Abstract
Tirapazamine (TPZ) [3-amino-1,2,4-benzotriazine 1,4-dioxide, SR4233, WIN 59075, and Tirazone™] is a novel anticancer drug that is selectively activated by the low oxygen environment in solid tumors. By killing the radioresistant hypoxic cells, TPZ potentiates the antitumor efficacy of fractionated irradiation of transplanted tumors in mice. As this cell kill is closely correlated with TPZ-induced DNA damage, we investigated whether human head and neck cancers would show DNA damage similar to that seen in mouse tumors following TPZ administration. TPZ-induced DNA damage in both transplanted tumors in mice and in neck nodes of 13 patients with head and neck cancer was assessed using the alkaline comet assay on cells obtained from fine-needle aspirates. The oxygen levels of the patients' tumors were also measured using a polarographic oxygen electrode. Cells from the patients' tumors showed DNA damage immediately following TPZ administration that was comparable to, or greater than, that seen with transplanted mouse tumors. The heterogeneity of DNA damage in the patients' tumors was greater than that of individual mouse tumors and correlated with tumor hypoxia. The similarity of TPZ-induced DNA damage in human and rodent tumors suggests that tirapazamine should be effective when added to radiotherapy or to cisplatin-based chemotherapy in head and neck cancers.
Keywords: tirapazamine, comet assay, hypoxia, eppendorf, radiotherapy
Introduction
Tirapazamine (TPZ) [3-amino-1,2,4-benzotriazine 1,4-dioxide, SR4233, WIN 59075, and Tirazone™ (Sanofi-Winthrop, GreatValley, PA)] is currently in Phase II and III clinical trials as an adjunct to radiotherapy and to cisplatin-based chemotherapy. The drug has a unique mechanism of action that depends on its metabolism to an active species that occurs only under low oxygenation [1]. Such low oxygenation, or hypoxia, does not occur in tissues under normal physiological conditions, but is common in mouse and human solid tumors [2,3]. The hypoxia-dependent metabolism of TPZ leads to two principal activities. First, it increases the activity of platinum-based chemotherapy both with experimental tumors transplanted into mice [4], and with hypoxic cells in vitro [5]. Though this potentiation of cisplatin cell kill depends on the TPZ exposure being under hypoxia, it can occur in the absence of any cytototoxicity by TPZ [5]. The clinical effectiveness of the drug in enhancing the efficacy of cisplatin has recently been demonstrated in a multicenter randomized Phase III study comparing cisplatin plus TPZ with cisplatin alone in advanced nonsmall cell lung cancer. In this trial, overall response rates were doubled and survival time was significantly longer when TPZ was added to cisplatin compared to cisplatin alone [6].
In addition to sensitizing tumors to cisplatin, TPZ is preferentially cytotoxic to hypoxic cells both in vitro [7] and in tumors in vivo [8,9]. Hypoxic cells are resistant to killing by ionizing radiation [10] and are associated with lower local control rates following radiotherapy [11–13], as well as with increased metastases [14,15]. Because of its selective toxicity to the radiation-resistant hypoxic cells in tumors, TPZ increases the efficacy of fractionated radiation to transplanted tumors in mice [16,17]. However, it has so far been tested with radiotherapy of human cancers only in a small Phase II study with head and neck cancer [18], from which it is not possible to conclude whether TPZ will be effective when combined with radiotherapy. Though this question can only be answered definitively with randomized clinical trials, the expense and time involved in such investigations make it attractive to ask whether a biologic endpoint could be used to indicate whether TPZ might be effective in human tumors.
