Abstract
The present study examined the expression and role of the thiazolidinedione (TZD)-activated transcription factor, peroxisome proliferator-activated receptor γ (PPARγ), in human bladder cancers. In situ hybridization shows that PPARγ mRNA is highly expressed in all human transitional epithelial cell cancers (TCCa's) studied (n=11). PPARγ was also expressed in five TCCa cell lines as determined by RNase protection assays and immunoblot. Retinoid X receptor α (RXRα), a 9-cis-retinoic acid stimulated (9-cis-RA) heterodimeric partner of PPARγ, was also co-expressed in all TCCa tissues and cell lines. Treatment of the T24 bladder cancer cells with the TZD PPARγ agonist troglitazone, dramatically inhibited 3H-thymidine incorporation and induced cell death. Addition of the RXRα ligands, 9-cis-RA or LG100268, sensitized T24 bladder cancer cells to the lethal effect of troglitazone and two other PPARγ activators, ciglitazone and 15-deoxy-Δ2,14-PGJ2 (15dPGJ2). Troglitazone treatment increased expression of two cyclin-dependent kinase inhibitors, P21WAF1/CIP1 and p16INK4, and reduced cyclin D1 expression, consistent with G1 arrest. Troglitazone also induced an endogenous PPARγ target gene in T24 cells, adipocyte-type fatty acid binding protein (A-FABP), the expression of which correlates with bladder cancer differentiation. In situ hybridization shows that A-FABP expression is localized to normal uroepithelial cells as well as some TCCa's. Taken together, these results demonstrate that PPARγ is expressed in human TCCa where it may play a role in regulating TCCa differentiation and survival, thereby providing a potential target for therapy of uroepithelial cancers.
Keywords: PPARγ, bladder cancer, cell proliferation, differentiation
Introduction
The peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear receptor superfamily and in combination with retinoid X receptor α (RXRα), a 9-cis-retinoic acid (9-cis-RA) receptor, forms a heterodimer which activates gene transcription and mediates terminal differentiation of adipocytes [1]. PPARγ is expressed in high levels not only in adipocytes, but in several other tissues, including kidney, ureter and urinary bladder, stomach, ileum, and spleen [2,3]. The expression of PPARγ in nonadipose tissues suggests additional roles for this nuclear receptor unrelated to adipogenesis. Since the discovery of specific ligands for PPARγ, including antidiabetic thiazolidinediones (TZD), 15-deoxy-Δ12,14-PGJ2 (15dPGJ2), nonsteroid anti-inflammatory drugs (NSAIDs), and some polyunsaturated fatty acids [4–6], evidence supporting a role for PPARγ in terminal differentiation of monocyte/macrophage [7] human liposarcoma and human breast cancer cells has been reported [8,9]. TZDs, such as pioglitazone, induce complete terminal differentiation of liposarcoma cells with withdrawal from cell cycle [8]. The PPARγ activators, troglitazone, and pioglitazone, have also recently been shown to induce differentiation and reduce growth rate in cultured breast and colon cancer cells and tumor-bearing mice [9–12]. These data suggest that PPARγ activation may play an important role in tumorigenesis in nonadipose cells.
Recently, we and others have reported that PPARγ is highly expressed in transitional epithelial cells of rabbit, mice, and human ureter and bladder [2,13]. Furthermore, the urinary tract is a rich source for endogenous prostaglandins, including the PPARγ ligands, PGJ2 and its precursor PGD2 [14–16]. Given its role as a differentiation factor, PPARγ may be important for maintaining integrity of the urinary tract epithelium. The presence of PPARγ in human ureter and bladder tissues may also have important pathophysiologic significance in bladder cancer. In this study, we examined the expression of PPARγ in human bladder cancer tissues and determined the effects of PPARγ ligands on transitional cancer cell growth.
Materials and Methods
Chemical Reagents and Cell Lines
Troglitazone (provided by Warner Lambert/Parke-Davis Pharmaceutical Research Co., Ann Arbor, MI), ciglitazone (purchased from BIOMOL®, Plymouth Meeting, PA), and LG100268 (provided by Dr. Richard Heymann, Ligand Pharmaceutics) were dissolved in DMSO at a concentration of 30 mM. 9-cis-retinoid acid (9-cis-RA) was purchased from Sigma Chemical Co. (St. Louis, MO) and dissolved in ethanol at a concentration of 10 mM. Human bladder cancer cell lines T24, HT-1376, RT-4, and 5637 and human ureter simian virus 40 (SV40)-transformed epithelial cell line (SV-HUC1) were obtained from American Type Culture Collection (ATCC) and cultured in the recommended media.
Preparation of Human cDNA Probes
A human PPARγ cDNA fragment was generated by reverse transcription-polymerase chain reaction (RT-PCR) from human kidney total RNA (Clontech, Palo Alto, CA) as previously described [2]. Human RXRα, cyclin D1, p16INK4, p21WAF1/CIP1, and adipocyte-type fatty acid binding-protein (A-FABP), 15-prostaglandin dehydrogenase (15-PGDH), and keratin-13 cDNA probes were amplified by RT-PCR from RNA of human kidney, ureter, liver, and human bladder cancer cell line, T24. The primers for PCR are shown in Table 1. After amplification, these fragments were subcloned into a vector (pBlueScript SK(-); Strategene, La Jolla, CA). Human β-actin (125 bp) was obtained from Ambion Inc. (Austin, TX). Antisense and sense riboprobes were synthesized in vitro using appropriate RNA polymerase (MAXI-script™ kit; Ambion) and 32P- or 35S-labeled UTP for RNase protection assays and in situ hybridization.
