Abstract
Schizosaccharomyces pombe rho1+ and rho2+ genes are involved in the control of cell morphogenesis, cell integrity, and polarization of the actin cytoskeleton. Although both GTPases interact with each of the two S. pombe protein kinase C homologues, Pck1p and Pck2p, their functions are distinct from each other. It is known that Rho1p regulates (1,3)β-d-glucan synthesis both directly and through Pck2p. In this paper, we have investigated Rho2p signaling and show that pck2Δ and rho2Δ strains display similar defects with regard to cell wall integrity, indicating that they might be in the same signaling pathway. We also show that Rho2 GTPase regulates the synthesis of α-d-glucan, the other main structural polymer of the S. pombe cell wall, primarily through Pck2p. Although overexpression of rho2+ in wild-type or pck1Δ cells is lethal and causes morphological alterations, actin depolarization, and an increase in α-d-glucan biosynthesis, all of these effects are suppressed in a pck2Δ strain. In addition, genetic interactions suggest that Rho2p and Pck2p are important for the regulation of Mok1p, the major (1–3)α-d-glucan synthase. Thus, a rho2Δ mutation, like pck2Δ, is synthetically lethal with mok1–664, and the mutant partially fails to localize Mok1p to the growing areas. Moreover, overexpression of mok1+ in rho2Δ cells causes a lethal phenotype that is completely different from that of mok1+ overexpression in wild-type cells, and the increase in α-glucan is considerably lower. Taken together, all of these results indicate the presence of a signaling pathway regulating α-glucan biosynthesis in which the Rho2p GTPase activates Pck2p, and this kinase in turn controls Mok1p.
INTRODUCTION
The fission yeast Schizosaccharomyces pombe undergoes morphogenetic changes during both the vegetative and sexual cell cycles that require asymmetric cell growth and actin cytoskeleton reorganizations (Mata and Nurse, 1997; Arellano et al., 1999a; Verde, 1998). Rho GTPases are critical modulators of these processes as in all other eukaryotes (Schmidt and Hall, 1998; Chant, 1999; Kaibuchi et al., 1999; Pruyne and Bretscher, 2000). In yeasts, these GTPases provide the coordinated regulation of cell wall biosynthetic enzymes and actin organization that is required to maintain cell integrity and polarized growth (Cabib et al., 1998; Arellano et al., 1999a).
The architecture of the fission yeast cell wall differs from that of Saccharomyces cerevisiae. The major S. pombe cell wall structural components are the (1–3)β-d-glucan (50–54% of total polysaccharides) and (1–3)α-d-glucan (28–32%) (Kopeckáet al., 1995; Manners and Meyer, 1977). This latter polymer is not present in S. cerevisiae. On the other hand, chitin, a major structural component of the S. cerevisiae cell wall, has not been detected in S. pombe, although a chitin synthase gene homologue has recently been described that is necessary for the formation of chitin or chitosan during spore cell wall maturation (Arellano et al., 2000). The catalytic subunit of the S. pombe (1–3)β-d-glucan synthase is encoded by the cps1+ gene, a homologue to FKS1 and FKS2 (Ishiguro et al., 1997). cps1+ is important for cytokinesis and cell polarity (Le Goff et al., 1999; Liu et al., 2000) and is part of a Wee1p-dependent septation checkpoint. At least three more genes similar to cps1+ are present in the S. pombe genome, but nothing is known yet about their function or regulation. The (1–3)α-d-glucan synthase is encoded by the mok gene family, which includes five members. The main one, ags1+/mok1+, is an essential gene (Hoschterbach et al., 1998; Katayama et al., 1999). Mok1p localizes to the growing tips and to the septum during cytokinesis, and it is regulated by Pck2p (Katayama et al., 1999).
S. pombe Rho1 GTPase was identified as a regulatory component of the (1–3)β-d-glucan synthase (Arellano et al., 1996) that is also required for maintenance of cell integrity and polarization of the actin cytoskeleton (Arellano et al., 1997; Nakano et al., 1997). We reported recently that GTP-bound Rho1p interacts with the two fission yeast PKC homologues, Pck1p and Pck2p, stabilizing both proteins (Arellano et al., 1999b). The interaction with Rho1p also allows the localization of pck2 to the cell growth areas (Sayers et al., 2000). Pck1p and Pck2p share overlapping roles in cell viability and partially complement each other (Toda et al., 1993, 1996). However, pck1Δ and pck2Δ cells have different phenotypes and differentially regulate cell wall integrity. Furthermore, GTP-bound Rho1p regulates (1–3)β-d-glucan cell wall biosynthesis and cell polarity mainly through Pck2p (Arellano et al., 1999b).
S. pombe rho2+ was isolated as a gene that causes lethality when overexpressed (Hirata et al., 1998). The Rho2p GTPase is localized to the sites of growth and seems to be involved in the control of cell polarity, reorganization of the actin cytoskeleton, and cell wall biosynthesis. Its disruption is not lethal but renders the cells rounded and hypersensitive to Aculeacin A, a specific (1–3)β-d-glucan synthase inhibitor, or treatment with glucanases (Hirata et al., 1998). This phenotype is only weakly suppressed by rho1+, indicating a minor functional overlap between the two GTPases (Hirata et al., 1998). rho2Δ cells are also hypersensitive to staurosporine, a potent inhibitor of protein kinase C, suggesting that Rho2p might be signaling through the PKC homologues. Moreover, GTP-bound Rho2p interacts with Pck1p and Pck2p in the two-hybrid system (Arellano et al., 1999b). Here we examine the role of Rho2p in cell wall biosynthesis and the function of the Rho2p interaction with Pck1p and Pck2p. We present genetic and biochemical evidence that Rho2p is a positive regulator of Mok1p, stimulating the biosynthesis of α-d-glucan and signaling through Pck2p.
