Abstract
The presence of radioresistant hypoxic cells in human brain tumors limits the overall effectiveness of conventional fractionated radiation therapy. Tumor-specific therapies that target hypoxic cells are clearly needed. We have investigated the expression of suicide genes under hypoxia by a hypoxia-responsive element (HRE), which can be activated through hypoxia-inducible factor-1 (HIF-1). We transfected plasmids containing multiple copies of HRE into U-87 MG and U-251 MG-NCI human brain tumor cells and tested their ability to induce LacZ gene expression under anoxia. Gene expression under anoxia versus oxia was increased about 12-fold for U-87 MG cells and about fourfold for U-251 MG-NCI cells. At intermediate hypoxic conditions, increased LacZ gene expression in U-87 MG cells was induced by the plasmid that contained three HREs, but not by the plasmid with two HREs. Lastly, when we placed a suicide gene BAX under the control of HREs, cells transfected with the BAX plasmids were preferentially killed through apoptosis under anoxia. Our studies demonstrate that HRE-regulated gene expression is active in brain tumor cells, and that the amount of increased gene expression obtained is dependent on the cell line, the HRE copy number, and the degree of hypoxia.
Keywords: hypoxia, brain tumor cells, hypoxia-responsive element, BAX, suicide gene
Introduction
Therapy for central nervous system malignancies, in particular malignant intracranial gliomas, currently relies on a multimodality approach using surgery, radiation therapy and chemotherapy. After surgery, radiation therapy is the most effective treatment for patients with malignant gliomas [1], and efficacy of the treatment increases with increasing radiation dose [2]. However, the dose-limiting tissue is the normal brain that surrounds the tumor, and the pivotal issue in the radiation therapy of gliomas is clearly a matter of therapeutic ratio — doses of radiation that are tolerable to the patient are insufficient to control the tumor. This dilemma will persist until we devise strategies that markedly improve the therapeutic ratio. To achieve greater efficacy, we need to exploit differences between normal and malignant tissues.
One important abnormal characteristic of almost all solid tumors, including human brain tumors, is the presence of regions with reduced oxygen concentrations (hypoxia) [3,4]. Chronic hypoxia can result from an insufficient blood supply, partly because tumor cells grow faster than the endothelial cells that make up the blood vessels, and partly because the newly formed vascular supply is disorganized [5]. Acute hypoxia in tumors is caused through transient, intermittent changes in blood flow [6,7]. Both conditions result in areas of acidosis and nutrient deprivation, as well as hypoxia. Hypoxia impacts on proliferation of cancer cells and on their sensitivity toward radiation therapy, because anoxic cells (pO2<0.5 mm Hg) require approximately three times the radiation dose needed to kill oxic cells (pO2≥20 mm Hg) [8]. Cells at intermediate oxygen levels (0.5 mm Hg<pO2<20 mm Hg) display intermediate sensitivities to radiation and therefore also play an important role in the overall efficacy of radiation therapy [9].
Studies have shown that regulation of gene expression by oxygen is an important feature of many biologic processes, and hypoxia is a powerful modulator of gene expression [10,11]. Therefore, it may be possible to target hypoxic cells by a gene therapy that uses plasmids containing suicide genes that are selectively expressed under hypoxic conditions. Genes that are regulated by oxygen include glycolytic and gluconeogenic enzymes in energy metabolism, vascular growth factors, transcription factors, glucose transporters, tyrosine hydroxylase, and erythropoietin (Epo), a hormone that regulates erythropoiesis in accordance with the oxygen-carrying capacity of the blood [10]. An important mediator of hypoxia-regulated gene expression is the interaction of a transcriptional complex termed hypoxia-inducible factor-1 (HIF-1) with its cognate DNA recognition site, the hypoxia-responsive element (HRE). HIF-1 is a heterodimeric basic helix-loop-helix (bHLH)-PAS domain transcription factor containing α and β subunits that is unique among mammalian transcription factors with respect to demonstrated specificity and sensitivity of induction by hypoxia [12]. Expression of the HIF-1α subunit, which is unique to HIF-1, is precisely regulated by cellular O2 concentration such that levels of HIF-1α protein and HIF-1 DNA-binding activity increase exponentially as O2 concentration decreases [13]. Gene knockout studies indicate that HIF-1α is essential for embryonic development, for solid tumor formation, and for tumor vascularization [14–16]. In contrast to the specificity of HIF-1α, the HIF-1β subunit, which was identified previously as the aryl hydrocarbon receptor nuclear translocator [17], heterodimerizes with several other mammalian bHLH-PAS proteins including the aryl hydrocarbon receptor [18–20].
