl pH 7.4, 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β-mercaptoethanol, 5 mM benzamidine, 0.1 mM AEBSF, 0.5 mg/ml leupeptin, 0.2 mg/ml chymostatin, 0.2 mg/ml pepstatin, 0.5 mg/ml soybean trypsin inhibitor and 1.4 TIU aprotinin) and subsequently homogenized with a Polytron tissuemizer on ice (3 times for 30 seconds each). The samples were centrifuged at 100g to remove particulate material and the resulting supernatant was analyzed as described below.
SDS-PAGE and Western Blot Analyses
For SDS-PAGE analysis, equivalent amounts (25–50 µg) of tissue extracts were resolved on 8% polyacrylamide gels and transferred to Immobilon-P using either a wet or semidry apparatus. Western blot analyses were performed on electroblots using commercially available antibodies for the PKA catalytic subunit and RI or using a polyclonal anti-RII antibody which we have developed in the laboratory. The protocols followed for the western blot analyses were exactly as described by the antibodies' manufacturer. For the RII westerns, electroblots were blocked for 1 hour in 1X Tris-buffered saline (TBS) containing 0.1% (v/v) Tween20 and 5% (w/v) nonfat dry milk (Blotto), then probed for 1 hour at room temperature in Blotto using a 1:2500 dilution of the RII antibody. The blots were washed six times for 5 minutes each time with 1X TBS/0.1% Tween20 and then probed with a 1:50,000 dilution of goat anti-rabbit HRP for 1 hour in Blotto at room temperature. This was followed by six 5-minute washes with 1X TBS/0.1% Tween20, and a final rinse with 1X TBS. All western blots were developed using standard enhanced chemiluminescent techniques.
PKA Catalytic Subunit Activity Assays
PKA catalytic subunit activity assays were performed on tissue extract supernatants using the SignaTECT cAMP-dependent protein kinase assay system according to the manufacturers instructions.
Protein Analysis
Protein content of tissue extract supernatants was determined with the BCA protein assay kit using BSA as a standard.
Statistical Analysis
Statistical differences between tumor and adjacent mucosa were determined using a Student's ttest or analysis of variance (ANOVA) with Dunnet's post-hoc analysis where appropriate. Values are presented as average ±SEM.
Results
Patient Demographics and Sample Procurement
Colorectal tumor samples as well as adjacent mucosa were surgically removed starting at the tumor margin and proceeding in 2-cm increments either proximal or distal to the tumor along the longest portion of the resected specimen. In some cases, the number of 2-cm increments of adjacent mucosa was limited to less than the full 12 cm by the size of the resected specimen. We examined specimens from 45 patients, 21 men and 24 women, with ages ranging from 23 to 90 years and consisting mainly of Caucasians and African-Americans (Table 1). The tumors represented an advanced disease sample group with the majority classified as Stage III or IV (85%) and approximately 37% having evidence of metastatic disease. Standard TNM staging revealed evidence of large locally advanced tumors with absent or early nodal metastases (Table 2). Approximately nine patients received either preoperative chemotherapy, radiation therapy, or both and four of these patients were unable to be staged.
Table 1.
Patient Demographics and Tumor Characteristics.
| Total Number of Patients | 45 |
| Average Age | 59.6 years (range 23–90) |
| Patient Gender | |
| Male | 21 |
| Female | 24 |
| Ethnicity | |
| Caucasian | 24 |
| African-American | 18 |
| Asian | 3 |
| Histological Classification | |
| Poorly differentiated | 13% |
| Moderately differentiated | 68% |
| Well differentiated | 19% |
Table 2.
TNM Staging of Tumors (41 Patients Staged).
