Abstract
The design of effective gene therapy strategies for brain tumors and other neurological disorders relies on the understanding of genetic and pathophysiological alterations associated with the disease, on the biological characteristics of the target tissue, and on the development of safe vectors and expression systems to achieve efficient, targeted and regulated, therapeutic gene expression. The herpes simplex virus type 1 (HSV-1) virion is one of the most efficient of all current gene transfer vehicles with regard to nuclear gene delivery in central nervous system-derived cells including brain tumors. HSV-1-related research over the past decades has provided excellent insight into the structure and function of this virus, which, in turn, facilitated the design of innovative vector systems. Here, we review aspects of HSV-1 structure, replication and pathogenesis, which are relevant for the engineering of HSV-1-based vectors.
Keywords: herpes simplex virus, gene therapy, recombinant HSV-1, amplicon
Introduction
In recent years, many neurological diseases have been characterized on a molecular level. The knowledge of the underlying genetic defect and the understanding of related pathophysiological alterations are the first steps toward the development of new treatment strategies based on gene therapy. This form of therapy can be defined as the introduction of exogenous DNA sequences into cells of a target tissue using recombinant DNA and vector technology. The design of effective gene therapy strategies relies on interdisciplinary attempts to: i) define the genetic and pathophysiological alterations associated with the disease; ii) understand the biological characteristics of the target tissue; and iii) develop safe vector and expression systems to achieve efficient, targeted and regulated gene expression. At present, many schemes for gene therapy of both hereditary and acquired diseases have been envisioned, but the logistics of bringing them to humans still needs much basic research [1]. Issues such as efficiency of gene delivery, vector toxicity, stability of transgene and transduced cell, choice of promoter, as well as dose, time and route of vector application must all be worked out individually for different applications.
In the field of neurology, research in gene therapy and vector technology concentrates on two basic aims, one of which is to achieve stable and non-toxic transduction of neurons and muscle cells for the treatment of neurode-generative and muscle dystrophic disease, for the alteration of neuronal physiology and conditions with chronic pain, for the control of dystonic movements and stimulation of nerve re-growth. The second aim is the selective and locally toxic transduction of brain tumor cells [2]. For these purposes, a number of different vector systems have been developed, including synthetic vectors, such as molecular conjugates and liposomes, and viral vectors with wide tropism, such as adenovirus (AdV), adeno-associated virus (AAV), retrovirus (RV) and herpes simplex virus type 1 (HSV-1). Synthetic vectors have low toxicity/immunogenicity but poor delivery efficiency, whereas virus vectors can exert some cytotoxicity/immunogenicity but are highly efficient vehicles.
The HSV-1 virion is one of the most efficient of all current gene transfer vehicles with regard to nuclear gene delivery in central nervous system-derived cells, including neurons, neural progenitor cells and gliomas. Many properties of HSV-1 are especially suitable for using this virus as a vector to treat diseases that affect the central nervous system (CNS), such as Parkinson's disease or malignant gliomas. These properties include: i) a high transduction efficiency; ii) a large genome (∼152 kb) and a large transgene capacity; iii) the ability of entering a state of latency in neurons; iv) the ability of some mutants to replicate specifically in dividing cells after deleting certain genes required for virus replication in non-dividing cells and, thereby, mediating selective oncolysis of gliomas (“virus therapy”). HSV-1-related research over the past decades has provided excellent insight into the structure and function of the virus, which, in turn, facilitated the design of innovative vector systems. However, to bring HSV-1 vector-mediated, targeted and regulatable gene transfer into clinical applications, more technical and logistical issues still need to be addressed.
Here, we review: i) the structure, replication and pathogenesis of HSV-1; and ii) HSV-1-based vector systems with their possible applications in experimental and clinical gene therapy protocols for neurological diseases and brain tumors (Part II).
Virus Structure
Genome
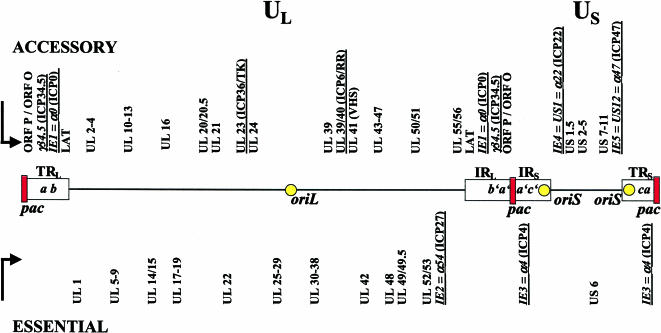
HSV-1 is a member of the Herpesviridae, a family of DNA viruses carrying a large, centrally located, linear, double-stranded DNA genome of ∼152 kb, which encodes ∼80 virus genes [3]. Approximately half of these genes are necessary for virus replication in cell culture. The other half encode accessory functions, which contribute to the virus life cycle in specific tissues or cell types (e.g., postmitotic neurons) of the host (Figure 1) [3–5]. The HSV-1 genome is composed of unique long (UL) and unique short (US) segments, which are both flanked by inverted repeats (R). The repeats of the L component are designated ab and b′a′; those of the S segment are a′c′ and ca. The HSV-1 genes fall into three categories depending on the kinetics of their transcription, which is tightly regulated in a cascade of three temporal phases: i) immediate early (IE or α); ii) early (E or β); and iii) late (L or γ) [6].
Figure 1.
HSV-1 genome structure. The virus genome is a linear, double-stranded DNA of ∼152 kb which encodes more than 80 genes. The genome is composed of unique long (UL) and unique short (US) segments, which are flanked by inverted repeats (R). IRL, internal repeat of the long segment; TRL, terminal repeat of the long segment; IRS, internal repeat of the short segment; TRS, terminal repeat of the short segment. The repeats of the L component are designated ab and b′a′; those of the S segment are a′c′ and ca. Pac signals are contained in the a sequences located at the junction between the long and short segments and at both termini. The HSV-1 genome contains two different origins of DNA replication, oriS and oriL. OriS is duplicated because it is located within the inverted repeats flanking US between the promoters of the IE3 and IE4/5 genes. OriL is located within UL and is flanked by transcriptional start sites of two E genes, which encode the single-stranded DNA binding protein, ICP8 (UL29), and the DNA polymerase (UL30). Approximately half of the genes are essential for virus replication in cell culture. The other half encode accessory functions, which contribute to the virus life cycle in specific tissues or cell types, e.g., postmitotic neurons. However, it can be assumed that the genes known to be dispensable for growth in cultured cells may be important for both optimal lytic replication and replication in vivo, contributing to pathogenesis, host range, latency, or spread in neurons. Genes underlined mark IE genes or genes which are relevant in certain recombinant HSV-1 mutants (see Part II).
Immediate early genes The immediate early (IE) genes, which encode the infected cell proteins (ICP) 0 (IE1=α0), ICP27 (IE2=UL54), ICP4 (IE3=α4), ICP22 (IE4=US1), and ICP47 (IE5=US12), map near the termini of UL and US or within the repeats. IE gene products have mostly regulatory functions and initiate expression of the early viral genes. The two open reading frames (ORF) P and ORF O are pre IE genes, map within the repeats, are antisense to the γ34.5 gene, and are expressed under conditions in which ICP4 is not functional.
Early genes The early (E) and late (L) genes are distributed throughout both unique sequences, UL and US, with only one exception, γ34.5, which is located in the repeats. Most E gene products are enzymes required for DNA metabolism and signal the onset of viral DNA replication. Seven E genes mapping in the L component (open reading frames UL5, 8, 9, 29, 30, 42 and 52) are required for synthesis of viral DNA starting at the origins of DNA replication (oriL and oriS). Other early gene products involved in nucleic acid metabolism include uracil DNA glycosylase (UL2), alkaline exonuclease (DNAse; UL12), thymidine kinase (TK; UL23), ribonucleotide reductase (RR; UL39–40), deoxyuridine triphosphate nucleotidohydrolase (dUTPase; UL50), and protein kinase (US3). A unique characteristic of TK is that it phosphorylates purine pentosides and a wide variety of nucleoside analogs that are not phosphorylated by cellular kinases [7,8]. This substrate specificity of TK is the basis for: i) the effectiveness of various nucleoside analogs in the treatment of HSV-1 infection [9]; ii) TK-mediated pro-drug activation as gene therapy of tumors [10]; and iii) the potential use of radiolabeled nucleoside analogs as “marker substrates” for the non-invasive assessment of TK expression by radionuclide imaging techniques (e.g., positron emission tomography [11–16]). Ribonucleotide reductase reduces ribonucleotides to deoxyribonucleotides, thereby creating a pool of substrates for DNA synthesis [17]. The uracil DNA glycosylase and the alkaline exonuclease encoded by HSV-1 are presumably involved in DNA repair and proofreading [18,19]. The dUTPase hydrolyses dUTP to dUMP, which prevents incorporation of dUTP into DNA and provides a pool of dUMP for conversion to dTMP by thymidylate synthetase.
Late genes The late (L) genes encode mainly structural components of the virion [20,21]. Eleven virion proteins (VPs) can be found on the surface of the virion, including glycoproteins (g) L (UL1), gM (UL10), gH (UL22), gB (UL27), gC (UL44), gK (UL53), gG (US4), gJ (US5), gD (US6), gI (US7), and gE (US8) [22]. These proteins are glycosylated and play important roles in virus attachment to target cells (gB, gC) [23], cell entry (gB, gD, gG, gH) [24], egress (gG, gH, gK) [25], cell-to-cell spread (gD, gE, gG, gH, gI) [26], and, from the host's point of view, in the induction of neutralizing antibodies (gD, gG, gH/gL) [27,28]. Moreover, gC and gE/gI mediate immune evasion in vivo: gC binds complement component C3, thereby inhibiting activation of the complement cascade [29–34]. Glycoproteins E and I form a complex to constitute a high affinity Fc receptor (FCγR) that binds the Fc domain of human anti-HSV IgG by a process called antibody bipolar bridging and inhibits Fc-mediated immune functions [35–41].
Three or more VPs are intrinsic envelope proteins encoded by UL20, UL24, and UL34. Seven VPs are found in the capsid in varying numbers, depending on the stage of capsid assembly: VP5 (UL19), VP21+VP22a+VP24 (UL26), VP26 (UL35), VP19C+VP23 (UL38), and VP22 [20,42–44]. Capsid proteins are not only of structural but also of functional importance during encapsidation of viral DNA, e.g., VP22a functions as a scaffolding protein for DNA packaging into capsids [45], and VP19C is thought to be involved in anchoring viral DNA in the capsid [46]. The major capsid protein, VP5, is the structural subunit of both the hexons and pentons comprising the capsomers [47]. The two minor capsid proteins, VP19C and VP23, make up trigonal nodules called triplexes (heterotrimers containing one copy of VP19C and two copies of VP23) found between adjacent capsomers [47,48]. The third minor capsid protein, VP26, is located at the outer tips of the hexons [49,50].
