Abstract
Increased expression of cyclooxygenase-2 (COX-2) expression has been observed in several human tumor types and in selected animal and cell culture models of carcinogenesis, including lung cancer. Increased expression of COX-2 and production of prostaglandins appear to provide a survival advantage to transformed cells through the inhibition of apoptosis, increased attachment to extracellular matrix, increased invasiveness, and the stimulation of angiogenesis. In the present studies, we found that transforming growth factor β1 (TGF-β1) and epidermal growth factor (EGF) synergistically induced the expression of COX-2 and prostaglandin E2 (PGE2) production in mink lung epithelial (Mv1Lu) cells. EGF, but not PDGF or IGF-1, was able to inhibit TGF-β1-induced apoptosis in Mv1Lu cells and this effect was blocked by NS-398, a selective inhibitor of COX-2 activity, suggesting a possible role for COX-2 in the anti-apoptotic effect of EGF receptor ligands. The combination of TGF-β1 and EGF also significantly induced COX-2 expression in rat intestinal epithelial (RIE-1) cells and completely prevented sodium butyrate (NaBu)-induced apoptosis. The synergistic induction of COX-2 by TGF-β1 and EGF was not observed in R1B-L17 cells, a line derived from Mv1Lu cells that lacks the TGF-β type-I receptor. AG1478, a selective inhibitor of EGF receptor tyrosine kinase activity, completely suppressed the induction of COX-2 expression by either EGF or TGF-β1+EGF. Also, PD98059, a specific inhibitor of MEK/ERK pathway, and SB203580, a specific inhibitor of p38 MAPK activity, significantly inhibited the induction of COX-2 in response to combined EGF and TGF-β1. These results suggest an important collaborative interaction of TGF-β1 and EGF signaling in the induction of COX-2 and prostaglandin production in Mv1Lu cells.
Keywords: COX-2, TGF-β, EGF, apoptosis
Introduction
Prostaglandins are a diverse group of autocrine and paracrine hormones that mediate many cellular and physiologic processes [1–3] such as cell proliferation, inflammatory and immune responses, bone development, wound healing, hemostasis, reproductive function, glomerular filtration and the production of extracellular matrix proteins [4,5]. Prostaglandin H2 (PGH2) is an intermediate in the formation of prostaglandins. Two prostaglandin synthases catalyze the formation of PGH2 from arachidonic acid, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) [2,3]. Although both proteins display similar enzymatic activity, they are products of separate genes [6,7]. COX-2 can be upregulated by various factors including cytokines, growth factors and tumor promoters [8,9]. On the other hand, COX-1 is constitutively expressed in most tissues and is thought to serve in general housekeeping functions, such as maintaining gastrointestinal mucosal integrity [1,10].
The overexpression of COX-2 has been most strongly associated with colorectal tumorigenesis and treatment with nonsteroidal anti-inflammatory agents that inhibit cyclooxygenase activity inhibits intestinal tumorigenesis in rodent models [11–14]. Several reports have demonstrated [15–18] significantly elevated levels of both COX-2 and prostaglandins in surgically excised colorectal tumors when compared to normal colonic mucosa. Increased tumor expression of COX-2 has also been reported in rodent models of colorectal carcinogenesis such as the multiple intestinal neoplasia mouse model and azoxymethane tumor induction models [14,19]. Similarly, abundant expression of both COX-2 mRNA and protein has been reported in human lung cancers, but not in the normal lung tissues [20]. Recent studies have suggested that expression of COX-2 and increased prostaglandin production by either tumor cells or by adjacent stromal or vascular endothelial cells may provide a survival advantage for transformed cells that promotes tumor growth. For example, increased expression of COX-2 enhances rat intestinal epithelial (RIE-1) cell adherence to extracellular matrix and inhibits apoptosis [3]. Further, cyclooxygenase expression appears to be important for the invasive and metastatic phenotype [21] and for the induction of angiogenesis [22].
Casey et al. [23] reported that epidermal growth factor (EGF) caused a significant induction of PGE2 release in human amnion cells only in the presence of arachidonic acid. Signaling through the EGF receptor as a consequence of binding of either EGF, TGF-α or amphiregulin stimulates the expression of COX-2 in intestinal epithelial (RIE-1) cells [24,25]. The combination of EGF and IL-1 resulted in enhanced COX-2 mRNA levels accompanied by synergistic induction of PGE2 in human gingival fibroblast [26]. The cytokines, IL-1β or TNF-α, induced COX-2 mRNA only in the presence of TGF-β1 in human lung fibroblast cells [27]. EGF and phorbol ester induced COX-2 mRNA and reversed the effect of NSAIDs in non-small cell lung cancer cells [28]. EGF also induced the release of PGE2 and arachidonic acid by increasing the activity of cytosolic phospholipase A2 (PLA2) in human squamous carcinoma (A431) cells [29] . We previously reported that TGF-β1 caused induction of COX-2 in RIE-1 cells [30]. TGF-β has been reported to augment COX-2 induction by IL-1β or the combination of phorbol ester and calcium ionophore in RIE-1 (IEC-6) cells [31] and to augment expression of COX-2 induced by mitogens in rodent fibroblast [32]. In contrast, TGF-β inhibited prostaglandin production in amnion and A431 cells [33] and inhibited endotoxin-induced COX-2 expression and prostaglandin synthesis in murine macrophages [34].