MATERIALS AND METHODS
Strains, Growth Conditions, and Genetic Methods
Standard S. pombe media and genetic manipulations were used (Moreno et al., 1991). All of the strains used were isogenic to wild-type strains h− 972 and h+ 975 and are described in Table 1.
Table 1.
List of strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h− leu1-32 | Moreno et al., 1991 |
| TP134-3B | h− pck1∷ura4+ ura4-D18 leu1-32 | Toda et al., 1993 |
| TP169-1C | h− pck2∷LEU2 leu1-32 ura4-D18 | Toda et al., 1993 |
| TP179-1Aa | h− sts6-8 leu1-32 ura4-D18 | Toda et al., 1993 |
| KN-1 | h− rho2∷ura4+ leu1-32 ura4-D18 ade6 | Hirata et al., 1998 |
| MA20 | h− P81nmt-pck1 leu1-32 kanr | Arellano et al., 1999a, b |
| MA21 | h− P81nmt-pck2 ura4-D18 kanr | Arellano et al., 1999a, b |
| TMC110b | h− P81nmt-pck1 rho2∷ura4+ leu1-32 ura4-D18 ade6 kanr | This work |
| TMC111b | h− P81nmt-pck2 rho2∷ura4+ ura4-D18 leu1-32 ade6 kanr | This work |
| DH664 | h− mok1-664 leu1-32 | Katayama et al., 1999 |
| SKP100 | h− mok1-664 pck2∷LEU2 leu1-32 | Katayama et al., 1999 |
| TMC112b | h− mok1-664 leu1-32 ura4-D18 | This work |
| TMC113b | h− mok1-664 rho2∷ura4+ ura4-D18 leu1-32 ade6 | This work |
| SKP103 | h− Pnmt1-mok1 leu1-32 kanr | Katayama et al., 1999 |
| SKP170 | h− Pnmt1-mok1 leu1-32 kanr pck2∷LEU2 | Katayama et al., 1999 |
| TMC114b | h− Pnmt1-mok1 leu1-32, ura4-D18 kanr | This work |
| TMC115b | h− Pnmt1-mok1 rho2∷ura4+ leu1-32 ura4-D18 ade6 kanr | This work |
| TMC116b | h− Pnmt1-mok1 pck1∷ura4+ leu1-32 ura4-D18 kanr | This work |
sts6-8 is a mutant allele of pck2 identified by its supersensitivity to staurosporine.
TMC strains were obtained by mating other haploid strains (or the h+ relatives) listed in this Table.
Yeast cells were grown in YES medium or minimal medium (EMM) supplemented with the necessary requirements. Echinocandin derivative LY280949 was from Lilly (Indianapolis, IN, and Papulacandin B was from Novartis (Basel, Switzerland). Incubations were performed at 28, 32, or 37°C. Growth was monitored by OD600 measurements.
Escherichia coli DH5α (Life Technologies, Gaithersburg, MD) was used as host for propagation of plasmids. Cells were grown in LB medium supplemented with 50 μg/ml ampicillin or 25 μg/ml kanamycin when appropriate. Solid-medium plates contained 2% agar.
Recombinant DNA Methods
All DNA manipulations were performed by established methods (Sambrook et al., 1989). Enzymes were used according to the recommendation of the suppliers. S. pombe was transformed by electroporation (Prentice, 1992) or by the lithium acetate method (Ito et al., 1983). The nmt promoter-containing vectors pREP1, pREP3X, pREP4X, pREP41X, and pREP42X (Forsburg, 1993) were used for the overexpression of pck2+ and rho2+. pREP1-pck2 has been described previously (Sayers et al., 2000). The rho2+ ORF was cloned in the SalI–BamHI sites of the vectors by PCR amplification from an S. pombe cDNA library using the following primers: 5′-ATATAGTCGACCATGG TG CAA TCT CAA CCG-3′ (Forward) and 5′-TATAT GGATCC TTA TGA AAT GAT GCA TTT TG-3′ (Backward), which contain SalI and BamHI sites (italics), respectively. The entire rho2+ ORF was confirmed by automated sequencing.
Microscopy Techniques
For Calcofluor staining, exponentially growing S. pombe cells cultivated at 32°C in minimal medium without thiamine were harvested, washed once, and resuspended in water with Calcofluor at 20 μg/ml final concentration for 5 min at room temperature.
For actin staining, cells were fixed in cold methanol for at least 15 min. Immunofluorescence was performed as described (Hagan and Hyams, 1988). The primary anti-actin antibody was the monoclonal N350 (Amersham, Arlington Heights, IL), and the secondary antibody was a sheep anti-mouse Cy3-conjugated F(ab′)2 fragment (Sigma, St. Louis, MO).
For Mok1p staining, purified rabbit polyclonal anti-mok1 antiserum (1:10) was used as primary antibody (Katayama et al., 1999), and FITC-conjugated goat anti-rabbit was used as secondary antibody.
Cells were immobilized on poly-l-lysine coverslips and observed using a confocal microscope (Zeiss MRC600).
Electron Microscopy
The procedure for electron microscopy observation was as described previously (Nakano et al., 1997). Briefly, cells were prefixed with 2.5% glutaraldehyde dissolved in 0.1 M sodium phosphate buffer, pH 7.0, at room temperature for 1 h and post-fixed with 1.5% potassium permanganate dissolved in distilled–deionized water at 4°C overnight. After dehydration in a graded series of acetone, samples were embedded in Quetol 812. Thin sections were cut with a Reichert Ultracut S microtome, stained in uranyl acetate and lead citrate, and examined with a JEOL-JEM1200EX electron microscope.