The present study was undertaken to determine whether TPZ-induced DNA damage could be used as such an endpoint for the activity of TPZ in human head and neck tumors. The logic for using such an assay is based on two findings. First, that potentiation of cell kill by fractionated radiation of mouse tumors is the result of TPZ killing the radioresistant hypoxic cells in the tumors [16]. Second, it is based on the finding that DNA damage measured by the comet assay is an excellent predictor of cell killing produced by TPZ both with cells in vitro [19], with spheroids in vitro and with transplanted tumors in mice [20]. We have also shown that DNA damage produced by TPZ in mouse tumors correlates both with tumor oxygenation and with radiation potentiation by TPZ [21]. Based on their data in mice, Olive et al. have suggested that the comet assay could be used to predict the efficacy of TPZ in clinical trials [20]. It was therefore of interest to ask whether measurable DNA damage would be produced by TPZ in human tumors, whether it would be comparable to that seen in mouse tumors, and whether it would show any relationship to tumor hypoxia. To date there is no information on the effect of TPZ on patients' tumors. In fact some findings have suggested that the efficacy of the drug might be less than that in mouse tumors, first because some mouse cell lines are more sensitive to TPZ-induced cell kill than human tumor cells [7,19], and second because mouse tumors are on the whole more hypoxic than human tumors [22]. This study therefore provided a means of assessing whether the TPZ doses were adequate, whether the human tumors were sufficiently hypoxic, and whether the cells had sufficient of the relevant reductive enzymes to metabolize TPZ to its DNA-damaging species. If any one of these were inadequate we would expect little or no DNA damage in the human tumors. However, comparable DNA damage by TPZ in mouse and human tumors would suggest that TPZ would be as effective when combined with radiotherapy of human tumors as it is with transplanted tumors in mice.
Materials and Methods
Patient Protocol
As part of an NCI-sponsored Phase II clinical trial for locally advanced squamous cell head and neck cancer, previously untreated patients with stage IV tumors and pathologically proven positive lymph node(s) were randomized to treatment with or without TPZ in combination with cisplatin-based induction chemotherapy followed by concurrent chemo-radiotherapy (Trial NCI-T94-01190). Before patient randomization, tissue oxygenation in the largest palpable lymph node was measured using the commercially available “pO2 Histograph” (Eppendorf, Hamburg, Germany). For these measurements, a stepper-motor-driven steel probe containing a glass-insulated micro-cathode (12 µm diameter) was inserted into the node along three tracks as previously described [23,24]. Approximately 60 individual oxygen measurements in the tumor were taken for each patient, from which median pO2 values and other estimates of tumor oxygenation were calculated. Those patients randomized to TPZ treatment also had fine-needle aspirates of the involved node both before and immediately after the first TPZ infusion (300 to 330 mg/m2) which was performed before any other treatment. Single-strand DNA breaks in the tumor cells from the aspirates were quantified with the comet assay (see below). The levels of single-strand DNA damage before and after TPZ in the same patients were compared to assess the tumor response to this drug. The patients described in this report represent the first 13 patients randomized into the TPZ-treatment arm. The Stanford University Institutional Review Board approved all the studies, and informed consent was obtained according to the Helsinki Declaration II.
Animals and Tumors
C3H/Km and immunodeficient (SCID) mice were bred and housed under specific pathogen-free conditions in the Stanford University Research Animal Facility. All experiments were conducted according to the guidelines and directives set forth by the Stanford University Administrative Panel on Laboratory Animal Care. The human tumor cell lines used were the human HT1080 fibrosarcoma, obtained from the American Type Culture Collection, and the colon adenocarcinoma HT29 cells, obtained from Dr. R. M. Sutherland (SRI International, Menlo Park, CA). The mouse tumor lines used were the RIF-1 fibrosarcoma and SCCVII squamous cell carcinoma, details of the derivation of which have been published [25,26]. Each tumor cell line was maintained in accordance with a strict in vitro/in vivo protocol to prevent any genetic drift over time [25]. From monolayer cultures, single-cell suspensions (human cells per injection: 1x106 and mouse cells per injection: 2x105) were inoculated intradermally in the lower back of each mouse approximately 2 cm from the tail. Experiments were conducted approximately 2 weeks later when the tumor volumes were 100 to 150 mm3.
