Abstract
Many properties of HSV-1 are especially suitable for using this virus as a vector to treat diseases affecting the central nervous system (CNS), such as Parkinson's disease or malignant gliomas. These advantageous properties include natural neurotropism, high transduction efficiency, large transgene capacity, and the ability of entering a latent state in neurons. Selective oncolysis in combination with modulation of the immune response mediated by replication-conditional HSV-1 vectors appears to be a highly promising approach in the battle against malignant glioma. Helper virus-free HSV/AAV hybrid amplicon vectors have great promise in mediating long-term gene expression in the PNS and CNS for the treatment of various neurodegenerative disorders or chronic pain. Current research focuses on the design of HSV-1-derived vectors which are targeted to certain cell types and support transcriptionally regulatable transgene expression. Here, we review the recent developments on HSV-1-based vector systems and their applications in experimental and clinical gene therapy protocols.
Keywords: glioma, gene therapy, recombinant HSV-1, amplicon
Recombinant HSV-1 Vectors
Recombinant herpes viruses were first constructed for the purpose of vaccination against HSV-1 and HSV-2 and to study the functions of individual virus genes. Only later were they considered suitable gene transfer vehicles, especially for use in the central nervous system (CNS). A protocol for site-specific modification of the virus genome was first reported by Post and Roizman [1] and is reviewed in detail elsewhere [2,3]. In principle, two types of recombinant HSV-1 vectors can be distinguished depending on the target tissue and purpose of gene delivery: i) for therapeutic gene transfer/replacement in neurons, mutation/deletion of some essential genes can serve to prevent the expression of toxic virus proteins and initiation of the lytic viral replication cycle (replication-defective HSV-1 mutants); ii) for therapeutic treatment of tumors (virus therapy), where toxicity is the main purpose, one or more genes are deleted which are essential for virus replication in nondividing cells of the surrounding tissue, but can be complemented by proliferating tumor cells (replication-conditional HSV-1 mutants). Both types of vectors can incorporate single or multiple therapeutic genes accounting for at least 30 kb of the 152-kb genome. In principle, recombinant HSV-1 mutants are generated by homologous recombination. The frequency of recombination is proportional to the length of the homologous flanking sequences and usually less than 5%.
Gene Transfer into Neurons
The wild-type (wt) HSV-1 genome can persist in neurons during latency in a nuclear life-long episomal location without disturbance of host-cell metabolism and without eliciting an immune response. This suggests that if viral neuropathogenesis and reactivation could be avoided by manipulation of the viral genome, it should be possible to express transgenes from a silent virus genome in a nerve cell-specific manner. However, several obstacles have to be overcome: i) replication-defective mutants of HSV-1 can cause cytopathic effects in primary cultures of neural cells and inflammatory responses in neural tissue in vivo; ii) most viral and nonviral promoters are silenced after injection into the brain; and iii) the activity of the LAT (latency associated transcript) promoter is weak in CNS neurons [4]. Therefore, the main focus in the development of new HSV-1-based vectors has been directed at achieving nontoxic, long-term gene expression in neurons.
Two main approaches have been taken to reduce toxicity: i) the engineering of mutants in which virus gene expression is blocked in an early stage; and ii) the use of mutants which lack the neurovirulence gene γ34.5. As the initiation of the viral replicative machinery depends on the concerted action of at least five viral proteins (VP) or infected cell proteins (ICP), including VP16, ICP 0, 4, 22, and 27, single or double deletion mutants involving various combinations (ICP 0 alone, ICP4 alone, ICP4+22, ICP4+27, ICP4+47) were initially created. Although expression of viral early and late genes is dramatically reduced in these mutant backgrounds, they still cause some toxicity and alteration of cell morphology and chromatin structure [5–9], most likely due to expression of remaining immediate-early (IE) genes. Supporting this hypothesis, mutations affecting the activation function of VP16 did reduce the toxicity of an ICP4- mutant significantly [10], probably due to the overall reduction in IE gene expression in the absence of VP16 transactivation. Substantially reduced vector cytotoxicity was also reported for replication-defective HSV-1 mutants that contain deletions in multiple genes [4,11–14]. These multiple deletions included the IE genes encoding ICP4, ICP22, ICP27, and ICP47 [11,12,14] and prevented early and late viral gene expression, but major histocompatibility complex (MHC) class I antigen expression remained intact in the absence of ICP47. Viral mutants deleted simultaneously for the IE genes encoding ICP4, ICP22, and ICP27 showed substantially reduced cytotoxicity when compared to viruses deleted for ICP4 alone or ICP4 in combination with either ICP22, ICP27 or ICP47 [14]. Infection of cultured neurons with these triple IE deletion mutants substantially improved cell survival and supported transgene expression for over 21 days [11,12]. However, infection of Vero and human embryonic lung cells leads to inhibition of host cell DNA synthesis and pronounced and distinct alterations of nuclear morphology, most likely due to the accumulation of ICP0 [14,15]. The elimination of ICP0 together with VP16 and ICP4 [16,17], or together with ICP4 and ICP27 [15], or together with all other IE genes [18], greatly reduced cytotoxicity, but also reduced the level of transgene expression. Therefore, transgenes inserted into the genome of these mutants may be expressed at reduced levels, depending on the promoter used to regulate them. Nevertheless, these types of vectors have been successfully used to express bcl-2 and to reduce 6-hydroxydopamine-induced apoptosis of neurons in the substantia nigra in experimental animal models [19].
The γ34.5 deletion mutants, where both copies of the γ34.5 gene are deleted, were first generated for the assessment of its phenotype [20], tested in animal models [21], and later adapted for virus-mediated therapy of brain tumors [22,23]. In proliferating cells, these mutants can replicate as efficiently as wt virus, whereas in most neurons, they are replication-defective, but still able to establish latency [24]. Delivery of a constitutively active form of a heat shock factor using this type of vector protected neurons from thermal and ischemic stress [25] as well as from apoptosis [26]. However, a mutant, where the lacZ reporter gene driven by the LAT promoter was inserted into a nonessential gene, UL43, of recombinant HSV-1 1716 (ICP34.5 double-deletion) failed to mediate transgene expression for more than 2 weeks after injection into the peripheral nervous system (PNS) and CNS [24]. The same vector did not transduce enteric neurons efficiently and, in addition, exhibited some toxicity [27].
The choice of the promoter driving the gene of interest is important to achieve long-term (several weeks to months) gene expression in neurons. In principle, three different types of promoters can be used: i) the HSV-1 LAT promoter; ii) other viral promoters; or iii) cellular promoters. The LAT promoter is of special interest, as it mediates specific gene expression and is active in latency in certain neurons [28,29]. Recombinants of HSV-1 that contained lacZ reporter genes placed under transcriptional control of the LAT promoter at both LAT loci, mediated synthesis of β-galactosidase in sensory neurons for 2 months [30], and in neurons located in facial and hypoglossal nerve nuclei and in the upper cervical spinal cord for up to 10 months [31]. Control of β-glucuronidase expression from the TATA box-containing latency promoter, LAP1, led to production of the enzyme for up to 4 months after inoculation of the vector into the trigeminal ganglia or brainstem of MPS VII mice [32]. Although the alternate LAT promoter, LAP2, is primarily responsible for LAT expression during lytic infection, LAP2 has also been shown to be transcriptionally active during latency [33]. LacZ mRNA could be detected in hippocampal neurons for up to 4 weeks after inoculation of recombinant HSV-1 vectors that express this reporter gene from LAP2 [7]. LAP2 was also capable of supporting long-term (4 weeks) expression of the gene for nerve growth factor, β-NGF, in latently infected neurons in vivo, providing protection of dorsal root ganglion neurons from peroxide toxicity [34]. HSV-1 recombinants that express the transgene from constitutive viral promoters, such as the retrovirus LTR or cytomegalovirus IE promoter, or even from neuron-specific promoters, support in general only short-term (several days) expression in the CNS [5–7,34–37]. Heterologous promoters introduced into the virus genome are prone to come under the influence of the transcriptional machinery of the virus, which can result in unspecific expression, at least in the CNS [7]. Moreover, the tightly condensed nucleosomal configuration of the HSV-1 genome in latency may restrict access of transcriptional activators. Two studies, however, demonstrated that the MoLMV LTR promoter can support lacZ gene expression in primary sensory neurons for up to 18 months [38,39].
Virus/Gene Therapy of Gliomas
HSV-1-based therapy of brain tumors relies on the selective killing of tumor cells by the virus replication per se and/or the virus-mediated expression of anti-tumor acting genes. Anti-tumor acting genes can encode: i) prodrug-activating enzymes (e.g., thymidine kinase [40], Escherichia coli cytosine deaminase [41,42], E. coli guanine phosphoribosyl transferase [43,44], cytochrome P450 [45–47], deoxycytidine kinase [48]); ii) cell-cycle regulating proteins (e.g., p53) [49]; iii) factors which inhibit angiogenesis; or iv) immunomodulating cytokines (reviewed in Ref. [50]).
For virus therapy of brain tumors, different single- or multiple-mutated replication-conditional HSV-1 vectors have been created, which have reduced neuronal toxicity and replicate selectively in dividing, and not in quiescent, cells. The deletions/mutations include the tk gene (e.g., HSV-1 RH105 [51]; dlsptk [52,53]; KOS-SB [54,55]; G92A [56,57]), the ribonucleotide reductase (RR) gene (e.g., HSV-1 hrR3 [58–62]), the γ34.5 gene (e.g., HSV-1 R3616 [20,22,23]; 1716 [63–65]) or the γ34.5 gene together with either the RR gene or the gene encoding uracyl N-glycosylase (HSV-1 G207 [66]; MGH-1 [67]; 3616UB [68]).
First generation HSV-1 mutants, such as the dlsptk vector, exhibited a substantial therapeutic effect in terms of killing glioma cells in culture and in vivo, but their application was limited for two reasons: first, the recombinant virus lacks a functional tk gene and, therefore, potential dlsptk-induced encephalitis cannot be controlled by ganciclovir (GCV); second, in some studies, intracranial inoculation caused fatal encephalitis at high vector doses [22,52,54]. Elimination of the neurovirulence gene, γ34.5, substantially reduced the risk of developing focal encephalitis, and still retained the therapeutic effect in primary glioma, medulloblastoma, and metastatic brain tumor models [22,23,69–72]. The LD50 in mice increased by a factor of ∼106 compared to wt HSV-1 [20,64]. Attempts to recover virus from brains of infected animals were either unsuccessful or yielded very small amounts of infectious particles [20,21]. Histopathological studies of immunocompetent mice which were injected into the left cerebral hemisphere with 1x106 PFU of 1716, a γ34.5 double-deletion mutant derived from HSV-1 strain syn17, exhibited a low grade meningoencephalitis with a limited inflammatory response at early times after inoculation [73]; virus gene expression was confined to the site of inoculation. By 28 days after inoculation, the CNS appeared histopathologically normal and virus-encoded antigens and virus-induced immune responses were no longer detectable. These findings demonstrate that infection of the CNS by γ34.5 null mutants results in a finite, self-limiting response and highlights the potential usefulness of these vectors for clinical protocols [73,74]. In a similar study [75], the recombinant HSV-1 R3616, a γ34.5 double-deletion mutant derived from HSV-1 strain F, has been shown to infect neurons, astrocytes, oligodendrocytes, and ependymal cells and, hence, did not discriminate at the level of infection among CNS cell types. The transduced cells have been cleared entirely from the nervous system by day 7 after infection [75]. This study supported the view that the neuroattenuation of γ34.5 deletion mutants is based on a gross reduction in the ability of the virus to replicate and spread cell-to-cell and not on a restricted host range in the CNS. However, other data suggest that this type of vector needs further attenuation before it can be used in clinical applications for the following reasons: i) the vector can replicate in and destroy the ependyma, leading to a persistent loss of the ependymal lining in mice [76]; ii) after inoculation into the CNS of rats or mice, the vector is able to spread to distant sites where it induces inflammatory reactions [77,78]; iii) the vector causes cytopathic effects in the immunocompromised host [79]. Moreover, HSV-1 strain-related differences of toxicity and the possibility of a second site suppressor mutation occurring in HSV-1 mutants, where a single gene is deleted or mutated, possibly affecting neurovirulence or other activities of the vector should be kept in mind when preparing an HSV-1 mutant for clinical use. Of note, the capacity of γ34.5 double-deletion mutants to kill human cells derived from glioblastoma multiforme, anaplastic astrocytoma, anaplastic glioma, gliosarcoma, and normal human astrocytes in culture varies [69]. Moreover, cultured astrocytes were two to three orders of magnitude less susceptible to killing than were malignant glia [69], indicating a cell-type dependent therapeutic effect. Insertion of different immunomodulating interleukin genes into the γ34.5 locus induced significant alterations of the host immune response and demonstrated its important role as part of the therapeutic effect of these vectors [80]. An enhanced anti-tumor effect of recombinant HSV-1 R3616 was also achieved by ionizing radiation, which caused a two- to five-fold enhanced replication in irradiated tumor cells, resulting in a significantly greater reduction in volume, or total regression, of tumors than either radiation or infection alone [81].