One example of hypoxia-regulated gene expression is provided by Epo [21]. Here, HIF-1 is activated in cells exposed to hypoxia and binds to the HRE consensus sequence in the 3′ enhancer region of the Epo gene, resulting in a significant increase of transcription [22]. In the present study, we show that the HRE of the human Epo gene can be used to regulate the expression of the LacZ reporter gene in response to hypoxia. However, the increase in HIF-1 regulated gene expression was cell line dependent. We also show that the responsiveness of HRE to different levels of hypoxia is dependent on the number of HRE copies incorporated in the plasmid.
BAX is a member of the Bel-2 family of proteins that can initiate apoptotic cell death in cell cultures [23,24] and in animals [25]. Overexpression of BAX protein is associated with loss of the mitochondrial membrane potential and with events typical of apoptosis, including cytosolic accumulation of cytochrome c, caspase activation, cleavage of poly (ADP-ribose)-polymerase, and DNA fragmentation [26,27]. Because of the strong apoptosis-triggering properties of the BAX protein, we decided to employ the BAX gene as a suicide gene under the regulation of the HIF-1/HRE system described above. We show here that activation of BAX gene expression under anoxia produced more than one log of cell killing in U-87 MG human glioma cells compared to oxia. We also show that the effect of cell killing was mainly mediated through apoptosis, consistent with the pro-apoptotic properties of BAX protein.
Materials and Methods
Plasmid Construction
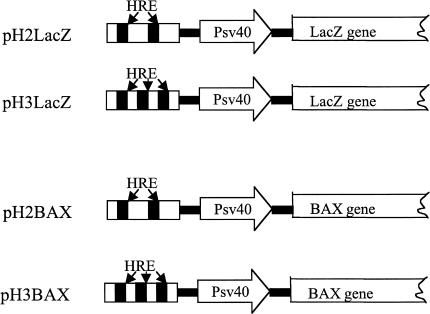
Based on the published HRE sequence from the 3′ enhancer region of the Epo gene [28], we designed two pairs of oligonucleotides that contain either two or three tandem repeats of the HRE (consensus sequence 5′-TACGTGCT-3′). These oligonucleotides were inserted into the multiple cloning sites of the mammalian expression vector pβgal-promoter (Clontech, Palo Alto, CA), which contains an enhancerless SV40 promoter situated upstream of the LacZ gene. The resulting constructs were named pH2LacZ and pH3LacZ, respectively. To construct pH2BAX and pH3BAX plasmids, the LacZ gene was deleted using HindIII and NdeI enzymes and replaced with a murine pro-apoptotic BAX cDNA fragment flanked by HindIII and NdeI sites.
Cell Culture
Human glioblastoma cell lines U-251 MG-NCI and U-87 MG were maintained in complete growth medium (CMEM), which consists of Eagle's minimum essential medium (MEM) supplemented with nonessential amino acids, glutamine and 10% fetal calf serum. Cultures were incubated in an humidified atmosphere containing 5% CO2 at 37°C.
Transient Transfection
Cells (4x105) were plated into 6-cm glass Petri dishes and grown for 16 to 18 hours until cell growth reached log phase and cell density was ∼50% confluent. Multiple sets of dishes in either duplicate or triplicate were prepared for each plasmid sample. Transfections were performed using 6 µl of lipofectamine (2 mg/ml, Gibco-BRL Life Technologies, Gaithersburg, MD), 5 µg of pH2LacZ or pH3LacZ plasmid and 0.5 µg of DNA containing the luciferase reporter gene (to correct for transfection efficiency) in 2 ml of serum-free CMEM for 5.5 hours. Then, the transfection solution in each dish was replaced with 4 ml of fresh CMEM and incubated at 37°C for 24 hours. One set of dishes was grown under normal oxic conditions and the other set(s) were subjected to different hypoxic conditions for 16 hours. Cells were then harvested and assayed for β-galactosidase (β-gal) activity by the enzymatic method described below.