Previous studies have implicated three different sub-families of mitogen-activated protein kinase (MAPK) as contributing to the induction of COX-2 in rodent fibroblasts and in human mammary epithelial cells. Activation of both the Ras/Raf1/mitogen-activated protein kinase kinase (MEK1 and MEK2)/extracellular signal-regulated (ERK) pathway and the Ras/MEKK-1/SEK-1 (SAPK/ERKkinase)/stress-activated protein kinase (SAPK)/Jun kinase (JNK) pathway is involved in the transcriptional induction of the COX-2 gene in response to v-src or platelet-derived growth factor [35,36]. Guan et al. [37] demonstrated that both JNK and p38 MAPK activation are important for COX-2 induction in NIH 3T3 cells. Overexpression of ERK1, JNK or p38 MAPK each can cause several- fold increases in COX-2 promoter activity and all three MAPK pathways appear to be important for induction of COX-2 via the ceramide signaling pathway [38]. We have recently reported that induction of activated Ha-RasVal 12 in Rat 1 fibroblasts resulted in both COX-2 transcriptional induction and mRNA stabilization, both of which were prevented by a specific inhibitor (PD98059) of MEK that prevents ERK1/2 activation [39]. We further demonstrated that ERK activity was necessary for COX-2 expression in response to either EGF or Ha-RasVal 12 in RIE-1 cells [39].
The biological significance of COX-2 induction by TGF-β1 is unknown. In the present study, we used mink lung epithelial (Mv1Lu) cells that are highly sensitive to TGF-β1 in both transcriptional and growth inhibition assays [40] to evaluate the effect of TGF-β1 alone and in combination with growth stimulatory growth factors on COX-2 expression and prostaglandin production. Further, TGF-β1 is known to induce apoptosis in several cell types, including the Mv1Lu cells [41]. We have examined the effect of TGF-β1 alone and in combination with the growth factors EGF, bFGF, PDGF or IGF-1 on COX-2 expression and prostaglandin production in the Mv1Lu cells. We found that COX-2 is synergistically induced by the combination of TGF-β1 and EGF. TGF-β1 also showed COX-2 induction by bFGF. In contrast, there was only minimal or no induction of COX-2 when Mv1Lu cells treated with any of the growth factors alone or when combinations of TGF-β1 and IGF-1 or TGF-β1 plus PDGF were used. Treatment of Mv1Lu cells with TGF-β1 alone resulted in apoptosis. The addition of either EGF or bFGF along with TGF-β1 protected the Mv1Lu cells against the apoptosis and this effect was abrogated by the addition of a selective COX-2 inhibitor (NS-398). Neither PDGF nor IGF-1 prevented TGF-β1-induced apoptosis in the Mv1Lu cells. The predominant prostaglandin produced after TGF-β1 and EGF treatment of Mv1Lu cells was PGE2. When PGE2 was added to Mv1Lu cells exposed to TGF-β1, apoptosis was inhibited. Further, TGF-β1 and EGF, in combination, inhibited sodium butyrate (NaBu)-induced apoptosis in RIE-1 cells.
Experimental Procedures
Cell Culture and Reagents
RIE-1 cells (a gift from KD Brown, Cambridge Research Station, Babraham, Cambridge, UK), Mv1Lu cells (from ATCC) and TGF-β type-I receptor-mutated mink lung epithelial cells (Mv1Lu/R1B-L17) (a generous gift from Dr. Massague [42] were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS. Human recombinant TGF-β1 was purchased from R&D Systems, EGF (Sigma), bFGF, PDGF and IGF-1 (Life Technologies) were used for cell treatment. Prostaglandin E2 (200 nM), arachidonic acid (15 µM) and a selective COX-2 inhibitor, NS-398, were purchased from Cayman Chemical, Ann Arbor, MI. Selective kinase inhibitors including PD98059, SB203580 and AG1478 were purchased from Calbiochem.
Immunoblot Analysis
Immunoblot analysis was performed as previously described [12]. Briefly, RIE-1, Mv1Lu and R1B cells were serum-free for 24 hours and treated with TGF-β1 (5 ng/ml), EGF (100 ng/ml), bFGF (10 ng/MI), PDGF (50 ng/ml), IGF-1 (75 ng/ml) separately and in combination and incubated at 37°C. At different times, the cells were lysed using NP-40 lysis buffer containing 50 mM Tris-Cl pH 7.8, 150 mM NaCl, 1% Nonidet P-40, 10 mM EDTA, 0.1% SDS, 10 mg/ml phenyl methyl sulphonyl fluoride, 20 µg/ml FOY-305 (Ono Pharmaceutical Co., Osaka, Japan), 1 mM sodium orthovanadate and 5 mM sodium fluoride. The cell lysates were clarified at 15,000 rpm for 10 min, denatured and fractionated by SDS-PAGE. After electrophoresis, the proteins were transferred to nitrocellulose membrane. The filters were then probed with the indicated antibodies, developed by enhanced chemiluminescence system (ECL, Amersham Pharmacia Biotech.) and exposed to X-AR5 film (Kodak). Quantitation was done using NIH image; version 1.61. The anti-COX-2 antibodies (N-20) and anti-cdk4 antibodies (C-22) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Quantitation of Eicosanoids
For these experiments, subconfluent (1x105 cells/plate) cell cultures (Mv1Lu) were established and serum starved for 24 hours. Serum-free cells were treated with TGF-β1 and EGF separately and in combination for 8 hours. In some experiments, cells were incubated with NS-398 (2 µM), a selective COX-2 antagonist, for 1 hour prior to the addition of growth factors. Serum-free DMEM with 15 µM arachidonic acid was applied 1 hour prior to collecting the conditioned medium. PGE2 release in the medium was measured by utilizing stable isotope dilution techniques employing gas chromatography negative ion chemical ionization mass spectrometry as described [24]. The results are expressed as nanograms of PGE2 per milliliter of medium. The limits of sensitivity for detection of PGE2 and other prostaglandins is 4 pg/ml.
Inhibition of MAPK and EGF Receptor-Mediated Signaling Pathways
PD98059, a specific inhibitor of mitogen-activated protein kinase kinase [43], SB203580, a selective inhibitor of p38 MAPK activity [44], and AG1478, a selective inhibitor of epidermal growth factor receptor tyrosine kinase activity [45], were used for this study. PD98059 (75 µM), AG1478 (50 µM) or SB203580 (10 µM) concentration was added to the serum-free culture media 1 hour prior to the treatment with TGF-β1 or EGF separately or TGF-β1+EGF for 8 hours. After incubation, protein lysates were collected and analyzed by immunoblotting for detection of COX-2 expression.