Other first-generation vectors, such as recombinant HSV-1 hrR3, bear a lacZ-insertional mutation within the large subunit of the gene encoding RR [61], thereby restricting replication of the vector to proliferating cells, e.g., tumor cells, which express complementary RR activity (Figure 1). This vector has been used for the selective destruction of tumor cells in different experimental tumor models in the brain and other organs [58,60,62,82–84]. For example, in in vivo studies, animals harboring human U-87MG gliomas were randomly divided and treated intraneoplastically with either 5x106 PFU of recombinant HSV-1 hrR3 or medium alone. The virus-treated animals showed significant inhibition of tumor growth [62]. The vector retains an intact HSV-1 tk gene, which can be used as “marker gene” to monitor vector replication and spread using non-invasive in vivo imaging methods [85] as well as to sensitize transduced cells to GCV. Administration of GCV to tumor-bearing animals treated with hrR3 reduced the vector-mediated inflammatory response in a disseminated brain tumor model [83] and served to increase the therapeutic capacity of the vector in animals bearing intracranial and intrathecal rat 9L gliosarcomas [60,83]. A similar observation was made with a variant of hrR3, rRp450, carrying a cytochrome P450 (CYP2B1) insertional mutation at the RR gene locus [86]. This mutant replicated selectively in dividing rat and human tumor cells which express high levels of mammalian RR, and addition of cyclophosphamide potentiated oncolytic effects against subcutaneous (s.c.) tumor xenografts established in athymic mice without compromising replication of the vector. In a similar experimental tumor model, injection of this vector with application of both cyclophosphamide and GCV resulted in enhanced tumor regression after prodrug activation [87]. In view of a potential clinical application of these vectors, it should be noted that pre-existing HSV-1 immunity decreases, but does not abolish HSV-1 vector-mediated gene transfer to experimental brain tumors [88]. Moreover, in two different latency models in rats, intracerebral injection of hrR3 did not reactivate latent wt HSV-1 in either the corneal or the cerebral model. This indicates that intracranial injection of partially defective recombinants of HSV-1 may bear little or no risk of reactivating latent wt virus present in sensory ganglia or brain [89], although it must be kept in mind that rats are, in general, less infectable with HSV-1 than humans.
Figure 1.
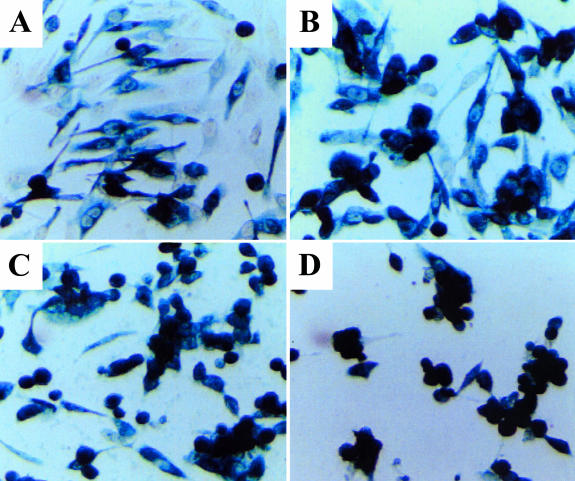
Time-course of cytopathic effect and lacZ gene expression mediated by replication-conditional HSV-1 mutant hrR3 [61] after infection of cultured rat 9L gliosarcoma cells. MOI=1.5PFU/cell. (A) 19 hours postinfection (p.i.); (B) 28 hours p.i.; (C) 42 hours p.i; (D) 50 hours p.i. Replication and spread of the recombinant leads to progressive cytopathic effects and, eventually, to death of all cells in the culture (D).
Second generation HSV-1 vectors, such as triple mutants carrying deletions at both γ34.5 gene loci and a transgene insertion that inactivates the ribonucleotide reductase gene (G207, MGH-1, and rRp450), were found to be especially suitable for treating human malignant brain tumors for several reasons [66,67,90]: i) high replication-competence in glioblastoma cells (and other dividing cells); ii) neuroattenuation; iii) temperature sensitivity; iv) GCV hypersensitivity [91]; and v) the presence of a detectable histochemical marker (lacZ) or therapeutic gene coding for a pro-drug activating enzyme. In nude mice harboring s.c. or intracerebral U-87MG gliomas, intraneoplastic inoculation with G207 caused decreased tumor growth and/or prolonged survival [66]. Similar therapeutic effects were observed in human F5 malignant meningioma [90], human malignant breast cancer [92], mouse CT26 colorectal carcinoma, and M3 melanoma [93], but not in rat 9L gliosarcoma cell lines [67], demonstrating again a cell-dependent therapeutic effect. Most importantly, such therapeutic vectors produce vi) a strong and highly tumor antigen-specific cytotoxic T-lymphocyte (CTL) response which seems to play a major role in the therapeutic effect of this vector in immune competent recipients [93]. The mutants are avirulent on intracerebral infection of both mice and highly HSV-sensitive nonhuman primates. They are therefore considered interesting candidate vectors for the clinical evaluation in the treatment of glioblastomas [94]. The presence of two mutations reduces the possibility of recombinational events in situ, thereby reducing the risk of generating virulent progeny during virus therapy [68].
HSV-1-Based Amplicon Vectors
Historical Background
The serial passage of HSV-1 at high multiplicities of infection results in the production of both wt and defective HSV-1 viruses [95]. The genomes of these naturally occurring defective viruses consist of multiple reiterations of specific HSV-1 sequences (repeat units), including a DNA cleavage/packaging signal (pac) and an origin of DNA replication (ori), arranged as head-to-tail concatemers comprising ∼152 kb of DNA, the size of the wt HSV-1 genome [95–97]. Based on these observations, Spaete and Frenkel [99] developed bacterial plasmids that contained single repeat units with ori and pac and demonstrated that, in mammalian cells in the presence of HSV-1 helper functions, the plasmids were replicated and packaged into virus particles. The genomes of these particles were composed of amplified head-to-tail-linked concatemers of the seed plasmid, which was therefore termed “amplicon” (Figure 2A) [98,99].
Figure 2.
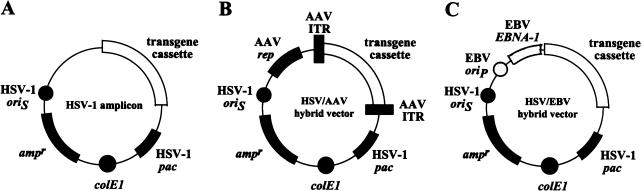
Amplicon structures. (A) The standard HSV-1 amplicon is composed of three types of genetic elements: i) sequences from bacteria, including an origin of DNA replication (colE1) and an antibiotic resistance gene (ampr), which allow propagation of plasmid DNA in E. coli; ii) sequences from HSV-1, in particular an origin of DNA replication (oriS) and a DNA cleavage/packaging signal (pac), which support replication of amplicon DNA and subsequent packaging into HSV-1 particles in mammalian cells in the presence of helper functions; and iii) a transgene cassette with one or more gene(s) of interest. (B) In addition to the standard amplicon elements, HSV/AAV hybrid amplicons contain the adeno-associated virus (AAV) rep gene and a transgene cassette that is flanked by AAV inverted terminal repeats (ITR). (C) HSV/EBV hybrid amplicons contain the EBV EBNA1 gene and the EBV latent origin of DNA replication oriP in addition to the standard amplicon elements.
The HSV-1 amplicon has several properties that make it a promising candidate gene transfer vehicle: i) The two HSV-1 sequence elements, ori and pac, that are sufficient to support replication and packaging into virions are smaller than 1 kb and do not encode any virus proteins. Consequently, the amplicon has the potential to accommodate large fragments of foreign DNA (theoretically up to 152 kb), including multiple and large transgenes or large cell type-specific promoters; ii) Depending on the size of the seed amplicon, several copies of the transgene can be packaged into a single vector particle, thereby increasing the transgene dose per infected cell; iii) HSV-1 can infect most mammalian cell types, including the nondividing cells of the nervous system; iv) The HSV-1 virion is quite nontoxic.
As a major disadvantage, however, replication and packaging of amplicons into HSV-1 particles depends on helper functions which have conventionally been provided by replication-conditional helper viruses (Figure 3A). Initially, a temperature-sensitive (ts) mutant, HSV-1 tsK [100], was employed as a helper virus to package amplicons into HSV-1 particles [101]. HSV-1 tsK carries a missense mutation in the essential IE3 gene which encodes a ts form of ICP4, allowing virus replication to proceed at 31°C, but not at 37°C. To prepare vector stocks, cells cultured at 31°C were transfected with the seed amplicon and then infected with the tsK helper virus, yielding vector stocks that contained both packaged amplicon vector and helper virus. Although replication of this helper virus is inhibited in target cells that are cultured at the restrictive temperature (37°C), HSV-1 tsK can still express many genes that are toxic and not compatible with cell survival. Moreover, reversion of ts ICP4 to wt phenotype occurs at a relatively high rate. Consequently, further developments were aimed at: i) reducing the frequency of reversion to wt HSV-1; ii) reducing the toxicity of the helper virus; and iii) increasing the proportion of vector particles relative to helper virus.
Figure 3.
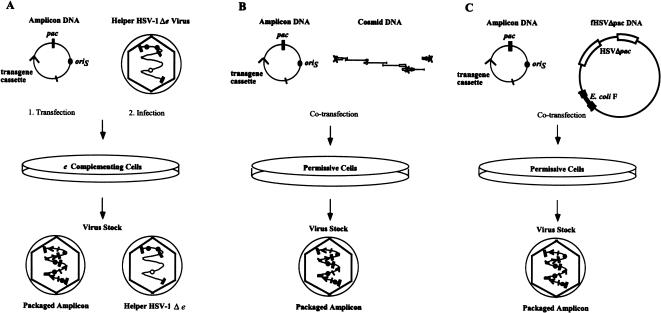
Packaging systems for HSV-1 amplicon vectors. (A) Helper virus-dependent packaging system using a replication-defective mutant of HSV-1 containing a deletion in an essential gene (Δe). Cells which complement the deleted virus gene (e), under transcriptional control of a VP16 responsive promoter, are transfected with amplicon DNA and then superinfected with the deletion-mutant helper virus. The resulting vector stocks contain both packaged amplicon vector and helper virus. (B) Cosmid-based, helper virus-free packaging system. Cells that are permissive for HSV-1 replication are cotransfected with amplicon DNA and DNA from cosmid set C6Δa48Δa. While helper HSV-1 genomes reconstituted from the cosmid clones are not packageable in the absence of pac signals, they still can provide all the trans-acting functions required for the replication and packaging of the co-transfected amplicon DNA. (C) F-plasmid-based helper virus-free packaging system. Permissive cells are co-transfected with amplicon DNA and fHSVΔpac plasmid DNA. The fHSVΔpac helper DNA is not packageable in the absence of pac signals, but it can still provide all the trans-acting functions required for the replication and packaging of co-transfected amplicon DNA.