Induction of Hypoxia in Tumor Cells
To achieve hypoxia, glass Petri dishes were placed into gas-tight aluminum gassing jigs. The jigs were subjected to five rounds of evacuation and flushing with either 95% air and 5% CO2 (oxic conditions) or 95% N2 and 5% CO2 (anoxic conditions) on a shaking platform at room temperature. Other hypoxic conditions were produced by using known mixtures of 95% air/5% CO2 and 95% N2/5% CO2 (Nellcor Puritan Bennett, Pleasanton, CA). After the last round of evacuation and flushing, the jigs were placed in a 37°C incubator and incubated for 16 hours.
β-Gal and Luciferase Assays
Transfected cells were washed with phosphate-buffered saline (PBS), removed from the glass surface using a rubber policeman and transferred into Eppendorf tubes. Cells were lysed using the “freeze-thaw” method, centrifuged and the supernatants were collected. The supernatants were assayed for β-gal activity using a chemiluminescent method according to manufacturer's instructions (Clontech). Briefly, an aliquot of supernatant was incubated with substrate at room temperature for 1 hour and the light intensity was measured using a Model 20e luminometer (Turner Designs, Sunnyvale, CA). The cotransfected luciferase activity of the extract was determined using a kit from Promega (Madison, WI). Briefly, an aliquot of the supernatant was incubated with the luciferase substrate luciferin, and the light intensity emitted by the sample was measured using the Model 20e luminometer. β-Gal activity was normalized to the cotransfected luciferase activity to standardize the efficiency of transient transfections. In some transfections, β-gal activity was normalized to cellular protein level, which was measured using the Bradford method (Bio-Rad, Hercules, CA).
Colony-Forming Efficiency Assay
To assay for clonogenic cell survival, cells were plated, transfected and held under either oxic or anoxic conditions for 16 hours as described above. On the day before assay, SF-126 cells were irradiated with 40 Gy and plated in six-well plates at a density of 5x104 cells/well to serve as a feeder layer. On the following day, we seeded the test cells to assay for clonogenic survival. Briefly, cells were trypsinized with 0.05% trypsin, counted, and plated into wells containing the irradiated feeder layer. Dishes were then incubated for 14 days. The colonies were stained with methylene blue (0.66% solution in 95% ethanol), and colonies containing at least 50 cells were counted. Plating efficiency was calculated as the ratio of the number of colonies formed to the number of cells seeded.
TUNEL Staining Assay
To detect apoptosis, we stained cells with the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method using the fluorescein in situ cell death detection kit (Boehringer Mannheim, Indianapolis, IN). Cells were then counterstained with fluorochrome 4′, 6-diamidino-2-phenylindole and visualized under fluorescent microscopy. The TUNEL method labels cells containing fragmented DNA, a hallmark of apoptosis. Cells undergoing apoptosis were recognized by an intensely fluorescent nucleus.
Results
HRE Mediates Reporter Gene Expression in Glioblastoma Cells under Anoxic Conditions
To evaluate the general feasibility of using the HIF-1/HRE system in human glioblastoma cells, we first determined whether HRE could initiate efficient transcription in human glioblastoma cells under hypoxia. Plasmids were constructed by inserting oligonucleotide pairs containing either two or three tandem repeats of HREs upstream of the enhancerless SV40 minimal promoter in a mammalian expression vector that contained the LacZ gene (Figure 1). We transiently transfected these plasmids into U-87 MG human glioblastoma cells using lipofectamine (Gibco-BRL, Gaithersburg, MD). To correct for variation in the efficiency of transfections, we included in the DNA/liposome complex a control plasmid containing the luciferase reporter gene. Twenty-four hours after transfection, cells were made anoxic for 16 hours. At the end of the 16-hour period, we collected protein extracts from transfected cells and assayed them for β-gal activity. The β-gal activity in cells that were maintained under oxic conditions served as a control. The β-gal activity was normalized by the cotransfected luciferase activity as described above. The luciferase activity was not affected by the reduced oxygen concentration. Compared with oxic control cells, lacZ gene expression in anoxic cells was increased 11.0- and 10.4-fold from plasmids pH2LacZ and pH3LacZ, respectively (Table 1). As a control, we also measured gene expression in cells that had been transfected with a plasmid containing the human β-actin promoter, pAcLacZ. This plasmid produced efficient β-gal expression under both oxic and anoxic conditions, indicating that the increased LacZ gene expression seen in the HRE-containing plasmids was specifically mediated through HREs. We repeated this experiment two additional times and consistently observed about a 13-fold increased LacZ gene expression from both pH2LacZ and pH3LacZ plasmids under anoxic conditions, compared to oxic conditions (Table 2).