All other VPs are found in the tegument, the space between capsid and envelope. The so-called tegument proteins VP1–2 (UL36), virion host shut off (VHS) protein (UL41), VP11–12 (UL46), VP13–14 (UL47), VP16 (α-TIF; UL48), and the product of the US11 gene play important roles in: i) the transport of viral capsids to the cell nucleus by aid of microtubules; ii) the release of virion DNA from the capsid into the nucleus (VP1–2); iii) the nonspecific degradation of mRNA and shut-off of macromolecular synthesis (VHS); and iv) the induction of IE genes (VP16) as the primary step of viral protein synthesis and replication [51,52]. The activity of VP16 is modulated by other tegument proteins (VP11–14).
The HSV-1 genome contains two different origins of DNA replication, oriL and oriS, neither of which is uniquely required for viral DNA replication [53,54]. OriS is duplicated because it is located within the inverted repeats flanking US, between the promoters of the IE3 and IE4/5 genes (Figure 1, [55]). OriL is located within UL and is flanked by transcriptional start sites of two E genes, which encode the single-stranded DNA-binding protein, ICP8 (UL29), and the DNA polymerase (UL30) [56]. OriL enables bidirectional DNA synthesis, whereas oriS initiates unidirectional DNA synthesis. The core component of oriS includes three binding sites for the viral origin binding proteins (OBP and OBPC) which partially overlap the binding site of a cellular factor, OF-1 [57–60]. The sequences between the oriS core and the IE3 and IE4/5 promoters contain numerous binding sites for transcription factors that play critical roles in the expression of IE3 and IE4/5 genes [61–63]. The interaction of cellular and viral transcription factors with these auxiliary regions can directly influence the efficiency of origin-dependent DNA replication, as well as transcription of genes flanking the origin [60].
The HSV-1 DNA cleavage/packaging signal (pac) is another essential cis-acting element which is required for the cleavage of the concatemeric products of HSV-1 genome replication into unit-length genomes following their packaging into capsids [64]. Pac signals are contained in the a sequences located at the junction between the long and short segment and at both termini (Figure 1).
The HSV-1 Virion
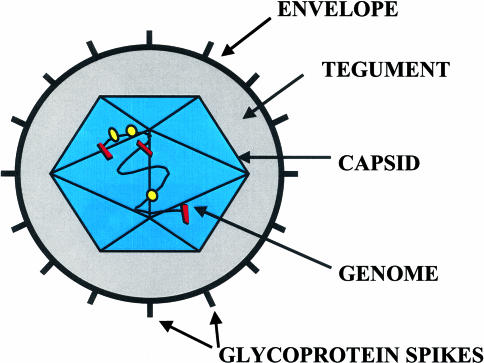
The architecture of the HSV-1 particle is depicted in Figures 2 and 3. The virion (diameter: 120 to 300 nm) consists of: i) the envelope; ii) the tegument; iii) the capsid; and iv) a core containing the virus genome. The double-stranded DNA genome is organized as regularly spaced (∼26 Å) concentric layers inside the capsid [65]. The capsid (diameter: ∼100 nm) [44] consists of 162 capsomers (150 hexons and 12 pentons) and is surrounded by the tightly adhering tegument. The various tegument proteins include the host shut-off protein, VHS, and the alpha-trans-inducing factor (α-TIF), VP16. The bulk of the tegument is not icosahedrally ordered. However, a small portion appears as filamentous structures around the pentons, interacting extensively with the capsid. Their locations and interactions with cellular transport proteins suggest multiple roles in guiding DNA transport into the nucleus [65]. The envelope consists of a lipid membrane, containing glycoprotein spikes on the surface [22], which vary in number and relative amounts, and also including several non-glycosylated viral proteins, lipids, and polyamines.
Figure 2.
Structure of the HSV-1 virion. The HSV-1 virion has a diameter of ∼120–300 nm and consists of an envelope, the tegument, the capsid, and a core containing the virus genome. The capsid has a diameter of ∼100 nm and is surrounded by tightly adhering tegument proteins. The envelope consists of a lipid membrane, containing glycoprotein spikes on the surface, which vary in number and relative amounts.
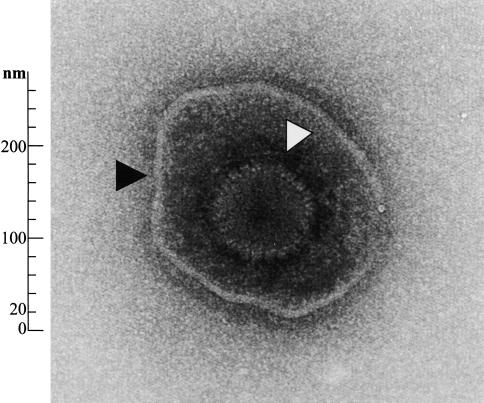
Figure 3.
Electronmicrograph of an HSV-1 virion. Envelope (▲) and capsid (△) are clearly delineated (a kind gift from Drs. Elisabeth Schraner and Peter Wild, University of Zürich).
HSV-1 Life Cycle
Lytic HSV-1 Infection
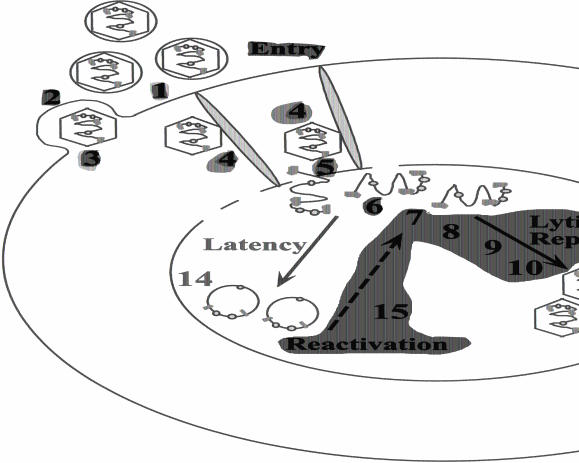
The steps of the productive HSV-1 infection include: i) attachment to heparin and related glycosaminoglycans with subsequent binding to specific cell-surface receptors; ii) fusion of the virion envelope with the plasma membrane; iii) transport of the capsid to the nuclear pores with release of the virion DNA into the nucleus; iv) transcription of IE and E genes; v) viral DNA synthesis; vi) transcription of L genes; vii) capsid assembly; viii) DNA packaging; ix) capsid envelopment; and x) virion egress. The individual steps in this cascade are tightly regulated [3], as depicted in Figure 4.
Figure 4.
Lytic and latent HSV-1 infection. The lytic HSV-1 life cycle takes ∼18 hours and the steps include: 1) attachment to heparan sulfate and cell-surface receptors; 2) fusion of the virion envelope with the plasma membrane; 3) release of the capsid into the cytoplasm and 4) active transport along microtubules to the nuclear pores with 5) release of the virion DNA into the nucleus; 6) α-TIF mediated induction of transcription of IE genes; 7) transcription of IE and E genes; 8) viral DNA synthesis; 9) transcription of L genes; 10) capsid assembly; 11) DNA packaging into preformed capsids; 12) capsid envelopment; and 13) virion egress. In sensory neurons, HSV-1 may enter a state of latency 14), which is characterized by the persistence of the HSV-1 genome as a concatemeric or circular molecule bound by nucleosomes that does not express viral genes other than the latency associated transcripts. Host cell, viral and external factors play a role in establishment and reactivation 15) of HSV-1 from latency.
As a first step to establish an infection, low-affinity attachment to the cell surface is mediated by an interaction between envelope glycoproteins C and B and, mainly, cell-surface heparan sulfate proteoglycan, but also dermatan sulfate [23,66–70]. Fusion of the virus envelope with the cell membrane requires at least four viral glycoproteins, gD, gB, and the gH/gL complex [24,71–75]. Two specific cell surface receptors which interact with gD have been identified and designated herpes virus entry mediator (Hve) A and HveC. HveA is a member of the tumor necrosis factor-nerve growth factor receptor superfamily [76–78], and HveC is the poliovirus receptor-related protein 1 [79–82]. HveA activates transcription factors κB, jun N-terminal kinase, and AP-1, indicating its involvement with signal transduction pathways that activate the immune response [83]. HveA mediates both entry of free virus and entry by cell-to-cell spread [77,84–86]. Moreover, it mediates HSV-1-induced cell fusion [87]. After interaction of viral gD with HveA, gB and the gH/gL complex act individually or in combination to trigger pH-independent fusion of the viral envelope with the host cell membrane [88–92].
The capsid is actively transported along the host cell microtubular cytoskeleton to the nuclear pores [93], where tegument proteins facilitate the release of the virus genome into the nucleus [94] (Figure 4). In the nucleus, the HSV-1 genome is circularized [95], and the virus genes are transcribed in a tightly regulated cascade with three temporal phases. One of the tegument proteins, VP16 (α-TIF or Vmw65 [96]), binds in the presence of cellular transcription factors to viral DNA at the consensus sequence 5′-GyATGnTAATGArATTCyTTGnGGG-3′ [97,98] and induces transcription of IE genes [51,96,99] by host RNA polymerase II. VP16 does not bind directly to the consensus sequence on IE promoters, but forms a multiprotein complex with two cellular proteins: the POU domain protein, Oct-1, and a host cell factor (HCF; also called C1, VCAF, or CFF) [63,100–106]. First, VP16 forms a complex with HCF. This association promotes interaction of the complex with Oct-1, which is bound to the TAATGARAT motif. The TAATGARAT motif in IE promoters can confer both positive and negative responses to cellular octamer-binding proteins, and the latter results in the absence of IE gene expression later in infection and during latency [107]. It should be noted that IE promoters also contain consensus sequences for other transcription factors. Like many other viral proteins, VP16 has more than one function. It acts both as a transactivator, which augments the basal expression of IE genes, and as an essential structural protein of the virion. Not only VP16, but also proteins involved in cell-cycle control, such as cyclin-dependent kinases (cdk), are important for transcriptional regulation of IE and E gene expression [108,109].
Immediate early gene products HSV-1 expresses five IE or α-genes, IE1, 2, 3, 4, 5 (peak rates: 2 to 4 hours post infection, p.i. [6]), which encode the infected cell proteins (ICP) 0, 27, 4, 22, and 47, respectively. Four of these proteins, ICP 0, 27, 4 and 22, regulate the productive cycle of the virus infection by initiating transcription of the E genes (peak rates: 5 to 7 hours, p.i.) [6]. ICP47 blocks the presentation of antigenic peptides on the infected cell surface [110–112].
ICP4 and ICP27 are absolutely essential for initiating and controlling the expression of early and also late genes through both transcriptional and posttranscriptional mechanisms [113–119]. ICP4, in concert with basal transcription factors, acts both as transactivator at low-affinity sites and as repressor at high-affinity sites at the transcription initiation signals of its own promoter and those of several other genes [114,120–124]. ICP4 has also been shown to have anti-apoptotic functions [125,126]. ICP27 acts predominantly at the posttranslational level by regulating the processing of viral and cellular mRNAs, thereby contributing to elevated levels of E gene products [127–132]. It also contributes to efficient L gene expression by acting as a transporter for late viral intronless mRNAs from the nucleus into the cytoplasm [133–135]. The efficiency of nuclear import of ICP27 is modulated by different cellular kinases, such as protein kinase A and C and casein kinase II [136].