To reduce the frequency of reversion to wt phenotype, HSV-1 tsK was replaced by a mutant of HSV-1 that contained a deletion in the essential IE3 gene (HSV-1 D30EBA [102], with packaging performed in IE3-complementing, M64 cells [103,104]). The reversion frequency was further reduced by replacing M64 cells with RR1 cells [8], which contain a smaller fragment of the HSV-1 genome carrying the IE3 gene than do M64 cells, thereby reducing the chance of homologous recombination and potential generation of replication competent virus. Mutants of HSV-1 that carry deletions in IE genes are useful helper viruses because virus replication in nonpermissive cells is blocked at a very early stage; however, of the five HSV-1 IE genes, only IE3 (ICP4) and IE2 (ICP27) are essential for HSV-1 replication. Lim et al. developed a packaging system with an IE2 deletion mutant helper virus (HSV-1 5dl1.2; [105]) using IE2-expressing 2-2 cells [106], which produced vector stocks with relatively high amplicon titers (106 to 107 transducing units, t.u., per milliliter), an acceptable vector to helper virus ratio (up to 1), and low levels (<10-7) of revertants with wt HSV-1 phenotype [107]. As a modification of the deletion-mutant packaging system, the so-called “piggyback” amplicon encodes a gene that complements a deletion-mutant helper virus, allowing packaging to be performed in any cell line that supports HSV-1 replication [108–110]. Moreover, replication and packaging of amplicon and helper virus mutually depend on each other, thereby eliminating helper viruses propagating independently and increasing the ratio of amplicon to helper virus.
Although the development of improved helper virus-dependent packaging systems has continuously increased the safety of the amplicon system, many problems associated with the helper virus still remained. These include: i) acute cytopathic effects and pronounced immune responses induced by gene expression from the helper virus; ii) reversion of the helper virus to wt HSV-1 phenotype; iii) potential interactions with endogenous viruses, such as reactivation and recombination; and iv) unreliability of transgene expression. Many of these problems have been eliminated by the recent development of helper virus-free packaging systems.
Helper virus-free packaging systems use replication-competent, packaging-defective genomes of HSV-1 to provide the functions necessary for replication and packaging of co-transfected amplicon DNA. The packaging of the HSV-1 helper DNA itself is prevented by deletion of pac signals, which are essential for entry of viral DNA into capsids, cleavage of the HSV-1 DNA, and closing of the capsid before virion production. In the initial helper virus-free packaging system, the helper functions were provided by a set of five overlapping cosmids that cover the HSV-1 genome [111], with pac signals deleted (cosmid set C6Δa48Δa; Figure 3B) [112]. On transfection into cells, the cosmids can form a complete, replication-competent HSV-1 genome, through homologous recombination between the individual clones, and provide all helper functions necessary for the replication and packaging of co-transfected amplicon DNA. However, the packaging of the reconstituted HSV-1 genome is blocked by virtue of absence of the pac signals. The resulting vector stocks have amplicon titers of up to 106 to 107 t.u./ml of cell culture medium, contain levels of helper virus less than 1 of 107 amplicon vectors, and can efficiently transduce many different cell types, including postmitotic neurons (Figure 4), while causing minimal to no cytopathic effects at MOIs≤50 [112–116]. To simplify this packaging system, the HSV-1 genome, deleted for the pac signals, has recently been cloned as a single-copy, F-plasmid-based bacterial artificial chromosome in E. coli (fHSVΔpac), which reduces the numbers of clones representing the HSV-1 genome from five (cosmid set C6Δa48Δa) to one (fHSVΔpac; Figure 3C) [117–119].
Figure 4.
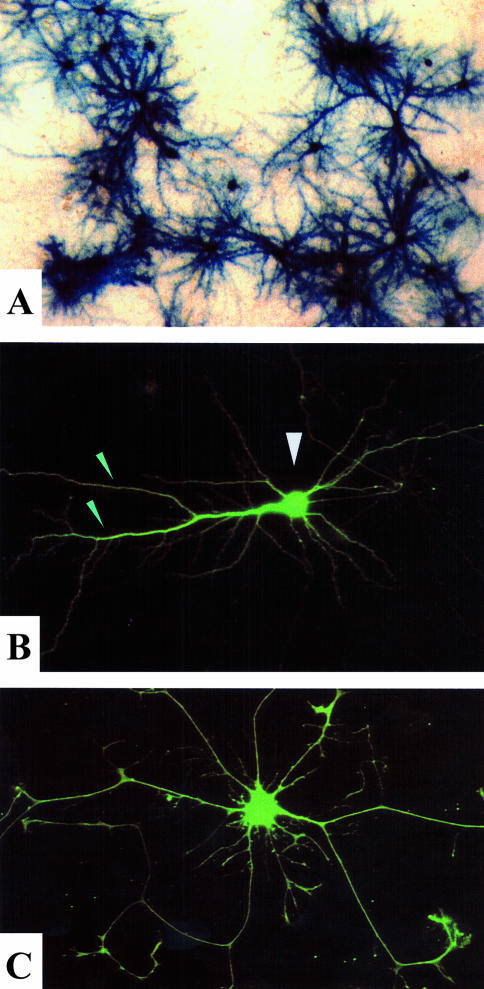
Helper virus-free amplicon mediated laZ (A) or gfp (B,C) expression in cultured rat cortical cells. Axons and dendrites (▼) and cell bodies (▽) are clearly delineated by expression of the reporter genes. The use of gfp as marker gene facilitates the assessment of the kinetics of transgene expression in living cells over time.
Amplicons and Hybrid Vectors
The recent development of improved packaging systems has greatly reduced the toxicity, but had little effect on extending the stability of amplicon-mediated transgene expression. A more fundamental improvement of long-term gene expression has been achieved by new vector designs, which included the use of cell type-specific promoters and the combination of components of the HSV-1 amplicon with genetic elements from other virus vectors.
Viral immediate-early promoters, such as the HSV-1 IE4/5 (induced by VP16) or CMV IE1 promoters that are typically used in amplicon vectors, support strong, but transient expression of the amplicon-delivered transgene in most target cells [112,114,120,121]. Neuron-specific promoters with large 5′ regulatory sequences, such as the 2.7-kb preproenkephalin (PPE) promoter and regulatory sequence, and the 9-kb tyrosine hydroxylase (TH) promoter and regulatory sequence, have been demonstrated to mediate long-term, cell type-specific gene expression from amplicon vectors in nondividing cells [122,123]. As one of the greatest attractions, the HSV-1 amplicon has a very large transgene capacity (theoretically up to ∼152 kb) and can easily accommodate large cell type-specific promoters, including their large 5′ regulatory sequences and intron elements. In practice, however, amplicons with inserts of >20 kb have not yet been described. Conventional amplicon vectors use plasmid backbones which allow medium to high copy-number plasmid propagation and are, therefore, not suitable to explore the full transgene capacity of HSV-1. The development of a single-copy, F-plasmid-based amplicon (famplicon) may increase the genetic stability and allow the packaging of very large inserts (unpublished material).
The large transgene capacity of the amplicon provides also the ability to introduce genetic elements from other viruses to achieve specific properties, including genetic stability and long-term gene expression. Optimally, such hybrid vectors combine the advantageous components of their parent virus vector systems without including the disadvantages. AAV vectors are nontoxic, have a broad host range, and can either persist in an episomal form or integrate site-specifically or randomly into the host cell genome, and support stable, long-term gene expression [124–129]. However, the transgene capacity of AAV vectors is less than 5 kb, which reduces their usefulness for many applications in gene therapy. HSV-1 amplicon vectors are also nontoxic in the absence of helper virus, also have a broad host range and, in addition, have a very large transgene capacity. As the major drawback, however, transgene expression from amplicon vectors is in general transient in dividing cells as vector DNA is lost during mitosis. The combination of the advantageous elements from these two virus vector systems resulted in the HSV/AAV hybrid vector [116]. In addition to the standard amplicon elements, HSV/AAV hybrid amplicons contain those elements from AAV that allow for stable maintenance of the transgene, in particular the rep gene and the ITR, which flank the transgene cassette (Figure 2B). Because hybrid amplicons are packaged into HSV-1 virions, the advantages of the amplicon system, including the availability of a helper virus-free packaging system and the large transgene capacity, are conserved. After delivery into the host cell genome, the hybrid vector has the potential to act like an AAV vector with rep-mediated amplification of the transgene cassette and genomic integration. The functionalities of the HSV-1 and AAV elements in the context of the hybrid vector have been demonstrated: the vector can i) be packaged into HSV-1 particles, ii) efficiently transduce many different cell types, and iii) support both amplification of the ITR-flanked transgene cassette and long-term gene expression in dividing and nondividing cells [114,116,130]. Moreover, 20% to 30% of 293 cells initially transduced with a hybrid vector, bearing a transgene cassette consisting of the genes for green fluorescent protein and neomycin resistance, formed stable, green fluorescent, neomycin resistant colonies. In comparison, only ∼4% of the cells transduced with a standard amplicon formed such colonies under these conditions (D.R.J., unpublished material).
Epstein-Barr virus (EBV)-based vectors, are bacterial plasmids that contain the EBV EBNA 1 gene and the EBV latent origin of DNA replication (oriP) which support episomal replication of the vector and segregation to daughter cells, and up to 180 kb of foreign DNA [131–135]. As a major disadvantage of this vector system, the host range of EBV is limited to human epithelial, muscle, and some hematopoietic cells, as well as B and T lymphocytes. By inserting the EBNA 1 gene and oriP into an HSV-1 amplicon vector, Wang and Vos have developed a hybrid vector that replicates episomally and supports long-term transgene expression in a broad range of host cells (Figure 2C) [136,137]. Taking advantage of the broad host range and retention properties of HSV/EBV hybrid amplicons, a new triple hybrid vector (HER) has been constructed, which contains additional retrovirus genes gag-pol and env (GPE), to efficiently convert human and dog primary glioma cells to retrovirus vector producer cells after single step transduction [138]. This system serves an intriguing approach to circumvent disadvantages of retroviral vectors, such as low transduction efficiency, vector instability and producer cell line immunogenicity, in gene therapy of brain tumors.
HSV-1 Amplicon Vector-Mediated Gene Transfer into Cells of the Nervous System
The large transgene capacity, which allows the use of cell type-specific promoters and/or the expression of multiple transgenes, as well as the natural tropism for the CNS, make the HSV-1 amplicon an ideal vector for CNS-directed gene transfer (Figure 4). Amplicon vectors have been employed to study neuronal physiology, such as the effect of expression of GAP43 or the low affinity nerve growth factor (NGF) receptor on morphology and growth of neurons [139,140]. In hippocampal slice cultures, amplicons have been used to mediate both kainate receptor-induced toxicity [141] and glucose transporter-induced protection of neurons [142]. Amplicon-mediated expression of brain-derived neurotrophic factor induced the growth of neuritic processes in cultures prepared from spiral ganglia of the murine ear [143], and promoted neuronal survival in dissociated avian cochlear cultures [144]. Amplicons have also been used to deliver various therapeutic genes in models of CNS disease in vivo. For example, expression of glucose transporter protected neurons in an induced seizure model and stroke [142,145,146], expression of bcl-2 rescued neurons after focal ischemia [147,148], and production of tyrosine hydroxylase-induced behavioral changes in parkinsonian rats [149].
An interesting approach by Brooks et al. employed amplicon vectors to generate mouse somatic mosaics [150]. Transgenic mice were developed that carried a NGF gene containing an inactivating insertional element, flanked by loxP sites, between promoter and transcript. The delivery of amplicon-encoded cre recombinase activated the expression of NGF in these animals at specific sites. The ability to induce conditional transgene expression in a spatial and temporal fashion will have broad applications for the generation of transgenic animals, especially with genes that encode toxic products or for which germline deletion is lethal.
Another property of HSV-1 relevant for CNS-directed gene transfer, is the ability of the virus particles to travel retrogradely along axons. The localization and spread of HSV-1 within the CNS has been investigated by using recombinants of HSV-1 or HSV-1 amplicon vectors that express the E. coli lacZ reporter gene [5,36,151,152]. After single injections into certain brain areas, transduced neurons and glia were detected at the injection site, but also at distant brain areas in neurons that make afferent connections with the cells at the primary site. For example, following striatal injection, amplicon vector-transduced cells have been demonstrated in both substantia nigra pars compacta and locus coeruleus [122,130].
Traditionally, the stability of transgene expression, as well as the toxicity and immunogenicity of the helper virus were the limiting factors for amplicon-mediated gene transfer to cells of the CNS in vivo [112,152–156]. However, the recent advancements discussed above have largely addressed these problems. Vector-associated toxicity and immune responses have been greatly reduced by eliminating the helper virus, and the stability of gene expression has been increased fundamentally by using hybrid vectors and/or neuron-specific promoters.