Figure 1.
The HRE/LacZ and HRE/BAX constructs. In pH2LacZ and pH3LacZ, either two or three tandem repeats of HRE were inserted into the multiple cloning sites in front of the SV40 minimal promoter in the mammalian expression vector pβgal-promoter. In pH2BAX and pH3BAX, the LacZ gene was replaced with a murine BAX cDNA.
Table 1.
Increased LacZ Gene Expression in Anoxic U-87 MG Cells.
| Treatment | Plasmid | Normalized β-gal Activity*,† (Relative Light Units) | Fold of Increase (Anoxic/Oxic) |
| Oxic | pH2LacZ | 86.0±23.5 | |
| pH3LacZ | 77.2±29.6 | ||
| pAcLacZ | 7558±2887 | ||
| Anoxic | pH2LacZ | 948.1±67.5 | 11.0 |
| pH3LacZ | 799.8±38.9 | 10.4 | |
| pAcLacZ | 10075±1905 | 1.3 | |
Normalized to the cotransfected luciferase activity.
Average±SD of three independent samples.
Table 2.
Summary of Independent Transfection Experiments in U-87 MG and U-251 MG-NCI Cells.
| Plasmid | Fold of Increase (Anoxic/Oxic) | |
| U-87 MG* | U-251 MG-NCI† | |
| pH2LacZ | 12.6±2.5 | 4.5±3.2 |
| pH3LacZ | 13.1±2.5 | 4.1±0.6 |
Average±SD from three independent experiments.
Average±SD from two independent experiments.
We next investigated whether activation of HRE under anoxia represents a general phenomenon in human glioblastoma cells by transiently transfecting pH2LacZ and pH3LacZ plasmids into another human glioblastoma cell line, U-251 MG-NCI. Transfected cells were then either made anoxic or left oxic for 16 hours, and β-gal activity was determined. We performed this experiment twice and observed an increase in β-gal expression from pH2LacZ and pH3LacZ under anoxia. Compared with control cells, anoxic treatment of transfected U-251 MG-NCI cells increased LacZ expression in both pH2LacZ and pH3LacZ plasmids about four- to fivefold (Table 2). As a control, β-gal expression from the control pAcLacZ plasmid remained constant under both oxic and anoxic conditions (data not shown).
Gene Expression at Intermediate Oxygen Levels Depends on HRE Copy Number
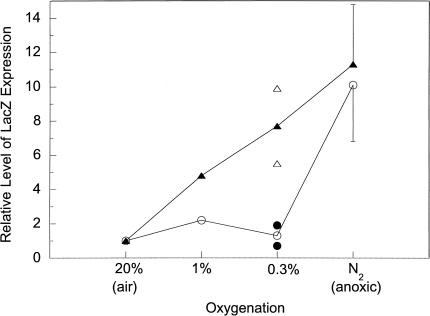
Previous studies have suggested that low radiation doses that are typical of those delivered in a daily radiotherapy protocol (1.8 to 2 Gy) produce a tumor response that is highly dependent on the tumor's oxygenation status [9]. In particular, hypoxic cells at oxygen levels intermediate between anoxia and full oxygenation are strong determinants of tumor response because many tumor cells fall within this range [7]. Therefore, in addition to killing anoxic cells, effective therapeutic agents aimed at hypoxic cells must have the ability to sensitize or kill cells at intermediate levels of oxygen. To determine the responsiveness of HRE under intermediate oxygen tensions, we measured β-gal expression at 0.3% and 1% oxygen levels in U-87 MG cells (Figure 2). Although the expression of LacZ from both pH2LacZ and pH3LacZ could be activated to similar extents under anoxic conditions, the fold of activation from these two plasmids showed a significant difference at intermediate oxygen tensions. Compared to the oxic condition, two copies of HRE showed almost no activation at either 1% or 0.3% oxygen levels. In contrast, when three copies of HRE were present, the expression of LacZ showed about a fivefold increase at 1% oxygen and a 7.5-fold increase at 0.3% oxygen.