| No. of Patients (% of Total) | |
| Tumor Size | |
| T1 | 2 (5%) |
| T2 | 2 (5%) |
| T3 | 20 (49%) |
| T4 | 17 (41%) |
| Node Status | |
| N0 | 14 (34%) |
| N1 | 12 (29%) |
| N2 | 10 (25%) |
| N3 | 2 (5%) |
| Nx (unknown) | 3 (7%) |
| Metastasis | |
| M0 | 24 (59%) |
| M1 | 15 (37%) |
| Mx (unknown) | 2 (4%) |
| Tumor Staging | |
| Stage 1 | 4% |
| Stage 2 | 10% |
| Stage 3 | 38% |
| Stage 4 | 47% |
Quantitation of PKA Regulatory Subunit Protein Levels
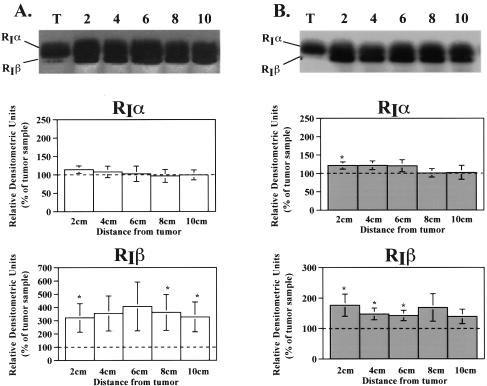
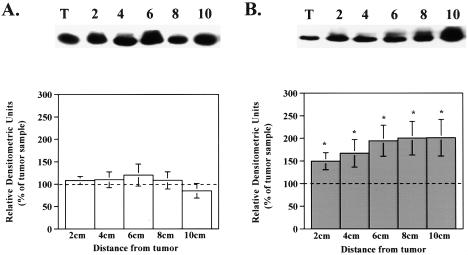
Because of the relative importance of RI and RII in the regulation of proliferation and differentiation, western blot analysis was performed on colorectal specimens to evaluate the amount of RIβ, RIα, and RII subunit protein in tumor compared to adjacent mucosa. In specimens from the ascending colon, RIβ was 200% lower in tumors compared to the levels in adjacent mucosa (Figure 1A). Similarly, in tumors from descending colon, RIβ subunit levels were 50% lower than in adjacent mucosa (Figure 1B). RIα protein levels in ascending colonic tumors showed no difference compared to adjacent mucosa. In contrast, RIα protein levels in tumors of the descending colon were significantly lower only when compared to the 2 cm adjacent mucosa sample (Figure B). More distant mucosa showed no significant difference between the amount of RIα present in tumor and mucosa (Figure 1B). Alterations in RII protein levels also differed according to the anatomic location of the neoplastic lesions. Although no alterations were observed in ascending colonic tumors, RII protein levels in the descending colon were 1.5- to 1.9-fold higher in the adjacent mucosa when compared to tumor (Figure 2, A and B).
Figure 1.
Alterations in type I PKA regulatory subunit protein levels in colorectal tumors. Cellular extracts of tissue homogenates were subjected to western blot analysis as described in Materials and Methods section. A representative western blot is shown above each bar graph. Bar graphs depict densitometric analysis of RIα and RIβ subunit protein levels in (A) ascending and (B) descending specimens. Data are presented as a percentage of the amount present in the tumor and are reported as the average±SEM. RIα (ascending), n=12 for 2, 6, 8 cm, 11 for 4 cm, and 10 for 10 cm; RIα (descending), n=19 for 2, 4, 6 cm, 16 for 8 cm, and 12 for 10 cm; *P<.05 when compared to tumor value. RIβ (ascending), n=8 for 2, 6, 8 cm, and 7 for 4, 10 cm; RIβ (descending), n=13 for 2, 4, 6 cm, 11 for 8 cm, and 8 for 10 cm; *P<.05 when compared to tumor value.
Figure 2.
Type II PKA regulatory subunit protein levels are lower in descending colorectal tumors compared to adjacent mucosa. Cellular extracts of tissue homogenates were subjected to western blot analysis as described in Materials and Methods section. A representative western blot is shown above each bar graph. Bar graphs depict densitometric analysis of RII subunit protein levels in (A) ascending and (B) descending specimens. Data are presented as a percentage of the amount present in the tumor and are reported as the average±SEM. (A) n=12 for 2, 4, 6, 8 cm and 10 for 10 cm; (B) n=21 for 2, 4, 6 cm, 19 for 8 cm, and 17 for 10 cm; *P<.05 when compared to tumor value.
PKA Catalytic Subunit Activity
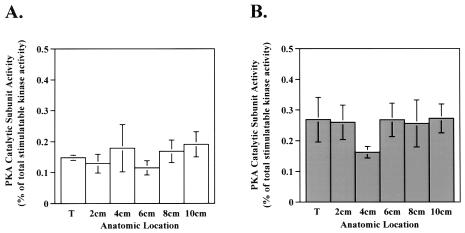
As mentioned above, cAMP and PKA catalytic subunit activity play pro-proliferative roles in many cell systems. Therefore, we next sought to measure the PKA catalytic subunit activity in tumor specimens to determine if the altered levels of PKA regulatory subunits resulted in different PKA catalytic subunit activity levels. Although significant alterations in RIβ, RIα, and RII levels were observed in tumors compared with adjacent mucosa, the relative PKA catalytic subunit activity (as a percent of the total stimulatable kinase activity) in the tumors did not differ significantly from that in adjacent mucosa (Figure 3).