HSV-1 Amplicon Vectors for Gene Therapy of Gliomas
We evaluated the efficiency of HSV-1 amplicon and HSV/AAV hybrid amplicon vectors expressing the tk gene to transduce and sensitize rat and human glioma cells to GCV in culture and in vivo [157]. The vectors were able to efficiently sensitize cultured cells to GCV in a vector dose-, GCV dose-, cell density- and time-dependent manner. However, transduction of s.c. rat 9L gliosarcoma tumor cell grafts in nude mice with 1x106 t.u. of vector particles did not result in consistent tumor regression, although the transduction rate was high around injection sites (1% to 10%) as assessed by immunohistochemistry (Figure 5). The efficiency of these vectors in vivo may be increased by developing methods that improve the distribution of vector particles throughout the tumor [158,159]. Similarly, replication-defective HSV-1 vectors that co-express tk and TNF-α also had little therapeutic effect in an intracerebral U-87 MG tumor model, also apparently due to low transduction efficiency (<1%) [160]. By contrast, expression of tk from an amplicon vector that was packaged by using a γ34.5 deletion mutant of HSV-1 (R3616) as the helper virus resulted in significant growth inhibition of s.c. tumors in a syngeneic, immune-competent mouse tumor model (GI261) [161]. In this case, the therapeutic effect was thought to be mediated by the combination of tk-mediated GCV sensitivity and the cytotoxicity of the helper virus, which is able to replicate in and spread within the tumor. However, no therapeutic effect was observed in human glioma cells (U87MG) grown in athymic mice, suggesting that the major factor responsible for tumor killing in the immune-competent animals may have been the immune response against the helper virus, possibly mediating enhanced immune recognition of tumor antigens. An intriguing approach would therefore be the boosting of tumor-specific immune responses by both cytotoxic, replication-conditional HSV-1 vectors and amplicon vectors encoding IL-12 [93,162].
Figure 5.
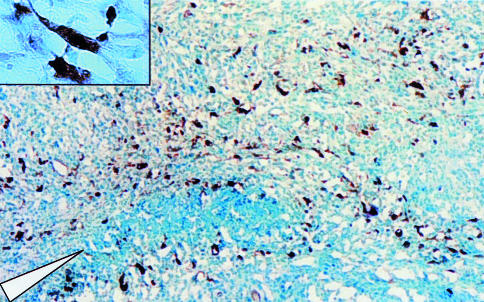
Immunohistochemical analysis of a rat 9L gliosarcoma grown s.c. in a nude mouse 2 days after direct injection of 1x106 t.u. of HSV/AAV hybrid amplicon vectors that express tk. The immunohistochemical staining for thymidine kinase (brown cells) reveals a transduction rate of approximately 1% to 10% around the injection site (▽).
Targeted and Regulated HSV-1 Vectors
Tissue- or cell-specific and regulatable targeting of vectors is critical to the overall success of gene therapy approaches. For HSV-1-derived vectors, this may be achieved by: i) modification of glycoprotein-mediated cell binding and entry; ii) gene complementation; iii) use of tissue-specific and/or regulatable promoters; and iv) improving the efficiency of physical vector transfer (reviewed in Ref. [163]).
The initial phase of virus attachment mediated by charge interaction between glycoproteins gB/gC and heparan sulfate moieties of the target cell membrane might be alterable by deletion or modification of essential glycoproteins. However, construction of targeted HSV-1 vectors is complicated by the highly complex process of infection. In addition, most of the glycoproteins have multiple functions and, therefore, some domains must be preserved while limiting the number that can be replaced by a new functional element. Laquerre et al. have constructed a recombinant HSV-1 vector for targeted binding to a non-HSV-1 cell surface receptor for erythropoietin by deletion of gC and the heparan sulfate binding domain of gB, and inclusion of chimeric proteins composed of mutated forms of gC and the erythropoietin hormone [164]. Moreover, an HSV-1 amplicon vector has been constructed expressing HSV-1 gC modified with His-tag replacing the heparan binding domain to achieve targeting vector toxicity to tumor cells [165]. These experiments demonstrate that targeted HSV-1 binding to a non-HSV-1 cell surface receptor is possible, in principle [164]. A similar approach has been very successful in targeting adenoviral vectors [166] either by direct genetic alterations of the HI loop of the adenoviral fiber knob [167,168] or by use of bifunctional antibody conjugates to adenoviral fiber and either epidermal growth factor receptor [169], folate receptor [170], or fibroblast growth factor receptor [171]. This approach can greatly increase the specificity and efficiency of gene transduction into appropriate cell types.
Replication-conditional HSV-1 vectors carrying mutations in genes that are neccessary for nucleic acid metabolism in nondividing cells, such as tk or RR, can be used to target proliferating tumor cells with complementary TK or RR activity [61]. A similar strategy has been employed to design an adenoviral vector for the selective killing of p53-deficient human tumor cells [172]. Apparently, the replication specificity of this vector does not reside entirely in the p53 status [173].
Transcriptional targeting can be achieved by the use of tissue-specific promoters. For example, incorporation of the glial fibrillary acidic protein (GFAP) promoter into HSV-1 recombinant 1716 yielded transgene (lacZ) expression predominantly in astrocytes both in culture and in vivo [174,175]. To target lytic virulence to gliomas, the recombinant HSV-1 Myb34.5 was engineered by deleting the RR gene and the two endogenous copies of the γ34.5 gene and by re-introducing one copy of γ34.5 under control of the E2F-responsive, cellular B-myb promoter [176]. Whereas neurovirulence of recombinant HSV-1 Myb34.5 (PFU/LD50=2.7x107) was similar to that of recombinant HSV-1 R3616 (PFU/LD50>1x107) after direct intracerebral inoculation into BALB/c mice, Myb34.5's oncolytic efficacy against a variety of human glioma cells in culture and in vivo was enhanced compared to that of R3616, and was similar to that of wild-type F strain and of HSV-1 mutants that possess a wild type γ34.5 gene [176]. These results indicate that transcriptional regulation of γ34.5 by cell cycle-regulated promoters may be used to target HSV-1 virulence toward tumors, while maintaining the desirable neuroattenuated phenotype of a γ34.5 mutant. Selective destruction of hepatocellular carcinoma cells by a replication-conditional HSV-1 vector was achieved by inserting an albumin enhancer/promoter-ICP4 transgene into the tk locus of HSV-1 mutant d120, which contains deletions in both copies of the ICP4 gene [56]. The hepatocyte-specific expression of the essential IE3 gene (ICP4) supported tissue-specific viral replication and cytolysis in dividing hepatoma cells, but not in the surrounding nondividing hepatocytes. In comparison, strategies for tumor-selective expression of therapeutic genes by adenoviral vectors make use of: i) an E2F-responsive promoter [177,178], presuming that tumor cells with mutations affecting the p16/cyclinD1/pRB/E2F pathway would have elevated levels of E2F; ii) a hypoxia-responsive promoter; or iii) a radiation inducible promoter. The different promoters direct selective transgene expression to cells with elevated E2F levels [179], hypoxemic cells [180], or to cells which have been radiosensitized [181]. All approaches result in increased control of tumor growth with limited damage to normal tissue [179,181].
Both constitutive (Gal4/VP16) and regulatable (RU486) transcriptional enhancers have been inserted into replication-conditional HSV-1 vectors. The Gal4/VP16 fusion protein consists of a DNA binding domain of the yeast transcriptional activator (Gal4) and the activation domain of the HSV-1 transactivator VP16 [182], which strongly activates promoters targeted by a Gal4-binding site [183]. Reporter gene expression was induced up to 35-fold in different models with the activator and the response promoter present either in the same or in different replication-conditional HSV-1 recombinant viruses [184,185]. Modification of this transactivation system produced a drug-inducible system, which uses a truncated form of the hormone binding domain (HBD) of the progesterone receptor fused to the Gal4/VP16 transactivator to form a HBD-Gal4/VP16 fusion protein. Binding of the specific activator RU486 to the modified HBD [186,187] alters the conformation of the fusion protein, which is necessary to allow binding to the Gal4 promoter elements with subsequent activation of transcription. Reporter gene expression was induced 30- to 150-fold by RU486, when the activator and the responsive promoter were placed on the same replication-conditional vector [188]. HSV-1 amplicon vectors containing tetracycline-regulatable promoter elements [189–192] have also been constructed [193,194]. These vectors express reporter genes from a combined minimal CMV promoter and a heptameric tetracycline operator. This promoter element is activated by a tetracycline-responsive hybrid protein which is also encoded by the vector. Maximal repression of reporter gene expression by tetracycline in hippocampal cultures was about 50-fold and withdrawal of tetracycline de-repressed gene expression, reaching maximal levels within 10 to 12 hours [193]. In adult rat hippocampus, reporter gene expression was repressed by tetracycline 9- to 60-fold [193]. In another report [194], after injection of amplicon vector stocks into the mouse hippocampus and administration of tetracycline, transgene expression could be repressed by a factor of 10 only. Moreover, the repression factor was reduced with increasing numbers of helper viruses present in the vector stock [194], and tetracycline-regulatable constructs in other amplicon vectors have proven leaky in the “off” state due to transcriptional override by HSV-1 elements and proteins [195].
The route of vector administration is crucial for the efficient killing of tumor cells in gene therapy protocols for malignant brain tumors. The delivery of viral vectors to the brain for treatment of intracerebral tumors is most commonly accomplished by stereotaxic inoculation directly into the tumor mass. However, diffusion of the vector is limited, which reduces the efficacy of viral therapy of large or disseminated tumors [157]. This obstacle can be overcome by intracarotid delivery of vector and concurrent, selective blood-tumor barrier disruption by bradykinin or RMP-27, as described previously [196–200]. The feasibility of this approach in the clinical application, however, needs to be demonstrated in the future. Other studies suggest that access of virus to tumors can be mediated through the cerebrospinal fluid [83,84] or through migratory neural progenitor cells [201,202].
Conclusions
HSV-1 structure and function has been studied extensively by others, which has facilitated the design and engineering of HSV-1-derived vectors as gene transfer vehicles for various treatment strategies in experimental gene therapy models. The natural neurotropism of HSV-1, as well as its large transgene capacity and high transduction efficiency, make HSV-1-derived vectors suitable for applications in the nervous system. Combined selective oncolysis and modulation of the immune response mediated by replication-conditional, multiple-mutated HSV-1 vectors appears to be a highly promising approach in the battle against malignant glioma. Helper virus-free HSV/AAV hybrid amplicon vectors have great promise in mediating long-term gene expression in the PNS and CNS for the treatment of neurodegenerative disorders, injury or chronic pain. The design of HSV-1-derived vectors, which are targeted to a certain cell population and support transcriptionally regulatable transgene expression, are the focus of present and future research. The importance of developing different modes of mechanical vector delivery, which enable sufficient vector distribution, cannot be underestimated. Gene therapy is a multi-variate approach with each variable being equally important for the overall success in a clinical situation. Therefore, the design of efficient gene therapy protocols relies on the concerted research and interaction of basic and clinical scientists.
Acknowledgements
We thank Petra Genutt and Mark Slack for critical reading of the manuscript.
Footnotes
Support to A.J. is from ZMMK-TV46 (Center of Molecular Medicine), grant 516-40000299 (Land NRW) and the Max-Planck-Society, Germany; X.O.B., NCI grant CA69246, NINDS grant NS24279; C.F., Swiss National Science Foundation.