Mouse Tirapazamine Studies
TPZ was kindly supplied by Sanofi-Winthrop (Great Valley, PA). The drug was dissolved in physiological saline at a concentration of 1 mg/ml and injected intraperitoneally according to body weight. Fine-needle aspirates were taken from the tumors to assess TPZ-induced DNA damage by the alkaline comet assay. Preliminary dose-response and time-course studies with the comet assay were conducted using the RIF-1 tumor. Based on these data and on previous experiments with the SCCVII tumor [21], further studies were conducted at a TPZ dose of 0.08 mmol/kg (=14.4 mg/kg), with fine-needle aspirates taken 45 minutes after TPZ injection to compare the DNA damage induced by TPZ in the HT1080, HT29, SCCVII, and RIF-1 tumors.
Comet Assay
Fine-needle aspirates from tumors in human patients and from mice were treated in the same manner. Single-cell suspensions were prepared by mixing the aspirates into calcium- and magnesium-free phosphate-buffered saline. A mixture of 0.5 ml cell suspension and 1.5 ml of a 1% solution of agarose containing 2% dimethyl sulfoxide was layered onto a microscope slide and allowed to cool on a cold block. For the comet assay [27], slides were placed in a lysis buffer consisting of 30 mM NaOH, 1 M NaCl and 0.1% N-lauroylsarcosine for 60 minutes. After three 20-minute washes in a buffer containing 30 mM NaOH and 2 mM EDTA, the slides were placed in a fresh solution of the same buffer for electrophoresis at 0.6 V/cm for 22 minutes. Thereafter, the slides were rinsed in distilled water for 15 minutes and then stained with propidium iodide (2.5 µg/ml) for 15 minutes. Slides were analyzed with a Nikon Optiphot microscope attached to an Ikegami 4612 CCD camera and fluorescence image analysis system. DNA single-strand breaks were measured using the tail moment parameter, the product of the percentage of DNA in the comet tail and the mean tail length, using specially designed software [28]. A total of 300 cells were scored per sample.
Results
TPZ-Induced DNA Damage in Mouse Tumors
Because we wished to use the alkaline comet assay to compare DNA damage induced by TPZ in transplanted tumors in mice with that induced in tumors in patients, we first obtained preliminary data on the time and dose needed to obtain maximum response in the mouse studies. As can be seen from Figure 1, maximum DNA damage, as assessed by median tail moment, is obtained roughly 30 to 45 minutes after a single intraperitoneal dose of 0.08 mmol/kg TPZ (Figure 1A. We also show that in these RIF-1 tumors DNA damage tends to plateau at doses above 0.08 mmol/kg (Figure 1B), as we previously observed with the SCCVII tumor [21].
Figure 1.
(A) Time course for TPZ-induced DNA damage in RIF-1 tumors in mice given a single intraperitoneal dose of 0.08 mmol/kg TPZ as measured with the alkaline comet assay. The data show the mean and standard errors for groups of three mice per time point. (B) Dose response for TPZ-induced DNA damage in RIF-1 tumors as measured with the alkaline comet assay. Fine-needle aspirates were taken from tumors 45 minutes after intraperitoneal injection of different TPZ doses. Means and standard errors for three to five tumors treated per point are shown.
Based on these data we chose 45 minutes after a single intraperitoneal dose of 0.08 mmol/kg as the dose and time to measure DNA damage in the four different transplanted tumors chosen for this study. These tumors were selected because we have previously shown that TPZ (0.08 to 0.12 mmol/kg per injection), when combined with each dose of a fractionated irradiation regimen (8x2.5 Gy), produced significant potentiation in all tumors except the human colon carcinoma HT-1080 [16,21]. The extent of potentiation of the two mouse (SCCVII and RIF-1), and the human colon carcinoma HT-29, was by dose modification factors of 1.5 to 1.7 (i.e., equivalent to giving 50% to 70% more radiation dose to the tumors) [21]. However we found no potentiation of the antitumor efficacy of fractionated radiation in the HT-1080 tumors by TPZ [21].