Glucanase Sensitivity Experiments
Glucanase sensitivity of S. pombe cells was evaluated following the procedure described previously (Shiozaki and Russell, 1995). Wild-type (HM123), rho2Δ (KN-1), and rho2Δ (KN-1) transformed with either pREP1-rho2 or pREP1-pck2 strains were grown to midlogarithmic phase in EMM medium with 5 μM thiamine at 30°C. The cells were harvested, washed in TE buffer, and resuspended at an OD600 of 1.0 in the same buffer containing 20 μg/ml β-glucanase (Zymolyase 100T; Seikagaku Kogio Co. Ltd., Tokyo, Japan). Cell suspensions were incubated at 30°C with shaking, and cell lysis was monitored by measuring the OD600.
Labeling and Fractionation of Cell Wall Polysaccharides
Labeling and fractionation of cell wall polysaccharides was performed as described (Arellano et al., 1997). Briefly, exponentially growing cultures of S. pombe wild-type and transformed cells were supplemented with [U-14C]glucose (1 μCi/ml) and incubated for an additional 4 h. Cells were harvested, and unlabeled cells were added to the radioactive samples as carriers. Total glucose incorporation was monitored by measuring the radioactivity in trichloroacetic acid-insoluble material. Mechanical breakage of cells was performed using prechilled glass beads added to the cells, and lysis was achieved in a Fast-Prep System FP120 (Bio 101, Savant, La Jolla, CA) using two 15 s intervals at 5.5 speed. Cell walls were pelleted at 1000 × g for 5 min and washed three times with 5% NaCl and three times with 1 mM EDTA. Aliquots (100 μl) of the total wall were incubated with 100 U Zymolyase 100T or Quantazyme (Quantum Biotechnologies Inc., Montreal, Quebec) for 36 h at 30°C. Aliquots without enzyme were included as control. The samples were centrifuged, and the supernatant and washed pellet were counted separately. The supernatants from the Zymolyase 100T reaction were considered β-glucan plus galactomannan, and the pellet was considered α-glucan. The supernatants from the Quantazyme reactions were considered (1–3)β-glucan, and the pellet was considered α-glucan plus galactomannan.
Immunoblot Analysis
Mok1p expressed in S. pombe cells was detected by immunoblotting. Approximately 1 × 108 cells growing exponentially in minimal medium with or without thiamine were harvested by brief centrifugation, washed once with lysis buffer (20 mM Tris, pH 8.0, 10 mM EDTA, 10% glycerol, 137 mM NaCl, and 1% Nonidet-P40 containing 1 mM p-aminophenyl methanesulfonyl fluoride, 2 μg/ml leupeptin, and 10 μg/ml aprotinin), and resuspended in 100 μl of the same buffer. Approximately 1 g of prechilled glass beads was added to the cells, and lysis was achieved in a Fast-Prep system (see above). The resulting homogenates were collected, and glass beads and large debris were removed by centrifugation for 5 min at 750 × g. One aliquot was diluted 2× with lysis buffer and subjected to 7.5% SDS-PAGE, and separated proteins were electrophoretically transferred to a nitrocellulose membrane sheet (Schleicher and Schuell, Keene, NH). The blot was processed to detect Mok1p with 1:1000 diluted anti-Mok1p rabbit polyclonal antiserum obtained as described (Katayama et al., 1999) and to detect tubulin with 1:1000 TAT1 anti-α-tubulin monoclonal (kindly provided by K. Gull, University of Manchester, United Kingdom) as primary antibodies. After they were washed several times, the membranes were incubated with HRP-conjugated antibodies, and bands were visualized by the luminol-based ECL detection kit (Amersham).
RESULTS
Rho2p Stimulates Cell Wall α-Glucan Biosynthesis
S. pombe Rho2p GTPase is involved in cell polarity and morphogenesis, but its function appears to be different from that of Rho1p. Microscopic examination of rho2+-overexpressing cells revealed a thicker cell wall than in wild-type cells (Hirata et al., 1998).
To investigate Rho2p function, we analyzed the cell wall composition of S. pombe rho2Δ mutants and cells overproducing Rho2p (Table 2). Incorporation of radioactive glucose into the cell wall was slightly lower in the rho2Δ cells, with no significant differences in cell wall composition; however, in cells transformed with pREP1-rho2 and grown in the absence of thiamine for 16 h, rho2+ overexpression caused an increase in the total cell wall incorporation (from 29.8 to 38.9%), resulting from a specific increase in α-glucan (from 10.0 to 17.2% of the total 14C-glucose incorporated into the cells). A similar increase in α-glucan has not been observed in Rho1p-overproducing cells (Arellano et al., 1996), which suggests that Rho2p has a role, distinct from Rho1p, as a positive regulator of α-glucan biosynthesis.
Table 2.
Incorporation of radioactivity from 14C-glucose into cell wall polysaccharides of different S. pombe strains grown in minimal medium without thiamine for 16 h
| Strain | Plasmid | Cell wall | Galactomannan | α-Glucan | β-Glucan |
|---|---|---|---|---|---|
| HM123 (wild-type) | 29.2 ± 1.8 | 2.3 ± 0.5 (8) | 9.5 ± 0.8 (33) | 17.4 ± 1.8 (60) | |
| KN-1 (rho2Δ) | 25.6 ± 0.4 | 2.3 ± 0.7 (9) | 7.6 ± 0.2 (30) | 15.7 ± 1.2 (61) | |
| TP179-1A (sts6–8) | 23.3 ± 3.7 | 3.5 ± 1.1 (15) | 6.8 ± 0.7 (29) | 13.0 ± 2.4 (56) | |
| TP169-1C (pck2Δ) | 24.7 ± 0.6 | 4.1 ± 0.9 (17) | 6.6 ± 0.4 (27) | 14.0 ± 1.0 (57) | |
| TP134-3B (pck1Δ) | 24.3 ± 2.1 | 3.9 ± 1.4 (16) | 8.2 ± 1.0 (34) | 12.2 ± 1.3 (50) | |
| HM123 | pREP4X | 29.8 ± 1.6 | 2.1 ± 0.8 (8) | 10.0 ± 0.8 (33) | 17.7 ± 1.5 (59) |
| HM123 | pREP4X-rho2 | 38.9 ± 3.3 | 1.5 ± 0.7 (4) | 17.2 ± 1.6 (44) | 20.1 ± 1.8 (52) |
| TP169-1C | pREP4X-rho2 | 23.3 ± 2.1 | 4.5 ± 1.2 (19) | 6.4 ± 0.8 (27) | 12.4 ± 0.9 (53) |
| TP134-3B | pREP3X-rho2 | 39.8 ± 2.2 | 4.1 ± 1.0 (10) | 16.8 ± 1.2 (42) | 18.9 ± 0.9 (47) |
14C-glucose was added 2 h before harvesting the cells.