Table 1.
PCR primers used for amplification of cDNA fragments.
| cDNA | Sense primer | Antisense primer | Predicted size |
| PPAFγ | 5′-CCCTCATGGCAATTGAATGTCGTG-3′ | 5′-TCGCAGGCTCTTTAGAAACTCCCT-3′ | 761 bp |
| RXRα | 5′-AGGAGCGGCAGCGTGGCAAGG-3′ | 5′-GATGGAGCGGTGGGAGAAGGA-3′ | 335 bp |
| cyclin D1 | 5′-CGCCCTCGGTGTCCTACTTCA-3′ | 5′-GGCATTTTGGAGAGGAAGTGT-3′ | 391 bp |
| p16 | 5′-CGCCGACCCCGCCACTCTCACC-3′ | 5′-GGTTGTGGCGGGGGCAGTTGT-3′ | 338 bp |
| p21 | 5′-AAAACGGCGGCAGACCAGCAT-3′ | 5′-CGGACAAGTGGGGAGGAGGAA-3′ | 420 bp |
| a-FABP | 5′-TCAGTGTGAATGGGGATGTGA-3′ | 5′-TCAACGTCCCTTGGCTTATGC-3′ | 463 bp |
| 15-PGDH | 5′-CGTGAACGGCAAAGTGGCGCTGGT-3′ | 5′-GCTAAAGATGACATATTGATAATG-3′ | 436 bp |
| keratin13 | 5′-ACCCGCGTGCTGGCAGAGATG-3′ | 5′-TACTCTTGGTTCTGGCACTCC-3′ | 353 bp |
In Situ Hybridization
In situ hybridization was performed as previously described [2]. The human ureter and bladder tissues were obtained from a normal male and 11 patients with transitional cell bladder cancer, respectively. Specimens of human bladder cancer were obtained from the Department of Pathology and Division of Urology, Vanderbilt University Medical Center and Veterans Administration Medical Center. Briefly, human tissue sections were deparaffinized, refixed in paraformaldehyde, treated with proteinase K (20 µg/ml), washed with phosphate-buffered saline (PBS) buffer, and treated with triethanolamine plus acetic anhydride (0.25% v/v). Finally, sections were dehydrated with 100% ethanol. 35S-labeled antisense and sense riboprobes from human PPARγ, RXRα, and A-FABP were hybridized to the sections at 55°C for 18 hours. After hybridization, the sections were washed at 65°C once in 5xSSC plus 10 mM β-mercaptoethanol (BME), once in 50% formamide, 2xSSC, and 100 mM BME for 30 minutes. After two additional washes in 10 mM Tris, 5 mM EDTA, 500 mM sodium chloride (TEN) at 37°C, sections were treated with RNase A (10 mg/ml) at 37°C for 30 minutes, followed by another wash in TEN at 37°C. Sections were then washed twice in 2xSSC, and twice in 0.1xSSC at 65°C. Slides were dehydrated with graded ethanol containing 300 mM ammonium acetate. Then, slides were dipped in emulsion (Ilford K5; Knutsford, Cheshire, England) diluted 1:1 with 2% glycerol and exposed for 4 to 5 days at 4°C. After developing in film (D-19; Kodak, New York, NY), slides were counterstained with hematoxylin. Photomicrographs were taken using a microscope (Zeiss Axioskop; Zeiss, Germany) with either dark-field or bright-field optics.
Solution Hybridization/RNase Protection Assays
RNase protection assays were performed as described previously [2,17]. Briefly, plasmids containing human cyclin D1, p16, p21, RXRα, A-FABP, 15-PGDH, keratin-13 and β-actin inserts described above were linearized with appropriate restriction enzymes. Radioactive riboprobes were synthesized from 1 µg of linearized plasmid in vitro, using MAXIscript™ kit (Ambion) for 1 hour at 37°C, in a total volume of 20 µl. The reaction buffer contained 10 mM dithiothreitol (DTT), 0.5 mM ATP, CTP, and GTP, 2.5 mM of UTP, and 5 µl of 800 Ci/mmol [α-32P] UTP at 10 mCi/ml (DuPont, NEN, Boston). Hybridization buffer included 80% deionized formamide, 100 mM sodium citrate, pH 6.4 and 1 mM EDTA (RPA II; Ambion). Twenty micrograms of total RNA, isolated by TRIZOL-REAGENT (Gibco BRL), was incubated at 45°C for 12 hours in hybridization buffer with 5x104 cpm labeled riboprobes. After hybridization, ribonuclease digestion was carried out at 37°C for 30 minutes, and precipitated, protected fragments were separated on 4% polyacrylamide gel at 200 V for 3 hours. The gel was exposed to XAR-5 film (Kodak) overnight at -80°C with intensifying screens.
