Abstract
Tumor cell invasion of basement membranes is one of the hallmarks of malignant transformation. Tumor cells secrete proteolytic enzymes known as matrix metalloproteinases (MMPs) which degrade extracellular matrix molecules. Increased expression of MMP-9 has been associated with acquisition of invasive phenotype in many tumors. However, multiple mechanisms for regulation of MMP-9 gene expression by tumor cell lines have been proposed. A number of transcription factor binding sites have been characterized in the upstream regulatory region of the MMP-9 gene, including those for AP-1. To determine how a specific AP-1 family member, c-fos, regulates MMP-9 promoter activity through these sites, we used an expression vector containing the c-fos coding region fused to the estrogen receptor (ER) ligand binding domain. This construct is activated upon binding estradiol. Stable expression of this construct in ER negative squamous cell carcinoma (SCC) lines produced an estradiol dependent decrease in the number of cells that migrated through a reconstituted basement membrane. This decreased invasiveness was accompanied by estradiol dependent downregulation of MMP-9 activity as determined by gelatin zymography. Estradiol also produced transcriptional downregulation of an MMP-9 promoter construct in cells transiently transfected with the c-fosER expression vector. This downregulation was mediated by the AP-1 site at -79 bp in the MMP-9 promoter. We concluded that the proximal AP-1 site mediated the transcriptional downregulation of the MMP-9 promoter by a conditionally activated c-fos fusion protein.
Keywords: invasion, matrix metalloproteinase, transcription factor, AP-1, extracellular matrix
Introduction
The ability of tumor cells to invade surrounding tissue is one of the most important features of the malignant phenotype [1]. Degradation of the basement membrane and invasion of underlying connective tissue have long been the histologic criteria for diagnosis of carcinoma [2]. Invading tumor cells must secrete proteolytic enzymes to degrade basement membranes [3]. Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that degrade specific basement membrane components. One member of this family, MMP-9, is upregulated in invasive cancers [4,5]. Previous studies demonstrated that highly invasive squamous cell carcinoma (SCC) lines expressed more than twice the MMP-9 activity of less invasive cells [6]. These studies illustrate the important role of MMPs in regulating tumor cell invasion.
The mechanisms by which tumor cells regulate MMP activity is the key to understanding the process of invasion. Regulation of MMP-9 activity has proven to be complex and controversial. Multiple signaling pathways upstream of the MMP-9 gene are involved in regulating its transcriptional activity. MMP-9 expression is activated in highly metastatic H-ras and v-myc transformed cells [7]. Epidermal growth factor induced MMP-9 gene transcription was also mediated by the ras protooncogene [8]. MMP-9 expression is also regulated by the jun amino terminal kinase and the extracellular signal regulated kinase cascades [9]. Additionally, inhibition of the p38 mitogen activated protein kinase pathway blocked induction of MMP-9 activity in SCC lines [10]. These studies demonstrate an important role for protein kinase signaling in regulation of MMP-9 gene expression.
At the transcriptional level, MMP-9 gene expression is regulated by binding of multiple cis acting factors to their cognate promoter elements [11]. These include two recognition sites for AP-1 which bind fos/jun family members and multiple PEA3 elements which are activated by ETS transcription factors [12]. Other sites include those for Sp-1 and NF-γB. Mutation analysis has revealed that the AP-1 site at -79 bp is not sufficient for stimulation by phorbol esters. This induction required cooperation with the upstream NF-γB or Sp-1 sites [13]. Activation of the MMP-9 promoter by tumor necrosis factor α required participation of AP-1, PEA3, NF-γB, and Sp-1 [14]. These studies point to the cooperativity of different response elements in MMP-9 transcriptional regulation.