Figure 3.
PKA catalytic subunit activity is unchanged in tumor compared to adjacent mucosa. PKA catalytic subunit activity was measured in cellular extracts of tissue homogenates as described in Materials and Methods section. Bar graphs depict PKA catalytic subunit activity as a percent of the total stimulatable kinase activity in tumor and adjacent mucosa in (A) ascending and (B) descending specimens. Data are presented as the average±SEM. (A) n=6 for tumor and 7 for 2, 4, 6, 8, and 10 cm; (B) n=16 for tumor, 15 for 2, 4, 6 cm, and 11 for 10 cm; *P<.05 when compared to tumor value.
Quantitation of PKA Catalytic Subunit Protein Levels
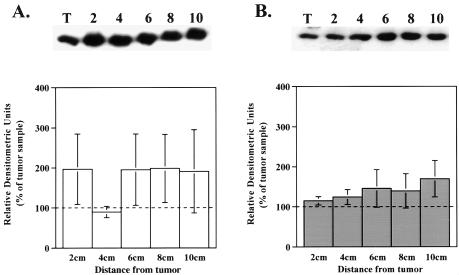
Based on similar PKA catalytic subunit activity in tumors and surrounding mucosa, one would assume that the absolute PKA catalytic subunit protein level would remain unchanged. Western blot analysis for the PKA catalytic subunit protein demonstrated that the relative amount of PKA catalytic subunit protein was similar in tumor compared to adjacent mucosa in both ascending and descending colonic specimens (Figure 4, A and B).
Figure 4.
Similar amounts of PKA catalytic subunit protein levels exist between tumor and adjacent mucosa samples in both ascending and descending specimens. Cellular extracts of tissue homogenates were subjected to western blot analysis as described in Materials and Methods section. A representative western blot is shown above each bar graph. Bar graphs depict densitometric analysis of PKA catalytic subunit protein levels in (A) ascending and (B) descending specimens. Data are presented as a percentage of the amount present in the tumor and are shown as the average±SEM. (A) n=9 for 2, 6, 8 and 9 for 10 cm; (B) n=21 for 2, 4 cm, 20 for 6 cm, 16 for 8 cm, and 11 for 10 cm; *P<.05 when compared to tumor value.
Discussion
Cell signaling events involving activation of the PKA enzyme by cAMP arise from a myriad of hormone-receptor interactions with such effectors as catecholamines, peptide hormones, and some prostaglandins [13]. These stimulants increase intracellular cAMP leading to activation of the PKA enzyme and propagation of the hormone-stimulated message. Alterations in these cell signaling pathways involving PKA play a role in the regulation of cellular proliferation both in vitro and in vivo. We focused our attention on an examination of human colorectal tumor specimens and adjacent mucosa either proximal or distal to the tumor site for alterations in cAMP-related signaling molecules.
We examined the levels of PKA regulatory subunits in tumors and adjacent mucosa because of the role of RI and RII in the regulation of proliferation and differentiation, respectively. A large decrease in the amount of RIβ protein was observed in both ascending and descending colonic tumor specimens when compared to adjacent mucosa. In descending colon specimens a decrease in the level of RIα in tumor compared to adjacent mucosa at 2 cm was observed. Interestingly, a similar trend was not observed in more distal mucosal samples. Our data showing alterations in RI protein levels are in direct contrast to the only other reported analysis of regulatory subunit expression in human colorectal tumor samples published by Bradbury et al. in 1994. They performed quantification of PKA RI and RII expression levels, examining 32 human colorectal tumor specimens [15]. They reported that RI was elevated and RII decreased in tumors compared to adjacent mucosa samples. However, they utilized [32P]8-azido-cAMP photoaffinity labeling of immunoprecipitated PKA subunit proteins to quantitate the PKA regulatory subunit protein levels. The theory behind this technique involves the quantitation of labeled cAMP binding to the regulatory subunits at the available cAMP-binding sites. Although a very powerful tool for the identification of cAMP-binding proteins as well as the determination of selective activation of PKA isoforms, this technique is influenced by the levels of endogenous cAMP in the samples. Thus, 8-azido-cAMP binding reflects the activation state of the PKA isoform and not necessarily the true PKA regulatory subunit protein level. We performed western blot analysis using polyclonal antibodies specific for the PKA regulatory subunits to circumvent this problem and avoid potential discrepancies.