ICP22, which shares the C-terminus with the US1.5 gene product [137], promotes efficient L gene expression and regulates the stability and splicing pattern of the IE1 mRNA [99,138,139]. It regulates viral gene transcription through modification (phosphorylation) of cellular RNA polymerase II [140]. Furthermore, ICP22 interacts with, and may be stabilized by cell cycle-dependent proteins, such as p78 and p60 [141,142]. The importance of ICP22 for L gene expression became apparent from studies showing that concurrent with the onset of viral DNA synthesis, ICP22 and ICP4 aggregate in nuclear structures with nascent viral DNA, RNA polymerase II, and other proteins, and that this aggregation is essential for late gene expression [143].
ICP0 is a potent transactivator of viral and cellular promoters and is required for efficient viral gene expression and virus replication [144–147]. ICP0 affects different aspects of the host cell metabolism, including cell cycle, proteolytic machinery, transcription and translation [148–151]. ICP0 i) stabilizes cell cycle regulatory proteins (e.g., cyclin D3) to maintain protein synthesis for virus replication [148]; ii) interferes with biochemical mechanisms relevant to both centromeres and ND10 nuclear structures [152,153]; iii) causes active degradation of the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) [154,155]; and iv) binds to a ubiquitin-specific protease, named HAUSP, which contributes to the role of ICP0 in activation of gene expression and stimulation of virus replication [156–158].
ICP47 binds to the peptide transporter, TAP. This interaction prevents the translocation of peptides into the endoplasmic reticulum (ER) and results in the downregulation of HLA classI/peptide complexes on the surface of infected cells [110–112]. Thus, by expressing ICP47 early in infection, HSV-1 evades detection by CD8+ cytotoxic T lymphocytes (CTL) [159] and prevents CTL-induced apoptosis as mechanisms of immune evasion [160] . In general, viruses have evolved mechanisms to block apoptosis in situations in which endogenously or exogenously induced apoptosis threatens the capacity of the cell to produce the required number and quality of infectious virus progeny [161,162].
Early gene products The appearance of the β-polypeptides such as ICP6 (UL39; large subunit of viral ribonucleotide reductase, RR), ICP8 (UL29; DNA binding protein), ICP36 (UL23; thymidine kinase, TK) and DNA polymerase (UL30) signals the onset of viral DNA synthesis. At this stage, cellular chromatin becomes degraded. DNA synthesis starts as early as 3 hours p.i. and continues for at least 12 hours. The bulk of the viral DNA is made relatively late in infection [54]. At least seven viral gene products are essential for viral DNA synthesis: i) the products of the UL5, UL8, and UL52 genes form a heterotrimeric helicase/primase complex [163]; ii) the products of the UL30 and UL42 genes form a heterodimer with processive DNA polymerase activity [164]; iii) the product of the UL29 gene is a single-stranded DNA binding protein [165]; iv) and the product of the UL9 gene is an origin binding protein which possesses limited helicase activity [166]. Specifically, the UL9 gene product interacts with DNA polymerase accessory proteins to provide a means for the ordered assembly of HSV-1 DNA replication proteins at origins of DNA replication, thereby forming a functional “replisome” for the initiation of viral DNA synthesis [53,167]. DNA replication takes place by a rolling-circle, or similar, mechanism [53,168,169], yielding long, head-to-tail linked concatemers of unit-length genomes. Recombination events play an important role in the generation of replication intermediates [170]. The newly synthesized DNA is not composed merely of linear concatemers, but also contains Y- and X-shaped branches [171].
Late gene products In parallel, a third round of transcription results in the production of the γ-proteins which are important for encapsidation of viral DNA and envelopment. The concatemeric products of HSV-1 genome replication are cleaved into unit-length genomes at the DNA cleavage/packaging signals (pac) after filling the preformed capsids [172–178]. DNA packaging requires products of the UL6, UL12, UL15, UL18, UL19, UL25, UL28, UL32, UL33 and UL36 genes [179–192]. In cells infected with viral mutants lacking functional UL6, UL15, UL28, UL32, or UL33 genes, unit-length genomes are not cleaved from concatemeric viral DNA [179,182,187,188,191,192]. The UL17 gene, which is located within the intron of the UL15 gene, has been demonstrated to encode a tegument protein and was the first tegument-associated protein shown to be required for cleavage and packaging of viral DNA [188]. The UL25 gene product is not required for DNA cleavage, but is needed for stable retention of DNA in capsids [186]. At 6 to 8 hours p.i., the major capsid protein, VP5, and at least some UL32 gene products, co-localize in the DNA replication compartment [193–195]. The UL32 gene product has been shown to guide pre-assembled capsids to the sites of DNA packaging [185]. At these early times, cleavage and packaging seem to occur within replication compartments. Later in infection, VP5 and some tegument proteins accumulate in intranuclear regions separate from the DNA replication compartments, the so-called assemblons [196]. During capsid formation, VP5 and the scaffolding protein, pre-VP22a, condense [197,198] and interact with preformed VP19C-VP232 heterotrimers to form procapsids [48,173,176] which mature to capsids.
Mature capsids bud through the nuclear membrane in areas where tegument proteins and glycoproteins have accumulated, thereby acquiring an envelope (Figure 4). The mode of virus egress is not entirely clear. Several models have been proposed, most of which suggest that capsids acquire the envelope at the inner nuclear membrane. Some models suggest that enveloped virions are transported from the nuclear membrane via the ER and Golgi apparatus to the surface without exchanging the envelope [199,200]. However, other models claim that capsids lose their initial envelope by fusion with the outer nuclear envelope (de-envelopment) and acquire a new envelope by budding into the Golgi apparatus (re-envelopment) [201–203]. The ability of HSV-1 gD to interact with mannose-6-phosphate receptors (MPRs) suggests that the intracellular traffic of gD-containing virions might be influenced by the ability of MPRs to direct proteins to endosomes. A vectorial transport of virions to the endosomal network, however, might direct egress to defined domains of the cell surface or promote re-envelopment of capsids within an endosomal compartment [204]. Whatever the mechanism of herpesvirus egress is, mature virions seem to be transported via the ER, the Golgi and trans-Golgi apparatus as well as endosomes into the extracellular space [204,205]. During transit through the Golgi apparatus, envelope glycoproteins are modified by glycosidases and mannosidases [206]. It should be noted, that the HSV-1 gE/gI complex not only constitutes a high affinity Fc receptor responsible for immune evasion, but also facilitates cell-to-cell spread within the mucosal site of primary infection and infection of sensory nerve terminals by interacting with components of cell junctions, such as β-catenin [26,207,208]. By spreading rapidly from cell-to-cell through a space that is isolated by tight junctions, HSV-1 races against the mounting immune response. This form of direct cell-to-cell spread is the primary mode of virus transmission and an important parameter of HSV-1 pathogenesis.
The entire life cycle of HSV-1 takes ∼18 to 20 hours, during which the infected cell undergoes major structural and biochemical alterations, ultimately resulting in its destruction. Recombinant HSV-1, which encodes capsid proteins such as VP26 (UL35) that are fused with the GFP reporter protein, allows monitoring of capsid assembly and virion formation in living cells over time [209]. An interesting, and potentially important finding is, that expression of HSV-1 glycoprotein gD can prevent re-infection of cells, particularly if the virus has been produced from these cells [210].
Neurovirulence
Any alteration that impairs virus replication reduces virulence and, therefore, all essential genes may be considered “neurovirulence” genes. However, the existence of a specific neurovirulence locus in the long repeat region of the HSV-1 genome is well-documented. This region contains the γ34.5 or RL1 gene which encodes a protein of 263-amino acids, designated ICP34.5 [211,212] (Figure 1). The C-terminal 70-amino acids are highly homologous to the mammalian growth arrest and DNA damage genes, GADD34 [213–215], and encode two functions. One of these functions enables the replication and spread of the virus in the CNS (“neurovirulence”), especially the maturation and egress from non-dividing cells [216–222]. Null-mutants fail to replicate productively and, hence, do not destroy neurons or cause encephalitis [218,220]. However, they are still capable of establishing and reactivating from latency [223], and retain their wild-type (wt) phenotype in permissive cells growing in culture [216,217]. The second function enables the interaction of ICP34.5 with cellular proteins, such as phosphatase 1α, which serves to dephosphorylate the α-subunit of eukaryotic translation initiation factor 2 (elF-2α). This interaction precludes the premature shut-off of protein synthesis by double-stranded RNA-activated protein kinase, PKR, and prevents apoptosis of infected cells, thus allowing continued virus replication [213–215,224–228]. Other neurovirulence factors include glycoproteins, such as gD, which mediates infection of neural cells, and enzymes involved in DNA metabolism, such as TK, RR, and dUTPase, since mutants in these enzymes cannot replicate in cells in G0 which have low levels of the complementing cellular enzymes.
Latency
The ability of HSV-1 to remain latent in sensory neurons innervating the primarily infected cells for the lifetime of the host is a unique property and is thought to mediate the perpetuation of the virus in the human population. The latent state is characterized by persistence of the virus genome as a non-integrated, concatemeric or circular molecule in the nucleus. During latency, transcription is limited to the latency-associated transcripts (LATs) and no viral proteins are synthesized [229–232]. Latently infected neurons are not rejected by the host immune response and appear to function normally. These properties make HSV-1 an interesting candidate vector for gene delivery to cells of the nervous system.
The exact mechanism of HSV-1 latency and reactivation is not known, and the following description can only serve as a model, which still has to be elucidated in detail [3]. Virus replication at the site of the primary HSV-1 infection supports entry of the virus into the nerve endings. From there, the virus capsids are transported retrogradely along microtubules to the sensory ganglia (migration rate ∼1 cm/hour [233]), where latency is established. There is a direct correlation between the amount of input virus and the number of neurons that become latently infected [234]. As mentioned above, the HSV-1 genome is transcriptionally silent during latency except for the LATs [235–241]. LAT has been suggested to act in an antisense manner to block ICP0 activity because the two genes overlap and are transcribed in opposite orientations [240]. However, the exact functions of the LATs during latency remain elusive. LAT expression is not required for establishment or maintenance of latency [242,243], but it serves to increase the number of neurons in which latency is established [244], and it is involved in the efficiency of reactivation [243,245–249].
The LAT region consists of several genetic elements [250]. Two promoter elements responsible for expression of LATs have been identified, LAP1 and LAP2 [251–255]. The LAP1 promoter, which includes a TATA-box, and USF, CRE, AP1, and POU binding domains, is primarily responsible for LAT expression during latency [256]. The combined deletion of USF, CRE, and TATA-box completely abolishes LAT transcription in the brain, identifying these elements as essential for the neuronal specificity of LAP1 during latency. In cell culture, LAP2 is primarily active following viral DNA synthesis and, hence, responsible for LAT expression during lytic infection [257]. The LAP2 promoter is located between LAP1 and the LAT intron and does not contain a TATA-box, but has homology to mammalian housekeeping gene promoters [254,255,257].
Both host cell- and viral factors seem to play a role in the establishment and maintenance of latency in that: i) neurons destined to harbor latent virus may not express Oct1 [258]; ii) ICP0 seems to play a role in establishment of latency [259] and reactivation [259,260]; iii) a NGF/FGF-inducible cellular factor may be responsible for the initiation of viral gene expression during reactivation when no ICP0 is present [261]; iv) deletion of the γ34.5 gene markedly impairs the ability of the virus to establish latency [262]; v) mutations that result in reduced efficiency of virus replication have a negative effect on both the establishment of latency and the ability to reactivate [144,146,263,264]; and vi) the number of HSV-1 genome copies within individual, latently infected neurons is regulated by viral genetic factors [265].