Analysis of Apoptosis
Apoptosis in Mv1Lu cells were assessed using DNA fragmentation (laddering) and chromatin staining. DNA laddering was performed according to the method described by Herrmann et al. [46]. For this experiment, subconfluent cell cultures were established in 100 mm plates. Serum-free cells were treated with TGF-β1, EGF, bFGF, PDGF,PGE2 and NS-398 as indicated for 24 hours. Both floating and attached cells were collected and lysed in lysis buffer (1% NP-40, 20 mM EDTA pH 7.5 and 50 mM Tris-Cl pH 7.5). The supernatant containing fragmented DNA was clarified by centrifugation for 5 minute at 1600g. Cell lysates were then digested with RNase A (5 mg/ml) at 56°C for 2 hours and with proteinase K (2.5 mg/ml) for at least 2 hours at 37°C. DNA was precipitated by adding 1/2 volume of 10 M ammonium acetate and 2.5 volume of 100% ethanol for overnight at -20°C, washed with 70% ethanol, quantitated and separated on 1.6% agarose gel. For chromatin staining, confluent cell cultures were established in 100 mm plates. Serum-free cells were treated with TGF-β1, EGF, FGF or PDGF individually and also in combinations as indicated for 24 hours. Both floating and attached cells were collected. After fixing with 1% glutaraldehyde for 10 minute at room temperature, the cells were washed with cold PBS and stained with a DNA-specific fluorochrome bis-benzamide trihydrochloride (Hoechst 33258, Sigma Chemical Co.). Cells with three or more nuclear chromatin fragments were considered positive for apoptosis [3].
Results
Synergistic Induction of COX-2 Protein by TGF-β1 and EGF
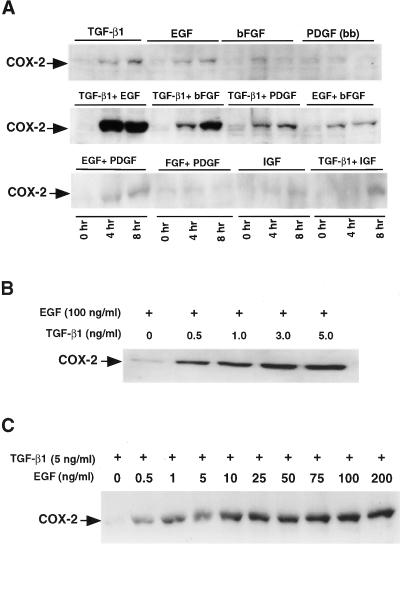
Mv1Lu cells are highly sensitive to TGF-β1 in transcriptional and growth inhibition assays. Several growth factors may induce COX-2 expression in a variety of cell types [9]. We evaluated COX-2 induction in response to EGF, bFGF, PDGF, TGF-β1 and IGF in Mv1Lu cells. COX-2 expression was induced five-fold and three-fold, respectively, by 8 hours when TGF-β1 or EGF was added alone; however, COX-2 expression increased synergistically by 25-fold (Figure 1A) by 8 hours after treatment with the combination of TGF-β1 and EGF. A significant (10-fold) induction of COX-2 expression was also observed after treatment with TGF-β1 and bFGF in combination. However, a slight induction of COX-2 expression was observed when PDGF was added in combination with TGF-β1. There was no significant induction of COX-2 in response to IGF-1, and IGF-1 in combination with TGF-β1 did not increase COX-2 levels over that observed after treatment with TGF-β1 alone.
Figure 1.
Growth factors mediated induction of COX-2 in mink lung epithelial cells. (A) Western blot analysis of COX-2 in Mv1Lu cells treated with different growth factors as indicated for 0, 4 and 8 hours. Mv1Lu cells were serum-free for 24 hours and treated with TGF-β1 (5 ng/ml), EGF (100 ng/ml), bFGF (50 ng/ml), PDGF (10 ng/ml) and IGF-1 (75 ng/ml) separately and in combination in serum-free medium and incubated at 37°C. An amount of 50 µg of each cell lysate was fractionated by SDS-PAGE. The filters were then probed with an antibody specific for COX-2. (B) Western blot analysis of dose-dependent induction of COX-2 by TGF-β1 against a fixed concentration of EGF (100 ng/ml) for 8 hours. (C) Dose-dependent induction of COX-2 by EGF against a fixed concentration of TGF-β1 (5 ng/ml) for 8 hours.
Dose- and Time-Dependent Induction of COX-2 by TGF-β1 and EGF
Preliminary analysis indicated that TGF-β1 and EGF increased the COX-2 expression synergistically (Figure 1A). We then examined the concentration-response relationship between TGF-β1 and EGF and COX-2 induction. As shown in Figure 1B, increasing concentrations of TGF-β1 were studied with a fixed concentration of EGF (100 ng/ml). In a reciprocal experiment (Figure 1C), the concentrations of EGF were varied and TGF-β1 was kept constant. These studies showed that 5 ng/ml of TGF-β1 and 100 ng/ml of EGF resulted in maximum induction of COX-2 by 8 hours.
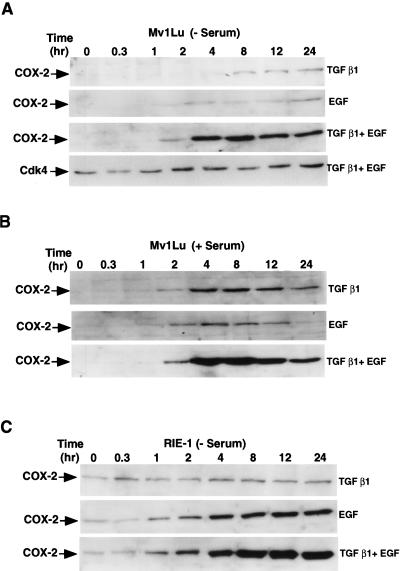
The temporal pattern of COX-2 induction by EGF and TGF-β1 was then assessed. EGF alone induced COX-2 expression approximately two-fold and TGF-β1 induced COX-2 expression 1.5-fold by 8 hours (Figure 2A). Maximum induction of COX-2 (25-fold) was observed by 8 hours when both TGF-β1 and EGF have been added in the serum-free medium and also in the presence of serum (30-fold) (Figure 2B). These results show that TGF-β1, in combination with EGF, causes synergistic induction of COX-2 expression in Mv1Lu cells.