Despite these studies, the role of specific transcription factors in controlling MMP-9 gene expression remains unclear. Although previous studies have shown that the AP-1 sites of the MMP-9 promoter are important transcriptional elements, the role of fos/jun family members in regulating expression of this gene has not been determined. To determine the effect of c-fos on regulation of the MMP-9 promoter through its AP-1 sites, we used a c-fos estrogen receptor fusion construct [15]. The complete coding sequence of c-fos was fused to the hormone binding domain of the human estrogen receptor. Previous studies have demonstrated that the transactivating function of c-fos was tightly regulated by estradiol using this construct [15]. Stable transfection of this construct into human SCC lines resulted in a marked decrease in MMP-9 activity upon estradiol stimulation. Decreased MMP-9 activity correlated with diminished ability of tumor cells to invade reconstituted basement membranes. Site directed mutagenesis studies of the MMP-9 promoter revealed that the decrease in activity was mediated at the transcriptional level through the AP-1 site at -79 bp. We concluded that conditional activation of c-fos repressed MMP-9 activity through the proximal AP-1 site of the promoter.
Materials and Methods
Cell Culture and Transfection
The human SCC lines used in this study have been described previously [6]. SCC9, SCC25, and SCC71 cells were grown in Dulbecco's modified Eagle medium (DMEM), 10% fetal bovine serum, and 40 µ/ml gentamicin at 37°C in a humidified atmosphere of 5% CO2. Cultures were transfected with 5 µg of the c-fosER expression vector or a neomycin resistance plasmid alone using LipofectAMINE according to manufacturer's recommendations (Life Technologies). Cells were selected with 500 µg/ml G418 for 2 weeks. Following selection, resistant clones were picked for expansion and characterization. Subsequent experiments except where indicated were conducted in phenol red free DMEM containing 1% charcoal-resin stripped fetal bovine serum to minimize activation of the fusion protein and endogenous c-fos respectively.
Western Blot
One hundred micrograms total cellular protein was separated by SDS-PAGE on 12% resolving gels under denaturing and reducing conditions. Separated proteins were electroblotted to PVDF membranes according to manufacturer's recommendations (Boehringer Mannheim). Blots were incubated with sheep anti-human c-fos antibody (Serotec) for 16 hours at 4°C. After washing in Tris buffered saline containing 0.1% Tween 20 (TBST, pH 7.4), blots were incubated for 30 minutes at room temperature with donkey anti-sheep IgG secondary antibody conjugated to horse-radish peroxidase (Serotec). Following extensive washing in TBST, bands were visualized by the enhanced chemiluminescence method (Boehringer Mannheim).
Gelatin Zymography
Neomycin resistant SCC9 cells or c-fosER expressing clones were plated in triplicate onto six-well tissue culture plates in serum free medium (KBM, Clonetics). Cells were treated with vehicle, 1 µM estradiol, or the estrogen receptor antagonist 4-hydroxytamoxifen. After 24 hours, conditioned medium was collected and subjected to gelatin zymography as previously described [5]. Unconditioned medium was used as a negative control. Protein concentrations were determined by Bradford assay according to manufacturer's recommendations (Bio-Rad). Conditioned media samples were separated on 10% SDS-PAGE gels containing 1 mg/ml gelatin under native conditions. Gels were washed in 2.5% Triton X-100 for 30 minutes at room temperature followed by incubation in 50 mM Tris-HCl (pH 7.5), 5 mM CaCl2 for 16 hours at 37°C. To visualize gelatinolytic bands, gels were stained with Coomassie blue for 1 hour at room temperature followed by extensive washing in 20% methanol, 5% acetic acid. Identification of the 92 kDa gelatinase activity as MMP-9 in these cells has been reported previously [6].
Invasion Assays
Neomycin resistant SCC9 clones (2x105) or c-fosER expressing cells were plated in triplicate into Matrigel invasion chambers according to manufacturer's recommendations (Becton Dickinson). Cells were treated with vehicle, 1 µM estradiol, or the estrogen receptor antagonist 4-hydroxytamoxifen. After one day, cells which had migrated through the reconstituted basement membrane were fixed in methanol, stained with hematoxylin, and counted.