The earliest molecular events in neurons that trigger reactivation of HSV-1 remain unclear, but may include altered expression of cellular factors such as the induction of transcriptional activators and downregulation of repressors. However, a temporal link between virus reactivation and induction of cellular IE genes encoding c-fos, c-jun, c-myc, Oct-1, TIS7, IFN, and IRF-1 has not yet been established [266–268]. The probability of virus reactivation increases with the number of latently infected neurons in the ganglia [269]. Well-known reactivation factors, such as physical and emotional stress, peripheral tissue and axonal damage, fever, UV light, hormonal imbalance, malignancy or immune suppression, may reactivate virus replication, followed by concurrent axonal transport of the virus progeny, usually to the site of the primary infection. Repeated reactivation events do not appear to kill the neuron, and thus, the extent of virus replication must be limited.
HSV-1 Pathogenesis
HSV-1 is transmitted from infected humans to susceptible individuals during close personal contact [270,271]. HSV-1 replicates initially in surface epithelial or mucosal cells, with subsequent spreading to cells of the nervous system via infection of nerve terminals that innervate the site of primary infection. Primary HSV-1 infection is usually established before the age of 5 years after an incubation period of several days (mean: 4 days). Great variability exists in the symptoms which may be subclinical or include variable combinations of fever and malaise, sore throat, gingivo-stomatitis and lymphadenopathy. Virus replication at the site of infection causes a localized vesicular or ulcerative lesion leading to edema, usually in the oropharyngeal mucosa. However, almost any organ can be infected with this virus. HSV-1 keratoconjunctivitis is a major cause of corneal blindness, and, even with appropriate antiviral therapy, healing of corneal ulcers may take several weeks. HSV-1 skin infections (eczema herpeticum) usually occur in patients with atopic dermatitis. The lesions can be either isolated or disseminated and may trigger an erythema multiforme. Especially in the immuno-compromised host, progressive disease may cause virus dissemination with infection of the skin, the respiratory tract, the esophagus, and the gastrointestinal tract. The clinical manifestations of neonatal HSV-1 infection are summarized elsewhere [271].
From the site of primary virus replication, nucleocapsids are transported via sensory neurons to the dorsal root ganglia (e.g., trigeminal ganglion Gasseri), where the virus enters either a lytic pathway with subsequent production of progeny virus particles or a latent state [233,272,273] (Figure 4). Certain stimuli can cause reactivation from latency with local virus replication within the ganglion, concurrent axonal transport of virions to the sites of mucosal membranes or the skin and subsequent recurrent infection (e.g., herpes labialis or keratoconjunctivitis). Clinical distinction should be drawn between intraoral gingival lesions, indicative of primary infection, and lip lesions, indicative of recurrent infection. More than a third of the human population have recurrent HSV-1 infections, which may even occur in the absence of clinical symptoms. Although usually the host-virus interaction leads to latency and recurrent infections within the periphery, rarely virions are transported into the CNS, and may cause life-threatening CNS infections, most commonly as hemorrhagic encephalitis of the temporal lobe. Symptoms of HSV-1 encephalitis include fever, altered consciousness, behavioral abnormalities and localized neurologic findings and seizures. Other manifestations include radiculitis, myelitis or meningitis similar to complications mediated by varicella zoster virus [274]. Thus, key elements in HSV-1 pathogenesis are the interaction of the virus with the nervous system and the immune system.
On average, virus shedding in mouth and stool occurs for 7 to 10 days. Neutralizing antibodies appear between 4 to 7 days after clinical onset and peak ∼2 weeks later. In addition, macrophages, natural killer cells, specific sub-populations of T-cells (CD4+, CD8+) and a variety of cytokines (e.g., type 1: IL-2, IFN-γ, IL-12; type 2: IL-4, IL-5, IL-10, IL-13) take part in the complex immune response against HSV-1 infection [275,276]. Humoral immunity does not prevent exogenous reinfection or recurrences, but does limit spread of the virus in the host.
The diagnosis of HSV-1 is made by virus isolation or detection of viral DNA by PCR from vesicles, nasopharynx, conjunctivae, cerebrospinal fluid, stool and urine, and by serology of rising specific antibodies. In the event of encephalitis, magnetic resonance imaging and electroencephalography reveal the structural and functional consequences of the destructive inflammation. Herpes encephalitis is treated with acyclovir, which can be effective if administration begins early (within 48 hours) in the course of CNS infection.
Conclusion
The mounting knowledge of HSV-1 structure and physiology has facilitated the development of tools for the rapid diagnosis and effective treatment of HSV-1 infections as well as the construction of HSV-1-derived vectors. There are two principle types of HSV-1-based vector systems: recombinant HSV-1 vectors and HSV-1 amplicon vectors. The engineering and possible applications of these vectors are discussed in detail in Part II of this review.
Acknowledgements
We thank Petra Genutt and Mark Slack for critical reading of the manuscript.
Footnotes
Support to A.J. is from ZMMK-TV46 (Center of Molecular Medicine), grant 516-40000299 (Land NRW) and the Max-Planck Society, Germany; X.O.B., NCI grant CA69246, NINDS grant NS24279; C.F., Swiss National Science Foundation.
References
- 1.Young AB. Neurological Disorders: An Overwiew. In: Chiocca EA, Breakefield XO, editors. Gene Therapy for Neurological Disorders and Brain Tumors. Humana Press; 1998. pp. 341–343. [Google Scholar]
- 2.Isacson O, Breakefield XO. Benefits and risks of hosting animal cells in the human brain. Nat Med. 1997;3:964–969. doi: 10.1038/nm0997-964. [DOI] [PubMed] [Google Scholar]
- 3.Roizman B, Sears AE. Herpes Simplex Viruses and Their Replication. In: Fields BN, Knipe DM, Howley PM, et al., editors. Fields Virology. Philadelphia, PA: Lippincott; 1996. pp. 2231–2295. [Google Scholar]
- 4.McGeoch DJ, Dolan A, Donald S, Rixon FJ. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 5.McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, Perry LJ, Scott JE, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 6.Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis: I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson AT, Gentry GA, Subak-Sharpe JH. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974;24:465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- 8.Jamieson AT, Subak-Sharpe JH. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974;24:481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffer HJ, Beauchamp L, de Miranda P, Elion GB, Bauer DJ, Collins P. 9-(2-Hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978;272:583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- 10.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 11.Gambhir SS, Barrio JR, Wu L, Iyer M, Namavari M, Satyamurthy N, Bauer E, Parrish C, MacLaren DC, Borghei AR, Green LA, Sharfstein S, Berk AJ, Cherry SR, Phelps ME, Herschman HR. Imaging of adenoviral-directed herpes simplex virus type 1 thymidine kinase reporter gene expression in mice with radiolabeled ganciclovir. J Nucl Med. 1998;39:2003–2011. [PubMed] [Google Scholar]
- 12.Gambhir SS, Barrio JR, Phelps ME, Iyer M, Namavari M, Satyamurthy N, Wu L, Green LA, Bauer E, MacLaren DC, Nguyen K, Berk AJ, Cherry SR, Herschman HR. Imaging adenoviral-directed reporter gene expression in living animals with positron emission tomography. Proc Natl Acad Sci USA. 1999;96:2333–2338. doi: 10.1073/pnas.96.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs A, Tjuvajev JG, Balatoni J, Herrlinger U, Joshi R, Pechan PA, Finn R, Chiocca EA, Breakefield XO, Blasberg RG. Imaging HSV-1 vector replication and gene delivery in vivo. J Gen Med. 1999;1:15. [Google Scholar]
- 14.Saito Y, Price RW, Rottenberg DA, Fox JJ, Su TL, Watanabe KA, Philips FS. Quantitative autoradiographic mapping of herpes simplex virus encephalitis with a radiolabeled antiviral drug. Science. 1982;217:1151–1153. doi: 10.1126/science.7112121. [DOI] [PubMed] [Google Scholar]
- 15.Tjuvajev JG, Stockhammer G, Desai R, Uehara H, Watanabe K, Gansbacher B, Blasberg RG. Imaging the expression of transfected genes in vivo. Cancer Res. 1995;55:6126–6132. [PubMed] [Google Scholar]
- 16.Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, Safer M, Beattie B, DiResta G, Daghighian F, Augensen F, Koutcher J, Zweit J, Humm J, Larson SM, Finn R, Blasberg R. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. [PubMed] [Google Scholar]
- 17.Bacchetti S, Evelegh MJ, Muirhead B. Identification and separation of the two subunits of the herpes simplex virus ribonucleotide reductase. J Virol. 1986;57:1177–1181. doi: 10.1128/jvi.57.3.1177-1181.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caradonna SJ, Cheng YC. Induction of uracil-DNA glycosylase and dUTP nucleotidohydrolase activity in herpes simplex virus-infected human cells. J Biol Chem. 1981;256:9834–9837. [PubMed] [Google Scholar]
- 19.Martinez R, Sarisky RT, Weber PC, Weller SK. Herpes simplex virus type 1 alkaline nuclease is required for efficient processing of viral DNA replication intermediates. J Virol. 1996;70:2075–2085. doi: 10.1128/jvi.70.4.2075-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heine JW, Honess RW, Cassai E, Roizman B. Proteins specified by herpes simplex virus: XII. The virion polypeptides of type 1 strains. J Virol. 1974;14:640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spear PG, Roizman B. Proteins specified by herpes simplex virus: V. Purification and structural proteins of the herpesvirion. J Virol. 1972;9:143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stannard LM, Fuller AO, Spear PG. Herpes simplex virus glycoproteins associated with different morphological entities projecting from the virion envelope. J Gen Virol. 1987;68:715–725. doi: 10.1099/0022-1317-68-3-715. [DOI] [PubMed] [Google Scholar]
- 23.Herold BC, WuDunn D, Soltys N, Spear PG. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller AO, Lee WC. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson L, Johnson DC. Herpes simplex virus glycoprotein K promotes egress of virus particles. J Virol. 1995;69:5401–5413. doi: 10.1128/jvi.69.9.5401-5413.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dingwell KS, Brunetti CR, Hendricks RL, Tang Q, Tang M, Rainbow AJ, Johnson DC. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicola AV, Peng C, Lou H, Cohen GH, Eisenberg RJ. Antigenic structure of soluble herpes simplex virus (HSV) glycoprotein D correlates with inhibition of HSV infection. J Virol. 1997;71:2940–2946. doi: 10.1128/jvi.71.4.2940-2946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng T, Ponce-de-Leon M, Jiang H, Dubin G, Lubinski JM, Eisenberg RJ, Cohen GH. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J Virol. 1998;72:65–72. doi: 10.1128/jvi.72.1.65-72.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman HM, Cohen GH, Eisenberg RJ, Seidel CA, Cines DB. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 30.Friedman HM, Wang L, Fishman NO, Lambris JD, Eisenberg RJ, Cohen GH, Lubinski J. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J Virol. 1996;70:4253–4260. doi: 10.1128/jvi.70.7.4253-4260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fries LF, Friedman HM, Cohen GH, Eisenberg RJ, Hammer CH, Frank MM. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986;137:1636–1641. [PubMed] [Google Scholar]
- 32.Hidaka Y, Sakai Y, Toh Y, Mori R. Glycoprotein C of herpes simplex virus type 1 is essential for the virus to evade antibody-independent complement-mediated virus inactivation and lysis of virus-infected cells. J Gen Virol. 1991;72:915–921. doi: 10.1099/0022-1317-72-4-915. [DOI] [PubMed] [Google Scholar]
- 33.Kostavasili I, Sahu A, Friedman HM, Eisenberg RJ, Cohen GH, Lambris JD. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J Immunol. 1997;158:1763–1771. [PubMed] [Google Scholar]