Oxygenation and TPZ-Induced DNA Damage in Human Tumors
Figure 2 shows the data obtained on tumor oxygenation and on TPZ-induced DNA damage from two patients to illustrate the type of data and the range of the values obtained. We calculated median pO2 for each tumor as well as the median tail moment and 20th and 80th percentile values from the fine-needle aspirates taken immediately before and after the TPZ infusion. We found extensive heterogeneity both in oxygenation within a single tumor as well as between tumors, with median pO2 values for individual tumors varying from 0.8 to 45.2 mm Hg in this series. This variation is much greater than that of transplanted mouse tumors, including those used in this study [22].
Figure 2.
Histograms from two patients (top and bottom rows) showing their tumor oxygen profiles (A and D) and TPZ-induced DNA damage measured by tail moments obtained from fine-needle aspirates taken immediately before (B and E) and after (C and F) the TPZ infusion. The data show that the more hypoxic tumor has the higher level of TPZ-induced DNA damage.
Figure 3 shows a summary of the median tail moments obtained from the four different transplanted tumors following a bolus injection of 0.08 mmol/kg TPZ and from the patients following an infusion of 300 to 330 mg/m2 TPZ. According to a recent analysis of total drug exposure in mice and humans [29] these clinical doses should give TPZ exposures approximately double those of the mice. In the case of the mice, a fine-needle aspirate from each of the tumors was taken immediately before and 45 minutes after the TPZ injection. For the patients, DNA damage was measured in aspirates taken immediately before and at the end of the infusion of TPZ given over a 1.5- to 2-hour period.
Figure 3.
Comparison of TPZ-induced DNA damage, measured by the comet assay, in patients' tumors and in human and murine tumors implanted in mice. Closed symbols show pretreatment median comet tail moments. In patients, open symbols indicate median comet tail moments immediately after the 1.5- to 2-hour intravenous infusion of TPZ (300 to 330 mg/m2). In mice, open symbols indicate median comet tail moments 45 minutes after a bolus injection of 0.08 mmol/kg TPZ.
The data show three important points. First, they show that the one tumor, HT 1080, that shows no potentiation of antitumor efficacy of fractionated radiation by TPZ also has very low levels of TPZ-induced DNA damage. The other three tumors show extensive DNA damage produced by TPZ. Second, all the patients' tumors show TPZ-induced DNA damage that is either comparable to, or greater than, that seen in the mouse tumors. Third, there is much greater interpatient variation in DNA damage induced by TPZ than between individual mouse tumors.
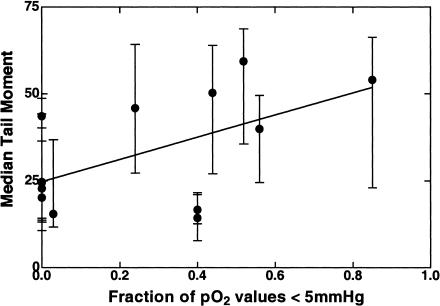
Because oxygen levels of all of the tumors in the clinical trial were measured using the Eppendorf pO2 electrode before randomization (and infusion of TPZ), we were able to test whether the variation in TPZ-induced DNA damage related to tumor oxygenation as has been seen for mouse tumors [21]. We found this to be the case. Patients in the lower half of the range of median tail moments of TPZ damage (i.e., below a median tail moment of 32) had better oxygenated tumors than those with higher levels of damage (mean±SE of median pO2 values=22.5±5.6 mm Hg, n=7, and 5.6±2.3 mm Hg, n=6, for the low-and high-damage groups respectively). The difference between the oxygen levels in the two groups is significant (P=0.02, two-tailed Student's t-test). Figure 4 shows the median tail moment of each tumor following TPZ as a function of the fraction of pO2 values less than 5 mm Hg (the pO2 value below which TPZ cell kill is close to maximum [30]). Although there is considerable scatter in the data (r2=0.32), the trend of increasing DNA damage as a function of tumor hypoxia is significant (P=0.04). A similar level of significance is obtained if the DNA damage data are plotted against median tumor pO2. Because of the extensive heterogeneity in the DNA damage from individual tumors we also analyzed the comet data by percentage of values with a tail moment of >20 because this criterion has been shown by Olive et al. to correlate best with the extent of TPZ-induced cell kill in mouse tumors [20]. However, although this also showed a trend with tumor hypoxia, the correlation was not significant, possibly because this artificially truncates the data at values more than 85% to 90% of values >20.