References
- 1.Post LE, Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 2.Herrlinger U, Jacobs A, Aghi M, Breakefield XO. HSV-1 Vectors for Therapy of Experimental CNS Tumors. In: Robins P, editor. Methods in Molecular Medicine, Gene Therapy Protocols. Totowa, NJ: Humana Press; 1999. in press. [DOI] [PubMed] [Google Scholar]
- 3.Krisky DM, Marconi PC, Oligino T, Rouse RJ, Fink DJ, Glorioso JC. Rapid method for construction of recombinant HSV gene transfer vectors. Gene Ther. 1997;4:1120–1125. doi: 10.1038/sj.gt.3300497. [DOI] [PubMed] [Google Scholar]
- 4.Fink DJ, Glorioso JC. Engineering herpes simplex virus vectors for gene transfer to neurons. Nat Med. 1997;3:357–359. doi: 10.1038/nm0397-357. [DOI] [PubMed] [Google Scholar]
- 5.Chiocca EA, Choi BB, Cai WZ, DeLuca NA, Schaffer PA, DiFiglia M, Breakefield XO, Martuza RL. Transfer and expression of the lacZ gene in rat brain neurons mediated by herpes simplex virus mutants. New Biol. 1990;2:739–746. [PubMed] [Google Scholar]
- 6.DeLuca NA, McCarthy AM, Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glorioso JC, DeLuca NA, Fink DJ. Development and application of herpes simplex virus vectors for human gene therapy. Annu Rev Microbiol. 1995;49:675–710. doi: 10.1146/annurev.mi.49.100195.003331. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PA, Miyanohara A, Levine F, Cahill T, Friedmann T. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol. 1992;66:2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell J, Stow ND, Stow EC, Preston CM. Herpes simplex virus genes involved in latency in vitro. J Gen Virol. 1987;68:3009–3018. doi: 10.1099/0022-1317-68-12-3009. [DOI] [PubMed] [Google Scholar]
- 10.Johnson PA, Wang MJ, Friedmann T. Improved cell survival by the reduction of immediate-early gene expression in replication-defective mutants of herpes simplex virus type 1 but not by mutation of the virion host shutoff function. J Virol. 1994;68:6347–6362. doi: 10.1128/jvi.68.10.6347-6362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krisky DM, Marconi PC, Oligino TJ, Rouse RJ, Fink DJ, Cohen JB, Watkins SC, Glorioso JC. Development of herpes simplex virus replication-defective multigene vectors for combination gene therapy applications. Gene Ther. 1998;5:1517–1530. doi: 10.1038/sj.gt.3300755. [DOI] [PubMed] [Google Scholar]
- 12.Krisky DM, Wolfe D, Goins WF, Marconi PC, Ramakrishnan R, Mata M, Rouse RJ, Fink DJ, Glorioso JC. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Ther. 1998;5:1593–1603. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- 13.Marconi P, Krisky D, Oligino T, Poliani PL, Ramakrishnan R, Goins WF, Fink DJ, Glorioso JC. Replication-defective herpes simplex virus vectors for gene transfer in vivo. Proc Natl Acad Sci USA. 1996;93:11319–11320. doi: 10.1073/pnas.93.21.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu N, Watkins SC, Schaffer PA, DeLuca NA. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70:6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaniego LA, Wu N, DeLuca NA. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston CM, Nicholl MJ. Repression of gene expression upon infection of cells with herpes simplex virus type 1 mutants impaired for immediate-early protein synthesis. J Virol. 1997;71:7807–7813. doi: 10.1128/jvi.71.10.7807-7813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preston CM, Mabbs R, Nicholl MJ. Construction and characterization of herpes simplex virus type 1 mutants with conditional defects in immediate early gene expression. Virology. 1997;229:228–239. doi: 10.1006/viro.1996.8424. [DOI] [PubMed] [Google Scholar]
- 18.Samaniego LA, Neiderhiser L, DeLuca NA. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada M, Oligino T, Mata M, Goss JR, Glorioso JC, Fink DJ. Herpes simplex virus vector-mediated expression of bcl-2 prevents 6-hydroxydopamine-induced degeneration of neurons in the substantia nigra in vivo. Proc Natl Acad Sci USA. 1999;96:4078–4083. doi: 10.1073/pnas.96.7.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to 34.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 21.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus 34.5 deletion mutants in rodent models. J Clin Invest. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markert JM, Malick A, Coen DM, Martuza RL. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Chambers R, Gillespie GY, Soroceanu L, Andreansky S, Chatterjee S, Chou J, Roizman B, Whitley RJ. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffin RS, MacLean AR, Latchman DS, Brown SM. Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther. 1996;3:886–891. [PubMed] [Google Scholar]
- 25.Wagstaff MJ, Smith J, Collaco-Moraes Y, de Belleroche JS, Voellmy R, Coffin RS, Latchman DS. Delivery of a constitutively active form of the heat shock factor using a virus vector protects neuronal cells from thermal or ischaemic stress but not from apoptosis. Eur J Neurosci. 1998;10:3343–3350. doi: 10.1046/j.1460-9568.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- 26.Wagstaff MJ, Collaco-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- 27.Howard MK, Coffin RS, MacLean AR, Brown SM, Bailey D, Anderson PN, Burnstock G, Latchman DS. Gene delivery to rat enteric neurons using herpes simplex virus-based vectors. J Mol Neurosci. 1997;9:65–74. doi: 10.1007/BF02736851. [DOI] [PubMed] [Google Scholar]
- 28.Batchelor AH, O'Hare P. Regulation and cell-type-specific activity of a promoter located upstream of the latency-associated transcript of herpes simplex virus type 1. J Virol. 1990;64:3269–3279. doi: 10.1128/jvi.64.7.3269-3279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwaagstra JC, Ghiasi H, Slanina SM, Nesburn AB, Wheatley SC, Lillycrop K, Wood J, Latchman DS, Patel K, Wechsler SL. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J Virol. 1990;64:5019–5028. doi: 10.1128/jvi.64.10.5019-5028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho DY, Mocarski ES. Herpes simplex virus latent RNA (LAT) is not required for latent infection in the mouse. Proc Natl Acad Sci USA. 1989;86:7596–7600. doi: 10.1073/pnas.86.19.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachmann RH, Efstathiou S. Utilization of the herpes simplex virus type 1 latency-associated regulatory region to drive stable reporter gene expression in the nervous system. J Virol. 1997;71:3197–3207. doi: 10.1128/jvi.71.4.3197-3207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe JH, Deshmane SL, Fraser NW. Herpesvirus vector gene transfer and expression of beta-glucuronidase in the central nervous system of MPS VII mice. Nat Genet. 1992;1:379–384. doi: 10.1038/ng0892-379. [DOI] [PubMed] [Google Scholar]
- 33.Goins WF, Sternberg LR, Croen KD, Krause PR, Hendricks RL, Fink DJ, Straus SE, Levine M, Glorioso JC. A novel latency-active promoter is contained within the herpes simplex virus type 1 UL flanking repeats. J Virol. 1994;68:2239–2252. doi: 10.1128/jvi.68.4.2239-2252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goins WF, Lee KA, Cavalcoli JD, O'Malley ME, DeKosky ST, Fink DJ, Glorioso JC. Herpes simplex virus type 1 vector-mediated expression of nerve growth factor protects dorsal root ganglion neurons from peroxide toxicity. J Virol. 1999;73:519–532. doi: 10.1128/jvi.73.1.519-532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersen JK, Frim DM, Isacson O, Breakefield XO. Herpesvirus-mediated gene delivery into the rat brain: specificity and efficiency of the neuron-specific enolase promoter. Cell Mol Neurobiol. 1993;13:503–515. doi: 10.1007/BF00711459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fink DJ, Sternberg LR, Weber PC, Mata M, Goins WF, Glorioso JC. In vivo expression of beta-galactosidase in hippocampal neurons by HSV-mediated gene transfer. Hum Gene Ther. 1992;3:11–19. doi: 10.1089/hum.1992.3.1-11. [DOI] [PubMed] [Google Scholar]
- 37.Lokensgard JR, Bloom DC, Dobson AT, Feldman LT. Long-term promoter activity during herpes simplex virus latency. J Virol. 1994;68:7148–7158. doi: 10.1128/jvi.68.11.7148-7158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpenter DE, Stevens JG. Long-term expression of a foreign gene from a unique position in the latent herpes simplex virus genome. Hum Gene Ther. 1996;7:1447–1454. doi: 10.1089/hum.1996.7.12-1447. [DOI] [PubMed] [Google Scholar]
- 39.Dobson AT, Margolis TP, Sedarati F, Stevens JG, Feldman LT. A latent, nonpathogenic HSV-1-derived vector stably expresses beta-galactosidase in mouse neurons. Neuron. 1990;5:353–360. doi: 10.1016/0896-6273(90)90171-b. [DOI] [PubMed] [Google Scholar]
- 40.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 41.Mullen CA, Kilstrup M, Blaese RM. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci USA. 1992;89:33–37. doi: 10.1073/pnas.89.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aghi M, Kramm CM, Chou TC, Breakefield XO, Chiocca EA. Synergistic anticancer effects of ganciclovir/thymidine kinase and 5-fluorocytosine/cytosine deaminase gene therapies. J Natl Cancer Inst. 1998;90:370–380. doi: 10.1093/jnci/90.5.370. [DOI] [PubMed] [Google Scholar]
- 43.Mroz PJ, Moolten FL. Retrovirally transduced Escherichia coli gpt genes combine selectability with chemosensitivity capable of mediating tumor eradication. Hum Gene Ther. 1993;4:589–595. doi: 10.1089/hum.1993.4.5-589. [DOI] [PubMed] [Google Scholar]
- 44.Tamiya T, Ono Y, Wei MX, Mroz PJ, Moolten FL, Chiocca EA. Escherichia coli gpt gene sensitizes rat glioma cells to killing by 6-thioxanthine or 6-thioguanine. Cancer Gene Ther. 1996;3:155–162. [PubMed] [Google Scholar]
- 45.Chen L, Waxman DJ. Intratumoral activation and enhanced chemotherapeutic effect of oxazaphosphorines following cytochrome P-450 gene transfer: development of a combined chemotherapy/cancer gene therapy strategy. Cancer Res. 1995;55:581–589. [PubMed] [Google Scholar]
- 46.Rainov NG, Dobberstein KU, Sena-Esteves M, Herrlinger U, Kramm CM, Philpot RM, Hilton J, Chiocca EA, Breakefield XO. New prodrug activation gene therapy for cancer using cytochrome P450 4B1 and 2-aminoanthracene/4-ipomeanol. Hum Gene Ther. 1998;9:1261–1273. doi: 10.1089/hum.1998.9.9-1261. [DOI] [PubMed] [Google Scholar]
- 47.Wei MX, Tamiya T, Chase M, Boviatsis EJ, Chang TK, Kowall NW, Hochberg FH, Waxman DJ, Breakefield XO, Chiocca EA. Experimental tumor therapy in mice using the cyclophosphamide-activating cytochrome P450 2B1 gene. Hum Gene Ther. 1994;5:969–978. doi: 10.1089/hum.1994.5.8-969. [DOI] [PubMed] [Google Scholar]
- 48.Manome Y, Wen PY, Dong Y, Tanaka T, Mitchell BS, Kufe DW, Fine HA. Viral vector transduction of the human deoxycytidine kinase cDNA sensitizes glioma cells to the cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nat Med. 1996;2:567–573. doi: 10.1038/nm0596-567. [DOI] [PubMed] [Google Scholar]
- 49.Roth JA, Nguyen D, Lawrence DD, Kemp BL, Carrasco CH, Ferson DZ, Hong WK, Komaki R, Lee JJ, Nesbitt JC, Pisters KM, Putnam JB, Schea R, Shin DM, Walsh GL, Dolormente MM, Han CI, Martin FD, Yen N, Xu K, Stephens LC, McDonnell TJ, Mukhopadhyay T, Cai D. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat Med. 1996;2:985–991. doi: 10.1038/nm0996-985. [DOI] [PubMed] [Google Scholar]
- 50.Chung RY, Chiocca EA. Gene therapy for tumors of the central nervous system. Surg Oncol Clin N Am. 1998;7:589–602. [PubMed] [Google Scholar]
- 51.Ho DY, Mocarski ES. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology. 1988;167:279–283. doi: 10.1016/0042-6822(88)90079-7. [DOI] [PubMed] [Google Scholar]