Measurement of DNA Synthesis
3H-thymidine incorporation was measured to determine the effect of troglitazone on DNA synthesis. The human bladder cancer cell line, T24, was maintained in McCoy's 5a medium supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, and 200 mg/l l-glutamine. Cells were cultured in a humidified atmosphere of 5% CO2 at 37°C. After achieving confluence, cells were cultured in serum-depleted medium for 48 hours. Cells were then stimulated by the addition of epidermal growth factor (EGF, 10-7 M) in the presence or absence of troglitazone (1 to 20 µM) for 24 hours. 3H-thymidine (1 mCi/ml) was added to the cells for the last hour of treatment. 3H-thymidine incorporation was detected by scintillation counting.
MTT Cell Viability Assay
A modified colorimetric assay based on the selective ability of living cells to reduce the yellow salt MTT (3-[4,5-dimethylthiozol-2, 5 diphenyl tetrazolium bromide) to formazan was used to quantitate cell viability [17]. Briefly, T24 cells were made quiescent by treatment in serum-free culture medium for 48 hours. Quiescent cells were then treated with troglitazone, ciglitazone or 15dPGJ2 with or without 9-cis-RA (or LG100268) for 24 hours; MTT at 5 mg/ml (Sigma Chemical Co.) was added to each well for a period of 4 hours. After formazan crystal formation, culture well supernatant was removed and the formazan precipitate was dissolved in 150 µl/well isopropanol. Absorbency measured at 570 nM using UV-VIS spectrophotometer (Shimadzu, Japan) is an indicator of cell viability.
Measurement of Apoptosis
DNA strand breaks were identified by terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assay using a DNA fragmentation assay kit (ApoAlert™, Clontech, Palo Alto, CA) as recommended by the supplier.
Immunoblot Analysis
Cells were harvested in SDS-PAGE sample buffer (120 mM Tris-HCl, pH 6.5, 4% SDS, 5 mM DTT, and 20% glycerol) followed by repetitive aspiration. This material was boiled for 3 minutes and protein concentration was measured by BCA protein assay (Pierce, Rockford). Ten micrograms of each protein sample was loaded onto 10% SDS-PAGE minigel and run at 100 V. Proteins were transferred to a nitrocellulose membrane at 22 V, overnight at 4°C. The nitrocellulose membranes were washed three times with PBS and incubated in blocking buffer (Tris-buffered saline which contained 150 mM NaCl, 50 mM Tris, 0.05% Tween 20 detergent, and 5% nonfat dry milk, pH 7.5) for 1 hour at room temperature. The membranes were then incubated in rabbit anti-mouse PPARγ1,2 polyclonal antibody (provided by Dr. Mitchell A. Lazar, University of Pennsylvania School of Medicine) diluted 1:2000 in blocking buffer for 2 hours at room temperature. After three washes in blocking buffer, the membranes were incubated with biotinylated anti-rabbit IgG antibody (1:2000; Vector Lab. Inc., Burlingame, CA) for 1 hour, followed by three 15-minute washings. Antibody labeling was visualized by addition of chemiluminescence reagent (DuPont, NEN, Boston) and exposing the membrane to XAR-5 film (Kodak).
Transient Transfections and Luciferase Reporter Assays
Human PPARγ cDNA was cloned into the pCB7 expression vector. PPARγ and PPRE3-tk-luciferase were provided by Dr. Raymond N. DuBois (Vanderbilt University) [12]. T24 bladder cancer cells were transfected with a mixture of PPRE3-tk-luciferase, pRL-SV40, and PPARγ expression vector using Effectene Transfection Reagent as recommended by the supplier (Qiagen Inc., Valencia, CA) for 8 hours. The transfection mixture was replaced with complete media containing either DMSO or troglitazone. After 14 hours, cells were harvested in 1xluciferase lysis buffer (Dual Luciferase Kit; Promega, China) and relative light units were determined using a luminometer (Mono light 2010; Analytical Luminescence Laboratory, San Diego, CA).
Results
PPARγ and RXRα Expression in Human Bladder Cancer Tissues
The localization of PPARγ mRNA in both histologically involved and uninvolved areas of human bladder cancers was examined using in situ hybridization. Transitional cell bladder cancers from 11 patients showed that PPARγ mRNA was expressed in all cases and was restricted to the epithelium with no expression detected in surrounding smooth muscle or interstitium (Figure 1a). PPARγ was expressed both in the uninvolved transitional epithelial cell layer (Figure 1a) and in submucosal infiltrating malignant transitional epithelial cells (Figure 1, b and c). Similarly, RXRα mRNA is highly expressed and localized in both normal and infiltrating malignant transitional bladder cancer cells. Little RXRα expression was observed in nonepithelial cells surrounding the infiltrating cancer (Figure 1, d–f).
Figure 1.
In situ hybridization showing distribution of PPARγ and RXRα mRNA in human bladder cancer tissue. (a) Dark-field illumination of uninvolved tissue of human bladder; white grains depict hybridization over urinary transitional epithelium; original magnification, x50. (b) Dark-field illumination of in situ hybridization for PPARγ over involved human bladder cancer tissue; white grains indicate hybridization over bladder epithelial cell layer and submucosai infiltrating malignant transitional cells (arrow); original magnification, x50. (c) Human bladder cancer tissue (bright field); black grains indicate hybridization over submucosai infiltrating malignant bladder cancer cells; original magnification, x400. (d) Dark-field illumination of uninvolved tissue of human bladder; white grains depict hybridization over urinary epithelial cells; original magnification, x10. (e) Dark-field illumination of in situ hybridization for PPARγ over involved human bladder cancer tissue; white dots indicate hybridization over bladder epithelial cell layer and submucosal infiltrating malignant transitional cells; original magnification, x20. (f) Human bladder cancer tissue (bright field); black grains indicate hybridization over infiltrating malignant bladder cancer cells; original magnification, x400.