Values indicate percentage of total 14C-glucose incorporated and are the mean ± SD calculated from at least four independent experiments. Values in parentheses are the percentage of the corresponding polysaccharide in the cell wall.
rho2Δ and pck2Δ Strains Have Similar Cell Wall Defects
rho2Δ cells are hypersensitive to staurosporine, a potent inhibitor of protein kinase C (Hirata et al., 1998). We have also reported that Rho2p physically interacts with Pck1p and Pck2p (Arellano et al., 1999b). To study the possible functional relationship between Rho2p and Pck2p, we first performed electron microscopy examination of rho2+-overexpressing cells, rho2::ura4+ (KN-1), and pck2 mutant (sts6–8) cells (strain TP179–1A) that show the same phenotype as pck2 disruptant cells (Toda et al., 1993). The cell wall of rho2+-overexpressing cells was thicker than that of wild-type cells (Figure 1, a and b, and Table 2) (Hirata et al., 1998), although the phenotype was moderate in comparison to that of rho1+- or pck2+-overexpressing cells (Arellano et al., 1996, 1999b; Nakano et al., 1997). In addition, whereas in wild-type cells membranous structures that have been proposed to be endoplasmic reticulum (Osumi, 1998) were seen beneath the cytoplasmic membrane (Figure 1a), in rho2+-overexpressing cells these structures were often heavily stacked at the cell ends and in septated regions (Figure 1b). In the pck2 mutant and the rho2Δ cells, the cell wall was thinner than in wild-type cells (Figure 1, c and d, and Table 2), and the outermost layer was less electron-dense. Interestingly, this outer layer was also absent in the α-d-glucan synthase mutant, ags1–1 (Hochsterbach et al., 1998).
Figure 1.
rho2Δ and pck2 mutant cells have similar cell wall defects. Shown are electron microscopy images of fission yeast cells expressing different levels of Rho2p. HM123 (wild-type) cells carrying pREP1 (a) or pREP1-rho2 (b); KN-1 (rho2Δ) (c), and TP179–1A (sts6–8=pck2) mutant cells (d) were grown at 30°C for 16 h in EMM liquid medium without thiamine before fixation. Arrows indicate membranous structure. N, nucleus, Mt, mitochondria. Twofold-magnified images are shown in insets. More than 30 cells of each sample were observed, and all display a similar phenotype. Bar, 1 μm.
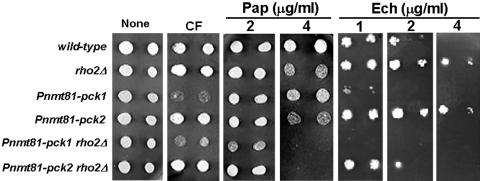
To corroborate the possible connections between Rho2p and Pck2p, we analyzed the sensitivity of mutant strains lacking these proteins to Calcofluor, a drug affecting cell wall integrity, and to the antibiotics Echinocandin and Papulacandin B, inhibitors of β-d-glucan biosynthesis (Perez et al., 1981). We used the previously generated MA20 (Pnmt81-pck1) and MA21 (Pnmt81-pck2) strains (Arellano et al., 1999a,b), in which each gene is under the control of the thiamine-repressible and reduced-expression–level promoter nmt81 (Forsburg, 1993). When grown in the presence of thiamine (repressed), MA20 was hypersensitive to Calcofluor, Papulacandin, and Echinocandin, suggesting a general defect in the cell wall. By contrast, neither KN-1 (rho2::ura4+) nor MA21 grown with thiamine was hypersensitive to Calcofluor, and both were more resistant to Echinocandin than wild-type cells (Figure 2). KN-1 and MA21 cells grown with thiamine were hypersensitive to Papulacandin B, and this might imply either a cell wall defect that increases the importance of β-glucan in maintaining cell wall integrity, such as a decrease in α-glucan, or an increase in cell wall permeability to the drug. The mutant strain TMC110 (rho2::ura4+ Pnmt81-pck1) grown with thiamine was more sensitive to Papulacandin B and Echinocandin than KN-1 or MA20 cells and was as hypersensitive to Calcofluor as MA20 cells, suggesting that Rho2p and Pck1p might act in different signaling pathways. The double-mutant TMC111 (rho2::ura4+ Pnmt81-pck2) grown with thiamine was not sensitive to Calcofluor, corroborating that Rho2p is not activating Pck1p, because if that were the case, the lack of Rho2p would cause the same effect as the lack of Pck1p. Interestingly, the TMC111 strain was not resistant to Echinocandin, suggesting that the lack of Rho2p and Pck2p at the same time might cause a different cell wall defect than the lack of Rho2p or Pck2p separately.
Figure 2.