- 34.Lubinski JM, Wang L, Soulika AM, Burger R, Wetsel RA, Colten H, Cohen GH, Eisenberg RJ, Lambris JD, Friedman HM. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J Virol. 1998;72:8257–8263. doi: 10.1128/jvi.72.10.8257-8263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baucke RB, Spear PG. Membrane proteins specified by herpes simplex viruses: V. Identification of an Fc-binding glycoprotein. J Virol. 1979;32:779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubin G, Frank I, Friedman HM. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J Virol. 1990;64:2725–2731. doi: 10.1128/jvi.64.6.2725-2731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubin G, Socolof E, Frank I, Friedman HM. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol. 1991;65:7046–7050. doi: 10.1128/jvi.65.12.7046-7050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank I, Friedman HM. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J Virol. 1989;63:4479–4488. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson DC, Frame MC, Ligas MW, Cross AM, Stow ND. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gl. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Vliet KE, De Graaf-Miltenburg LA, Verhoef J, Van Strijp JA. Direct evidence for antibody bipolar bridging on herpes simplex virus-infected cells. Immunology. 1992;77:109–115. [PMC free article] [PubMed] [Google Scholar]
- 41.Nagashunmugam T, Lubinski J, Wang L, Goldstein LT, Weeks BS, Sundaresan P, Kang EH, Dubin G, Friedman HM. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J Virol. 1998;72:5351–5359. doi: 10.1128/jvi.72.7.5351-5359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen GH, Ponce dL, Diggelmann H, Lawrence WC, Vernon SK, Eisenberg RJ. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980;34:521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preston VG, Coates JA, Rixon FJ. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983;45:1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrag JD, Prasad BV, Rixon FJ, Chiu W. Three-dimensional structure of the HSV-1 nucleocapsid. Cell. 1989;56:651–660. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- 45.Newcomb WW, Brown JC. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991;65:613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braun DK, Batterson W, Roizman B. Identification and genetic mapping of a herpes simplex virus capsid protein that binds DNA. J Virol. 1984;50:645–648. doi: 10.1128/jvi.50.2.645-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newcomb WW, Trus BL, Booy FP, Steven AC, Wall JS, Brown JC. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J Mol Biol. 1993;232:499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 48.Trus BL, Booy FP, Newcomb WW, Brown JC, Homa FL, Thomsen DR, Steven AC. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. J Mol Biol. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 49.Booy FP, Trus BL, Newcomb WW, Brown JC, Conway JF, Steven AC. Finding a needle in a haystack: detection of a small protein (the 12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus) Proc Natl Acad Sci USA. 1994;91:5652–5656. doi: 10.1073/pnas.91.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wingfield PT, Stahl SJ, Thomsen DR, Homa FL, Booy FP, Trus BL, Steven AC. Hexon-only binding of VP26 reflects differences between the hexon and penton conformations of VP5, the major capsid protein of herpes simplex virus. J Virol. 1997;71:8955–8961. doi: 10.1128/jvi.71.12.8955-8961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batterson W, Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983;46:371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, McKnight JL. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J Virol. 1993;67:1482–1492. doi: 10.1128/jvi.67.3.1482-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boehmer PE, Lehman IR. Herpes simplex virus DNA replication. Annu Rev Biochem. 1997;66:347–384. doi: 10.1146/annurev.biochem.66.1.347. [DOI] [PubMed] [Google Scholar]
- 54.Igarashi K, Fawl R, Roller RJ, Roizman B. Construction and properties of a recombinant herpes simplex virus 1 lacking both S-component origins of DNA synthesis. J Virol. 1993;67:2123–2132. doi: 10.1128/jvi.67.4.2123-2132.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stow ND, McMonagle EC. Characterization of the TRS/IRS origin of DNA replication of herpes simplex virus type 1. Virology. 1983;130:427–438. doi: 10.1016/0042-6822(83)90097-1. [DOI] [PubMed] [Google Scholar]
- 56.Weller SK, Spadaro A, Schaffer JE, Murray AW, Maxam AM, Schaffer PA. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985;5:930–942. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baradaran K, Hardwicke MA, Dabrowski CE, Schaffer PA. Properties of the novel herpes simplex virus type 1 origin binding protein, OBPC. J Virol. 1996;70:5673–5679. doi: 10.1128/jvi.70.8.5673-5679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dabrowski CE, Carmillo PJ, Schaffer PA. Cellular protein interactions with herpes simplex virus type 1 oriS. Mol Cell Biol. 1994;14:2545–2555. doi: 10.1128/mcb.14.4.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elias P, Gustafsson CM, Hammarsten O. The origin binding protein of herpes simplex virus 1 binds cooperatively to the viral origin of replication oriS. J Biol Chem. 1990;265:17167–17173. [PubMed] [Google Scholar]
- 60.Wong SW, Schaffer PA. Elements in the transcriptional regulatory region flanking herpes simplex virus type 1 oriS stimulate origin function. J Virol. 1991;65:2601–2611. doi: 10.1128/jvi.65.5.2601-2611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones KA, Tjian R. Sp1 binds to promoter sequences and activates herpes simplex virus “immediate-early” gene transcription in vitro. Nature. 1985;317:179–182. doi: 10.1038/317179a0. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen-Huynh AT, Schaffer PA. Cellular transcription factors enhance herpes simplex virus type 1 oriS-dependent DNA replication. J Virol. 1998;72:3635–3645. doi: 10.1128/jvi.72.5.3635-3645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preston CM, Frame MC, Campbell ME. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988;52:425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 64.Deiss LP, Frenkel N. Herpes simplex virus amplicon: cleavage of concatemeric DNA is linked to packaging and involves amplification of the terminally reiterated a sequence. J Virol. 1986;57:933–941. doi: 10.1128/jvi.57.3.933-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou ZH, Chen DH, Jakana J, Rixon FJ, Chiu W. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J Virol. 1999;73:3210–3218. doi: 10.1128/jvi.73.4.3210-3218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banfield BW, Leduc Y, Esford L, Schubert K, Tufaro F. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J Virol. 1995;69:3290–3298. doi: 10.1128/jvi.69.6.3290-3298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herold BC, Visalli RJ, Susmarski N, Brandt CR, Spear PG. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 68.Laquerre S, Argnani R, Anderson DB, Zucchini S, Manservigi R, Glorioso JC. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shieh MT, WuDunn D, Montgomery RI, Esko JD, Spear PG. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai WH, Gu B, Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988;62:2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiang HY, Cohen GH, Eisenberg RJ. Identification of functional regions of herpes simplex virus glycoprotein gD by using linker-insertion mutagenesis. J Virol. 1994;68:2529–2543. doi: 10.1128/jvi.68.4.2529-2543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Highlander SL, Cai WH, Person S, Levine M, Glorioso JC. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988;62:1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ligas MW, Johnson DC. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roop C, Hutchinson L, Johnson DC. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 77.Nicola AV, Ponce dL, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol. 1998;72:3595–3601. doi: 10.1128/jvi.72.5.3595-3601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitbeck JC, Peng C, Lou H, Xu R, Willis SH, Ponce dL, Peng T, Nicola AV, Montgomery RI, Warner MS, Soulika AM, Spruce LA, Moore WT, Lambris JD, Spear PG, Cohen GHn, Eisenberg RJ. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpesvirus Ig-like receptor (HlgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci USA. 1998;95:15700–15705. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 81.Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Warner MS, Geraghty RJ, Martinez WM, Montgomery Rl, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 83.Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 84.Rux AH, Willis SH, Nicola AV, Hou W, Peng C, Lou H, Cohen GH, Eisenberg RJ. Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to the herpes-virus entry mediator. J Virol. 1998;72:7091–7098. doi: 10.1128/jvi.72.9.7091-7098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willis SH, Rux AH, Peng C, Whitbeck JC, Nicola AV, Lou H, Hou W, Salvador L, Eisenberg RJ, Cohen GH. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance. J Virol. 1998;72:5937–5947. doi: 10.1128/jvi.72.7.5937-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roller RJ, Rauch D. Herpesvirus entry mediator HVEM mediates cell cell spread in BHK(TK-) cell clones. J Virol. 1998;72:1411–1417. doi: 10.1128/jvi.72.2.1411-1417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Terry-Allison T, Montgomery RI, Whitbeck JC, Xu R, Cohen GH, Eisenberg RJ, Spear PG. HveA (herpes virus entry mediator A), a co-receptor for herpes simplex virus entry, also participates in virus-induced cell fusion. J Virol. 1998;72:5802–5810. doi: 10.1128/jvi.72.7.5802-5810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson AC, Johnson DC. A novel herpes simplex virus glycoprotein, gl_, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66:2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McClain DS, Fuller AO. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology. 1994;198:690–702. doi: 10.1006/viro.1994.1081. [DOI] [PubMed] [Google Scholar]
- 90.Peng T, Ponce dL, Novotny MJ, Jiang H, Lambris JD, Dubin G, Spear PG, Cohen GH, Eisenberg RJ. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J Virol. 1998;72:6092–6103. doi: 10.1128/jvi.72.7.6092-6103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72:873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wittels M, Spear PG. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 1991;18:271–290. doi: 10.1016/0168-1702(91)90024-p. [DOI] [PubMed] [Google Scholar]
- 93.Sodeik B, Ebersold MW, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Newcomb WW, Brown JC. Induced extrusion of DNAfrom the capsid of herpes simplex virus type 1. J Virol. 1994;68:433–440. doi: 10.1128/jvi.68.1.433-440.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garber DA, Beverley SM, Coen DM. Demonstration of circularization of herpes simplex virus DNA following infection using pulsed field gel electrophoresis. Virology. 1993;197:459–462. doi: 10.1006/viro.1993.1612. [DOI] [PubMed] [Google Scholar]
- 96.Campbell ME, Palfreyman JW, Preston CM. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984;180:1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- 97.Gaffney DF, McLauchlan J, Whitton JL, Clements JB. A modular system for the assay of transcription regulatory signals: the sequence TAATGARAT is required for herpes simplex virus immediate early gene activation. Nucleic Acids Res. 1985;13:7847–7863. doi: 10.1093/nar/13.21.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mackem S, Roizman B. Regulation of alpha genes of herpes simplex virus: the alpha 27 gene promoter-thymidine kinase chimera is positively regulated in converted L cells. J Virol. 1982;43:1015–1023. doi: 10.1128/jvi.43.3.1015-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Post LE, Mackem S, Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981;24:555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- 100.Kristie TM, LeBowitz JH, Sharp PA. The octamer-binding proteins form multiprotein-DNA complexes with the HSV alpha TIF regulatory protein. EMBO J. 1989;8:4229–4238. doi: 10.1002/j.1460-2075.1989.tb08608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kristie TM, Sharp PA. Purification of the cellular C1 factor required for the stable recognition of the Oct-1 homeodomain by the herpes simplex virus alpha-trans-induction factor (VP16) J Biol Chem. 1993;268:6525–6534. [PubMed] [Google Scholar]