Previously reported studies have noted the role of increased levels of RII in the induction of growth inhibition and differentiation in cultured cells [14]. We did detect elevated RII levels in adjacent mucosa compared to tumors, supporting the in vitro findings of Cho-Chung et al. who demonstrated an association between increases in RII protein levels and decreases in the proliferative capacity of a cell [14]. This elevated level of RII protein observed in adjacent mucosa compared to tumors supports the findings of Bradbury et al. [15]. However, we observed that the decrease in RII protein levels in tumors compared to adjacent mucosa was localized to descending but not ascending colon specimens. These results suggest a differential regulation of this PKA regulatory subunit in different anatomic regions of the colon.
Considerable research has been devoted to studying the relationship between the anatomic location of colorectal tumors and their genotype. Familial neoplasms not associated with adenomatous polyposis syndromes, such as those in the hereditary nonpolyposis colorectal cancer syndrome, as well as 15% to 20% of sporadic neoplasms appear to arise through a pathway characterized by microsatellite instability and defective mismatch repair [16]. Tumors demonstrating microsatellite instability are poorly differentiated, show less evidence of invasion, and predominately are located in the proximal colon. Microsatellite instability also has been negatively associated with mutations in K-ras and p53 [16] which suggests that differential expression/function of these genetic markers also exists throughout the colon. In addition, colorectal cancers with microsatellite instability and defective mismatch repair also exhibit a reduced level of cyclooxygenase-2 (COX-2) [16]. However, COX-2 expression is elevated in 50% of adenomas and 80% of colorectal carcinomas [17]. Whether COX-2 expression in tumor and adjacent mucosa is differentially expressed throughout the anatomic regions of the colon is not known at this time. The potential dissimilarity in anatomic location among colorectal tumors possessing APC gene mutations, microsatellite instability, defective mismatch repair, decreased COX-2 expression, with those demonstrating decreased RII levels is quite intriguing. We therefore postulate that alterations in RII, observed only in the descending colon, may be associated with the expression of one or more of these genetic markers.
The model for genetic changes leading to colorectal carcinogenesis posited by Kinzler and Vogelstein [18] proposed a series of seven insults leading to malignancy. The inciting event leading to carcinogenesis often is a mutation in APC, followed by mutations in tumor suppressor genes such as p53, and accompanied by mutations in other genes allowing progression of an adenoma to carcinoma. In our characterization of colorectal tumors exhibiting either reduced expression of RIβ or differential expression of RII we have not noted any type of relationship with regard to histology, staging, or depth of invasion. However, that does not preclude the possibility that alterations in PKA regulatory subunit expression levels represent a new molecular marker in the seven-step progression from adenoma to carcinoma.
The primary role of the PKA regulatory subunits is to inhibit catalytic subunit activity. Thus, the alterations in regulatory subunit protein levels observed in both ascending and descending tumors should have led to increased unbound PKA catalytic subunit and therefore an increase in its activity. Surprisingly, both the ascending and descending colon specimens showed neither an alteration in the catalytic subunit activity, nor a change in the quantity of catalytic subunit protein present in either location. We do not know at this time why alterations in these regulatory subunit protein levels do not result in an increase in catalytic subunit activity in the face of unchanged catalytic subunit protein levels. Therefore, identifying the mechanism by which decreased levels of RIβ protein, and/or RII in the descending colon, serve as part of a signaling pathway independent of its ability to inhibit catalytic subunit activity is critical if we are to understand the hyperproliferative process in colorectal neoplasms.
It is important to note that the alterations in cAMP-related signaling molecules that we observed in this study were due to the presence of cancer, and not a result of diverticulitis, ischemic colitis, gastrointestinal bleeding, or preoperative neoadjuvant therapy. In addition, these differences between neoplastic and normal colorectal tissues could serve not only as potential therapeutic targets for chemotherapeutic agents, but also modalities for the identification of new mechanisms regulating cellular proliferation in the neoplastic colon.