PPARγ and RXRα Expression in Human Transitional Cell Cancer Cell Lines
Nuclease protection assays and immunoblots were used to examine the expression of PPARγ in four human transitional bladder cancer cell lines (RT-4, T24, 5637, and HT-1376) and one transformed human ureter epithelial cell line (SV-HUC-1). As shown in Figure 2, all these cell lines displayed the expected protected fragment of 350 bp for PPARγ mRNA and a ∼55 kDa band recognized by an anti-PPARγ antibody for PPARγ protein. Human bladder cancer cell lines express high levels of PPARγ, comparable to that of adipose tissue. RXRα mRNA was also examined by nuclease protection assay. A 335-bp protected band was observed in all four human bladder cancer cell lines (RT-4, T24, 5637, and HT-1376) and the ureter cell line (SV-HUC-1) (Figure 2). The T24 cell line was selected for further investigation.
Figure 2.
Upper panel, RNase protection showing RXRα mRNA expression (335-bp protected band) in four human bladder cancer cell lines (RT-4, T24, 5637, and HT-1376) and one transformed human ureter epithelial cell line (SV-HUC-1). Middle and lower panels, immunoblot and nuclease protection showing PPARγ protein expression in five transitional cell lines and PPARγ mRNA in five human transitional cell lines, kidney, and adipose tissue. Lower panel, a 350-bp protected band was detected in all cell lines. Middle panel, PPARγ protein was also detected by immunoblot with a doublet at ∼55 kDa.
Effect of Troglitazone on 3H-Thymidine Incorporation in T24 Cells
3H-thymidine incorporation was assessed in T24 cells to estimate DNA synthetic rates. Troglitazone caused dose-dependent inhibition of 3H-thymidine incorporation in T24 bladder cancer cells of 1.5-, 2.6-, and 9.1-fold (P<0.05) at concentrations of 10, 15, and 20 µM, respectively. This inhibition was associated with a dose-dependent decrease in cell viability as assessed by the MTT assay (r=0.98, P<0.001) (Figure 3). At concentrations exceeding 15 µM, cell troglitazone alone also caused death through apoptosis as assessed by the TUNEL assay (Figure 4, A and B). Similar effects of troglitazone on cell viability and 3H-thymidine incorporation were observed in two other bladder cancer cell lines, HT-1376 and 5637 (28.9% and 43.7% inhibition of 3H-thymidine incorporation and 12.5% and 55.6% suppression of cell viability at 15 µM, respectively). Ciglitazone, another PPARγ ligand, also induced cell death in these cell lines (data not shown).
Figure 3.
Troglitazone inhibits 3H-thymidine incorporation and decreases viability of T24 human bladder cancer cells. Upper panel, concentration-dependent effects of troglitazone on 3H-thymidine incorporation in T24 cells. 3H-thymidine incorporation was measured as described in Materials and Methods section. Results are expressed as percentage inhibition compared to untreated cells. Error bars represent standard errors of mean (SEM) (n=16; *P<0.05, **P<0.01). Data are from a single experiment and are representative of four independent experiments. Lower panel, concentration-dependent effects of troglitazone on viability of T24 cells were determined by the MTT assay. Values represent the mean±SEM of 12 wells from a single experiment representative of three independent experiments (n=12; *P<0.05, **P<0.01).
Figure 4.
(A) Representative photomicrographs showing morphology of T24 bladder cancer cells before treatment (left panel) and after a 24-hour treatment with 15 µM troglitazone (right panel). (B) Induction of apoptosis in T24 bladder cancer cells treated with 15 µM troglitazone for 1 day. The TUNEL assay was performed, labeling the apoptosis-specific DNA strand breaks with green fluorescent label as described in Materials and Methods section. Left panel, T24 cells treated with DMSO. Right panel, T24 cells treated with troglitazone (15 µM) for 24 hours.
RXRα Ligands Sensitize T24 Cells to PPARγ Ligand-Induced Cell Death
T24 cells were treated with increasing concentrations of troglitazone in the presence or absence of the RXRα ligands 9-cis-RA or LG100268 for 24 hours. Cell viability was assessed using the MTT assay. Neither LG100268, 9-cis-RA (5 µM) or troglitazone (10 µM) alone caused cell death, but combination of troglitazone with either of these two RXRα ligands resulted in a significant decrease in cell viability. In the presence of 9-cis-RA, troglitazone concentrations ranging from 0.1 to 15 µM induced 1.4- to 3.3-fold more cell death than troglitazone alone (Figure 5). LG100268, another specific ligand for RXRα, sensitized T24 cells to troglitazone-induced cell death by 1.2- to 9.6-fold at concentration from 1 to 15 µM (Figure 5). Synergy between troglitazone and 9-cis-RA was observed in another bladder cancer cell line, 5637 cells (data not shown). The endogenous PPARγ ligand 15dPGJ2 (Figure 5) also caused cell death. 15dPGJ2 killing of T24 cells at concentrations between 5 and 15 µM was completely dependent on the presence of 9-cis-RA. Another TZD ciglitazone (data not shown) also killed T24 cells in a dose-dependent manner (2.4%, 5.1 %, 15.9%, and 98.3% cell death at 0.1, 1, 5, and 10 µM, respectively) and displayed synergy with 9-cis-RA (4.4%, 19.1%, 63.8%, and 97.4% cell death at 0.1, 1, 5, and 10 µM, respectively). Thus, three different PPARγ ligands induced T24 cell death synergistically in the presence of RXRα ligands.