- 102.Lu R, Yang P, Padmakumar S, Misra V. The herpesvirus transactivator VP16 mimics a human basic domain leucine zipper protein, luman, in its interaction with HCF. J Virol. 1998;72:6291–6297. doi: 10.1128/jvi.72.8.6291-6297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKnight JL, Kristie TM, Roizman B. Binding of the virion protein mediating alpha gene induction in herpes simplex virus 1-infected cells to its cis site requires cellular proteins. Proc Natl Acad Sci USA. 1987;84:7061–7065. doi: 10.1073/pnas.84.20.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O'Hare P, Goding CR. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988;52:435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- 105.Stern S, Tanaka M, Herr W. The Oct-1 homeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989;341:624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- 106.Wilson AC, LaMarco K, Peterson MG, Herr W. The VP16 accessory protein HCF is a family of polypeptides processed from a large precursor protein. Cell. 1993;74:115–125. doi: 10.1016/0092-8674(93)90299-6. [DOI] [PubMed] [Google Scholar]
- 107.Thomas S, Coffin RS, Watts P, Gough G, Latchman DS. The TAATGARAT motif in the herpes simplex virus immediate-early gene promoters can confer both positive and negative responses to cellular octamer-binding proteins when it is located within the viral genome. J Virol. 1998;72:3495–3500. doi: 10.1128/jvi.72.4.3495-3500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schang LM, Phillips J, Schaffer PA. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schang LM, Rosenberg A, Schaffer PA. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999;73:2161–2172. doi: 10.1128/jvi.73.3.2161-2172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert PM, Tampe R, Peterson PA, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 111.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 112.York IA, Roop C, Andrews DW, Riddell SR, Graham FL, Johnson DC. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 113.DeLuca NA, McCarthy AM, Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DeLuca NA, Schaffer PA. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985;5:1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kristie TM, Roizman B. Alpha 4, the major regulatory protein of herpes simplex virus type 1, is stably and specifically associated with promoter-regulatory domains of alpha genes and of selected other viral genes. Proc Natl Acad Sci USA. 1986;83:3218–3222. doi: 10.1073/pnas.83.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McCarthy AM, McMahan L, Schaffer PA. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA-deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Michael N, Spector D, Mavromara-Nazos P, Kristie TM, Roizman B. The DNA-binding properties of the major regulatory protein alpha 4 of herpes simplex viruses. Science. 1988;239:1531–1534. doi: 10.1126/science.2832940. [DOI] [PubMed] [Google Scholar]
- 118.Sacks WR, Greene CC, Aschman DP, Schaffer PA. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985;55:796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watson RJ, Clements JB. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980;285:329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- 120.Everett RD. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984;3:3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gu B, Rivera-Gonzalez R, Smith CA, DeLuca NA. Herpes simplex virus infected cell polypeptide 4 preferentially represses Sp1-activated over basal transcription from its own promoter. Proc Natl Acad Sci USA. 1993;90:9528–9532. doi: 10.1073/pnas.90.20.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O'Hare P, Hayward GS. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J Virol. 1985;56:723–733. doi: 10.1128/jvi.56.3.723-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carrozza MJ, DeLuca NA. Interaction of the viral activator protein ICP4 with TFIID through TAF250. Mol Cell Biol. 1996;16:3085–3093. doi: 10.1128/mcb.16.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith CA, Bates P, Rivera-Gonzalez R, Gu B, DeLuca NA. ICP4, the major transcriptional regulatory protein of herpes simplex virus type 1, forms a tripartite complex with TATA-binding protein and TFIIB. J Virol. 1993;67:4676–4687. doi: 10.1128/jvi.67.8.4676-4687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leopardi R, Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McMahan L, Schaffer PA. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J Virol. 1990;64:3471–3485. doi: 10.1128/jvi.64.7.3471-3485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rice SA, Su LS, Knipe DM. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. J Virol. 1989;63:3399–3407. doi: 10.1128/jvi.63.8.3399-3407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Samaniego LA, Webb AL, DeLuca NA. Functional interactions between herpes simplex virus immediate-early proteins during infection: gene expression as a consequence of ICP27 and different domains of ICP4. J Virol. 1995;69:5705–5715. doi: 10.1128/jvi.69.9.5705-5715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Uprichard SL, Knipe DM. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J Virol. 1996;70:1969–1980. doi: 10.1128/jvi.70.3.1969-1980.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hardy WR, Sandri-Goldin RM. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sandri-Goldin RM, Hibbard MK. The herpes simplex virus type 1 regulatory protein ICP27 co-immunoprecipitates with anti-Sm antiserum, and the C terminus appears to be required for this interaction. J Virol. 1996;70:108–118. doi: 10.1128/jvi.70.1.108-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mears WE, Rice SA. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology. 1998;242:128–137. doi: 10.1006/viro.1997.9006. [DOI] [PubMed] [Google Scholar]
- 134.Sandri-Goldin RM. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Soliman TM, Sandri-Goldin RM, Silverstein SJ. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol. 1997;71:9188–9197. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhi Y, Sandri-Goldin RM. Analysis of the phosphorylation sites of herpes simplex virus type 1 regulatory protein ICP27. J Virol. 1999;73:3246–3257. doi: 10.1128/jvi.73.4.3246-3257.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ogle WO, Roizman B. Functional anatomy of herpes simplex virus 1 overlapping genes encoding infected-cell protein 22 and US1.5 protein. J Virol. 1999;73:4305–4315. doi: 10.1128/jvi.73.5.4305-4315.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carter KL, Roizman B. Alternatively spliced mRNAs predicted to yield frame-shift proteins and stable intron 1 RNAs of the herpes simplex virus 1 regulatory gene alpha 0 accumulate in the cytoplasm of infected cells. Proc Natl Acad Sci USA. 1996;93:12535–12540. doi: 10.1073/pnas.93.22.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Purves FC, Ogle WO, Roizman B. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rice SA, Long MC, Lam V, Schaffer PA, Spencer CA. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J Virol. 1995;69:5550–5559. doi: 10.1128/jvi.69.9.5550-5559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bruni R, Roizman B. Herpes simplex virus 1 regulatory protein ICP22 interacts with a new cell cycle-regulated factor and accumulates in a cell cycle-dependent fashion in infected cells. J Virol. 1998;72:8525–8531. doi: 10.1128/jvi.72.11.8525-8531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bruni R, Fineschi B, Ogle WO, Roizman B. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J Virol. 1999;73:3810–3817. doi: 10.1128/jvi.73.5.3810-3817.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leopardi R, Ward PL, Ogle WO, Roizman B. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the U(L)13 protein kinase. J Virol. 1997;71:1133–1139. doi: 10.1128/jvi.71.2.1133-1139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cai W, Astor TL, Liptak LM, Cho C, Coen DM, Schaffer PA. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J Virol. 1993;67:7501–7512. doi: 10.1128/jvi.67.12.7501-7512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jordan R, Schaffer PA. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J Virol. 1997;71:6850–6862. doi: 10.1128/jvi.71.9.6850-6862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Samaniego LA, Wu N, DeLuca NA. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kawaguchi Y, Bruni R, Roizman B. Interaction of herpes simplex virus 1 alpha regulatory protein ICP0 with elongation factor 1delta: ICP0 affects translational machinery. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kawaguchi Y, Van Sant C, Roizman B. Eukaryotic elongation factor 1 delta is hyperphosphorylated by the protein kinase encoded by the U(L)13 gene of herpes simplex virus 1. J Virol. 1998;72:1731–1736. doi: 10.1128/jvi.72.3.1731-1736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kawaguchi Y, Matsumura T, Roizman B, Hirai K. Cellular elongation factor 1delta is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J Virol. 1999;73:4456–4460. doi: 10.1128/jvi.73.5.4456-4460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Everett RD, Maul GG. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Everett RD, Earnshaw WC, Findlay J, Lomonte P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 1999;18:1526–1538. doi: 10.1093/emboj/18.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lees-Miller SP, Long MC, Kilvert MA, Lam V, Rice SA, Spencer CA. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J Virol. 1996;70:7471–7477. doi: 10.1128/jvi.70.11.7471-7477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Parkinson J, Lees-Miller SP, Everett RD. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J Virol. 1999;73:650–657. doi: 10.1128/jvi.73.1.650-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Everett RD, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically-associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16:1519–1530. doi: 10.1093/emboj/16.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Everett RD, Orr A, Preston CM. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 1998;17:7161–7169. doi: 10.1093/emboj/17.24.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Everett RD, Meredith M, Orr A. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J Virol. 1999;73:417–426. doi: 10.1128/jvi.73.1.417-426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koelle DM, Tigges MA, Burke RL, Symington FW, Riddell SR, Abbo H, Corey L. Herpes simplex virus infection of human fibroblasts and keratinocytes inhibits recognition by cloned CD8+ cytotoxic T lymphocytes. J Clin Invest. 1993;91:961–968. doi: 10.1172/JCI116317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jerome KR, Tait JF, Koelle DM, Corey L. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J Virol. 1998;72:436–441. doi: 10.1128/jvi.72.1.436-441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Galvan V, Brandimarti R, Roizman B. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol. 1999;73:3219–3226. doi: 10.1128/jvi.73.4.3219-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Crute JJ, Tsurumi T, Zhu LA, Weller SK, Olivo PD, Challberg MD, Mocarski ES, Lehman IR. Herpes simplex virus 1 helicase-primase: a complex of three herpes-encoded gene products. Proc Natl Acad Sci USA. 1989;86:2186–2189. doi: 10.1073/pnas.86.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gottlieb J, Marcy AI, Coen DM, Challberg MD. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J Virol. 1990;64:5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weller SK, Lee KJ, Sabourin DJ, Schaffer PA. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983;45:354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Olivo PD, Nelson NJ, Challberg MD. Herpes simplex virus DNA replication: the UL9 gene encodes an origin-binding protein. Proc Natl Acad Sci USA. 1988;85:5414–5418. doi: 10.1073/pnas.85.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Monahan SJ, Grinstead LA, Olivieri W, Parris DS. Interaction between the herpes simplex virus type 1 origin-binding and DNA polymerase accessory proteins. Virology. 1998;241:122–130. doi: 10.1006/viro.1997.8953. [DOI] [PubMed] [Google Scholar]