Figure 2.
Kinetic study of COX-2 induction by TGF-β1 and EGF in Mv1Lu and RIE-1 cells. Both Mv1Lu and RIE-1 cells were incubated for 0–24 hours in the presence of TGF-β1 (5 ng/ml), EGF (100 ng/ml) alone or in combinations. For all experiments, 50 µg of each cell lysate was used for immunoblotting. (A) Mv1Lu cells were incubated in the absence (B) presence of serum. (C) RIE-1 cells were incubated in the absence of serum.
We also observed a significant induction of COX-2 expression (Figure 2C) in RIE cells when incubated with TGF-β1+EGF in serum-free conditions. The maximum induction of COX-2 expression in RIE-1 cells was 13-fold by 12 hours after the addition of TGF-β1+EGF. CDK4 levels remained relatively constant under these conditions in Mv1Lu cells and therefore, CDK4 immunoblotting is provided as a loading control (Figure 2A).
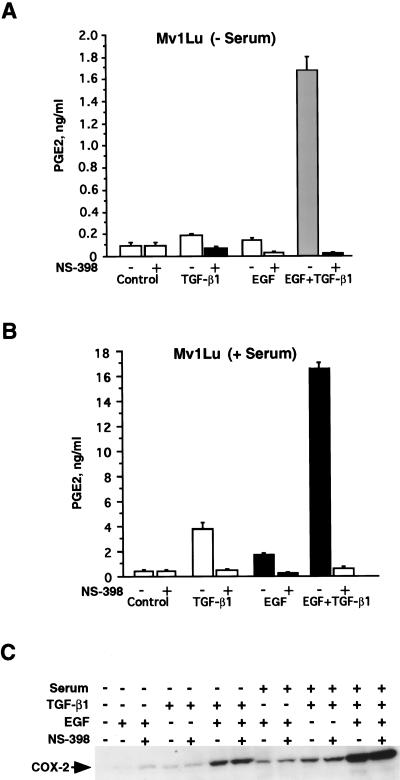
COX-2 Induction and Prostaglandin Production in Mv1Lu Cells
To determine whether increased COX-2 synthesis was associated with increased formation of prostaglandins, we evaluated prostaglandin E2 (PGE2) release in response to EGF, TGF-β1 or TGF-β1+EGF in arachidonate-supplemented Mv1Lu cells. We measured PGE2 release into the medium at 0 and 8 hours after the addition of growth factors. Besides PGE2, other prostaglandins released by these cells in lesser amounts were PGFα, PGI2, thromboxane B2 and PGD2 (data not shown). Figure 3 shows PGE2 release (ng/ml) in the absence (Figure 3A) or presence (Figure 3B) of serum. In both cases, EGF or TGF-β1 individually had a very little effect on PGE2 release by 8 hours after treatment. PGE2 release was increased from 0.15 to 1.7 ng/ml (11.3-fold) over the period of 8 hours in response to the combination of TGF-β1 and EGF in the absence of serum (Figure 3A) and from 0.8 to 17 ng/ml (21.3-fold) over the same interval in the presence of serum (Figure 3B). The increased formation of PGE2 corresponded to the increase in COX-2 expression in the Mv1Lu cells. Addition of NS-398 (2 µM) along with TGF-β1, EGF or TGF-β1+EGF completely inhibited the prostaglandin release in both the absence and presence of serum (Figure 3A–B). Immunoblot analysis showed that NS-398 caused no change in the level of COX-2 expression (Figure 3C).
Figure 3.
COX-2 induction and prostaglandin production in Mv1Lu cells. (A) Analysis of PGE2 release in serum-free condition. For this experiment, subconfluent (1x105 cells/plate) cell cultures (Mv1Lu) were established. The serum-free cells were treated with TGF-β1 and EGF separately and in combination for 7 hours. After treatment, medium was replaced with fresh serum-free DMEM containing 15 µM arachidonic acid 1 hour prior to collection. The PGE2 release in the medium was measured by utilizing stable isotope dilution techniques employing gas chromatography negative ion chemical ionization mass spectrometry. The results are expressed as nanograms of PGE2 per milliliter of medium. In similar experiments, NS-398 (2 µM) was also added into the medium 1 hour prior to the addition of growth factors. (B) Analysis of PGE2 release in serum-supplemented condition. (C) Western blot analysis of COX-2 in response to growth factors and NS-398 in the presence and absence of serum.
Both TGF-β1 and EGF Signaling are Needed for Synergistic Induction of COX-2
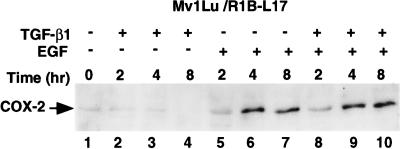
In order to further examine the signaling pathways involved in induction of COX-2 expression, we used the R1B-L17 cells that lack functional TGF-β type-I receptor. The R1B-L17 cells were derived from Mv1Lu cells by chemical mutagenesis [42]. As shown in Figure 4, COX-2 was not induced in response to TGF-β1 treatment (Figure 4, lanes 2–4), whereas modest induction of COX-2 expression was observed by 4 and 8 hours after the treatment with EGF (Figure 4, lanes 5–7). No further induction of COX-2 expression was observed when TGF-β1 and EGF were added together, indicating that TGF-β1 signaling via the type-I receptor is required for the synergistic response (Figure 4, lanes 8–10).