Transient Transfection and CAT Assay
SCC9 cells were plated in triplicate onto six-well tissue culture plates. Cells were transiently transfected with 5 µg of the MMP-9 promoter construct pMMP-9-CAT [12] using LipofectAMINE (Life Technologies). Identical cultures were transiently transfected with the promoter construct containing a 5′-TGAGTtg-3′ mutation at the -79 or -533 AP-1 sites. Two micrograms β-galactosidase expression plasmid (Vical) was used to normalize for transfection efficiency. Cells were treated with vehicle, 1 µM estradiol, or the estrogen receptor antagonist 4-hydroxytamoxifen. After 48 hours, CAT activity was assayed using a commercially available kit (Promega). Levels of CAT activity were normalized to levels of β-galactosidase in each sample.
Results
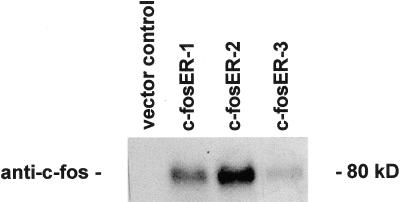
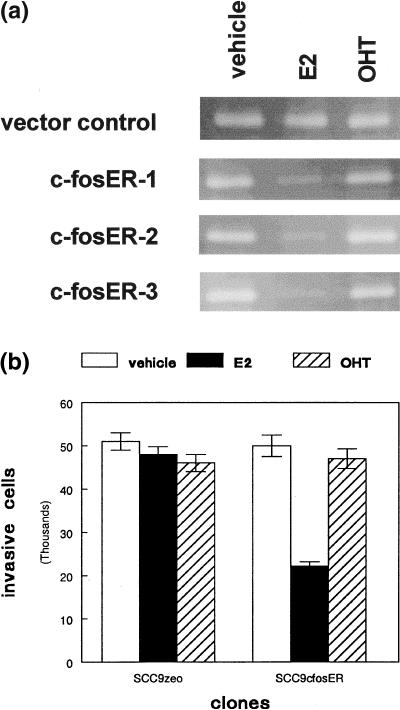
To determine the effects of c-fos expression on MMP-9 promoter activity, we first created stable clones from three cell lines which expressed the c-fosER fusion protein. Expression of the 80 kD fusion protein in three SCC9 clones compared to the vector control is shown in Figure 1. To determine the effects of c-fos expression on MMP-9 activity, conditioned medium was collected from G418 resistant clones and c-fosER cells treated with vehicle, estradiol, or 4-hydroxytamoxifen. All c-fosER expressing clones exhibited estradiol dependent reduction (∼70%) in MMP-9 activity as determined by gelatin zymography (Figure 2A). This effect was not observed with the ER antagonist 4-hydroxytamoxifen. No effect of estradiol or tamoxifen was observed in the vector transfected control cells. Similar results were obtained with two other SCC lines. These results indicate that estradiol dependent activation of a c-fosER fusion protein downregulates MMP-9 activity in human SCC lines.
Figure 1.
Expression of the c-fosER fusion protein in SCC9 stable transfectants. SCC9 cells were transfected with the c-fosER expression plasmid or the G418 resistance vector alone (control) as described in Materials and Methods. Resistant clones were subjected to western blot analysis using an anti-c-fos antibody. Expression of the 80 kD fusion protein was detected in three c-fosER stable clones but not in control cells. A representative blot is shown.
Figure 2.
Estradiol-dependent downregulation of MMP-9 activity by the c-fosER fusion protein correlates with inhibition of tumor cell invasion. (a) Serum free conditioned medium from G418 resistant SCC9 cells (vector control) or three c-fosER expressing clones treated with vehicle, estradiol (E2), or tamoxifen (OHT) was subjected to gelatin zymography as described in Materials and Methods section. Representative gels are shown. This experiment was performed three times with similar results. (b) G418 resistant SCC9 clones (neo) or c-fosER expressing cells were plated into matrigel invasion chambers and treated with vehicle or 1 µM estradiol (E2) or tamoxifen (OHT) as described in Materials and Methods section. The number of cells that migrated through the reconstituted basement membrane were counted after 24 hours. Error bars represent SEM. This experiment was performed three times with similar results.