- 168.Jacob RJ, Morse LS, Roizman B. Anatomy of herpes simplex virus DNA: XII. Accumulation of head-to-tail concatemers in nuclei of infected cells and their role in the generation of the four isomeric arrangements of viral DNA. J Virol. 1979;29:448–457. doi: 10.1128/jvi.29.2.448-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Skaliter R, Makhov AM, Griffith JD, Lehman IR. Rolling circle DNA replication by extracts of herpes simplex virus type 1-infected human cells. J Virol. 1996;70:1132–1136. doi: 10.1128/jvi.70.2.1132-1136.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weber PC, Challberg MD, Nelson NJ, Levine M, Glorioso JC. Inversion events in the HSV-1 genome are directly mediated by the viral DNA replication machinery and lack sequence specificity. Cell. 1988;54:369–381. doi: 10.1016/0092-8674(88)90200-0. [DOI] [PubMed] [Google Scholar]
- 171.Severini A, Scraba DG, Tyrrell DL. Branched structures in the intracellular DNA of herpes simplex virus type 1. J Virol. 1996;70:3169–3175. doi: 10.1128/jvi.70.5.3169-3175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Deiss LP, Chou J, Frenkel N. Functional domains within the a sequence involved in the cleavage-packaging of herpes simplex virus DNA. J Virol. 1986;59:605–618. doi: 10.1128/jvi.59.3.605-618.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Newcomb WW, Homa FL, Thomsen DR, Booy FP, Trus BL, Steven AC, Spencer JV, Brown JC. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J Mol Biol. 1996;263:432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- 174.Newcomb WW, Homa FL, Thomsen DR, Trus BL, Cheng N, Steven A, Booy F, Brown C. Assembly of the herpes simplex virus procapsid from purified components and identification of small complexes containing the major capsid and scaffolding proteins. J Virol. 1999;73:4239–4250. doi: 10.1128/jvi.73.5.4239-4250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rixon FJ, Addison C, McGregor A, Macnab SJ, Nicholson P, Preston VG, Tatman JD. Multiple interactions control the intracellular localization of the herpes simplex virus type 1 capsid proteins. J Gen Virol. 1996;77:2251–2260. doi: 10.1099/0022-1317-77-9-2251. [DOI] [PubMed] [Google Scholar]
- 176.Spencer JV, Newcomb WW, Thomsen DR, Homa FL, Brown JC. Assembly of the herpes simplex virus capsid: preformed triplexes bind to the nascent capsid. J Virol. 1998;72:3944–3951. doi: 10.1128/jvi.72.5.3944-3951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Varmuza SL, Smiley JR. Signals for site-specific cleavage of HSV DNA: maturation involves two separate cleavage events at sites distal to the recognition sequences. Cell. 1985;41:793–802. doi: 10.1016/s0092-8674(85)80060-x. [DOI] [PubMed] [Google Scholar]
- 178.Vlazny DA, Kwong A, Frenkel N. Site-specific cleavage/packaging of herpes simplex virus DNA and the selective maturation of nucleocapsids containing full-length viral DNA. Proc Natl Acad Sci USA. 1982;79:1423–1427. doi: 10.1073/pnas.79.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Addison C, Rixon FJ, Preston VG. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J Gen Virol. 1990;71:2377–2384. doi: 10.1099/0022-1317-71-10-2377. [DOI] [PubMed] [Google Scholar]
- 180.Al-Kobaisi MF, Rixon FJ, McDougall I, Preston VG. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology. 1991;180:380–388. doi: 10.1016/0042-6822(91)90043-b. [DOI] [PubMed] [Google Scholar]
- 181.Ali MA, Forghani B, Cantin EM. Characterization of an essential HSV-1 protein encoded by the UL25 gene reported to be involved in virus penetration and capsid assembly. Virology. 1996;216:278–283. doi: 10.1006/viro.1996.0061. [DOI] [PubMed] [Google Scholar]
- 182.Baines JD, Cunningham C, Nalwanga D, Davison A. The U(L)15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the U(L)15 gene product. J Virol. 1997;71:2666–2673. doi: 10.1128/jvi.71.4.2666-2673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Koslowski KM, Shaver PR, Casey JT, Wilson T, Yamanaka G, Sheaffer AK, Tenney DJ, Pederson NE. Physical and functional interactions between the herpes simplex virus UL15 and UL28 DNA cleavage and packaging proteins. J Virol. 1999;73:1704–1707. doi: 10.1128/jvi.73.2.1704-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lamberti C, Weller SK. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology. 1996;226:403–407. doi: 10.1006/viro.1996.0668. [DOI] [PubMed] [Google Scholar]
- 185.Lamberti C, Weller SK. The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments. J Virol. 1998;72:2463–2473. doi: 10.1128/jvi.72.3.2463-2473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McNab AR, Desai P, Person S, Roof LL, Thomsen DR, Newcomb WW, Brown JC, Homa FL. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J Virol. 1998;72:1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Patel AH, Rixon FJ, Cunningham C, Davison AJ. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology. 1996;217:111–123. doi: 10.1006/viro.1996.0098. [DOI] [PubMed] [Google Scholar]
- 188.Salmon B, Cunningham C, Davison AJ, Harris WJ, Baines JD. The herpes simplex virus type 1 U(L)17 gene encodes virion tegument proteins that are required for cleavage and packaging of viral DNA. J Virol. 1998;72:3779–3788. doi: 10.1128/jvi.72.5.3779-3788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Salmon B, Baines JD. Herpes simplex virus DNA cleavage and packaging: association of multiple forms of U(L)15-encoded proteins with B capsids requires at least the U(L)6, U(L)17, and U(L)28 genes. J Virol. 1998;72:3045–3050. doi: 10.1128/jvi.72.4.3045-3050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Taus NS, Salmon B, Baines JD. The herpes simplex virus 1 UL17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology. 1998;252:115–125. doi: 10.1006/viro.1998.9439. [DOI] [PubMed] [Google Scholar]
- 191.Tengelsen LA, Pederson NE, Shaver PR, Wathen MW, Homa FL. Herpes simplex virus type 1 DNA cleavage and encapsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J Virol. 1993;67:3470–3480. doi: 10.1128/jvi.67.6.3470-3480.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yu D, Weller SK. Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging. J Virol. 1998;72:7428–7439. doi: 10.1128/jvi.72.9.7428-7439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lukonis CJ, Burkham J, Weller SK. Herpes simplex virus type 1 prereplicative sites are a heterogeneous population: only a subset are likely to be precursors to replication compartments. J Virol. 1997;71:4771–4781. doi: 10.1128/jvi.71.6.4771-4781.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Phelan A, Dunlop J, Patel AH, Stow ND, Clements JB. Nuclear sites of herpes simplex virus type 1 DNA replication and transcription co-localize at early times postinfection and are largely distinct from RNA processing factors. J Virol. 1997;71:1124–1132. doi: 10.1128/jvi.71.2.1124-1132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Quinlan MP, Chen LB, Knipe DM. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984;36:857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- 196.Ward PL, Ogle WO, Roizman B. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hong Z, Beaudet-Miller M, Durkin J, Zhang R, Kwong AD. Identification of a minimal hydrophobic domain in the herpes simplex virus type 1 scaffolding protein which is required for interaction with the major capsid protein. J Virol. 1996;70:533–540. doi: 10.1128/jvi.70.1.533-540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhou ZH, Macnab SJ, Jakana J, Scott LR, Chiu W, Rixon FJ. Identification of the sites of interaction between the scaffold and outer shell in herpes simplex virus-1 capsids by difference electron imaging. Proc Natl Acad Sci USA. 1998;95:2778–2783. doi: 10.1073/pnas.95.6.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Campadelli-Fiume G, Farabegoli F, Di Gaeta S, Roizman B. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J Virol. 1991;65:1589–1595. doi: 10.1128/jvi.65.3.1589-1595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Torrisi MR, Di Lazzaro C, Pavan A, Pereira L, Campadelli-Fiume G. Herpes simplex virus envelopment and maturation studied by fracture label. J Virol. 1992;66:554–561. doi: 10.1128/jvi.66.1.554-561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Alconada A, Bauer U, Sodeik B, Hoflack B. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J Virol. 1999;73:377–387. doi: 10.1128/jvi.73.1.377-387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Browne H, Bell S, Minson T, Wilson DW. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for re-envelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.van Genderen IL, Brandimarti R, Torrisi MR, Campadelli G, van Meer G. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology. 1994;200:831–836. doi: 10.1006/viro.1994.1252. [DOI] [PubMed] [Google Scholar]
- 204.Brunetti CR, Dingwell KS, Wale C, Graham FL, Johnson DC. Herpes simplex virus gD and virions accumulate in endosomes by mannose 6-phosphate-dependent and -independent mechanisms. J Virol. 1998;72:3330–3339. doi: 10.1128/jvi.72.4.3330-3339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Avitabile E, Di Gaeta S, Torrisi MR, Ward PL, Roizman B, Campadelli-Fiume G. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J Virol. 1995;69:7472–7482. doi: 10.1128/jvi.69.12.7472-7482.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Campadelli G, Brandimarti R, Di Lazzaro C, Ward PL, Roizman B, Torrisi MR. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci USA. 1993;90:2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dingwell KS, Doering LC, Johnson DC. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dingwell KS, Johnson DC. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Desai P, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998;72:7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Campadelli-Fiume G, Qi S, Avitabile E, Foa-Tomasi L, Brandimarti R, Roizman B. Glycoprotein D of herpes simplex virus encodes a domain which precludes penetration of cells expressing the glycoprotein by superinfecting herpes simplex virus. J Virol. 1990;64:6070–6079. doi: 10.1128/jvi.64.12.6070-6079.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ackermann M, Chou J, Sarmiento M, Lerner RA, Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J Virol. 1986;58:843–850. doi: 10.1128/jvi.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McKie EA, Hope RG, Brown SM, MacLean AR. Characterization of the herpes simplex virus type 1 strain 17+ neurovirulence gene RL1 and its expression in a bacterial system. J Gen Virol. 1994;75:733–741. doi: 10.1099/0022-1317-75-4-733. [DOI] [PubMed] [Google Scholar]
- 213.Brown SM, MacLean AR, McKie EA, Harland J. The herpes simplex virus virulence factor ICP34.5 and the cellular protein MyD116 complex with proliferating cell nuclear antigen through the 63-amino-acid domain conserved in ICP34.5, MyD116, and GADD34. J Virol. 1997;71:9442–9449. doi: 10.1128/jvi.71.12.9442-9449.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chou J, Roizman B. Herpes simplex virus 1 gamma(1)34.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc Natl Acad Sci USA. 1994;91:5247–5251. doi: 10.1073/pnas.91.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.He B, Chou J, Liebermann DA, Hoffman B, Roizman B. The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the gamma(1)34.5 gene of herpes simplex virus to preclude the premature shut-off of total protein synthesis in infected human cells. J Virol. 1996;70:84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brown SM, Harland J, MacLean AR, Podlech J, Clements JB. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J Gen Virol. 1994;75:2367–2377. doi: 10.1099/0022-1317-75-9-2367. [DOI] [PubMed] [Google Scholar]
- 217.Brown SM, MacLean AR, Aitken JD, Harland J. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J Gen Virol. 1994;75:3679–3686. doi: 10.1099/0022-1317-75-12-3679. [DOI] [PubMed] [Google Scholar]
- 218.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma(1)34.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 219.Dolan A, McKie E, MacLean AR, McGeoch DJ. Status of the ICP34.5 gene in herpes simplex virus type 1 strain 17. J Gen Virol. 1992;73:971–973. doi: 10.1099/0022-1317-73-4-971. [DOI] [PubMed] [Google Scholar]