Figure 5.
The RXRα ligand, 9-cis -RA, sensitizes T24 bladder cancer cells to troglitazone or 15dPGJ2-induced cell death. Upper panel, quiescent T24 cells were treated with troglitazone (0.1, 1, 5, 10, 15, and 20 µM) in the presence or absence of 9-cis -RA LG100268 (5 µM) for 24 hours. MTT assay was performed to measure cellular viability. Values represent the percentage of inhibition of control culture (treated with vehicle alone) from five independent experiments. Error bars are standard errors of mean (SEM) (n=20; *P<0.05, **P<0.01). Lower panel, concentration-dependent effects of 15dPQJ2 on T24 cell viability. Study was performed in the presence or absence of 9-cis -RA (5 µM). Values represent the percentage of control culture (treated with vehicle alone) values from three independent experiments. Error bars are standard errors of mean (SEM) (n=12; *P<0.05, **P<0.01).
Effects of Troglitazone on mRNA Expression of Cell Cycle Proteins
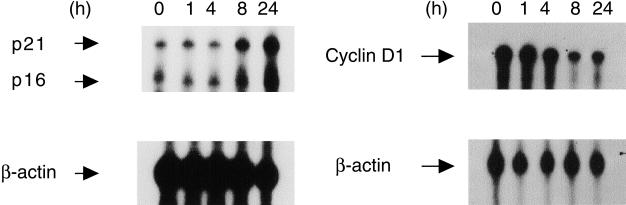
RNase protection assay was performed to determine the effects of troglitazone (10 µM) on expression of cell cycle regulatory proteins in T24 cells. As shown in Figure 6, troglitazone causes time-dependent inhibition of cyclin D1 mRNA expression. Maximal inhibition of cyclin D1 mRNA was observed after an 8-hour treatment with troglitazone. In contrast, troglitazone induced mRNA expression for two cyclin-dependent kinase inhibitors, p16INK4 and p21WAF1/CIP1, in a time-dependent manner.
Figure 6.
Nuclease protection assay showing effects of troglitazone on mRNA expression of cell cycle regulatory proteins in T24 bladder cancer cells. Quiescent T24 cells were treated with troglitazone (10 µM) for 1, 4, 8, and 24 hours. Total RNA was extracted and analyzed as described under Materials and Methods section. Upper panels, nuclease protection for cyclin-dependent kinase inhibitor, p16INK4 (338 bp), p21WAF1/CIP1 (420 bp), and cyclin D1 (391 bp). Lower panels, RNA loading was assessed by simultaneous use of a β-actin (125 bp) riboprobe.
Functional Transcriptional Activity of PPARγ in T24 Bladder Cancer Cells
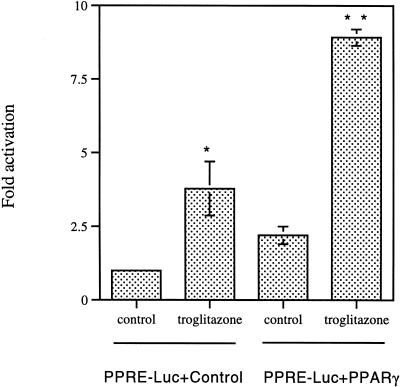
PPARγ activity in T24 cells was determined using a reporter construct containing three peroxisome proliferator response elements or PPREs (PPRE3-Luc) and an expression vector for human PPARγ. In T24 cells transfected with PPRE3-Luc alone, treatment with troglitazone resulted in a significant increase of luciferase activity (Figure 7). When cells were cotransfected with PPRE3-Luc and exogenous PPARγ expression vector, activation of luciferase activity was further enhanced in troglitazone-treated cells as compared with the control (Figure 7). Ciglitazone and 15dPGJ2 treatment had a similar effect on PPARγ reporter activity (data not shown).
Figure 7.
PPARγ is functionally active in T24 bladder cancer cells. Cells were transfected with PPRE3-Luc with control vector or PPARγ expression vector and treated with 10 µM troglitazone or control buffer for 14 hours. Results are mean±SEM of n=7, representative of three independent experiments. Control values are normalized to 1.0. Asterisks indicate significant difference from the control (*P<0.05, **P<0.01).
Expression of an Endogenous PPARγ Target Gene A-FABP
A-FABP is an endogenous gene transcriptionally activated by PPARγ in adipocytes. The effect of troglitazone treatment on A-FABP mRNA expression in T24 cells was examined by RNase protection assay. Expression of AFABP was markedly enhanced following 7 days of troglitazone (10 µM) treatment (Figure 8A). Increased expression of A-FABP was also observed after ciglitazone treatment in T24 cells. A-FABP, 15-PGDH, and keratin-13 have been suggested to be markers of transitional epithelial cell differentiation [18]. In contrast to A-FABP, there was no expression of 15-PGDH or keratin-13 mRNA in T24 cells and troglitazone did not upregulate the expression of these genes (data not shown).
Figure 8.