Figure 4.
TGF-β1- and EGF-mediated induction COX-2 in T(I)R mutated Mv1Lu cells (RIB/L17). Experimental conditions were same as mentioned in Figure 1A. RIB cells were treated with growth factors as indicated and incubated for 0–24 hours. After treatment, cells were lysed and 50 µg of each cell lysate was used for immunoblotting.
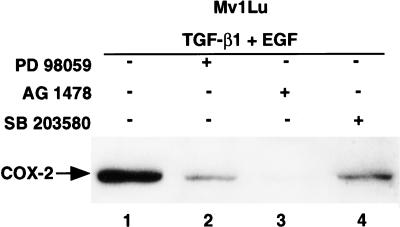
Signaling by EGF and TGF-β1 leads to activation of multiple signaling pathways. We therefore utilized several selective kinase inhibitors including PD98059 [43], SB203580 [44] and AG1478 [45] to explore potential pathways that are needed for synergistic induction of COX-2 (Figure 5). AG1478, an EGF receptor tyrosine kinase inhibitor, at 50 µM almost completely inhibited (>95%) the COX-2 expression at 8 hours in response to either EGF or TGF-β1+EGF (Figure 5, lane 3). PD98059 (a MEK inhibitor), at 75 µM, inhibited the induction of COX-2 expression by 80% (Figure 5, lane 2), whereas addition of SB203580 (a p38 MAPK inhibitor) at 10 µM inhibited TGF-β+EGF-mediated induction of COX-2 expression by 60% (Figure 5, lane 4). These data suggest an important collaborative interaction of TGF-β1 and EGF involving MEK-ERK and p38 MAPK signaling in the induction of COX-2 and prostaglandin production in Mv1Lu cells.
Figure 5.
TGF-β1- and EGF-mediated induction of COX-2 is inhibited by PD98059 (75 µM), SB203580 (10 µM) and AG1478 (50 µM) in Mv1Lu cells. PD98059, SB203580 and AG1478 compounds were added in the serum-free media 1 hour prior to the addition of TGF-β1 and EGF. After 8 hours of treatment with the growth factors, cells were lysed and 50 µg of each cell lysate was used for immunoblotting.
Overexpression of COX-2 and Apoptosis
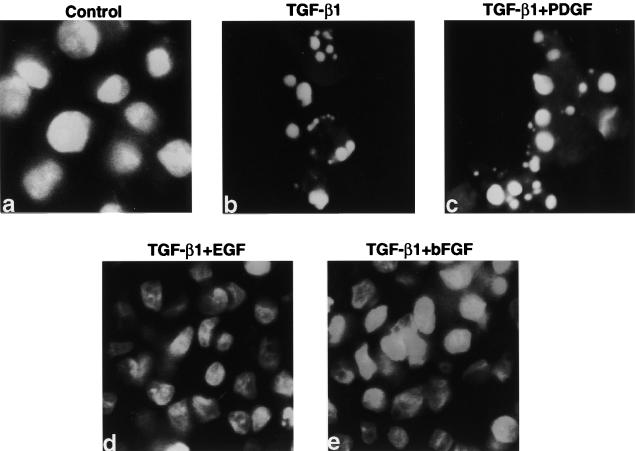
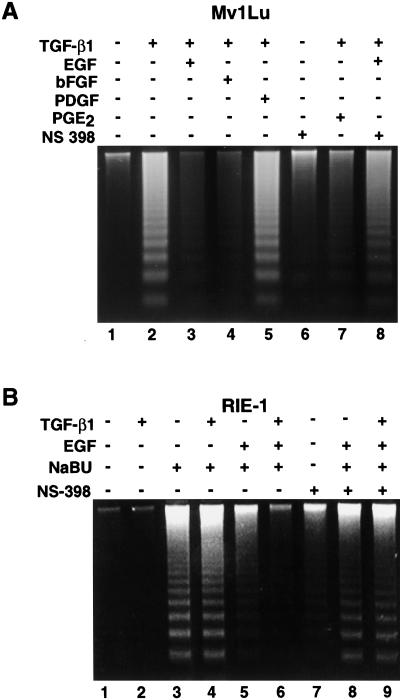
In order to evaluate the biological significance of the marked induction of COX-2 expression by TGF-β1 and EGF, we analyzed the effect of growth factors individually and in combination on apoptosis in both Mv1Lu and RIE-1 cells. It has been reported that TGF-β1 induces apoptosis in Mv1Lu cells [41]. We also assessed the apoptosis in Mv1Lu cells using a DNA-specific fluorochrome bis-benzamide trihydrochloride. Extensive chromatin fragmentation was observed in the TGF-β1-treated cells (Figure 6B), whereas no such nuclear chromatin fragmentation was visible in the control, TGF-β1+EGF- or TGF-β1+bFGF-treated cells (Figure 6A, D, and E). PDGF did not prevent TGF-β1-mediated chromatin fragmentation in Mv1Lu cells (Figure 6C). Cells with three or more nuclear chromatin fragments were considered positive for apoptosis [3]. We also evaluated DNA fragmentation as a measure of apoptosis. There was extensive DNA fragmentation when TGF-β1 was added into the medium (Figure 7A, lane 2). TGF-β1-mediated apoptosis was completely inhibited when either EGF or bFGF was added (Figure 7A, lanes 3–4). Exogenously added PGE2 (200 nm) also significantly inhibited the TGF-β1-induced apoptosis but did not completely reverse it (Figure 7A, lane 7). Neither PDGF (Figure 7A, lane 5) nor IGF treatment (data not shown) prevented TGF-β1-mediated apoptosis in Mv1Lu cells. When the COX-2 antagonist, NS-398, was added alone to the culture media, there was no significant induction of apoptosis (Figure 7A, lane 6). However, NS-398 blocked the protective effect of EGF when it was added in combination with TGF-β1 (Figure 7A, lane 8).