To determine if estradiol dependent down regulation of MMP-9 activity by the c-fosER fusion protein correlated with decreased tumor cell invasion, we plated SCC9 vector transfected control cells and c-fosER expressing clones into Matrigel (Becton Dickinson) invasion chambers. Estradiol treatment produced a 50% reduction in the number of cells which penetrated the reconstituted basement membrane (Figure 2B). This effect was not observed with the ER antagonist 4-hydroxytamoxifen. No effect of estradiol or tamoxifen was observed in the vector transfected control cells. Similar results were obtained with two other SCC lines. These results indicate that estradiol dependent reduction of MMP-9 activity in human SCC lines by the c-fosER fusion protein correlated with reduced tumor cell invasion.
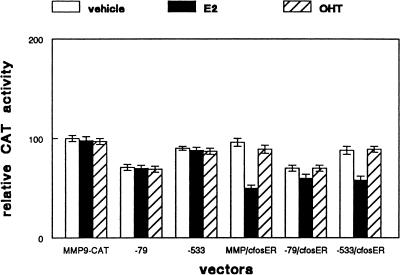
The activity of the MMP-9 promoter is regulated by AP-1 sequences at -79 bp and -533 bp relative to the transcriptional start site [12]. To determine which AP-1 site was involved in regulation of MMP-9 promoter activity by c-fosER, we transiently transfected SCC9 cells with intact pMMP-9-CAT or the reporter construct containing mutations at either of these two sites. Empty vector or the c-fosER expression construct was cotransfected with the reporter and the cells were treated with vehicle, estradiol, or tamoxifen. As shown in Figure 3, estradiol treatment produced a 50% reduction in MMP-9 promoter activity in cells transfected with the c-fosER expression vector. However, mutation of the AP-1 site at -79 bp blocked estradiol dependent reduction of MMP-9 promoter activity in c-fosER cells. Mutation of the AP-1 site at -533 bp failed to abrogate the estradiol dependent reduction of MMP-9 promoter activity in c-fosER cells. These effects were not observed when cells were treated with tamoxifen. Cotransfection with empty vector instead of the c-fosER expression plasmid produced no estradiol dependent effects on MMP-9 promoter activity. These results indicate the AP-1 site at -79 bp in the MMP-9 promoter is required for estradiol dependent down regulation by the c-fosER fusion protein.
Figure 3.
Transcriptional downregulation of the MMP-9 promoter by c-fosER is mediated by the proximal AP-1 site. Triplicate cultures of SCC9 cells were transiently transfected with either the MMP-9-CAT reporter vector or the same plasmid containing mutations of the AP-1 sites at -79 bp or -533 bp. Cells were cotransfected with either c-fosER expression plasmid or blank vector. Expression of a CMV-β-galactosidase plasmid was used to correct for transfection efficiency. After 16 hours, cultures were treated with vehicle, 1 µM estradiol (E2), or 1 µM tamoxifen (OHT) for 24 hours prior to determination of CAT activity. Error bars represent SEM. This experiment was performed three times with similar results.
Discussion
The role of specific transcription factors in regulation of MMP expression has only recently been investigated. Although the potential contributions of putative transcription factor binding sites to MMP promoter activity have been examined, few have correlated activation of a specific factor with changes in enzyme activity and tumor cell phenotype. In the current study, we demonstrated that activation of c-fos in the form of an estrogen receptor fusion protein can reduce MMP-9 activity which correlated with decreased tumor cell invasion in vitro. The effect of c-fosER on MMP-9 activity was mediated at the transcriptional level by the proximal AP-1 site of the promoter. The correlation between MMP-9 activity and tumor cell invasion and metastasis has been the focus of other studies [16]. Ras and myc transformed cell lines which were highly metastatic overexpressed MMP-9 mRNA and protein [17]. Ribozyme mediated degradation of MMP-9 mRNA eliminated the metastatic capability of these cells. These data point to the importance of MMP-9 regulation in the invasive phenotype of tumor cells.