Acknowledgements
Daniel T. Dransfield is supported by grants from the American Digestive Health Foundation and the Medical College of Georgia Research Institute. The authors thank James R. Goldenring for his critical review of this manuscript.
Abbreviations
- PKA
protein kinase A
References
- 1.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Eng J Med. 1988;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 2.Hecht JR. Genetics, epidemiology, prevention, and early detection of colorectal cancer. Curr Opin Gastroenterol. 1997;13(1):5–10. [Google Scholar]
- 3.Withers DJ, Coppock HA, Surfferlein T, Smith DM, Bloom SR, Rozengurt E. Adrenomedullin stimulates DNA synthesis and cell proliferation via elevation of CAMP in Swiss 3T3. FEBS Lett. 1996;378(1):83–87. doi: 10.1016/0014-5793(95)01427-6. [DOI] [PubMed] [Google Scholar]
- 4.Grieco D, Porcellini A, Avvedimento EV, Gottesman ME. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science. 1996;271(5256):1718–1723. doi: 10.1126/science.271.5256.1718. [DOI] [PubMed] [Google Scholar]
- 5.Ward MP, Gimeno CJ, Fink GR, Garrett S. SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol Cell Bio. 1995;15(12):6853–6854. doi: 10.1128/mcb.15.12.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sewing A, Miller R. Protein kinase A phosphorylates cyclin D1 at three distinct sites within the cyclin box and at the C-terminus. Oncogene. 1994;9(9):2733–2736. [PubMed] [Google Scholar]
- 7.Coulonval K, Maenhaut C, Dumont JE, Lam YF. Phosphorylation of the three Rb protein family members is a common step of the cAMP-, the growth factor, and the phorbol ester-mitogenic cascades but is not necessary for the hypertrophy induced by insulin. Exp Cell Res. 1997;233:395–398. doi: 10.1006/excr.1997.3582. [DOI] [PubMed] [Google Scholar]
- 8.Russel DH. Type I cyclic AMP-dependent protein kinase as a positive effector of growth. Adv Cyclic Nucleotide Res. 1978;9:493–506. [PubMed] [Google Scholar]
- 9.Cho-Chung YS. Hypothesis: cyclic AMP and its receptor protein in tumor growth regulation on vivo. J Cyclic Nucleotide Res. 1980;6:163–177. [PubMed] [Google Scholar]
- 10.Yokozaki H, Budillon A, Tortora G, Meissner S, Beaucage S, Miki K, Cho-Chung YS. An antisense oligodeoxynucleotide that depletes RIα subunit of cyclic AMP-dependent protein kinase induces growth inhibition in human cancer cells. Cancer Res. 1993;53:868–872. [PubMed] [Google Scholar]
- 11.Cho-Chung YS, Clair T. The regulatory subunit of cAMP-dependent protein kinase as a target for chemotherapy of cancer and other cellular dysfunctional-related diseases. Pharmacol Ther. 1994;60:265–288. doi: 10.1016/0163-7258(93)90010-b. [DOI] [PubMed] [Google Scholar]
- 12.Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott JD. Cyclic nucleotide-dependent protein kinases. Pharmacol Ther. 1992;50:123–145. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- 14.Nesterova M, Yokozaki H, McDuffie E, Cho-Chung YS. Overexpression of RIIβ regulatory subunit of protein kinase A in human colon carcinoma cell induces growth arrest and phenotypic changes that are abolished by site-directed mutation of RIIβ. Eur J Biochem. 1996;235:486–494. doi: 10.1111/j.1432-1033.1996.00486.x. [DOI] [PubMed] [Google Scholar]
- 15.Bradbury AW, Carter DC, Miller WR, Cho-Chung YS, Clair T. Protein kinase A (PK-A) regulatory subunit expression in cancer and related mucosa. Br J Cancer. 1994;69:738–742. doi: 10.1038/bjc.1994.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karnes WE, Jr, Shattuck-Brandt R, Burgart LJ, DuBois RN, Tester DJ, Cunningham JM, Kim CY, McDonnell SK, Schaid DJ, Thibodeau SN. Reduced COX-2 protein in colorectal cancer with defective mismatch repair. Cancer Res. 1998;58:5473–5477. [PubMed] [Google Scholar]
- 17.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 18.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]