Figure 6.
Nuclear condensation in Mv1Lu cells. For chromatin staining, subconfluent cell cultures were established. Serum-free cells were treated with TGF-β1, EGF, FGF, PDGF individually and also in combinations as indicated for 24 hours. Both floating and attached cells were collected. After fixing with 1% glutaraldehyde for 10 minutes at room temperature, the cells were washed with cold PBS and stained with a DNA-specific fluorochrome bis-benzamide trihydrochloride. Cell with three or more nuclear chromatin fragments were considered positive for apoptosis. (a) Control, (b) TGF-β1, (c) TGF-β1+PDGF, (d) TGF-β1+EGF, (e) TGF-β1+bFGF.
Figure 7.
DNA fragmentation in response to growth factors, NS-398 and NaBu in Mv1Lu and RIE cells. (A) Mv1Lu cells were grown up to 90% confluency and serum-free for 24 hours. Cells were then treated with TGF-β1 (5 ng/ml), EGF (100 ng/ml), bFGF (50 ng/ml), PGE2 (200 nM), PDGF (50 ng/ml) and NS-398 (30 µM) as indicated in serum-free medium and incubated at 37°C for another 24 hours. NS-398 was added 1 hour prior to the addition of TGF-β1 and EGF. Both floating and attached cells were collected and lysed. The fragmented DNA was separated on 1.6% agarose gel. (B) RIE cells were grown up to 90% confluency and serum-free for 24 hours. TGF-β1 (5 ng/ml), EGF (100 ng/ml), NaBu (5 mM) and NS-398 (5 µM) were added as indicated. NS-398 was added 1 hour prior to the addition of growth factors and incubated at 37°C for 24 hours. Both floating and attached cells were collected, lysed and followed by agarose (1.6%) gel electrophoresis.
We also determined whether EGF alone or EGF+TGF-β1 could inhibit NaBu-induced apoptosis in RIE-1 cells. NaBu at 5 mM induced apoptosis in RIE-1 cells (Figure 7B, lane 3). TGF-β1 alone had no effect on DNA fragmentation and did not alter NaBu-induced apoptosis in RIE-1 cells (Figure 7B, lane 2,4). EGF decreased the amount of DNA fragmentation (Figure 7B, lane 5) and EGF combined with TGF-β1 completely abrogated NaBu-induced apoptosis in this cell line (Figure 7B, lane 6). In contrast, neither EGF alone nor TGF-β1+EGF prevented NaBu-induced apoptosis in RIE-1 cells in the presence of NS-398 (Figure 7B, lanes 8–9).
Discussion
The present studies demonstrate synergistic induction of COX-2 expression and prostaglandin production by TGF-β1 and EGF, and this effect is independent of serum. A selective inhibitor (NS-398) of COX-2 completely inhibited PGE2 release without altering the expression of COX-2 under similar conditions. This synergistic induction of COX-2 expression was not observed in Mv1Lu/R1B-L17 cells that lack the TGF-β type-I receptor. Addition of several selective kinase inhibitors including AG1478, PD98059 and SB203580 significantly inhibited the COX-2 expression. We also observed that EGF and bFGF completely inhibited the TGF-β1-induced apoptosis in Mv1Lu cells, whereas PDGF and IGF were unable to prevent apoptosis induced by TGF-β1 and had no significant effect on the induction of COX-2. The combination of TGF-β1 and EGF also completely inhibited the NaBu-induced apoptosis in RIE-1 cells and significantly induced the COX-2 expression. This protective effect was inhibited by the addition of a COX-2 antagonist.
Synergistic stimulation of prostaglandin production by growth factors and cytokines has been reported in several cell lines [47–50]. Comparison of the sequences of COX-2 from several species including mink [51] demonstrate that the domains of COX-2 associated with biological activities are highly conserved in mink. In our studies, we have tested several growth factors including TGF-β1, EGF, PDGF and IGF-1 alone and in different combinations. We found that only the combination of TGF-β1 and EGF synergistically induced COX-2 expression and PGE2 production, whereas the effect of TGF-β1 and bFGF on COX-2 expression and PGE2 production was additive in Mv1Lu cells. The combination of TGF-β1 and EGF also additively induced COX-2 expression in RIE-1 cells.
TGF-β1 induces apoptosis in several epithelial cell types such as HT-29 (colon adenocarcinoma), Mv1Lu (mink lung), DU-145 (human prostate carcinoma), MCF-7 (human breast adenocarcinoma), an effect that has been characterized by internucleosomal DNA fragmentation resulting from the activation of endonuclease(s) [41]. Duffy et al. [52] reported that the apoptosis induced by TGF-β1 in head and neck squamous cell carcinoma was inhibited by endonuclease inhibitor aurinetricarboxylic acid (ATA). However, Oberhammer et al. [53] reported that the induction of apoptosis by TGF-β1 in cultured hepatocytes and also in regressing liver occurs without the activation of endonuclease. We confirmed that TGF-β1 induced DNA fragmentation and apoptosis in the Mv1Lu cells but not in RIE-1 cells.
EGF modulates apoptosis and proliferation in several different ways. STATs (signal transducers and activators of transcription) are known to be activated by EGF and these have been shown to induce Bcl-2 and Bcl-xL expression in ras-transformed RIE (IEC) cells [54]. EGF may also mediate survival signals by activating ERK1/2, which then increases the level of c-jun mRNA [55]. It has also been reported that EGF may prevent apoptosis by inhibiting the induction of c-fos in osteoblast cells [56]. Like EGF, bFGF activates specific tyrosine kinase receptors. Interruption of bFGF signaling, a potent mitogen and survival factor, results in apoptosis in vascular smooth muscle cells due to the inappropriate entry into the S phase [57]. FGF has also been reported to suppress TNF-α-mediated apoptosis in L929 cells by activating the Raf/MEK/MAPK pathway [58]. Both EGF and bFGF inhibited TGF-β1-induced apoptosis in the Mv1Lu cells in the present study.