The MMP-9 promoter has several transcription factor binding motifs, including those for AP-1, ETS family members, Sp-1, and NF-γB [18,19]. Coordinate activation of these sites was required for regulation of promoter activity by tumor necrosis factor or phorbol esters [12,13]. Both ETS and AP-1 sites were required for ras-induced upregulation of MMP-9 promoter activity [8]. However, these factors activate a variety of signaling pathways upstream of the MMP-9 promoter, making it difficult to determine the precise role of a particular binding site in regulating expression of this gene. In the present study, we took advantage of the conditional activation of the c-fosER fusion protein by estradiol to examine the effects of this particular transcription factor on MMP-9 activity and tumor cell invasion. Our results indicated that the effects of the c-fosER fusion protein on downregulation of MMP-9 activity were mediated by the AP-1 site at -79 bp in the promoter of this gene (Figure 3). This construct should be useful in examining the effects of c-fos activation on other cellular processes and for elucidating the contribution of potential AP-1 sites in promoter regulation.
Differential regulation of c-fosER and c-mycER by estradiol and tamoxifen has also been reported. Both estradiol and tamoxifen activated c-fosER in mouse mammary epithelial cells [15]; however, in a colon carcinoma line only estradiol was able to activate the construct [20]. Conversely, in human epidermal stem cells only tamoxifen was able to activate a c-mycER construct [21]. We reported here that estradiol was able to activate c-fosER in human carcinoma lines; however, no activation was seen with ER antagonist tamoxifen. The ability of the c-fosER construct to activate the MMP-9 promoter is in part limited by transfection techniques. Depending on the culture model used, AP-1 site deletions have variable effects on MMP-9 promoter activity [12,14]. These data suggest that the effects of estradiol and tamoxifen on ER fusion proteins are cell-type dependent, perhaps requiring additional factors present in certain cells for activation by specific ligands.
In summary, c-fosER activation produced MMP-9 down-regulation and concomitant reduction in tumor cell invasion. The reduction in MMP-9 activity was mediated at the transcriptional level by the proximal AP-1 site of the promoter. Future experiments will use similar approaches to examine the role of other transcription factors in regulation of the MMP-9 promoter and the invasive phenotype.
Acknowledgements
We thank Ernst Reichmann for the c-fosER expression vector, Hiroshi Sato for the MMP-9 promoter construct, and Valentino Santos for technical assistance. This study was supported by National Institutes of Health grant DE10966 to DLC.