Panel A, effect of troglitazone on expression of A-FABP in bladder cancer cells. Cells were treated with troglitazone (10 µM) for 7 days and mRNA expression was determined by RNase protection. Lane 1, probes for β-actin and A-FABP, vehicle alone, Lane 2, troglitazone (10 µM). Total RNA (5 µg) was used for detection and the β-actin was the control for the amount of loaded RNA. Panel B, in situ hybridization showing distribution of A-FABP mRNA (white grains) in normal uroepithelial cell layer (original magnification, x40, top panel) and in submucosal infiltrating bladder cancer cells (original magnification, x400, lower panel).
The expression of A-FABP in human TCCas was also examined by in situ hybridization. While PPARγ and RXRα were widely expressed in both malignant and normal transitional epithelial cells, A-FABP was uniformly expressed only in histologically normal transitional epithelium and only focally expressed in malignant TCCa cells (Figure 8B).
Discussion
PPARγ was originally described as a nuclear receptor which plays an important role in adipocyte differentiation. PPARγ-mediated gene transcription in adipocytes requires hetero-dimerization with another nuclear receptor, RXRα, which is activated by its ligand 9-cis-RA [19,20]. Several recent reports have demonstrated the expression of PPARγ in normal urinary transitional epithelial cells of human, mouse, and rabbit [2,13]. The role of PPARγ in these epithelial cells remains uncharacterized. The present study now shows for the first time that both PPARγ and RXRα are also expressed in human transitional bladder cancer tissues and transformed uroepithelial cell lines. The expression of both PPARγ and RXRα in cultured uroepithelial cancer cells allowed us to examine the functional consequences of their activation and identify a gene transcriptionally activated by PPARγ in these cells.
Three distinct PPARγ ligands exhibited lethal effects on transitional bladder cancer cells. The effective concentrations of troglitazone and 15dPGJ2 were concentration-dependent in the range of 1 to 20 µM. These concentrations are similar to the effective range seen by other investigators who examined the inhibitory effect of these compounds on growth of cultured breast and colon cancer cells [10,21]. These concentrations are in the range of the measured binding affinity of these compounds for PPARγ, their pharmacologic target (IC50 of 3 to 4 µM for troglitazone and 2.5 µM for 15dPGJ2) [22,23]. Furthermore, when administered together with the RXRα selective ligands 9-cis-RA or LG100268, the minimal effective concentration of troglitazone was reduced to 0.1 µM. Since peak concentrations following intermediate doses of troglitazone range from 3 to 7 µM, the present effects are near the clinically relevant pharmacologic plasma concentration range for troglitazone (RezulinΔ packet insert and Ref. [24]).
TZDs induced transitional cancer cell death through apoptosis as demonstrated by DNA fragmentation using TUNEL assay, similar to reports in other cancers including prostate and breast cancers [9,10,25]. In addition to the common effect of three different PPARγ ligands, a role for PPARγ in mediating TZD-induced transitional epithelial cell death is supported by the finding of synergy with the RXRα ligands, 9-cis-RA, and LG100268 [20,26]. Addition of 9-cis-RA at a concentration which, by itself, had no effect on cell viability, increased the lethal potency of troglitazone by 10- to 100-fold. Similarly, 9-cis-RA increased the lethal potency of 15dPGJ2, a structurally dissimilar PPARγ ligand. Synergy between TZDs and RXRα ligand has been demonstrated for genes regulated by PPARγ both in vivo and in vitro [20,26]. Taken together, the present results support a role for PPARγ activation in decreasing bladder cancer cell growth and inducing apoptosis.
Accumulating evidence suggests a tight association between cell cycle status and cell death [25]. Cyclins and cyclin-dependent kinases (cdks) provide critical check points, regulating cell cycle in eukaryotes [26]. Cyclin-cdk complexes drive cells through the cell cycle, but can be inhibited by several cdk inhibitors, including p15INK4B, p16INK4A, p21WAF1/CIP1, p27KIP1, and P57KIP2 [27]. In T24 bladder cancer cells, TZD-induced cell death was associated with induction of mRNA for two cdk inhibitors, p16INK4A and p21WAF1/CIP1. This was accompanied by inhibition of cyclin D1 expression. The observation that troglitazone suppresses the expression of G1 cyclin (cyclin D1) and induces expression of G1 cdk inhibitors (p16INK4A and p21WAF1/CIP1) suggests that troglitazone inhibits the proliferation of T24 bladder cancer cells by arresting cells in G1 phase. This is consistent with the observation that troglitazone decreased 3H-thymidine incorporation, suggesting a block before S phase.
The present study is, to our knowledge, the first to examine the effects of PPARγ ligand on bladder cancer cell viability. In contrast, the use of retinoids as differentiating agents for chemoprevention of bladder cancer has been previously examined [27]. The synthetic retinoid, 13-cis-RA, reduced the incidence and extent of bladder cancer by approximately 50% in a carcinogen-induced rat model [28]. Similarly, megadose vitamin A resulted in a significant reduction in recurrence rates for bladder cancer in man [29]. The molecular targets mediating these effects remain unclear. Two families of nuclear retinoid receptors exist: RXRs (α, β, and γ) which preferentially bind 9-cis-RA, and RARs (α, β, and γ) which bind both all-trans RA (atRA) and 9-cis-RA. Recently, Anzano et al. have demonstrated that 9-cis-RA has superior efficacy over atRA as a chemopreventive agent in NMU-induced rat mammary carcinoma model [30] and suggested that activation of the RXRs may contribute to the superior activity of 9-cis-RA in this model. This was supported by Gottardis et al. in which LGD1069 (Targretin), an RXR-selective ligand, can act as a highly effective and benign chemopreventive agent for mammary carcinoma [31]. The present study supports a role for retinoids acting through RXRα in bladder cancer therapy and further suggests that both RXRα and PPARγ ligands might be used together advantageously.