Figure 2.
Differential responsiveness of HREs under intermediate levels of oxygen tension. U-87 MG cells were transfected with either pH2LacZ (-○-) or pH3LacZ (-▴-), and then subjected to various oxygen levels for 16 hours as described in the Materials and Methods section. The relative level of LacZ expression in oxic cells was designated as 1.0. The error bars at anoxic conditions represent the standard deviations of three independent experiments. The actual values obtained from two independent experiments at 0.3% oxygen level are shown.
Expression of BAX in Anoxic U87-MG Cells Produced Significant Cell Killing through Apoptosis
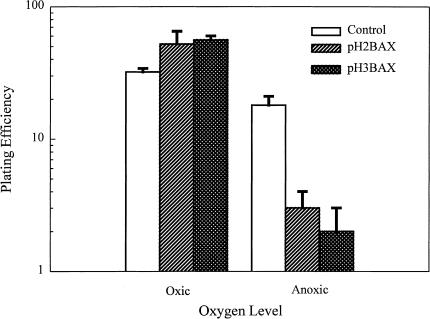
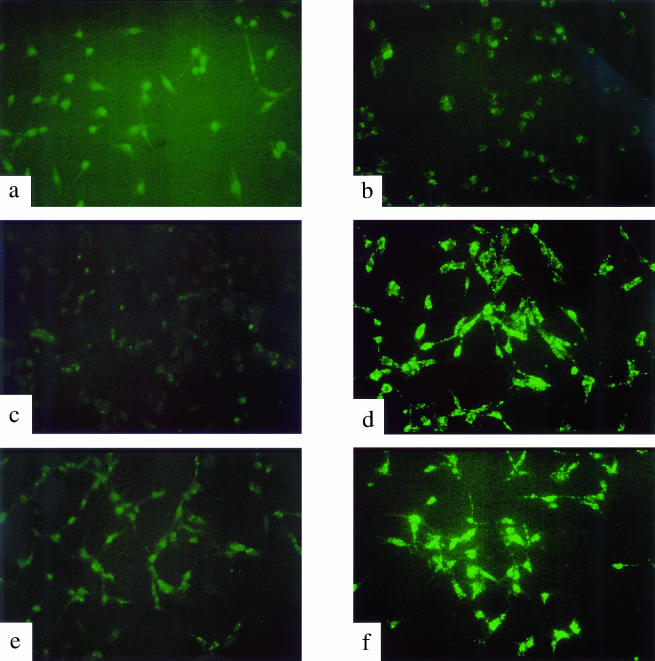
One objective of the present study was to determine whether expression of a suicide gene through the HIF-1/HRE pathway would be effective at specifically killing hypoxic tumor cells. For this purpose, we selected the mammalian pro-apoptotic BAX gene as the candidate gene. The pH2BAX and pH3BAX plasmids (Figure 1) were transfected into U-87 MG; transfection with lipofectamine alone was used as the control. In the initial experiments, the efficiency of transfection in U-87 MG cells was about 70% to 80% (data not shown), therefore we transfected U-87 MG cells and assayed the transfectants for cell survival without selecting for stably transfected clones. Following transfection, cells were grown for 16 hours in either oxic or anoxic conditions. Transfected cells were then collected and assayed for cell survival using a colony-forming efficiency assay. As shown in Figure 3, control U-87 MG cells were inherently sensitive to anoxia; the 16-hour exposure produced about 50% cell killing. However, induced expression of BAX under anoxia in U-87 MG cells that had been transfected with either plasmid produced more than one additional log of cell killing. Furthermore, under oxic conditions, BAX-transfected U-87 MG cells survived as well as control cells. We have repeated this experiment and obtained similar results (data not shown). Taken together, these results demonstrated that HRE-regulated expression of BAX could specifically kill hypoxic cells in vitro. To determine whether this BAX-mediated cell killing was triggered through cell apoptosis, we measured apoptosis in BAX-transfected U-87 MG cells under either oxic or anoxic conditions using the TUNEL assay. As shown in Figure 4, anoxia-induced expression of BAX in both pH2BAX- and pH3BAX-transfected U-87 MG cells caused a significant amount of apoptosis, as manifested by the intense fluorescent labeling of fragmented DNA (Figure 4, panels d and f). In contrast, control U-87 MG cells and BAX-transfected cells under oxic conditions showed little, if any, evidence of apoptosis (Figure 4, panels a–c and e).