- 52.Markert JM, Coen DM, Malick A, Mineta T, Martuza RL. Expanded spectrum of viral therapy in the treatment of nervous system tumors. J Neurosurg. 1992;77:590–594. doi: 10.3171/jns.1992.77.4.0590. [DOI] [PubMed] [Google Scholar]
- 53.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 54.Jia WW, McDermott M, Goldie J, Cynader M, Tan J, Tufaro F. Selective destruction of gliomas in immunocompetent rats by thymidine kinase-defective herpes simplex virus type 1. J Natl Cancer Inst. 1994;86:1209–1215. doi: 10.1093/jnci/86.16.1209. [DOI] [PubMed] [Google Scholar]
- 55.Jia WW, Tan J, Redekop GJ, Goldie JH. Toxicity studies in thymidine kinase-deficient herpes simplex virus therapy for malignant astrocytoma. J Neurosurg. 1996;85:662–666. doi: 10.3171/jns.1996.85.4.0662. [DOI] [PubMed] [Google Scholar]
- 56.Miyatake S, Iyer A, Martuza RL, Rabkin SD. Transcriptional targeting of herpes simplex virus for cell-specific replication. J Virol. 1997;71:5124–5132. doi: 10.1128/jvi.71.7.5124-5132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaplitt MG, Tjuvajev JG, Leib DA, Berk J, Pettigrew KD, Posner JB, Pfaff DW, Rabkin SD, Blasberg RG. Mutant herpes simplex virus induced regression of tumors growing in immunocompetent rats. J Neurooncol. 1994;19:137–147. doi: 10.1007/BF01306455. [DOI] [PubMed] [Google Scholar]
- 58.Boviatsis EJ, Scharf JM, Chase M, Harrington K, Kowall NW, Breakefield XO, Chiocca EA. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1:323–331. [PubMed] [Google Scholar]
- 59.Boviatsis EJ, Chase M, Wei MX, Tamiya T, Hurford RKJ, Kowall NW, Tepper RI, Breakefield XO, Chiocca EA. Gene transfer into experimental brain tumors mediated by adenovirus, herpes simplex virus, and retrovirus vectors. Hum Gene Ther. 1994;5:183–191. doi: 10.1089/hum.1994.5.2-183. [DOI] [PubMed] [Google Scholar]
- 60.Boviatsis EJ, Park JS, Sena-Esteves M, Kramm CM, Chase M, Efird JT, Wei MX, Breakefield XO, Chiocca EA. Long-term survival of rats harboring brain neoplasms treated with ganciclovir and a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994;54:5745–5751. [PubMed] [Google Scholar]
- 61.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mineta T, Rabkin SD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54:3963–3966. [PubMed] [Google Scholar]
- 63.Brown SM, Harland J, MacLean AR, Podlech J, Clements JB. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J Gen Virol. 1994;75:2367–2377. doi: 10.1099/0022-1317-75-9-2367. [DOI] [PubMed] [Google Scholar]
- 64.MacLean AR, ul-Fareed M, Robertson L, Harland J, Brown SM. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J Gen Virol. 1991;72:631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 65.McKie EA, MacLean AR, Lewis AD, Cruickshank G, Rampling R, Barnett SC, Kennedy PG, Brown SM. Selective in vitro replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants in primary human CNS tumours—evaluation of a potentially effective clinical therapy. Br J Cancer. 1996;74:745–752. doi: 10.1038/bjc.1996.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 67.Kramm CM, Chase M, Herrlinger U, Jacobs A, Pechan PA, Rainov NG, Sena-Esteves M, Aghi M, Barnett FH, Chiocca EA, Breakefield XO. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997;8:2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- 68.Pyles RB, Warnick RE, Chalk CL, Szanti BE, Parysek LM. A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 69.Andreansky S, Soroceanu L, Flotte ER, Chou J, Markert JM, Gillespie GY, Roizman B, Whitley RJ. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- 70.Kesari S, Randazzo BP, Valyi-Nagy T, Huang QS, Brown SM, MacLean AR, Lee VM, Trojanowski JQ, Fraser NW. Therapy of experimental human brain tumors using a neuroattenuated herpes simplex virus mutant. Lab Invest. 1995;73:636–648. [PubMed] [Google Scholar]
- 71.Lasner TM, Kesari S, Brown SM, Lee VM, Fraser NW, Trojanowski JQ. Therapy of a murine model of pediatric brain tumors using a herpes simplex virus type-1 ICP34.5 mutant and demonstration of viral replication within the CNS. J Neuropathol Exp Neurol. 1996;55:1259–1269. doi: 10.1097/00005072-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 72.Randazzo BP, Kesari S, Gesser RM, Alsop D, Ford JC, Brown SM, MacLean A, Fraser NW. Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus 1 mutant. Virology. 1995;211:94–101. doi: 10.1006/viro.1995.1382. [DOI] [PubMed] [Google Scholar]
- 73.McKie EA, Brown SM, MacLean AR, Graham DI. Histopathological responses in the CNS following inoculation with a non-neurovirulent mutant (1716) of herpes simplex virus type 1 (HSV-1): relevance for gene and cancer therapy. Neuropathol Appl Neurobiol. 1998;24:367–372. doi: 10.1046/j.1365-2990.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 74.Rampling R, Cruickshank G, MacLean A, Brown M. Therapeutic replication-competent herpes virus. Nat Med. 1998;4:133. doi: 10.1038/nm0298-133c. [DOI] [PubMed] [Google Scholar]
- 75.Markovitz NS, Baunoch D, Roizman B. The range and distribution of murine central nervous system cells infected with the gamma(1)34.5-mutant of herpes simplex virus 1. J Virol. 1997;71:5560–5569. doi: 10.1128/jvi.71.7.5560-5569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kesari S, Lasner TM, Balsara KR, Randazzo BP, Lee VM, Trojanowski JQ, Fraser NW. A neuroattenuated ICP34.5-deficient herpes simplex virus type 1 replicates in ependymal cells of the murine central nervous system. J Gen Virol. 1998;79:525–536. doi: 10.1099/0022-1317-79-3-525. [DOI] [PubMed] [Google Scholar]
- 77.McMenamin MM, Byrnes AP, Charlton HM, Coffin RS, Latchman DS, Wood MJ. A gamma 34.5 mutant of herpes simplex 1 causes severe inflammation in the brain. Neuroscience. 1998;83:1225–1237. doi: 10.1016/s0306-4522(97)00513-7. [DOI] [PubMed] [Google Scholar]
- 78.McMenamin MM, Byrnes AP, Pike FG, Charlton HM, Coffin RS, Latchman DS, Wood MJ. Potential and limitations of a gamma 34.5 mutant of herpes simplex 1 as a gene therapy vector in the CNS. Gene Ther. 1998;5:594–604. doi: 10.1038/sj.gt.3300639. [DOI] [PubMed] [Google Scholar]
- 79.Lasner TM, Tal-Singer R, Kesari S, Lee VM, Trojanowski JQ, Fraser NW. Toxicity and neuronal infection of a HSV-1 ICP34.5 mutant in nude mice. J Neurovirol. 1998;4:100–105. doi: 10.3109/13550289809113487. [DOI] [PubMed] [Google Scholar]
- 80.Andreansky S, He B, van Cott J, McGhee J, Markert JM, Gillespie GY, Roizman B, Whitley RJ. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5:121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 81.Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, Weichselbaum RR. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- 82.Carroll NM, Chiocca EA, Takahashi K, Tanabe KK. Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virus. Ann Surg. 1996;224:323–329. doi: 10.1097/00000658-199609000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kramm CM, Rainov NG, Sena-Esteves M, Barnett FH, Chase M, Herrlinger U, Pechan PA, Chiocca EA, Breakefield XO. Long-term survival in a rodent model of disseminated brain tumors by combined intrathecal delivery of herpes vectors and ganciclovir treatment. Hum Gene Ther. 1996;7:1989–1994. doi: 10.1089/hum.1996.7.16-1989. [DOI] [PubMed] [Google Scholar]
- 84.Kramm CM, Rainov NG, Sena-Esteves M, Chase M, Pechan PA, Chiocca EA, Breakefield XO. Herpes vector-mediated delivery of marker genes to disseminated central nervous system tumors. Hum Gene Ther. 1996;7:291–300. doi: 10.1089/hum.1996.7.3-291. [DOI] [PubMed] [Google Scholar]
- 85.Jacobs A, Tjuvajev JG, Balatoni J, Herrlinger U, Joshi R, Pechan PA, Finn R, Chiocca EA, Breakefield XO, Blasberg RG. Imaging HSV-1 vector replication and gene delivery in vivo. J Gene Med. 1999;01:15. [Google Scholar]
- 86.Chase M, Chung RY, Chiocca EA. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998;16:444–448. doi: 10.1038/nbt0598-444. [DOI] [PubMed] [Google Scholar]
- 87.Aghi M, Chou TC, Suling K, Breakefield XO, Chiocca EA. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/CYP2B1 and ganciclovir/HSV-TK gene therapies. Cancer Res. 1999 in press. [PubMed] [Google Scholar]
- 88.Herrlinger U, Kramm CM, Aboody-Guterman KS, Silver JS, Ikeda K, Johnston KM, Pechan PA, Barth RF, Finkelstein D, Chiocca EA, Louis DN, Breakefield XO. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998;5:809–819. doi: 10.1038/sj.gt.3300643. [DOI] [PubMed] [Google Scholar]
- 89.Wang Q, Guo J, Jia W. Intracerebral recombinant HSV-1 vector does not reactivate latent HSV-1. Gene Ther. 1997;4:1300–1304. doi: 10.1038/sj.gt.3300535. [DOI] [PubMed] [Google Scholar]
- 90.Yazaki T, Manz HJ, Rabkin SD, Martuza RL. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Res. 1995;55:4752–4756. [PubMed] [Google Scholar]
- 91.Coen DM, Goldstein DJ, Weller SK. Herpes simplex virus ribonucleotide reductase mutants are hypersensitive to acyclovir. Antimicrob. Agents Chemother. 1989;33:1395–1399. doi: 10.1128/aac.33.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toda M, Rabkin SD, Martuza RL. Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1. Hum Gene Ther. 1998;9:2177–2185. doi: 10.1089/hum.1998.9.15-2177. [DOI] [PubMed] [Google Scholar]
- 93.Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 94.Martuza RL. Act locally, think globally. Nat Med. 1997;3:1323. doi: 10.1038/nm1297-1323. [DOI] [PubMed] [Google Scholar]
- 95.Frenkel N, Locker H, Batterson W, Hayward GS, Roizman B. Anatomy of herpes simplex virus DNA. VI. Defective DNA originates from the S component. J Virol. 1976;20:527–531. doi: 10.1128/jvi.20.2.527-531.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Locker H, Frenkel N. Structure and origin of defective genomes contained in serially passaged herpes simplex virus type 1 (Justin) J Virol. 1979;29:1065–1077. doi: 10.1128/jvi.29.3.1065-1077.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Locker H, Frenkel N, Halliburton I. Structure and expression of class II defective herpes simplex virus genomes encoding infected cell polypeptide number 8. J Virol. 1982;43:574–593. doi: 10.1128/jvi.43.2.574-593.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwong AD, Frenkel N. The herpes simplex virus amplicon. IV. Efficient expression of a chimeric chicken ovalbumin gene amplified within defective virus genomes. Virology. 1985;142:421–425. doi: 10.1016/0042-6822(85)90351-4. [DOI] [PubMed] [Google Scholar]
- 99.Spaete RR, Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982;30:295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- 100.Preston CM. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979;29:275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988;241:1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paterson T, Everett RD. A prominent serine-rich region in Vmw175, the major transcriptional regulator protein of herpes simplex virus type 1, is not essential for virus growth in tissue culture. J Gen Virol. 1990;71:1775–1783. doi: 10.1099/0022-1317-71-8-1775. [DOI] [PubMed] [Google Scholar]
- 103.Davidson I, Stow ND. Expression of an immediate early polypeptide and activation of a viral origin of DNA replication in cells containing a fragment of herpes simplex virus DNA. Virology. 1985;141:77–88. doi: 10.1016/0042-6822(85)90184-9. [DOI] [PubMed] [Google Scholar]