The specific genes activated by PPARγ in transitional bladder epithelial remain poorly characterized. It has been reported that TZD-induced terminal differentiation of adipocytes and breast cancer cells is accompanied by accumulation of lipid droplets [1,9]. We did not observe lipid accumulation in bladder cancer cells treated with troglitazone. However, troglitazone did markedly increase the expression of the A-FABP gene. Interestingly, expression of this protein has recently been reported to be a marker of bladder cancer differentiation [18,32]. The present study demonstrates for the first time that A-FABP mRNA is selectively expressed in transitional epithelial cells of both normal and malignant bladders, rather than the stromal or interstitial cells. Members of the A-FABP family have been shown to act as tumor suppressors [33–35]. A-FABP mRNA expression is known to be regulated by a PPRE in adipocytes and we now present evidence that induction of A-FABP expression by TZDs in T24 cells may similarly be mediated through endogenous PPARγ-mediated gene transcription. Activation of exogenous PPRE-driven luciferase expression by troglitazone supports the presence of PPARγ transcriptional activity in T24 cells. The recent report of correlation between decreased A-FABP expression and worsened grade and stage of human bladder cancer [18], together with the present study showing that troglitazone induces A-FABP gene expression in T24 cells, suggests that PPARγ activation may promote differentiation of transitional cancer cells similar to its effects on liposarcoma cells [8]. Arguing against this is our finding that two other markers of human bladder cancer, 15-PGDH and keratin-13 [18], which were also correlated with tumor grade were not induced by treatment of T24 cells with troglitazone. It may be that these genes are regulated by other differentiating factors.
In summary, the present study demonstrates that PPARγ and RXRα are co-expressed in human transitional cell bladder cancers. Simultaneous activation of PPARγ and RXRα promote death of transitional bladder cancer cells. The lethal effects of PPARγ activators are accompanied by increased expression of cell cycle inhibitors, upregulation of A-FABP, and apoptosis. These results suggest that PPARγ may be a potential therapeutic target in human bladder cancer.
Acknowledgements
The authors thank the expert help of Jorge Fortune and discussion with Rajnish A. Gupta. We are grateful to Mitchell A. Lazar for providing the PPARγ antibody. M. Breyer is a recipient of the VA Clinical Investigator Career Development Award.
Abbreviations
- PPARγ
peroxisome proliferator-activated receptor γ
- RXRα
retinoid X receptor α
- 15-PGDH
15-prostaglandin dehydrogenase
- A-FABP
adipocyte-type fatty acid binding protein
Footnotes
This study was supported by funds from a Vanderbilt Cancer Center Pilot grant (IP30-CA68485) and the NIDDK (2P01-DK38226). Funds were also provided by a Veterans Administration Merit Award.
References
- 1.Brun RP, Tontonoz P, Forman BM, Ellis R, Chen J, Evans RM, Spiegelman BM. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996;10:974–984. doi: 10.1101/gad.10.8.974. [DOI] [PubMed] [Google Scholar]
- 2.Guan Y, Zhang Y, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am J Physiol. 1997;273:F1013–F1022. doi: 10.1152/ajprenal.1997.273.6.F1013. [DOI] [PubMed] [Google Scholar]
- 3.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang C, Ting AT, Seed B. PPARg agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors a and g are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Blol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 6.Forman B, Tontonoz P, Chen J, Brun R, Spiegelman B, Evans R. 15-deoxy-12, 14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPAR-gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 7.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARg promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 8.Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CDM, Brun PP, Mueller E, Altiok S, Oppenheim H, Evans RM, Spiegelman BM. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor g and the retinoid X receptor. Proc Natl Acad Sci USA. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM. Terminal differentiation of human breast cancer through PPARg. Mol Cell. 1998;1:465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 10.Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP. Ligands for peroxisome proliferator-activated receptor g and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuBois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA. The nuclear eicosanoid receptor, PPARg, is aberrantly expressed in colonic cancers. Carcinogenesis. 1998;19:49–53. doi: 10.1093/carcin/19.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Brockman JA, Gupta RA, DuBois RN. Activation of PPARg leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115:1049–1055. doi: 10.1016/s0016-5085(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 13.Jain S, Pulikuri S, Zhu Y, Qi C, Kanwar YS, Yeldandi AV, Rao MS, Reddy JK. Differential expression of the peroxisome proliferator-activated receptor g (PPARg) and its coactivators steroid receptor coactivator-1 and PPAR-binding protein PBP in the brown fat, urinary bladder, colon, and breast of the mouse. Am J Pathol. 1998;153:349–354. doi: 10.1016/s0002-9440(10)65577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farman N, Pradelles P, Bonvalet JP. PGE2, PGF2a, 6-keto-PGE1a, and TxB2 synthesis along the rabbit nephron. Am J Physiol. 1987;252:F53–F59. doi: 10.1152/ajprenal.1987.252.1.F53. [DOI] [PubMed] [Google Scholar]