Figure 3.
Clonogenic survival of oxic and anoxic U-87 MG cells transfected with pH2BAX and pH3BAX. U-87 MG cells were transfected with either pH2BAX and pH3BAX, followed by exposure for 16 hours to either oxic or anoxic conditions. Cells were then collected and assayed for cell survival using a colony-forming efficiency assay as described in the Materials and Methods section.
Figure 4.
Apoptosis in oxic and anoxic U-87 MG cells transfected with pH2BAX and pH3BAX. Cells were transfected with either pH2BAX or pH3BAX and grown in either oxic or anoxic conditions as described in the Materials and Methods section. The TUNEL assay revealed fragmented DNA as intensely fluorescent, which is characteristic of apoptosis. Panels a and b are U-87 MG cells under oxic and anoxic conditions, respectively. Panels c and d are pH2BAX-transfected U-87 MG cells under oxic and anoxic conditions, respectively. Panels e and f are pH3BAX-transfected U-87 MG cells under oxic and anoxic conditions, respectively.
Discussion
Solid tumors are heterogeneous and typically composed of physiologically distinct subpopulations. Over 40 years ago, the pioneering work by Thomlinson and Gray demonstrated that the sensitivity of tumor cells to radiation damage depended on the presence of oxygen in the tumor microenvironment [29]. Since their discovery, many subsequent studies have confirmed the presence of radioresistant hypoxic cells in tumors, including brain tumors [3,4]. Radiation therapy can effectively kill the oxic tumor cells; however, the hypoxic cells pose an obstacle in attempts to cure brain tumors with radiation. Clearly, we need to devise new therapeutic strategies to selectively target these hypoxic tumor cells.
The presence of hypoxic cells in solid tumors can also provide a means for selectively targeting cancer for treatment because normal tissues are well oxygenated [30,31]. One study by Dachs et al. [32] tested the ability of hypoxia to selectively control gene expression and increase cell killing. They placed a CD2 marker gene or a gene that produces the enzyme cytosine deaminase under the control of HRE, and showed that CD2 was induced in vitro only when oxygen concentrations were <2%. These investigators also showed that activation of cytosine deaminase under hypoxic conditions caused significant cell killing when the prodrug 5-fluorocytosine was present. Recently, Shibata et al. used multiple copies of HRE derived from the human vascular endothelial growth factor gene to achieve hypoxia-specific expression of therapeutic genes. When cells were transfected with an Escherichia coli nitroreductase gene (which can activate an anticancer prodrug CB1954) under the regulation of HRE, marked growth inhibition was specifically achieved under hypoxic conditions [33]. Other studies on the molecular mechanisms of hypoxia-regulated gene expression provide strong evidence that hypoxia induces gene expression through the cis-acting DNA sequence HRE, which interacts with a transcriptional activator HIF-1 [21]. Our current work demonstrates that such an HRE is functional under hypoxia in at least two human glioblastoma cell lines.
Our studies on the response of HREs under hypoxia reveal several interesting findings. First, although both two and three copies of HREs can activate gene expression to the same extent under anoxia (Table 1), gene expression under intermediate hypoxic conditions occurs only when the HRE copy number is three (Figure 2). This could have important implications for HRE-mediated gene therapy of human tumors, which contain cells under a wide range of oxygen concentrations. Further experiments are needed to determine the optimal HRE copy number that engenders sufficient levels of activation of suicide genes at intermediate levels of oxygen to result in effective cell killing, yet still does not cause significant cytotoxicity in well-oxygenated cells. In addition, it may be important to compare different HRE sequences, such as those from the vascular endothelial growth factor gene [34] and the phosphoglycerate kinase-1 gene [35], to determine an optimal choice for the suicide gene therapy.