Figure 4.
The median tail moments after TPZ in the 13 human tumors in this investigation as a function of the fraction of pO2 values less than 5 mm Hg. The mean tail moment as well as the 20th and 80th percentile values of individual tumor cells are shown as an indication of the heterogeneity of the distribution of tail moments within each tumor.
Discussion
In this study we show that DNA damage induced in human head and neck tumors by the new anticancer drug tirapazamine is similar to, or greater than, that produced in human and murine tumors transplanted in mice. We have previously shown with these transplanted tumor models that TPZ, at drug and radiation doses comparable to those given clinically, produces a significant tumor-specific enhancement of radiation damage [16,21,31]. This potentiation of radiation damage is the result of TPZ killing the hypoxic (and therefore radiation-resistant) cells in the tumor [16,32]. We and Olive et al. have also shown that TPZ-induced DNA damage measured by the comet assay is closely correlated with TPZ-induced cell kill [19,20], and is therefore a possible surrogate assay for its ability to enhance the efficacy of radiotherapy.
In order for TPZ to improve the outcome of radiotherapy three important criteria must be met. First, the dose of TPZ must be sufficient. Previous measurements of the total drug exposure (area under the curve) for TPZ obtained from Phase I studies have suggested that dose levels predicted to be active in potentiating radiation are reached in the clinic [29,33]. Second, levels of the intranuclear enzyme(s) that metabolize TPZ to produce the DNA damage leading to hypoxic cell kill [34] must be adequate. Up to the present study there have been comparisons of DNA damage and cytotoxicity of human and mouse tumors only with established cell lines in vitro [1,7,19,21]. These studies have suggested that human cells may not be as sensitive to killing by TPZ under hypoxia as are rodent cells [7,19]. Third, human tumors must be sufficiently hypoxic for TPZ activation. Although there is abundant evidence from oxygen electrode measurements that many human tumors have hypoxic regions [3,24,35], we have recently shown, using electrode measurements, that transplanted mouse tumors are significantly more hypoxic than human tumors [22]. Thus, until the present study there was reason to suspect that TPZ might not be as active in human tumors as in mouse tumors.
Assuming that DNA damage measured by the alkaline comet assay correlates with hypoxic cell kill, our data suggest that all three of the above criteria for killing of the hypoxic cells in the tumor are met in these human tumors from patients treated with 300 to 330 mg/m2 of TPZ. These data, therefore, provide support for the hypothesis that TPZ will potentiate radiation therapy in these human head and neck tumors.
The fact that nodes of human head and neck cancers show a wide range of tumor oxygenation [13,24,35,36] allowed us to make a further test of the hypothesis that hypoxia is important for the activation of TPZ. We would expect in our series that there would be a correlation between TPZ damage and tumor hypoxia. This was the case: we found a significant association of TPZ-induced DNA damage with levels of tumor hypoxia. This is expected from our earlier studies of mouse tumors in which we modified tumor oxygenation by the mice breathing different oxygen concentrations. We found that the level of TPZ-induced DNA damage assayed by the alkaline comet assay correlated closely with tumor hypoxia [21]. However, it should be noted that Olive et al. found that with mouse tumors there was too much heterogeneity of damage to distinguish between hypoxic and aerobic cells in tumors on the basis of TPZ-induced damage [20]. This is likely because there is a continuous gradient of oxygen levels in tumors [37] as well as the fact that cell killing by TPZ (and probably DNA damage) is a continuous function of oxygen concentration [30]. It is also apparent from the data of TPZ-induced DNA damage as a function of tumor hypoxia (Figure 4) that, despite the significant trend, there is considerable variability with only 32% of the variation in comet median tail moment being attributable to measured tumor oxygenation. There are multiple possible reasons for this, including the fact that the pO2 probe may overestimate cellular hypoxia by measuring necrotic areas, contamination of the fine-needle aspirates with leukocytes (see below), variable levels of TPZ metabolizing enzymes in the individual tumors, and the fact that DNA damage induced by TPZ could be low in transiently hypoxic cells, the numbers of which could vary between tumors.