- 104.Geller AI, Keyomarsi K, Bryan J, Pardee AB. An efficient deletion mutant packaging system for defective herpes simplex virus vectors: potential applications to human gene therapy and neuronal physiology. Proc Natl Acad Sci USA. 1990;87:8950–8954. doi: 10.1073/pnas.87.22.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCarthy AM, McMahan L, Schaffer PA. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith IL, Hardwicke MA, Sandri-Goldin RM. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 107.Lim F, Hartley D, Starr P, Lang P, Song S, Yu L, Wang Y, Geller AI. Generation of high-titer defective HSV-1 vectors using an IE 2 deletion mutant and quantitative study of expression in cultured cortical cells. Biotechniques. 1996;20:460–469. doi: 10.2144/19962003460. [DOI] [PubMed] [Google Scholar]
- 108.Berthomme H, Fournel S, Epstein AL. Increased transcomplementation properties of plasmids carrying HSV-1 origin of replication and packaging signals. Virology. 1996;216:437–443. doi: 10.1006/viro.1996.0081. [DOI] [PubMed] [Google Scholar]
- 109.Pechan PA, Fotaki M, Thompson RL, Dunn R, Chase M, Chiocca EA, Breakefield XO. A novel ‘piggyback’ packaging system for herpes simplex virus amplicon vectors. Hum Gene Ther. 1996;7:2003–2013. doi: 10.1089/hum.1996.7.16-2003. [DOI] [PubMed] [Google Scholar]
- 110.Pechan PA, Herrlinger U, Aghi M, Jacobs A, Breakefield XO. Combined HSV-1 recombinant and amplicon piggyback vectors: replication-competent and defective forms, and therapeutic efficacy for experimental gliomas. J Gene Med. 1999;1:176–185. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<176::AID-JGM35>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 111.Cunningham C, Davison AJ. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 112.Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller AI. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J Virol. 1996;70:7190–7197. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aboody-Guterman KS, Pechan PA, Rainov NG, Sena-Esteves M, Jacobs A, Snyder EY, Wild P, Schraner E, Tobler K, Breakefield XO, Fraefel C. Green fluorescent protein as a reporter for retrovirus and helper virus-free HSV-1 amplicon vector-mediated gene transfer into neural cells in culture and in vivo. NeuroReport. 1997;8:3801–3808. doi: 10.1097/00001756-199712010-00029. [DOI] [PubMed] [Google Scholar]
- 114.Fraefel C, Jacoby DR, Lage C, Hilderbrand H, Chou JY, Alt FW, Breakefield XO, Majzoub JA. Gene transfer into hepatocytes mediated by helper virus-free HSV/AAV hybrid vectors. MolMed. 1997;3:813–825. [PMC free article] [PubMed] [Google Scholar]
- 115.Fraefel C, Breakefield XO, Jacoby DR. HSV-1 Amplicon. In: Chiocca EA, Breakefield XO, editors. Gene Therapy for Neurological Disorders and Brain Tumors. Totowa, NJ: Humana Press; 1998. pp. 63–82. [Google Scholar]
- 116.Johnston KM, Jacoby D, Pechan PA, Fraefel C, Borghesani P, Schuback D, Dunn RJ, Smith FI, Breakefield XO. HSV/AAV hybrid amplicon vectors extend transgene expression in human glioma cells. Hum Gene Ther. 1997;6:359–370. doi: 10.1089/hum.1997.8.3-359. [DOI] [PubMed] [Google Scholar]
- 117.Saeki Y, Ichikawa T, Saeki A, Chiocca EA, Tobler K, Ackermann M, Breakefield XO, Fraefel C. Herpes simplex virus type 1 DNA amplified as bacterial artificial chromosome in Escherichia coli: rescue of replication-competent virus progeny and packaging of amplicon vectors. Hum Gene Ther. 1998;9:2787–2794. doi: 10.1089/hum.1998.9.18-2787. [DOI] [PubMed] [Google Scholar]
- 118.Saeki Y, Fraefel C. Herpes Simplex Virus Type 1-Based Amplicon and Hybrid Amplicon Vector Systems. In: Cid-Arregui A, Garcia A, editors. Viral Vectors: Basic Science and Gene Therapy. Natick: BioTechniques Books; 1999. in press. [Google Scholar]
- 119.Stavropoulos TA, Strathdee CA. An enhanced packaging system for helper-dependent herpes simplex virus vectors. J Virol. 1998;72:7137–7143. doi: 10.1128/jvi.72.9.7137-7143.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ho DY, Mocarski ES, Sapolsky RM. Altering central nervous system physiology with a defective herpes simplex virus vector expressing the glucose transporter gene. Proc Natl Acad Sci USA. 1993;190:3655–3659. doi: 10.1073/pnas.90.8.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith RL, Geller AI, Escudero KW, Wilcox CL. Long-term expression in sensory neurons in tissue culture from herpes simplex virus type 1 (HSV-1) promoters in an HSV-1-derived vector. J Virol. 1995;69:4593–4599. doi: 10.1128/jvi.69.8.4593-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jin BK, Belloni M, Conti B, Federoff HJ, Starr R, Son JH, Baker H, Joh TH. Prolonged in vivo gene expression driven by a tyrosine hydroxylase promoter in a defective herpes simplex virus amplicon vector. Hum Gene Ther. 1996;7:2015–2024. doi: 10.1089/hum.1996.7.16-2015. [DOI] [PubMed] [Google Scholar]
- 123.Kaplitt MG, Kwong AD, Kleopoulos SP, Mobbs CV, Rabkin SD, Pfaff DW. Preproenkephalin promoter yields region-specific and long-term expression in adult brain after direct in vivo gene transfer via a defective herpes simplex viral vector. Proc Natl Acad Sci USA. 1994;91:8979–8983. doi: 10.1073/pnas.91.19.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balague C, Kalla M, Zhang WW. Adeno-associated virus Rep78 protein and terminal repeats enhance integration of DNA sequences into the cellular genome. J Virol. 1997;71:3299–3306. doi: 10.1128/jvi.71.4.3299-3306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Flotte TR, Afione SA, Zeitlin PL. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am J Respir Cell Mol Biol. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- 126.Kotin RM, Linden RM, Berns KI. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Linden RM, Ward P, Giraud C, Winocour E, Berns KI. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1996;93:11288–11294. doi: 10.1073/pnas.93.21.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Russell DW, Miller AD, Alexander IE. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Surosky RT, Urabe M, Godwin SG, McQuiston SA, Kurtzman GJ, Ozawa K, Natsoulis G. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J Virol. 1997;71:7951–7959. doi: 10.1128/jvi.71.10.7951-7959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Constantini LC, Jacoby DR, Wang S, Fraefel C, Breakefield XO, Isacson O. Gene transfer to the nigrostriatal system by hybrid HSV/AAV amplicon vectors. Hum Gene Ther. 1999 doi: 10.1089/10430349950016825. in press. [DOI] [PubMed] [Google Scholar]
- 131.Gahn TA, Schildkraut CL. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989;58:527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- 132.Middleton T, Sugden B. Retention of plasmid DNA in mammalian cells is enhanced by binding of the Epstein-Barr virus replication protein EBNA1. J Virol. 1994;68:4067–4071. doi: 10.1128/jvi.68.6.4067-4071.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun TQ, Livanos E, Vos JM. Engineering a mini-herpesvirus as a general strategy to transduce up to 180 kb of functional self-replicating human mini-chromosomes. Gene Ther. 1996;3:1081–1088. [PubMed] [Google Scholar]
- 134.Wohlgemuth JG, Kang SH, Bulboaca GH, Nawotka KA, Calos MP. Long-term gene expression from autonomously replicating vectors in mammalian cells. Gene Ther. 1996;3:503–512. [PubMed] [Google Scholar]
- 135.Yates J, Warren N, Reisman D, Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci USA. 1984;81:3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang S, Vos JM. A hybrid herpesvirus infectious vector based on Epstein-Barr virus and herpes simplex virus type 1 for gene transfer into human cells in vitro and in vivo. J Virol. 1996;70:8422–8430. doi: 10.1128/jvi.70.12.8422-8430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang S, Di S, Young WB, Jacobson C, Link CJJ. A novel herpesvirus amplicon system for in vivo gene delivery. Gene Ther. 1997;4:1132–1141. doi: 10.1038/sj.gt.3300523. [DOI] [PubMed] [Google Scholar]
- 138.Sena-Esteves M, Saeki Y, Camp SM, Chiocca EA, Breakefield XO. Single-step conversion of cells to retrovirus vector producers with HSV/EBV hybrid amplicons. J Virol. 1999 doi: 10.1128/jvi.73.12.10426-10439.1999. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Battleman DS, Geller Al, Chao MV. HSV-1 vector-mediated gene transfer of the human nerve growth factor receptor p75hNGFR defines high-affinity NGF binding. J Neurosci. 1993;13:941–951. doi: 10.1523/JNEUROSCI.13-03-00941.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neve RL, Ivins KJ, Benowitz LI, During MJ, Geller AI. Molecular analysis of the function of the neuronal growth-associated protein GAP-43 by genetic intervention. Mol Neurobiol. 1991;5:131–141. doi: 10.1007/BF02935542. [DOI] [PubMed] [Google Scholar]
- 141.Bergold PJ, Casaccia-Bonnefil P, Zeng XL, Federoff HJ. Transsynaptic neuronal loss induced in hippocampal slice cultures by a herpes simplex virus vector expressing the GluR6 subunit of the kainate receptor. Proc Natl Acad Sci USA. 1993;90:6165–6169. doi: 10.1073/pnas.90.13.6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ho DY, Saydam TC, Fink SL, Lawrence MS, Sapolsky RM. Defective herpes simplex virus vectors expressing the rat brain glucose transporter protect cultured neurons from necrotic insults. J Neurochem. 1995;65:842–850. doi: 10.1046/j.1471-4159.1995.65020842.x. [DOI] [PubMed] [Google Scholar]
- 143.Geschwind MD, Hartnick CJ, Liu W, Amat J, Van De Water TR, Federoff HJ. Defective HSV-1 vector expressing BDNF in auditory ganglia elicits neurite outgrowth: model for treatment of neuron loss following cochlear degeneration. Hum Gene Ther. 1996;7:173–182. doi: 10.1089/hum.1996.7.2-173. [DOI] [PubMed] [Google Scholar]
- 144.Garrido JJ, Alonso MT, Lim F, Carnicero E, Giraldez F, Schimmang T. Defining responsiveness of avian cochlear neurons to brain-derived neurotrophic factor and nerve growth factor by HSV-1-mediated gene transfer. J Neurochem. 1998;70:2336–2346. doi: 10.1046/j.1471-4159.1998.70062336.x. [DOI] [PubMed] [Google Scholar]
- 145.Lawrence MS, Ho DY, Dash R, Sapolsky RM. Herpes simplex virus vectors overexpressing the glucose transporter gene protect against seizure-induced neuron loss. Proc Natl Acad Sci USA. 1995;92:7247–7251. doi: 10.1073/pnas.92.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lawrence MS, Sun GH, Kunis DM, Saydam TC, Dash R, Ho DY, Sapolsky RM, Steinberg GK. Over-expression of the glucose transporter gene with a herpes simplex viral vector protects striatal neurons against stroke. J Cereb Blood Flow Metab. 1996;16:181–185. doi: 10.1097/00004647-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 147.Lawrence MS, McLaughlin JR, Sun GH, Ho DY, McIntosh L, Kunis DM, Sapolsky RM, Steinberg GK. Herpes simplex viral vectors expressing Bcl-2 are neuroprotective when delivered after a stroke. J Cereb Blood Flow Metab. 1997;17:740–744. doi: 10.1097/00004647-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 148.Linnik MD, Zahos P, Geschwind MD, Federoff HJ. Expression of bcl-2 from a defective herpes simplex virus-1 vector limits neuronal death in focal cerebral ischemia. Stroke. 1995;26:1670–1674. doi: 10.1161/01.str.26.9.1670. [DOI] [PubMed] [Google Scholar]
- 149.During MJ, Naegele JR, O'Malley KL, Geller AI. Long-term behavioral recovery in parkinsonian rats by an HSV vector expressing tyrosine hydroxylase. Science. 1994;266:1399–1403. doi: 10.1126/science.266.5189.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brooks AI, Muhkerjee B, Panahian N, Cory-Slechta D, Federoff HJ. Nerve growth factor somatic mosaicism produced by herpes virus-directed expression of cre recombinase. Nat Biotechnol. 1997;15:57–62. doi: 10.1038/nbt0197-57. [DOI] [PubMed] [Google Scholar]