Hypersensitivity to cell wall inhibitors of different S. pombe strains. Wild-type (HM123), rho2Δ (KN-1), Pnmt81-pck1 (MA20), Pnmt81-pck2 (MA21), Pnmt81-pck1 rho2Δ (TMC110), and Pnmt81-pck2 rho2Δ (TMC111) cells were grown at 28°C in EMM with thiamine, spotted on EMM with thiamine plates at 4 × 106 and 106 cells/ml, and incubated 2–3 d at 28°C. Drug concentrations were as follows: Calcofluor (CF) (0.5 mg/ml), Papulacandin B (Pap) (2 and 4 μg/ml); and Echinocandin (Ech) (1, 2, and 4 μg/ml).
Overproduction of Pck2p Suppresses the Hypersensitivity to β-Glucanases of rho2Δ Cells
The cell wall defect of rho2 disruptant cells was also revealed by testing the resistance to lysis during treatment with a β-glucanase complex that digests the cell wall. KN-1 cells were very sensitive to β-glucanase treatment (Figure 3), corroborating the observed hypersensitivity to the β-d-glucan synthase inhibitor Papulacandin B (Figure 2). We also analyzed whether a mild overexpression of pck2+ was able to suppress the rho2 disruptant phenotype. rho2Δ cells bearing pREP1, pREP1-rho2, or pREP1-pck2 were grown in EMM with 5 μM thiamine. In such conditions, the strong nmt1 promoter is not totally repressed (Forsburg, 1993). As shown in Figure 3, overproduction of either Rho2p or Pck2p largely reduced the lysis rate of KN-1 cells caused by β-glucanase treatment, strongly suggesting a functional relationship between Rho2p and Pck2p.
Figure 3.
Hypersensitivity of rho2Δ cells to glucanases is suppressed by pck2+ overexpression. S. pombe wild-type (⋄) and rho2Δ (KN-1) cells transformed with the plasmids pREP1 (□), pREP1-rho2 (○), and pREP1-pck2 (▵) were grown at 32°C in minimal medium with 5 μM thiamine for 20 h and resuspended in TE buffer with 20 mg/ml β-glucanase complex (Zymolyase 100T). Cell lysis was monitored by measuring optical density at 600 nm as a function of time. Values were calculated from at least four independent experiments. Means and SDs are shown.
The Morphological Effects Caused by Rho2p Overproduction Require the Presence of Pck2p
In addition to the formation of a thick cell wall (Figure 1b) and the increase in α-glucan content (Table 2), rho2+ overexpression is lethal and causes a rounded morphology and actin depolarization (Hirata et al., 1998). To further analyze whether Pck1p or Pck2p is a functional effector protein of Rho2p, we overproduced the GTPase in HM123, TP134–3B (pck1Δ), and TP169–1C (pck2Δ) strains. High levels of Rho2p were lethal in HM123 or TP134–3B but not in TP169–1C cells (Figure 4A). Moreover, the actin depolarization and rounded morphology caused by rho2+ overexpression in the wild-type strain was suppressed in the pck2Δ cells (Figure 4B), suggesting that Rho2p signaling requires Pck2p. Cell wall analysis of these cells revealed that the increase in α-glucan caused by overexpressing rho2+ occurred in pck1Δ cells but not in pck2Δ cells (Table 2), indicating that pck2p kinase might also be required for the stimulating effect of Rho2p on the biosynthesis of cell wall α-glucan. In summary, these results demonstrate that the main effects of rho2+ overexpression, lethality, round morphology, actin depolarization, and increase in α-glucan, are mediated by Pck2p but not by Pck1p.
Figure 4.
rho2+ overexpression does not cause any effects in the absence of Pck2p. (A) Growth of wild-type (HM123), pck1Δ (TP134–3B), and pck2Δ (TP169–1C) cells transformed with pREP3X, pREP4X, pREP3X-rho2, or pREP4X-rho2. Cells were grown on EMM plates without thiamine. (B) Localization of F-actin in (a) HM123, (b) TP169–1C, (c) HM123 transformed with pREP3X-rho2, (d) TP169–1C transformed with pREP3X-rho2. Bar, 5 μm.
Functional Relationships among rho2+, pck2+, and mok1+
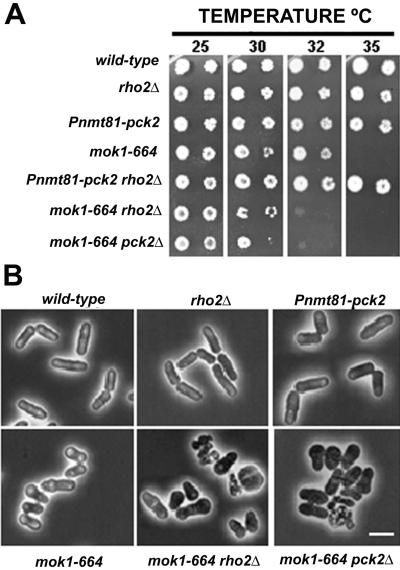
Mok1p is responsible for the biosynthesis of α-glucan in S. pombe and is directly regulated by Pck2p (Katayama et al., 1999). To analyze the functional relationship between rho2+ and mok1+, we constructed the double-mutant strain TMC113 (rho2::ura4+ mok1–664), which grew slowly at 30°C and could not form colonies at 32°C (Figure 5A), whereas each single mutant could grow under these conditions. This synthetic lethal interaction was similar to that observed between mok1–664 and pck2Δ (Figure 5A) (Katayama et al., 1999), and the morphology of the cells was also similar in both double mutants (Figure 5B).
Figure 5.