Despite these caveats, the present data suggest that the alkaline comet assay has the potential of being a surrogate endpoint for cell kill by agents that produce DNA single strand breaks. The fact that such breaks can be measured both in mouse and human tumors provides a means of assessing whether a particular treatment, or a modifier of treatment, is likely to be effective in human tumors. We have previously demonstrated that stable translocations determined by fluorescence in situ hybridization could be used to predict the efficacy of a modifier of radiation treatment in human tumor xenografts in mice [38]. In the present study we show that DNA damage assessed by the comet assay from a fine-needle aspirate may be able to predict the efficacy of a cancer treatment that produces DNA strand breaks. The assay is extremely useful in this regard, first, because a fine-needle aspirate yields an adequate number of cells for the assay, and second, because by measuring DNA damage in individual cells, heterogeneity in response can be evaluated [28]. Nor is the comet assay limited to assessing anticancer agents that kill cells by forming DNA strand breaks such as radiation or topoisomerase inhibitors [39]. It could theoretically be used for measuring the efficacy of agents that kill cells by forming interstrand crosslinks such as bifunctional alkylating agents or cisplatin [27,40].
One potential problem with the assay, however, is that only in cases in which the DNA content of the tumor cells is markedly different from diploid can normal cells within the tumor be excluded from the analysis. This may not be a problem with normal cells resident in the tumors as Olive et al. have shown that tumor-associated macrophages have a similar distribution of oxygen levels as do tumor cells [28]. However, fine-needle aspirates such as performed here can be contaminated with circulating leukocytes that were not in the tumor during all the time of exposure to TPZ. These may be removed before analysis using magnetic immunobeads coated with antibodies against leukocyte surface antigens [41], but this was not done in the present investigation.
In summary, we show using the alkaline comet assay, which measures DNA single-strand breaks in individual tumor cells, that tirapazamine produces comparable damage in human tumors in patients given tolerated doses of drug as it does with tumors transplanted into mice. This provides encouragement that TPZ may be as effective in potentiating the efficacy of radiotherapy of human head and neck cancers as it is in potentiating fractionated radiation of the majority of transplanted tumors in mice.
Abbreviations
- TPZ
tirapazamine
Footnotes
This work was supported by US Public Health Service grant P01 CA 67166.