- 15.Hirata Y, Hayashi H, Ito S, Kikawa Y, Ishibashi M, Sudo M, Miyazaki H, Fukushima M, Narumiya S, Hayashi O. Occurrence of 9-deoxy-D9,12-dihydroprostaglandin D2 in human urine. J Biol Chem. 1988;263:16619–16625. [PubMed] [Google Scholar]
- 16.Danon A, Zenser TV, Thomasson DL, Davis BB. Eicosanoid synthesis by cultured human urothelial cells: potential role in bladder cancer. Cancer Res. 1986;46:5676–5681. [PubMed] [Google Scholar]
- 17.Law RE, Meehan WP, Xi X-P, Graf K, Wuthrich DA, Coats W, Faxon D. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996;98:1897–1905. doi: 10.1172/JCI118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celis JE, Stergaard M, Basse B, Celis A, Lauridsen JB, Ratz GP, Andersen I, Hein B, Wolf H, Rntoft TF, Rasmussen HH. Loss of adipocyte-type fatty acid binding protein and other protein biomarkers is associated with progression of human bladder transitional cell carcinomas. Cancer Res. 1996;56:4782–4790. [PubMed] [Google Scholar]
- 19.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis-RA and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulman IG, Shao G, Heyman RA. Transactivation by retinoid X receptor-peroxisome proliferator-activated receptor g (PPARg) heterodimers: intermolecular synergy requires only the PPARg hormone-dependent activation function. Mol Cell Biol. 1998;18:3489–3494. doi: 10.1128/mcb.18.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM. Differentiation and reversal of malignant changes in colon cancer through PPARgamma [see comments] Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 22.Elbrecht A, Chen Y, Cullinan C, Hayes N, Leibowitz M, Moller D, Berger J. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors g1 and g2. Biochem Biophys Res Commun. 1996;224:431–437. doi: 10.1006/bbrc.1996.1044. [DOI] [PubMed] [Google Scholar]
- 23.Kliewer S, Lenhard J, Wilson T, Patel I, Morris D, Lehmann J. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 24.Young MA, Eckland DJA, Eastmond R, Lettis S. Establishing the dose response curve for metabolic control with troglitazone, an insulin action enhancer, in type 2 diabetes patients. Ann Med. 1998;30:206–212. doi: 10.3109/07853899808999405. [DOI] [PubMed] [Google Scholar]
- 25.Kubuta T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP. Ligand for peroxisome proliferator-activated receptor g (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 26.Mukherjee R, Davies PJ, Crombier DL, Bischoff ED, Cesario RM, Jow L, Hamanns LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Jr, Heyman RA. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386:407–414. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 27.Trump D. Retinoids in bladder, testis, and prostate cancer: epidemiologic, preclinical and clinical observations. Leukemia. 1994;8(suppl 3):S50–S54. [PubMed] [Google Scholar]
- 28.Sporn M, Squire R, Brown C, Smith J. 13-cis-RA: inhibition of bladder carcinogenesis in the rat. Science. 1977;195:487–489. doi: 10.1126/science.835006. [DOI] [PubMed] [Google Scholar]
- 29.Lamm D, Riggs D, Shriver J, vanGilder P, Rach J, DeHaven J. Megadose vitamins in bladder cancer: a double blind clinical trial. J Urol. 1994;151:21–26. doi: 10.1016/s0022-5347(17)34863-2. [DOI] [PubMed] [Google Scholar]
- 30.Anzano MA, Byers SW, Smith JM, Peer CW, Mullen LT, Brown CC, Roberts AB, Sporn MB. Prevention of breast cancer in the rat with 9-cis-RA as a single agent and in combination with tamoxifen. Cancer Res. 1994;54:4614–4617. [PubMed] [Google Scholar]
- 31.Gottardis MM, Bischoff ED, Shirley MA, Wagoner MA, Lamph WW, Heyman RA. Chemoprevention of mammary carcinoma by LGD1069 (Targretin): an RXR-selective ligand. Cancer Res. 1996;56:5566–5570. [PubMed] [Google Scholar]
- 32.Gromova I, Gromov P, Wolf H, Celis JE. Protein abundancy and mRNA levels of the adipocyte-type fatty acid binding protein correlated in non-invasive and invasive bladder transitional cell carcinomas. Int J Oncol. 1998;13:379–383. doi: 10.3892/ijo.13.2.379. [DOI] [PubMed] [Google Scholar]
- 33.Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty-acid binding protein [PA-FABP]) that is highly up-regulated in psoriasis skin and that shares similarity to fatty-acid binding proteins. J Invest Dermatol. 1992;99:299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- 34.Huynh HT, Larsson C, Narod S, Pollak M. Tumor suppressor activity of the gene encoding mammary-derived growth inhibitor. Cancer Res. 1995;55:2225–2231. [PubMed] [Google Scholar]
- 35.Yang Y, Spitzer E, Kenney N, Zschiesche W, Li M, Kromminga A, Muller T, Spener F, Lezius A, Veerkamp JH, Smith GH, Salomon DS, Grosse R. Members of the fatty acid binding protein family are differentiation factors for the mammary gland. J Cell Biol. 1994;127:1097–1109. doi: 10.1083/jcb.127.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]