- 220.MacLean AR, ul-Fareed M, Robertson L, Harland J, Brown SM. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the “a” sequence. J Gen Virol. 1991;72:631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 221.McKay EM, McVey B, Marsden HS, Brown SM, MacLean AR. The herpes simplex virus type 1 strain 17 open reading frame RL1 encodes a polypeptide of apparent M(r) 37K equivalent to ICP34.5 of herpes simplex virus type 1 strain F. J Gen Virol. 1993;74:2493–2497. doi: 10.1099/0022-1317-74-11-2493. [DOI] [PubMed] [Google Scholar]
- 222.Thompson RL, Stevens JG. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983;131:171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- 223.Robertson LM, MacLean AR, Brown SM. Peripheral replication and latency reactivation kinetics of the non- neurovirulent herpes simplex virus type 1 variant 1716. J Gen Virol. 1992;73:967–970. doi: 10.1099/0022-1317-73-4-967. [DOI] [PubMed] [Google Scholar]
- 224.Cassady KA, Gross M, Roizman B. The second-site mutation in the herpes simplex virus recombinants lacking the gamma(1)34.5 genes precludes shut-off of protein synthesis by blocking the phosphorylation of elF-2alpha. J Virol. 1998;72:7005–7011. doi: 10.1128/jvi.72.9.7005-7011.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cassady KA, Gross M, Roizman B. The herpes simplex virus US11 protein effectively compensates for the gamma(1)34.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J Virol. 1998;72:8620–8626. doi: 10.1128/jvi.72.11.8620-8626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chou J, Roizman B. The gamma(1)34.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chou J, Chen JJ, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor elF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5- mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1 alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Deshmane SL, Fraser NW. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol. 1989;63:943–947. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Efstathiou S, Minson AC, Field HJ, Anderson JR, Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J Virol. 1986;57:446–455. doi: 10.1128/jvi.57.2.446-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mellerick DM, Fraser NW. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987;158:265–275. doi: 10.1016/0042-6822(87)90198-x. [DOI] [PubMed] [Google Scholar]
- 232.Rock DL, Fraser NW. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983;302:523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- 233.Cook ML, Stevens JG. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973;7:272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sawtell NM. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997;71:5423–5431. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Croen KD, Ostrove JM, Dragovic LJ, Smialek JE, Straus SE. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene “anti-sense” transcript by in situ hybridization. New Engl J Med. 1987;317:1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- 236.Deatly AM, Spivack JG, Lavi E, O'Boyle DR, Fraser NW. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988;62:749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Farrell MJ, Dobson AT, Feldman LT. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci USA. 1991;88:790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fraser NW, Block TM, Spivack JG. The latency-associated transcripts of herpes simplex virus: RNA in search of function. Virology. 1992;191:1–8. doi: 10.1016/0042-6822(92)90160-q. [DOI] [PubMed] [Google Scholar]
- 239.Spivack JG, Fraser NW. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987;61:3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 241.Stevens JG, Haarr L, Porter DD, Cook ML, Wagner EK. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988;158:117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- 242.Ho DY, Mocarski ES. Herpes simplex virus latent RNA (LAT) is not required for latent infection in the mouse. Proc Natl Acad Sci USA. 1989;86:7596–7600. doi: 10.1073/pnas.86.19.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Steiner I, Spivack JG, Lirette RP, Brown SM, MacLean AR, Subak-Sharpe JH, Fraser NW. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989;8:505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Thompson RL, Sawtell NM. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bloom DC, Hill JM, Devi-Rao G, Wagner EK, Feldman LT, Stevens JG. A 348-base-pair region in the latency-associated transcript facilitates herpes simplex virus type 1 reactivation. J Virol. 1996;70:2449–2459. doi: 10.1128/jvi.70.4.2449-2459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hill JM, Maggioncalda JB, Garza HHJ, Su YH, Fraser NW, Block TM. In vivo epinephrine reactivation of ocular herpes simplex virus type 1 in the rabbit is correlated to a 370-base-pair region located between the promoter and the 5′ end of the 2.0 kilobase latency-associated transcript. J Virol. 1996;70:7270–7274. doi: 10.1128/jvi.70.10.7270-7274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hill JM, Garza HHJ, Su YH, Meegalla R, Hanna LA, Loutsch JM, Thompson HW, Varnell ED, Bloom DC, Block TM. A 437-base-pair deletion at the beginning of the latency-associated transcript promoter significantly reduced adrenergically induced herpes simplex virus type 1 ocular reactivation in latently infected rabbits. J Virol. 1997;71:6555–6559. doi: 10.1128/jvi.71.9.6555-6559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Perng GC, Chokephaibulkit K, Thompson RL, Sawtell NM, Slanina SM, Ghiasi H, Nesburn AB, Wechsler SL. The region of the herpes simplex virus type 1 LAT gene that is co-linear with the ICP34.5 gene is not involved in spontaneous reactivation. J Virol. 1996;70:282–291. doi: 10.1128/jvi.70.1.282-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sawtell NM, Thompson RL. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lokensgard JR, Berthomme H, Feldman LT. The latency-associated promoter of herpes simplex virus type 1 requires a region downstream of the transcription start site for long-term expression during latency. J Virol. 1997;71:6714–6719. doi: 10.1128/jvi.71.9.6714-6719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Batchelor AH, O'Hare P. Localization of cis-acting sequence requirements in the promoter of the latency-associated transcript of herpes simplex virus type 1 required for cell-type-specific activity. J Virol. 1992;66:3573–3582. doi: 10.1128/jvi.66.6.3573-3582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Dobson AT, Sederati F, Devi-Rao G, Flanagan WM, Farrell MJ, Stevens JG, Wagner EK, Feldman LT. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J Virol. 1989;63:3844–3851. doi: 10.1128/jvi.63.9.3844-3851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dobson AT, Margolis TP, Gomes WA, Feldman LT. In vivo deletion analysis of the herpes simplex virus type 1 latency-associated transcript promoter. J Virol. 1995;69:2264–2270. doi: 10.1128/jvi.69.4.2264-2270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Goins WF, Sternberg LR, Croen KD, Krause PR, Hendricks RL, Fink DJ, Straus SE, Levine M, Glorioso JC. A novel latency-active promoter is contained within the herpes simplex virus type 1 UL flanking repeats. J Virol. 1994;68:2239–2252. doi: 10.1128/jvi.68.4.2239-2252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nicosia M, Deshmane SL, Zabolotny JM, Valyi-Nagy T, Fraser NW. Herpes simplex virus type 1 latency-associated transcript (LAT) promoter deletion mutants can express a 2-kilobase transcript mapping to the LAT region. J Virol. 1993;67:7276–7283. doi: 10.1128/jvi.67.12.7276-7283.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Soares K, Hwang DY, Ramakrishnan R, Schmidt MC, Fink DJ, Glorioso JC. cis-acting elements involved in transcriptional regulation of the herpes simplex virus type 1 latency-associated promoter 1 (LAP1) in vitro and in vivo. J Virol. 1996;70:5384–5394. doi: 10.1128/jvi.70.8.5384-5394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chen X, Schmidt MC, Goins WF, Glorioso JC. Two herpes simplex virus type 1 latency-active promoters differ in their contributions to latency-associated transcript expression during lytic and latent infections. J Virol. 1995;69:7899–17908. doi: 10.1128/jvi.69.12.7899-7908.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wheatley SC, Dent CL, Wood JN, Latchman DS. A cellular factor binding to the TAATGARAT DNA sequence prevents the expression of the HSV immediate-early genes following infection of nonpermissive cell lines derived from dorsal root ganglion neurons. Exp Cell Res. 1991;194:78–82. doi: 10.1016/0014-4827(91)90132-e. [DOI] [PubMed] [Google Scholar]
- 259.Wilcox CL, Smith RL, Everett RD, Mysofski D. The herpes simplex virus type 1 immediate-early protein ICP0 is necessary for the efficient establishment of latent infection. J Virol. 1997;71:6777–6785. doi: 10.1128/jvi.71.9.6777-6785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhu XX, Chen JX, Young CS, Silverstein S. Reactivation of latent herpes simplex virus by adenovirus recombinants encoding mutant IE-0 gene products. J Virol. 1990;64:4489–4498. doi: 10.1128/jvi.64.9.4489-4498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jordan R, Pepe J, Schaffer PA. Characterization of a nerve growth factor-inducible cellular activity that enhances herpes simplex virus type 1 gene expression and replication of an ICP0 null mutant in cells of neural lineage. J Virol. 1998;72:5373–5382. doi: 10.1128/jvi.72.7.5373-5382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma(1)34.5 deletion mutants in rodent models. J Clin Invest. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Katz JP, Bodin ET, Coen DM. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kosz-Vnenchak M, Jacobson J, Coen DM, Knipe DM. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J Virol. 1993;67:5383–5393. doi: 10.1128/jvi.67.9.5383-5393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sawtell NM, Poon DK, Tansky CS, Thompson RL. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol. 1998;72:5343–5350. doi: 10.1128/jvi.72.7.5343-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tal-Singer R, Lasner TM, Podrzucki W, Skokotas A, Leary JJ, Berger SL, Fraser NW. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J Virol. 1997;71:5268–5276. doi: 10.1128/jvi.71.7.5268-5276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tal-Singer R, Podrzucki W, Lasner TM, Skokotas A, Leary JJ, Fraser NW, Berger SL. Use of differential display reverse transcription-PCR to reveal cellular changes during stimuli that result in herpes simplex virus type 1 reactivation from latency: upregulation of immediate-early cellular response genes TIS7, interferon, and interferon regulatory factor-1. J Virol. 1998;72:1252–1261. doi: 10.1128/jvi.72.2.1252-1261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Valyi-Nagy T, Deshmane S, Dillner A, Fraser NW. Induction of cellular transcription factors in trigeminal ganglia of mice by corneal scarification, herpes simplex virus type 1 infection, and explantation of trigeminal ganglia. J Virol. 1991;65:4142–4152. doi: 10.1128/jvi.65.8.4142-4152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sawtell NM. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol. 1998;72:6888–6892. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Corey L, Spear PG. Infections with herpes simplex viruses (1) New Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- 271.Whitley RJ. Herpes Simplex Viruses. In: Fields BN, Knipe DM, Howley PM, et al., editors. Fields Virology. Philadelphia, PA: Lippincott; 1996. pp. 2297–2342. [Google Scholar]
- 272.Baringer JR, Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. New Engl J Med. 1973;288:648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- 273.Hill TJ. Herpes Simplex Virus Latency. In: Roizman B, editor. The Herpesviruses. New York: Plenum; 1985. pp. 175–240. [Google Scholar]
- 274.Jacobs A, Bamborschke S, Szelies B, Lanfermann H, Schroder R, Heiss WD. Varicella-zoster-virus myelitis without herpes. An important differential diagnosis of the radicular syndrome. Dtsch Med Wochenschr. 1996;121:331–335. doi: 10.1055/s-2008-1043009. [DOI] [PubMed] [Google Scholar]
- 275.Lopez Z, Arvin AM, Ashley R. Immunity to Herpesvirus Infections in Humans. In: Roizman B, Whitley RJ, Lopez C, editors. The Human Herpesviruses. New York: Raven; 1993. pp. 397–425. [Google Scholar]
- 276.Posavad CM, Koelle DM, Corey L. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]