Our results also indicate that the magnitude of gene expression that occurs under hypoxic conditions is different for the two brain tumor cell lines used in our studies. Whereas anoxic U-87 MG cells produce more than a 10-fold increase in gene expression, the increase in gene expression in anoxic U-251 MG-NCI cells is only about fourfold. Such differences may reflect differences in HIF-1 activity among cell lines, although we have not yet tested this possibility. Because of differences in hypoxia-induced gene expression among different brain tumor cells, the efficacy of the hypoxia-targeted gene therapy will be dependent on the responsiveness of the particular brain tumor cells under hypoxia. Therefore, a successful strategy will probably rely on the ability of the gene therapy to kill tumor cells despite their different responsiveness under hypoxia. One requirement for such gene therapy may be to select an efficient suicide gene that becomes active and causes selective cell killing at low levels of hypoxia-induced expression. A therapy employing such a suicide gene may result in the same degree of cell killing among different brain tumor cells with various levels of responsiveness under hypoxia.
One candidate suicide gene is BAX, a member of the BCL-2 gene family. We have shown that BAX can be used as a suicide gene to trigger cellular apoptosis in U-87 MG cells under hypoxia when regulated by HREs (Figure 3). It should be noted that the extent of cell killing by BAX under hypoxia may be an underestimate in our experimental system because only 70% to 80% of the cells were transfected with the BAX construct. Therefore, BAX may be more effective in killing cells than we have determined. In addition, it will be interesting to examine whether BAX can kill U-251 MG-NCI cells under hypoxic conditions, despite the decreased amount of hypoxia-induced gene expression that occurs compared to U-87 MG cells. A positive result will indicate that BAX is functional in cell killing even when increase of expression of BAX in U-251 MG-NCI is as low as four- to five-fold.
In gene therapy, it is unlikely that all tumor cells in vivo will be transfected with the suicide gene. Therefore, the bystander effect may play an important role in determining the outcome of the gene therapy. A bystander effect has often been observed in the traditional enzyme/prodrug system (e.g., thymidine kinase/gancyclovir). This effect is generally attributed to the metabolic cooperation between cells and/or the release of toxic compounds from dead cells into neighboring cells [36]. However, there is increasing evidence to indicate that the immune system plays an important role in the bystander effect in vivo [37]. Although it is unlikely that the cell killing caused by BAX will exert any significant bystander effect through metabolic cooperation or release of toxic compounds, BAX may elicit a bystander effect in vivo through an immunologic component. We have not yet tested this possibility.
In conclusion, the presence of hypoxic cells in solid tumors is an important factor leading to resistance to radiation therapy and these cells form a major obstacle in attempts to cure brain tumors with radiation. Recently emerging results have clearly demonstrated hypoxia-targeted gene therapy as an exciting innovative strategy for targeting hypoxic cells in tumors. Potential application of this gene therapy, especially when combined with radiation, could greatly improve the therapeutic outcome for brain tumors. Our studies have a number of implications for cancer gene therapy. First, in agreement with the earlier reports [32,33], our results support the notion that the HIF-1/HRE system might be useful in gene therapy to provide a selective means of controlling gene expression based on the lower oxygen levels in solid tumors compared with normal tissues. In addition, our results point out that the ultimate use of the HIF-1/HRE system will depend on the ability to obtain appropriate levels of induced gene expression at all levels of reduced oxygenation. Finally, our results demonstrate that BAX can be used as a suicide gene to trigger cell apoptosis under anoxia, providing the potential for the BAX gene to be used in gene therapy as a new class of “efficient” suicide genes.
Acknowledgements
The authors thank Mark Israel for providing the murine BAX cDNA. We also thank Tomoko Ozawa and Michael Zhang for helpful discussions.
Abbreviations
- Epo
erythropoietin
- β-gal
β-galactosidase
- HIF-1
hypoxia-inducible factor-1
- HRE
hypoxia-responsive element
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
Footnotes
This work was supported by NIH grant CA-13525. H. R. was supported by NIH grant CA-09215. C.-S. L. was supported by the California Breast Cancer Research Program.