References
- 1.Brown JM. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer. 1993;67:1163–1170. doi: 10.1038/bjc.1993.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moulder JE, Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984;10:695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- 3.Vaupel PW, Hockel M. Oxygenation status of human tumors: a reappraisal using computerized pO2 histography. In: Vaupel PW, Kelleher DK, Gunderoth M, editors. Tumor Oxygenation. Stuttgart: Gustav Fischer Verlag; 1995. pp. 219–232. [Google Scholar]
- 4.Dorie MJ, Brown JM. Tumor-specific, schedule-dependent interaction between tirapazamine (SR 4233) and cisplatin. Cancer Res. 1993;53:4633–4636. [PubMed] [Google Scholar]
- 5.Kovacs MS, Hocking DJ, Evans JW, Siim BG, Wouters BG, Brown JM. Cisplatin anti-tumour potentiation by tirapazamine results from a hypoxia-dependent cellular sensitization to cisplatin [In Process Citation] Br J Cancer. 1999;80:1245–1251. doi: 10.1038/sj.bjc.6690492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Pawel J, von Roemeling R. Survival benefit from tirazone (tirapazamine) and cisplatin in advanced non-small cell lung cancer (NSCLC) patients: final results from the international phase III CATAPULT 1 trial. Proc Amer Soc Clin Oncol. 1998;17 454a Abstr #1749. [Google Scholar]
- 7.Zeman EM, Brown JM, Lemmon MJ, Hirst VK, Lee WW. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986;12:1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- 8.Kim IH, Brown JM. Reoxygenation and rehypoxiation in the SCCVII mouse tumor. Int J Radiat Oncol Biol Phys. 1994;29:493–497. doi: 10.1016/0360-3016(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 9.Durand RE, Olive PL. Physiological and cytotoxic effects of tirapazamine in tumor-bearing mice. Radiat Oncol Invest. 1997;5:213–219. doi: 10.1002/(SICI)1520-6823(1997)5:5<213::AID-ROI1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. Concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Brit J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 11.Gatenby RA, Kessler HB, Rosenblum JS, Coia LR, Moldofsky PJ, Hartz WH. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988;14:831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- 12.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–40. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 13.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 14.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 15.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 16.Brown JM, Lemmon MJ. Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res. 1990;50:7745–7749. [PubMed] [Google Scholar]
- 17.Brown JM, Lemmon MJ. Tumor hypoxia can be exploited to preferentially sensitize tumors to fractionated irradiation. Int J Radiat Oncol Biol Phys. 1991;20:457–461. doi: 10.1016/0360-3016(91)90057-b. [DOI] [PubMed] [Google Scholar]
- 18.Lee D-J, Trotti ASS, Rostock R, Fisher C, von Roemeling R, Harvey E, Groves E. Concurrent tirapazamine and radiotherapy for advanced head and neck carcinomas: a phase II study. Int J Radiat Oncol Biol Phys. 1998;42:811–815. doi: 10.1016/s0360-3016(98)00310-1. [DOI] [PubMed] [Google Scholar]
- 19.Siim BG, van Zijl PL, Brown JM. Tirapazamine-induced DNA damage measured using the comet assay correlates with cytotoxicity toward hypoxic tumour cells in vitro. Br J Cancer. 1996;73:952–960. doi: 10.1038/bjc.1996.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olive PL, Vikse CM, Banath JP. Use of the comet assay to identify cells sensitive to tirapazamine in multicell spheroids and tumors in mice. Cancer Res. 1996;56:4460–4463. [PubMed] [Google Scholar]
- 21.Siim BG, Menke DR, Dorie MJ, Brown JM. Tirapazamine-induced cytotoxicity and DNA damage in transplanted tumors: relationship to tumor hypoxia. Cancer Res. 1997;57:2922–2928. [PubMed] [Google Scholar]
- 22.Adam MF, Dorie MJ, Brown JM. Oxygen tension measurements of tumors growing in mice. Int J Rad Oncol Biol Phys. 1999;45:171–180. doi: 10.1016/s0360-3016(99)00157-1. [DOI] [PubMed] [Google Scholar]