- 151.Huang Q, Vonsattel JP, Schaffer PA, Martuza RL, Breakefield XO, DiFiglia M. Introduction of a foreign gene (Escherichia coli lacZ) into rat neostriatal neurons using herpes simplex virus mutants: a light and electron microscopic study. Exp Neurol. 1992;115:303–316. doi: 10.1016/0014-4886(92)90196-w. [DOI] [PubMed] [Google Scholar]
- 152.Wood MJ, Byrnes AP, Kaplitt MG, Pfaff DW, Rabkin SD, Charlton HM. Specific patterns of defective HSV-1 gene transfer in the adult central nervous system: implications for gene targeting. Exp Neurol. 1994;130:127–140. doi: 10.1006/exnr.1994.1192. [DOI] [PubMed] [Google Scholar]
- 153.Ho DY, Fink SL, Lawrence MS, Meier TJ, Saydam TC, Dash R, Sapolsky RM. Herpes simplex virus vector system: analysis of its in vivo and in vitro cytopathic effects. J Neurosci Methods. 1995;57:205–215. doi: 10.1016/0165-0270(94)00150-f. [DOI] [PubMed] [Google Scholar]
- 154.Pakzaban P, Geller Al, Isacson O. Effect of exogenous nerve growth factor on neurotoxicity of and neuronal gene delivery by a herpes simplex amplicon vector in the rat brain. Hum Gene Ther. 1994;5:987–995. doi: 10.1089/hum.1994.5.8-987. [DOI] [PubMed] [Google Scholar]
- 155.Wood MJ, Byrnes AP, Pfaff DW, Rabkin SD, Charlton HM. Inflammatory effects of gene transfer into the CNS with defective HSV-1 vectors. Gene Ther. 1994;1:283–291. [PubMed] [Google Scholar]
- 156.Wood MJ, Byrnes AP, Rabkin SD, Pfaff DW, Charlton HM. Immunological consequences of HSV-1-mediated gene transfer into the CNS. Gene Ther. 1994;1(suppl 1):S82. [PubMed] [Google Scholar]
- 157.Jacobs A, Jacoby DR, Sena-Esteves M, Aghi M, Herrlinger U, Wang S, Johnston KM, Aboody-Guterman KS, Fraefel C, Breakefield XO. Gene delivery by helper virus-free defective herpes viral vectors. J Gene Med. 1999;01:66. [Google Scholar]
- 158.Brooks AI, Halterman MW, Chadwick CA, Davidson BL, Haak-Frendscho M, Radel C, Porter C, Federoff HJ. Reproducible and efficient murine CNS gene delivery using a microprocessor-controlled injector. J Neurosci Methods. 1998;80:137–147. doi: 10.1016/s0165-0270(97)00207-0. [DOI] [PubMed] [Google Scholar]
- 159.Voges J, Reszka R, Sturm V, Jacobs A, Heiss WD. Stereotactic intracerebral catheter implantation and continuous intratumoral infusion of the liposome associated HSV-tk gene in patients with recurrent, unresectable glioblastoma multiforme—Clinical protocol for a phase I/II study. Keystone. 1998 [Google Scholar]
- 160.Moriuchi S, Oligino T, Krisky D, Marconi P, Fink D, Cohen J, Glorioso JC. Enhanced tumor cell killing in the presence of ganciclovir by herpes simplex virus type 1 vector-directed coexpression of human tumor necrosis factor-alpha and herpes simplex virus thymidine kinase. Cancer Res. 1998;58:5731–5737. [PubMed] [Google Scholar]
- 161.Miyatake S, Martuza RL, Rabkin SD. Defective herpes simplex virus vectors expressing thymidine kinase for the treatment of malignant glioma. Cancer Gene Ther. 1997;4:222–228. [PubMed] [Google Scholar]
- 162.Toda M, Martuza RL, Kojima H, Rabkin SD. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J Immunol. 1998;160:4457–4464. [PubMed] [Google Scholar]
- 163.Spear MA, Herrlinger U, Rainov N, Pechan P, Weissleder R, Breakefield XO. Targeting gene therapy vectors to CNS malignancies. J Neurovirol. 1998;4:133–147. doi: 10.3109/13550289809114514. [DOI] [PubMed] [Google Scholar]
- 164.Laquerre S, Anderson DB, Stolz DB, Glorioso JC. Recombinant herpes simplex virus type 1 engineered for targeted binding to erythropoietin receptor-bearing cells. J Virol. 1998;72:9683–9697. doi: 10.1128/jvi.72.12.9683-9697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spear M, Schuback D, Sun F, Brandt CR, Breakefield XO. HSV-1 gC modified with his-tag replacing heparan sulfate binding domain is expressed in an HSV-1 amplicon vector targeting system. ASGT. 1999 [Google Scholar]
- 166.Bilbao G, Gomez-Navarro J, Curiel DT. Targeted adenoviral vectors for cancer gene therapy. Adv Exp Med Biol. 1998;451:365–374. doi: 10.1007/978-1-4615-5357-1_57. [DOI] [PubMed] [Google Scholar]
- 167.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miller CR, Buchsbaum DJ, Reynolds PN, Douglas JT, Gillespie GY, Mayo MS, Raben D, Curiel DT. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998;58:5738–5748. [PubMed] [Google Scholar]
- 170.Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 171.Goldman CK, Rogers BE, Douglas JT, Sosnowski BA, Ying W, Siegal GP, Baird A, Campain JA, Curiel DT. Targeted gene delivery to Kaposi's sarcoma cells via the fibroblast growth factor receptor. Cancer Res. 1997;57:1447–1451. [PubMed] [Google Scholar]
- 172.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 173.Rothmann T, Hengstermann A, Whitaker NJ, Scheffner M, zur Hansen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brenner M. Structure and transcriptional regulation of the GFAP gene. Brain Pathol. 1994;4:245–257. doi: 10.1111/j.1750-3639.1994.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 175.McKie EA, Graham DI, Brown SM. Selective astrocytic transgene expression in vitro and in vivo from the GFAP promoter in a HSV RL1 null mutant vector—potential glioblastoma targeting. Gene Ther. 1998;5:440–450. doi: 10.1038/sj.gt.3300621. [DOI] [PubMed] [Google Scholar]
- 176.Chung RY, Saeki Y, Chiocca EA. B-myb promoter re-targeting of herpes simplex virus' γ34.5-mediated virulence towards tumor and cycling cells. J Virol. 1999 doi: 10.1128/jvi.73.9.7556-7564.1999. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Adams PD, Kaelin WGJ. Transcriptional control by E2F. Semin Cancer Biol. 1995;6:99–108. doi: 10.1006/scbi.1995.0013. [DOI] [PubMed] [Google Scholar]
- 178.Sellers WR, Kaelin WGJ. Role of the retinoblastoma protein in the pathogenesis of human cancer. J Clin Oncol. 1997;15:3301–3312. doi: 10.1200/JCO.1997.15.11.3301. [DOI] [PubMed] [Google Scholar]
- 179.Parr MJ, Manome Y, Tanaka T, Wen P, Kufe DW, Kaelin WGJ, Fine HA. Tumor-selective transgene expression in vivo mediated by an E2F-responsive adenoviral vector. Nat Med. 1997;3:1145–1149. doi: 10.1038/nm1097-1145. [DOI] [PubMed] [Google Scholar]
- 180.Dachs GU, Patterson AV, Firth JD, Ratcliffe PJ, Townsend KM, Stratford IJ, Harris AL. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–520. doi: 10.1038/nm0597-515. [DOI] [PubMed] [Google Scholar]
- 181.Hallahan DE, Mauceri HJ, Seung LP, Dunphy EJ, Wayne JD, Hanna NN, Toledano A, Hellman S, Kufe DW, Weichselbaum RR. Spatial and temporal control of gene therapy using ionizing radiation. Nat Med. 1995;1:786–791. doi: 10.1038/nm0895-786. [DOI] [PubMed] [Google Scholar]
- 182.Sadowski I, Ma J, Triezenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 183.Axelrod JD, Reagan MS, Majors J. GAL4 disrupts a repressing nucleosome during activation of GAL1 transcription in vivo. Genes Dev. 1993;7:857–869. doi: 10.1101/gad.7.5.857. [DOI] [PubMed] [Google Scholar]
- 184.Fink DJ, Poliani PL, Oligino T, Krisky DM, Goins WF, Glorioso JC. Development of an HSV-based vector for the treatment of Parkinson's disease. Exp Neurol. 1997;144:103–121. doi: 10.1006/exnr.1996.6395. [DOI] [PubMed] [Google Scholar]
- 185.Oligino T, Poliani PL, Marconi P, Bender MA, Schmidt MC, Fink DJ, Glorioso JC. In vivo transgene activation from an HSV-based gene therapy vector by GAL4:vp16. Gene Ther. 1996;3:892–899. [PubMed] [Google Scholar]
- 186.Vegeto E, Allan GF, Schrader WT, Tsai MJ, McDonnell DP, O'Malley BW. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- 187.Wang Y, O'Malley BWJ, Tsai SY, O'Malley BW. A regulatory system for use in gene transfer. Proc Natl Acad Sci USA. 1994;91:8180–8184. doi: 10.1073/pnas.91.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oligino T, Poliani PL, Wang Y, Tsai SY, O'Malley BW, Fink DJ, Glorioso JC. Drug inducible transgene expression in brain using a herpes simplex virus vector. Gene Ther. 1998;5:491–496. doi: 10.1038/sj.gt.3300612. [DOI] [PubMed] [Google Scholar]
- 189.Baron U, Gossen M, Bujard H. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res. 1997;25:2723–2729. doi: 10.1093/nar/25.14.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Freundlieb S, Baron U, Bonin AL, Gossen M, Bujard H. Use of tetracycline-controlled gene expression systems to study mammalian cell cycle. Methods Enzymol. 1997;283:159–173. doi: 10.1016/s0076-6879(97)83014-5. [DOI] [PubMed] [Google Scholar]
- 191.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 193.Ho DY, McLaughlin JR, Sapolsky RM. Inducible gene expression from defective herpes simplex virus vectors using the tetracycline-responsive promoter system. Brain Res Mol Brain Res. 1996;41:200–209. doi: 10.1016/0169-328x(96)00097-6. [DOI] [PubMed] [Google Scholar]
- 194.Fotaki ME, Pink JR, Mous J. Tetracycline-responsive gene expression in mouse brain after amplicon-mediated gene transfer. Gene Ther. 1997;4:901–908. doi: 10.1038/sj.gt.3300487. [DOI] [PubMed] [Google Scholar]
- 195.Herrlinger U, Pechan P, Jacobs A, Woiciechowski Ch, Rainov N, Fraefel C, Paulus W, Breakefield XO. HSV-1 infected-cell proteins influence the expression of a tetracycline-regulated transgene. Hum Gene Ther. 1999 doi: 10.1002/1521-2254(200009/10)2:5<379::AID-JGM126>3.0.CO;2-G. submitted. [DOI] [PubMed] [Google Scholar]
- 196.Barnett FH, Rainov NG, Ikeda K, Schuback DE, Elliott P, Kramm CM, Chase M, Qureshi NH, Harsh G, Chiocca EA, Breakefield XO. Selective delivery of herpes virus vectors to experimental brain tumors using RMP-7. Cancer Gene Ther. 1999;6:14–20. doi: 10.1038/sj.cgt.7700003. [DOI] [PubMed] [Google Scholar]
- 197.Muldoon LL, Nilaver G, Kroll RA, Pagel MA, Breakefield XO, Chiocca EA, Davidson BL, Weissleder R, Neuwelt EA. Comparison of intracerebral inoculation and osmotic blood-brain barrier disruption for delivery of adenovirus, herpesvirus, and iron oxide particles to normal rat brain. Am J Pathol. 1995;147:1840–1851. [PMC free article] [PubMed] [Google Scholar]
- 198.Nilaver G, Muldoon LL, Kroll RA, Pagel MA, Breakefield XO, Davidson BL, Neuwelt EA. Delivery of herpesvirus and adenovirus to nude rat intracerebral tumors after osmotic blood-brain barrier disruption. Proc Natl Acad Sci USA. 1995;92:9829–9833. doi: 10.1073/pnas.92.21.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rainov NG, Zimmer C, Chase M, Kramm CM, Chiocca EA, Weissleder R, Breakefield XO. Selective uptake of viral and monocrystalline particles delivered intra-arterially to experimental brain neoplasms. Hum Gene Ther. 1995;6:1543–1552. doi: 10.1089/hum.1995.6.12-1543. [DOI] [PubMed] [Google Scholar]
- 200.Rainov NG, Dobberstein KU, Heidecke V, Dorant U, Chase M, Kramm CM, Chiocca EA, Breakefield XO. Long-term survival in a rodent brain tumor model by bradykinin-enhanced intra-arterial delivery of a therapeutic herpes simplex virus vector. Cancer Gene Ther. 1998;5:158–162. [PubMed] [Google Scholar]
- 201.Aboody K, Rainov NG, Small JE, Liu S, Bower KA, Herrlinger U, Black P, Breakefield XO, Snyder EY. Neural stem cells migrate throughout and express foreign genes within experimental gliomas—a potential gene therapy approach to brain tumors. Nat Med. 1999 submitted. [Google Scholar]
- 202.Herrlinger U, Woiciechowski Ch, Aboody-Guterman K, Jacobs A, Rainov N, Snyder EY, Breakefield XO. C17-2 neural progenitor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Gene Ther. 1999 doi: 10.1006/mthe.2000.0046. submitted. [DOI] [PubMed] [Google Scholar]