Synthetic lethality of rho2Δ and mok1–664 mutations. (A) Wild-type (HM123), rho2Δ (KN-1), Pnmt81-pck2 (MA21), mok1–664 (DH664), Pnmt81-pck2 rho2Δ (TMC111), mok1–664 rho2Δ (TMC113), and mok1–664 pck2Δ (SKP100) cells were grown in YES medium at 28°C, spotted on YES solid-medium plates, and incubated for 3 d at different temperatures. (B) Phase-contrast micrographs of the same strains grown in YES liquid medium for 20 h at 32°C. Bar, 10 μm.
Overexpression of mok1+ is lethal in wild-type cells and doubles the amount of cell wall α-glucan. We used strain SKP103, carrying the integrated Pnmt1–mok1 (Katayama et al., 1999), to construct different mutant strains that allowed the overexpression of mok1+ in the absence of Rho2p (strain TMC115), Pck1p (strain TMC116), or Pck2p (strain SKP170) (Katayama et al., 1999). As described previously (Katayama et al., 1999), mok1+ overexpression was not lethal in SKP170 cells (Figure 6A). In contrast, a significant toxicity was observed in TMC116 cells, where Pnmt1-driven mok1+ was deleterious even in the presence of thiamine. Interestingly, overexpression of mok1+ in TMC115 cells was toxic, but the observed lethal phenotype was delayed as compared with SKP103 cells (Figure 6A). Under these conditions, the levels of Mok1p, analyzed by immunoblot with anti-Mok1p antiserum, were similar in SKP103, TMC115, and SKP170 (Figure 6B), but the morphologies of the cells were different (Figure 6C). A possible explanation for the observation that strong Mok1p overproduction is still lethal in rho2Δ cells could be that in the absence of Rho2p, Rho1p partially substitutes for this protein, and it signals through Pck2p not only to regulate the (1,3)β-glucan-synthase but also to regulate Mok1p.
Figure 6.
Tolerance of rho2Δ, pck2Δ, and pck1Δ strains to mok1+ overexpression. (A) Strains SKP103, TMC115, SKP170, and TMC116 (corresponding to wild-type, rho2Δ, pck2Δ, and pck1Δ cells carrying the integrated Pnmt1–mok1+ gene, respectively) were streaked on minimal medium in the presence (bottom plate) or absence (top plate) of thiamine and incubated at 28° for 3 d. (B) Mok1p levels in SKP103 (1, 4), TMC115 (2,5), and SKP170 (3,6) cells. Total cell extracts (5 μg) were prepared from cells grown for 14 h in medium with (1–3) or without (4—6) thiamine, run on 7.5% SDS-PAGE, and immunoblotted with anti-Mok1p antiserum and TAT1 anti-tubulin monoclonal antibody. (C) Cell morphologies and Calcofluor staining of the same S. pombe strains grown in EMM without thiamine for 14 h. Bar, 8 μm. (D) Morphologies of SKP103, TMC115, SKP170, and TMC116 cells grown in EMM with 5 μM thiamine for 48 h. Bar, 10 μm. (E) Cell wall composition of different S. pombe strains analyzed for 14C-glucose radioactivity incorporated into each cell wall polysaccharide. Strains HM123, SKP103, TMC115, and SKP170 were grown in the presence or absence of thiamine for 18 h, and 14C-glucose was added 2 h before the cells were harvested. Values are the means of three independent experiments with duplicated samples. SDs for the total carbohydrate values are shown.
The different strains carrying Pnmt1–mok1 were also grown in the presence of 5 μM thiamine in the expectation of producing only a mild increase in the Mok1p levels. Under these conditions, Mok1p caused drastic morphologic defects in the wild-type and pck1Δ cells, whereas those defects were not observed in the pck2Δ or rho2Δ cells (Figure 6D).
Biochemical analysis of cell wall composition was also performed in cells overexpressing mok1+ to establish whether Rho2p regulates the function of this protein. As shown in Figure 6E, high levels of Mok1p caused an increase in the α-glucan fraction that was considerably reduced in the rho2Δ cells as compared with the wild-type strain. Indeed, the level of α-glucan in the rho2Δ strain was close to that in the pck2Δ strain.
We therefore analyzed Mok1p localization in rho2Δ cells to determine whether there was a defect similar to that observed in pck2Δ cells (Katayama et al., 1999). The normal specific localization of Mok1p to the growth areas (Figure 7, left panel) was impaired in 75–80% of the rho2Δ cells (Figure 7, center panel). This defect was less pronounced than in pck2Δ cells (Figure 7, right panel) in which a dispersed pattern was observed in 100% of the cells.
Figure 7.
Mok1p localization in wild-type, rho2Δ, and pck2Δ cells. Exponentially growing wild-type (HM123), rho2Δ (KN-1), and pck2Δ (TP169–1C) cells were fixed and stained for immunofluorescence microscopy using affinity-purified anti-Mok1 antibody. More than 100 cells of each sample were observed. Bar, 5 μm.
In summary, the results obtained by overexpressing mok1+ and localizing Mok1p in different backgrounds indicate that Rho2p and Pck2p, but not Pck1p, regulate Mok1p in a similar manner.
DISCUSSION
S. pombe Rho1p and Rho2p GTPases appear to have different functions. Both affect the morphology of the cells; however, Rho1p is essential and required for the maintenance of (1–3)β-D-glucan synthase activity (Arellano et al., 1997), whereas Rho2p is not essential and does not affect this enzyme (Hirata et al., 1998). As shown in this study, rho2Δ cells maintain the normal proportions of all three major cell wall polymers, and the overall incorporation of glucose into this structure is only slightly lower than in a wild-type strain. These results suggest that Rho1p is sufficient to maintain cell wall integrity and can partially substitute for Rho2p (Hirata et al., 1998), whereas Rho2p cannot substitute for Rho1p, because rho1Δ cells are inviable. In addition, overexpression of rho1+ is deleterious but not lethal and causes a general increase in cell wall biosynthesis, mainly in the β-glucan content (Arellano et al., 1996). In contrast, Rho2p overexpression is lethal and increases the level of α-glucan, whereas it barely changes the β-d-glucan level. Therefore, Rho2p seems mainly to regulate α-d-glucan synthesis. Furthermore, the results suggest that a high increase in the level of this polymer might be lethal for the cells, because overproduction of the α-d-glucan synthase is also lethal (Katayama et al., 1999).