- 23.Terris DJ, Dunphy EP. Oxygen tension measurements of head and neck cancers. Arch Otolaryngol Head Neck Surg. 1994;120:283–287. doi: 10.1001/archotol.1994.01880270031006. [DOI] [PubMed] [Google Scholar]
- 24.Adam M, Gabalski EC, Bloch DA, Oehlert JW, Brown JM, Elsaid AA, Pinto HA, Terris DJ. Tissue oxygen distribution in head and neck cancer patients. Head and Neck. 1999;21:146–153. doi: 10.1002/(sici)1097-0347(199903)21:2<146::aid-hed8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Twentyman PR, Brown JM, Gray JW, Franko AJ, Scoles MA, Kallman RF. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980;64:595–604. [PubMed] [Google Scholar]
- 26.Hirst DG, Brown JM, Hazlehurst JL. Effect of partition coefficient on the ability of nitroimidazoles to enhance the cytotoxicity of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Cancer Res. 1983;43:1961–1965. [PubMed] [Google Scholar]
- 27.Olive PL, Wlodek D, Durand RE, Banath JP. Factors influencing DNA migration from individual cells subjected to gel electrophoresis. Exp Cell Res. 1992;198:259–267. doi: 10.1016/0014-4827(92)90378-l. [DOI] [PubMed] [Google Scholar]
- 28.Olive PL, Banath JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat Res. 1990;122:86–94. [PubMed] [Google Scholar]
- 29.Brown JM, Wang LH. Tirapazamine: laboratory data relevant to clinical activity. Anticancer Drug Des. 1998;13:529–539. [PubMed] [Google Scholar]
- 30.Koch CJ. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 1993;53:3992–3997. [PubMed] [Google Scholar]
- 31.Brown JM, Lemmon MJ. SR 4233: A tumor specific radiosensitizer active in fractionated radiation regimes. Radiother. Oncol. 1991;20:151–156. doi: 10.1016/0167-8140(91)90203-s. [DOI] [PubMed] [Google Scholar]
- 32.Brown JM, Koong A. Therapeutic advantage of hypoxic cells in tumors: a theoretical study. J Natl Cancer Inst. 1991;83:178–185. doi: 10.1093/jnci/83.3.178. [DOI] [PubMed] [Google Scholar]
- 33.Graham MA, Senan S, Robin H, Eckhardt N, Lendrem D, Hincks J, Greenslade D, Rampling R, Kaye SB, von Roemeling R, Workman P. Pharmacokinetics of the hypoxic cell cytotoxic agent tirapazamine and its major bioreductive metabolites in mice and humans: retrospective analysis of a pharmacokinetically guided dose escalation strategy in a phase I trial. Cancer Chemother Pharmacokinetics. 1997;40:1–10. doi: 10.1007/s002800050617. [DOI] [PubMed] [Google Scholar]
- 34.Evans JE, Yudoh K, Delahoussaye YM, Brown JM. Tirapazamine is metabolized to its DNA damaging radical by intranuclear enzymes. Cancer Res. 1998;58:2098–2101. [PubMed] [Google Scholar]
- 35.Nordsmark M, Bentzen SM, Overgaard J. Measurement of human tumour oxygenation status by a polarographic needle electrode. Acta Oncol. 1994;33:383–389. doi: 10.3109/02841869409098433. [DOI] [PubMed] [Google Scholar]
- 36.Lartigau E, Le Ridant A-M, Lambin P, Weeger P, Martin L, Sigal R, Lusinchi A, Luboinski B, Eschwege F, Guichard M. Oxygenation of head and neck tumors. Cancer. 1993;71:2319–2325. doi: 10.1002/1097-0142(19930401)71:7<2319::aid-cncr2820710724>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 37.Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat Res. 1997;147:541–550. [PubMed] [Google Scholar]
- 38.Kovacs MS, Yudoh K, Evans JW, Menke D, Brown JM. Stable translocations detected by fluorescence in situ hybridization: a rapid surrogate end point to evaluate the efficacy of a potentiator of tumor response to radiotherapy. Cancer Res. 1997;57:672–677. [PubMed] [Google Scholar]
- 39.Olive PL, Banath JP, Durand RE. Detection of etoposide resistance by measuring DNA damage in individual Chinese hamster cells. J Natl Cancer Inst. 1990;82:779–783. doi: 10.1093/jnci/82.9.779. [DOI] [PubMed] [Google Scholar]
- 40.Pfuhler S, Wolf HU. Detection of DNA-crosslinking agents with the alkaline comet assay. Environ Mol Mutagen. 1996;27:196–201. doi: 10.1002/(SICI)1098-2280(1996)27:3<196::AID-EM4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 41.Strauss GH, Peters WP, Everson RB. Measuring DNA damage in individual cells of heterogeneous mixtures: a novel application of an immunological typing technique. Mutat Res. 1994;304:211–216. doi: 10.1016/0027-5107(94)90213-5. [DOI] [PubMed] [Google Scholar]