We observed that the treatment with EGF, bFGF and also PGE2 inhibited the TGF-β1-induced apoptosis in Mv1Lu cells. The observation, that the protective effect was abrogated by the COX-2 antagonist NS-398, strongly suggests that the inhibition of apoptosis was the result of the synergistic increase in COX-2 expression and PGE2 production. The biological significance of this synergistic effect is further supported by the observations in the RIE cells. TGF-β1 caused a modest increase in COX-2 and did not cause apoptosis in this cell line. The modest induction of COX-2 by TGF-β1 alone was insufficient to inhibit NaBu-induced apoptosis in RIE-1 cells, whereas EGF alone partially inhibited the apoptotic response. In contrast, the combination of TGF-β1 and EGF completely prevented the NaBu-induced apoptosis. Further, this combination resulted in a significantly greater increase in COX-2 expression and prostaglandin production than either agent alone. The protective effect of TGF-β1 and EGF was also abrogated by the COX-2 inhibitor NS-398, again suggesting that the apoptosis protection resulted from the increase in COX-2 and prostaglandin production. Tsujii and DuBois [3] previously reported that forced overexpression of COX-2 resulted in the inhibition of apoptotic responses in RIE-1 cells. In the current study, the involvement of COX-2 and prostaglandins in the protection from apoptosis was further supported by the observation that PGE2 inhibited the TGF-β1-mediated apoptosis in the Mv1Lu cells. The exact mechanism by which this occurs is unknown. Ottonello et al. [59] reported in neutrophils that PGE2 upregulated the cAMP level and thereby delayed the apoptosis. We also previously reported that exogenously added PGE2 enhances the survival of human colon cancer cells (HCA-7) by inducing the expression of Bcl-2 without altering Bcl-x or Bax expression [60].
Our data support the concept that increased COX-2 expression and prostaglandin production are important for survival of epithelial cells and demonstrate that the combination of growth factors likely to be present in the areas of neoplasia or inflammation may act synergistically to cause an increase in COX-2 and prostaglandins. The present study also suggests that the induction of apoptosis by COX-2 antagonists may not be entirely due to the inhibition of prostaglandin production. This is based on our observations that near-complete inhibition of PGE2 release into the media resulted from treatment with NS-398 at a 2-µM concentration, whereas 30 µM was required to abrogate the protective effects TGF-β1 and EGF in the Mv1Lu cells. This is consistent with the previous reports showing that the concentration of COX-2 antagonist required to significantly inhibit prostaglandin synthesis was less than that required to induce apoptosis [60,61], or to inhibit DNA synthesis [24] and cell growth [60]. Ballif et al. [62] have reported that both COX isoenzymes may modulate apoptosis by interacting with the apoptosis/autoimmunity-associated protein Nuc to retain it in the endoplasmic reticulum. This effect may occur independently of prostaglandin production.
To determine whether signaling from the previously characterized type-I TGF-β receptor is required for this synergistic induction of COX-2 and production of prostaglandins, we examined Mv1Lu/R1B-L17 cells lacking a functional TGF-β type-I receptor [42]. We observed no synergistic induction of COX-2 expression in Mv1Lu/R1B-L17 cells in response to the combination of TGF-β1 and EGF. EGF treatment alone did cause a modest induction of COX-2, indicating that COX-2 gene remained functional in these cells. These data demonstrate that signaling from the type-I TGF-β receptor is required for the synergistic induction of COX-2 expression and prostaglandin production.
EGF exerts its actions by binding to its receptor (EGF-R), which is a transmembrane protein tyrosine kinase. Binding of EGF to its receptor triggers receptor dimerization and autophosphorylation and recruitment of kinase substrates. These events lead to the activation of Ras and multiple signaling pathways including the mitogen-activated protein kinase (MEK/ERK) pathway. In the present studies, we used AG1478, a specific inhibitor of EGF receptor tyrosine kinase which almost completely (>95%) blocked the TGF-β1+EGF-mediated induction of COX-2. PD98059, a specific inhibitor of mitogen-activated protein kinase kinase (MEK), has been shown to inhibit the activation of MEK both in vivo and in vitro [43,63]. In our study, we observed that addition of PD98059 significantly prevented (80%) the induction of COX-2 expression in response to the combination of TGF-β1 and EGF. Previously, we reported that in Rat 1 fibroblasts transformed by Ha-Ras (V12), PD98059 blocked the activity of ERK1/2 by inhibiting MEK and thereby prevented the expression of COX-2 [19]. These data suggest that the synergistic induction of COX-2 by TGF-β1+EGF requires both functional EGF receptor tyrosine kinase activity and MAP kinase signaling cascades.
Recent studies have revealed that mitogen-activated protein kinase (MAPK) consists of at least three subfamilies, namely, classical MAPK (also known as ERK), stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK), and the p38 kinase pathways [64]. TGF-β-activating kinase (TAK)-1 is reported to stimulate both the p38-mitogen-activated protein kinase (MAPK) pathway and JNK [65]. In our present study, we found that SB203580, an inhibitor of p38 MAPK, significantly (60%) inhibited the induction of COX-2 expression in response to the combination of TGF-β1 and EGF. Currently, we are conducting a more detailed examination of signal transduction pathways that contribute to the COX-2 expression.
In summary, our observations indicate that TGF-β1 may collaborate with other growth factors to synergistically induce COX-2 and prostaglandin synthesis. Our data support the hypothesis that augmented expression of COX-2 and increased prostaglandin production that occur as the result of combinations of growth factors that are commonly present in tumors may provide a significant survival advantage for the cells that are exposed to those factors and may contribute to tumor progression. Targeting therapy toward inhibiting the COX-2 activity may prove beneficial both for prevention of tumors and for treatment of established tumors.