How does Rho2p signal to stimulate the biosynthesis of α-d-glucan? Our previous results suggested that Pck2p plays crucial roles in the regulation of both (1–3)β-D-glucan synthase and α-D-glucan synthase (Arellano et al., 1999b; Katayama et al., 1999; Sayers et al., 2000). Examination of rho2Δ and pck2Δ strains by transmission electron microscopy showed similar defects in the cell walls that were indistinguishable in their thickness and staining. The similar phenotypes of rho2Δ and pck2Δ strains were also revealed using cell wall inhibitors. Although cells lacking Pck1p were hypersensitive to Calcofluor, Papulacandin B, and Echinocandin, both rho2 and pck2 null strains were hypersensitive to Papulacandin B but not to Calcofluor, and they were resistant to Echinocandin. Interestingly, rho2::ura4+ Pnmt81-pck2 cells grown in the presence of thiamine were not resistant to Echinocandin. If the absence of Rho2p is partially complemented by Rho1p, that might cause a decrease in the available GTP-Rho1p required to directly activate the (1,3)-β-d-glucan synthase. In the absence of Pck2p, that effect would be more dramatic, because this kinase also regulates the (1,3)-β-d-glucan synthase (Arellano et al., 1999b), resulting in a decrease of (1,3)-β-d-glucan that would make the cells more sensitive to Echinocandin. This explanation is consistent with previous data showing that rho1+ overexpression can partially complement the sensitivity of rho2Δ cells to Aculeacin A, an antibiotic with an effect similar to that of Papulacandin B (Hirata et al., 1998). The possibility that Rho2p also signals through another effector is not excluded, but Pck1p is unlikely to be that effector because neither rho2Δ cells nor the mutant strain TMC111, lacking both Rho2p and Pck2p when grown in thiamine, is hypersensitive to Calcofluor as would be expected if the Pck1p signaling pathway were impaired. The experiments based on β-glucanase treatment provided confirmatory results for the hypothesis that Rho2p and Pck2p are in the same signaling pathway. Finally, the dramatic changes in actin distribution and cell morphology caused by rho2+ overexpression in wild-type or pck1Δ cells were not observed in pck2Δ cells. Moreover, rho2+ overexpression is not lethal and does not have any significant effect on the cell wall of pck2Δ cells, supporting the hypothesis that Pck2p is the major Rho2p effector.
The genetic experiments shown in this study also revealed the functional relationship between Rho2p and Mok1p, the major α-glucan synthase. Thus, the double-mutant rho2Δ mok1–664 showed a synthetic lethal interaction in which the cells were round and lysed spontaneously when grown at 32°C, as occurs also in the double-mutant pck2Δ mok1–664 (Katayama et al., 1999). In addition, although a high level of Mok1p was deleterious in rho2Δ cells, it caused a different phenotype than overexpression in a wild-type background. The lethality was delayed, the morphology of the cells was different, and the level of α-glucan in the walls was not increased to the same level as when mok1+ was overexpressed in a rho2+ background.
None of the genetic interactions observed between mok1 and rho2 or pck2 could be reproduced with pck1, indicating that Pck1p is not involved in α-d-glucan biosynthesis and signals in a different pathway.
Taking together all the available data, we propose that Rho1p regulates (1–3)β-d-glucan biosynthesis in a dual manner, directly and also through Pck2p (Arellano et al., 1999b; Sayers et al., 2000). On the other hand, Rho2p regulates the biosynthesis of α-d-glucan exclusively through Pck2p, because Rho2p overproduction results in an increase of the cell wall α-d-glucan only when Pck2p is present. Rho1p interacts with, stabilizes, and localizes Pck2p to the growth areas (Arellano et al., 1999b, Sayers et al., 2000). Rho2p is also localized to the growth areas, interacts with, and signals to Mok1p through Pck2p. It thus appears that both GTPases use the same kinase to regulate coordinately the biosynthesis of the two main S. pombe cell wall polymers.
The regulation of Pck1p and its role in cell wall integrity remain to be established. Besides being able to partially substitute for Pck2p, this kinase seems to be involved in cell integrity through a different pathway, because pck1+ also showed genetic interactions with ras1+ and ral1+ (Arellano et al., 1999b). In addition, at least two more GTPases belonging to the Rho family are present in S. pombe, and both are able to interact with Pck1p and Pck2p in the two-hybrid assay (our unpublished results). Clarification of the relationships between these proteins and their direct effectors will require further studies and will help to elucidate the regulation of cell wall assembly and the maintenance of cell integrity.
ACKNOWLEDGMENTS
We thank Dr. Ribas, Dr. Valdivieso, and Dr. Durán for their help with this manuscript and for stimulating discussions. We thank Dr. Heidi Browning for correcting this manuscript. T.M.C. acknowledges support from a fellowship granted by Ministerio de Educación y Ciencia, Spain. S.K. is supported by the Japan Society for the Promotion of Science postdoctoral fellowships for research abroad. This work was supported by grants BIO98-0814-C02-01 and IFD97-1570-C02-01 from the Comision Interministerial de Ciencia y Tecnología, Spain; by grant CSI1/99 from the Junta de Castilla y León, Spain; by grant 10213202 from the Ministry of Education, Science and Culture of Japan; and by a contract with the company Lilly S.A. Spain.