Abbreviations
- MMP
matrix metalloproteinase
- ER
estrogen receptor
- AP-1
activator protein 1
- SCC
squamous cell carcinoma
- CAT
chloramphenicol acetyl transferase
- PVDF
polyvinylidene difluoride
References
- 1.Crowe DL, Shuler CF. Regulation of tumor cell invasion by extracellular matrix. Histol Histopathol. 1999;14:665–671. doi: 10.14670/HH-14.665. [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA. Tumor invasion and metastases—role of the extracellular matrix. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 3.Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. [PubMed] [Google Scholar]
- 4.Garbisa S, Negro A, Kalbic T, Pozzatti R, Muschel R, Saffiotti U, Liotta LA. Type IV collagenolytic activity linkage with the metastatic phenotype induced by ras transfection. Adv Exp Med Biol. 1988;233:179–186. doi: 10.1007/978-1-4899-5037-6_20. [DOI] [PubMed] [Google Scholar]
- 5.Davies B, Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR. Activity of type IV collagenases in benign and malignant breast disease. Br J Cancer. 1993;67:1126–1131. doi: 10.1038/bjc.1993.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vo H, Lee MK, Crowe DL. α2β1 integrin signaling via the mitogen activated protein kinase (MAPK) pathway modulates retinoic acid (RA) dependent downregulation of tumor cell invasion and transcriptional downregulation of matrix metalloproteinase 9 activity. Int J Oncol. 1998;13:1127–1134. doi: 10.3892/ijo.13.6.1127. [DOI] [PubMed] [Google Scholar]
- 7.Himelstein BP, Lee EJ, Sato H, Seiki M, Muschel RJ. Transcriptional activation of the matrix metalloproteinase 9 gene in an H-ras and v-myc transformed rat embryo cell line. Oncogene. 1997;14:1995–1998. doi: 10.1038/sj.onc.1201012. [DOI] [PubMed] [Google Scholar]
- 8.Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, Boyd D. Stimulation of 92 kDa gelatinase B promoter activity by ras is mitogen activated protein kinase kinase 1 independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem. 1996;271:10672–10680. doi: 10.1074/jbc.271.18.10672. [DOI] [PubMed] [Google Scholar]
- 9.Gum R, Wang H, Lengyel E, Juarez J, Boyd D. Regulation of 92 kDa type IV collagenase expression by the jun amino terminal kinase- and the extracellular signal regulated kinase dependent signaling cascades. Oncogene. 1997;14:1481–1493. doi: 10.1038/sj.onc.1200973. [DOI] [PubMed] [Google Scholar]
- 10.Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen activated protein kinase by SB203580 blocks PMA induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res. 1998;58:1135–1139. [PubMed] [Google Scholar]
- 11.Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 12.Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- 13.Sato H, Kita M, Seiki M. V-Src activates the expression of 92 kDa type IV collagenase gene through the AP-1 site and the GT box homologous to retinoblastoma control elements. A mechanism regulating gene expression independent of that by inflammatory cytokines. J Biol Chem. 1993;268:23460–23468. [PubMed] [Google Scholar]
- 14.Lauricella-Lefebvre MA, Castronovo V, Sato H, Seiki M, French DL, Merville MP. Stimulation of the 92 kD type IV collagenase promoter and enzyme expression in human melanoma cells. Invasion Metastasis. 1993;13:289–300. [PubMed] [Google Scholar]
- 15.Reichmann E, Schwarz H, Deiner EM, Leitner I, Eilers M, Berger J, Busslinger M, Beug H. Activation of an inducible c-fosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992;71:1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- 16.Bernhard EJ, Muschel RJ, Hughes EN. Mr 92,000 gelatinase release correlates with the metastatic phenotype in transformed rat embryo cells. Cancer Res. 1990;50:3872–3877. [PubMed] [Google Scholar]
- 17.Hua J, Muschel RJ. Inhibition of matrix metalloproteinase 9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996;56:5279–5284. [PubMed] [Google Scholar]
- 18.Fini ME, Bartlett JD, Matsubara M, Rinehart WB, Mody MK, Girard MT, Rainville M. The rabbit gene for 92 kDa matrix metalloproteinase. Role of AP-1 and AP-2 in cell type specific transcription. J Biol Chem. 1994;269:28620–28628. [PubMed] [Google Scholar]
- 19.Huhtala P, Chow LT, Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990;265:11077–11082. [PubMed] [Google Scholar]
- 20.Preston GA, Lyon TT, Yin Y, Lang JE, Solomon G, Annab L, Srinivasan DG, Alcorta DA, Barrett JC. Induction of apoptosis by c-fos protein. Mol Cell Biol. 1996;16:211–218. doi: 10.1128/mcb.16.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandarillas A, Watt FM. C-myc promotes differentiation of human epidermal stem cells. Genes Dev. 1997;11:2869–2882. doi: 10.1101/gad.11.21.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]