References
- 1.Walker MD, Alexander EJ, Hunt WE, MacCarty CS, Mahaley MS, Mealey JJ, Norell HA, Owens G, Ransohoff J, Wilson CB. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 3.Kayama T, Yoshimoto T, Fujimoto S, Sakurai Y. Intratumoral oxygen pressure in malignant brain tumor. J Neurosurg. 1991;74:55–59. doi: 10.3171/jns.1991.74.1.0055. [DOI] [PubMed] [Google Scholar]
- 4.Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994;29:427–431. doi: 10.1016/0360-3016(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 6.Brown JM. Evidence for acutely hypoxic cells in mouse tumors and a possible mechanism for reoxygenation. Br J Radiol. 1979;52:650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 7.Chaplin DJ, Durand RE, Olive PL. Acute hypoxia in tumors: implications for modifiers of radiation effects. Int J Radiat Oncol Biol Phys. 1986;12:1279–1282. doi: 10.1016/0360-3016(86)90153-7. [DOI] [PubMed] [Google Scholar]
- 8.Hall EJ. Radiobiology for the Radiologist. Philadelphia, PA: J.B. Lippincott Co.; 1993. [Google Scholar]
- 9.Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat Res. 1997;147:541–550. [PubMed] [Google Scholar]
- 10.Semenza GL. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J Lab Clin Med. 1998;131:207–214. doi: 10.1016/s0022-2143(98)90091-9. [DOI] [PubMed] [Google Scholar]
- 11.Ratcliffe PJ, O'Rourke JF, Maxwell PH, Pugh CW. Oxygen sensing: hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J Exp Biol. 1998;201:1153–1162. doi: 10.1242/jeb.201.8.1153. [DOI] [PubMed] [Google Scholar]
- 12.Wang GL, Jiang B-H, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang B-H, Zheng JZ, Leung SW, Roe R, Semenza GL. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 14.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes & Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshet E. HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 16.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman EC, Reyes H, Chu F-F, Sander F, Conley LH, Brooks BA, Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 18.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kurijama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS 1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 20.Swanson HI, Chan WK, Bradfield CA. DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J Biol Chem. 1995;270:26292–26302. doi: 10.1074/jbc.270.44.26292. [DOI] [PubMed] [Google Scholar]
- 21.Bunn HF, Gu J, Huang LE, Park J-W, Zhu H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassouna I, Wickert H, Zimmermann M, Gillardon F. Increase in BAX expression in substantia nigra following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment of mice. Neurosci Lett. 1996;204:85–88. doi: 10.1016/0304-3940(96)12323-5. [DOI] [PubMed] [Google Scholar]
- 24.Pastorino JG, Chen S-T, Tafani M, Snyder JW, Farber JL. The overexpression of BAX produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 25.Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. BAX suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 26.Adams JM, Cory S. The Bcl-2 protein family: arbitors of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 27.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 28.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bremmer JC. Assessing the bioreductive effectiveness of the nitroimidazole RSU1069 and its prodrug RB6145: with particular reference to in vivo methods of evaluation. Cancer Metastasis Rev. 1993;12:177–193. doi: 10.1007/BF00689809. [DOI] [PubMed] [Google Scholar]
- 31.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 32.Dachs GU, Patterson AV, Firth JD, Ratcliffe PJ, Townsend KM, Stratford IJ, Harris AL. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–520. doi: 10.1038/nm0597-515. [DOI] [PubMed] [Google Scholar]
- 33.Shibata T, Giaccia AJ, Laderoute KR, Brown JM. Tumor-specific gene therapy using hypoxia-responsive gene expression. Proc 90th Annu Meet Am Assoc Cancer Res. 1999;40:632. [Google Scholar]
- 34.Forsythe JA, Jiang B, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen regulated elements in the phosphoglycerate kinase-1 and lactate dehydrogenase A genes: Similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pope IM, Poston GJ, Kinsella AR. The role of the bystander effect in suicide gene therapy. Euro J Cancer. 1997;33:1005–1016. doi: 10.1016/s0959-8049(96)00483-2. [DOI] [PubMed] [Google Scholar]
- 37.Gagandeep S, Brew R, Green B, Christmas SE, Klatzmann D, Poston J, Kinsella AR. Prodrug-activated gene therapy: involvement of an immunological component in the bystander effect. Cancer Gene Ther. 1996;3:83–88. [PubMed] [Google Scholar]