REFERENCES
- Arellano M, Duran A, Pérez P. Rho1 GTPase activates the (1–3)β-d-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996;15:4584–4591. [PMC free article] [PubMed] [Google Scholar]
- Arellano M, Duran A, Pérez P. Localization of the Schizosaccharomyces pombe Rho1p GTPase and its involvement in the organization of the actin cytoskeleton. J Cell Sci. 1997;110:2547–2555. doi: 10.1242/jcs.110.20.2547. [DOI] [PubMed] [Google Scholar]
- Arellano M, Coll PM, Pérez P. Rho GTPases and its involvement in the organization of the actin cytoskeleton. Microsc Res Tech. 1999a;47:51–60. doi: 10.1002/(SICI)1097-0029(19991001)47:1<51::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Arellano M, Valdivieso MH, Calonge MT, Coll PM, Duran A, Pérez P. Schizosaccharomyces pombe protein kinase C homologues, Pck1p and Pck2p, are targets of Rho1p and Rho2p and differentially regulate cell integrity. J Cell Sci. 1999b;112:3569–3578. doi: 10.1242/jcs.112.20.3569. [DOI] [PubMed] [Google Scholar]
- Arellano M, Cartagena-Lirola A, Nasser-Hajibagheri MH, Durán A, Valdivieso MH. Proper ascospore maturation requires the chs1+ chitin synthase gene in Schizosaccharomyces pombe. Mol Microbiol. 2000;35:79–90. doi: 10.1046/j.1365-2958.2000.01678.x. [DOI] [PubMed] [Google Scholar]
- Cabib E, Drgonovâ J, Drgon T. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu Rev Biochem. 1998;67:307–333. doi: 10.1146/annurev.biochem.67.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J. Cell polarity in yeast. Annu Rev Cell Dev Biol. 1999;15:365–391. doi: 10.1146/annurev.cellbio.15.1.365. [DOI] [PubMed] [Google Scholar]
- Forsburg SL. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan IM, Hyams JS. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hirata D, Nakano K, Fukui M, Takenaka H, Miyakawa T, Mabuchi I. Genes that cause aberrant cell morphology by overexpression in fission yeast: a role of a small GTP-binding protein Rho2 in cell morphogenesis. J Cell Sci. 1998;111:149–159. doi: 10.1242/jcs.111.2.149. [DOI] [PubMed] [Google Scholar]
- Hochsterbach F, Klis FM, van den Ende E, van Donselar PJP, Klausner RD. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc Natl Acad Sci USA. 1998;95:9161–9166. doi: 10.1073/pnas.95.16.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro J, Saitou A, Duran A, Ribas JC. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to Cyclosporin A and Papulacandin B. J Bacteriol. 1997;179:7653–7662. doi: 10.1128/jb.179.24.7653-7662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Katayama S, Hirata D, Arellano M, Perez P, Toda T. Fission yeast α-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J Cell Biol. 1999;144:1173–1186. doi: 10.1083/jcb.144.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecká M, Fleet GH, Phaff HF. Ultrastructure of the cell wall of Schizosaccharomyces pombe following treatment with various glucanases. J Struct Biol. 1995;114:140–152. doi: 10.1006/jsbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Le Goff X, Woollard A, Simanis V. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol Gen Genet. 1999;262:163–172. doi: 10.1007/s004380051071. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, Balasubramanian MK. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J Cell Sci. 2000;113:1223–1230. doi: 10.1242/jcs.113.7.1223. [DOI] [PubMed] [Google Scholar]
- Manners DJ, Meyer MT. The molecular structures of some glucans from the cell wall of Schizosaccharomyces pombe. Carbohydr Res. 1977;57:189–203. [Google Scholar]
- Mata J, Nurse P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast. Cell. 1997;89:939–950. doi: 10.1016/s0092-8674(00)80279-2. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakano K, Arai R, Mabuchi I. The small GTP-binding protein Rho1 is a multifunctional protein that regulates actin localization, cell polarity, and septum formation in the fission yeast Schizosaccharomyces pombe. Genes Cells. 1997;2:679–694. doi: 10.1046/j.1365-2443.1997.1540352.x. [DOI] [PubMed] [Google Scholar]
- Osumi M. The ultrastructure of yeast: cell wall structure and formation. Micron. 1998;29:207–233. doi: 10.1016/s0968-4328(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Pérez P, Varona R, Garcia-Acha I, Durán A. Effect of Papulacandin B and Aculeacin A on β(1,3)-glucan synthase from Geotrichum lactis. FEBS Lett. 1981;12:249–252. [Google Scholar]
- Prentice HL. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1992;20:621. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. J Cell Sci. 2000;113:365–375. doi: 10.1242/jcs.113.3.365. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sayers LG, Katayama S, Nakano K, Mellor H, Mabuchi I, Toda T, Parker P. Rho-dependence of Schizosaccharomyces pombe pck2. Genes Cells. 2000;5:17–27. doi: 10.1046/j.1365-2443.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Counteractive roles of protein phosphatase 2C (PP2C) and a MAP kinase kinase homolog in the osmoregulation of fission yeast. EMBO J. 1995;14:492–502. doi: 10.1002/j.1460-2075.1995.tb07025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Dhut S, Superti-Furga G, Gotoh Y, Nishida E, Sugiura R, Kuno T. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol Cell Biol. 1996;16:6752–6764. doi: 10.1128/mcb.16.12.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Shimanuki M, Yanagida M. Two novel protein kinase C-related genes of fission yeast are essential for cell viability and implicated in cell shape control. EMBO J. 1993;12:1987–1995. doi: 10.1002/j.1460-2075.1993.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F. On growth and form: control of cell morphogenesis in fission yeast. Curr Opin Microbiol. 1998;1:712–718. doi: 10.1016/s1369-5274(98)80120-3. [DOI] [PubMed] [Google Scholar]