Abbreviations
- COX-2
cyclooxygenase-2
- PG
prostaglandins
- EGF
epidermal growth factor
- bFGF
basic fibroblast growth factor
- TGF-β
transforming growth factor-β
- MAPK/ERK
mitogen-activated protein kinase
- MEK MAPK
kinase
- CDK4
cyclin dependent kinase 4
- JNK
c-jun N-terminal kinase
- NaBu
Sodium butyrate
Footnotes
This work was supported by NIH grant CA69457, CA77839 and DK52334 (to R.D.B.); NIH grant DK48831, GM42056, GM15431, DK26637 and CA77839 (to J.D.M.); and NIH grant CA 42572 (to H.L.M.) and the Vanderbilt Cancer Center support grant CA68485. J.D.M. is the recipient of a Burroughs-Wellcome fund clinical scientist award in translational research.
References
- 1.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jannet JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption cause severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 2.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethocin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 3.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 4.Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 5.Marnett LJ. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992;52:5575–5589. [PubMed] [Google Scholar]
- 6.Yokoyama C, Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem Biophysics Res Commun. 1989;165:888–894. doi: 10.1016/s0006-291x(89)80049-x. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher BS, Kujubu DA, Perrin DM, Hershman HR. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J Biol Chem. 1992;267:4338–4344. [PubMed] [Google Scholar]
- 8.Jones DA, Carlton D, McIntyre MT, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993;268:9049–9054. [PubMed] [Google Scholar]
- 9.Williams CS, DuBois RN. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol. 1996;270:G393–G400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993;330:379–384. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- 11.Jacoby RF, Marshall DJ, Newton MA, Novakovic K, Tutsch K, Cole CE, Lubet RA, Kelloff GJ, Verma A, Moser AR, Dove WF. Chemoprevention of spontaneous intestinal adenomas in the ApcMin mouse model. Cancer Res. 1996;56:710–714. [PubMed] [Google Scholar]
- 12.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 13.Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996;56:4566–4569. [PubMed] [Google Scholar]
- 14.Williams CW, Luongo C, Radhika A, Zhang T, Lamps L, Nanney LB, Beauchamp RD, DuBois RN. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology. 1996;111:1134–1140. doi: 10.1016/s0016-5085(96)70083-5. [DOI] [PubMed] [Google Scholar]
- 15.Ebehart CW, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Upregulation of cyclooxygenase-2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 16.Kargman S, O'Neill G, Vickers P, Evans J, Mancini J, Jothy S. Expression of prostaglandin G/H synthase-1 and-2 in human colorectal cancer. Cancer Res. 1995;55:2556–2559. [PubMed] [Google Scholar]
- 17.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 18.Kutchera W, Jones DA, Matsunami N, Groden J, McIntyre TM, Zimmerman GA, White RL, Prescott SM. Prostaglandin H synthase-2 is expressed abnormally in human colon cancer: evidence for a transcriptional effect. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh J, Hamid R, Reddy BS. Dietary fat and colon cancer: modulation of cyclooxygenase-2 by types and amount of dietary fat during the post-initiation stage of colon carcinogenesis. Cancer Res. 1997;57:3465–3470. [PubMed] [Google Scholar]
- 20.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomo T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase-2 occurs frequently in human lung cancers, specially in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 21.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 23.Casey ML, Korte K, MacDonald PC. Epidermal growth factor stimulation of prostaglandin E2 biosynthesis in amnion cells. Induction of prostaglandin H2 synthase. J Biol Chem. 1988;263:7846–7854. [PubMed] [Google Scholar]
- 24.Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsy PJ, Chinnery R, Kirkland SC, DuBois RN, Jetton TL, Morrow JD. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci USA. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DuBois RN, Awad J, Morrow J, Roberts LJ, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-α and phorbol ester. J Clin Invest. 1994;93:493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yucel-Lindberg T, Ahola H, Carlstedt-Duke J, Modeer T. Involvement of tyrosine kinases on cyclooxygenase expression and prostaglandin E2 production in human gingival fibroblasts stimulated with interleukin-1 beta and epidermal growth factor. Biochem Biophys Res Commun. 1999;257:528–532. doi: 10.1006/bbrc.1999.0523. [DOI] [PubMed] [Google Scholar]
- 27.Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA. Differential regulation of cyclooxygenases 1 and 2 by interleukin-1 beta, tumor necrosis factor-alpha, and transforming growth factor-beta 1 in human lung fibroblasts. Exp Cell Res. 1998;241:222–229. doi: 10.1006/excr.1998.4050. [DOI] [PubMed] [Google Scholar]
- 28.Hida T, Leyton J, Makheja AN, Ben-Av P, Hla T. Non-small cell lung cancer cyclooxygenase activity and proliferation are inhibited by non-steroidal anti-inflammatory drugs. Anticancer Res. 1998;18:775–782. [PubMed] [Google Scholar]
- 29.Sato T, Nakajima H, Fujio K, Mori Y. Enhancement of prostaglandin E2 production by epidermal growth factor requires the coordinate activation of cytosolic phospholipase A2 and cyclooxygenase 2 in human squamous carcinoma A431 cells. Prostaglandins. 1997;53:355–369. doi: 10.1016/0090-6980(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 30.Sheng H, Shao J, Hooton EB, Tsujii M, DuBois RN, Beauchamp RD. Cyclooxygenase-2 induction and transforming growth factor beta growth inhibition in rat intestinal epithelial cells. Cell Growth Differ. 1997;8:463–470. [PubMed] [Google Scholar]
- 31.Gilbert RS, Reddy ST, Targan S, Harschman HR. TGF-β1 augments expression of the TIS10/prostaglandin synthase-2 gene in intestinal epithelial cells. Cell Mol Biol Res. 1994;40:653–660. [PubMed] [Google Scholar]
- 32.Gilbert RS, Reddy ST, Kujubu DA, Xie W, Luner S, Herschman HR. Transforming growth factor beta 1 augments mitogen-induced prostaglandin synthesis and expression of the TIS10/prostaglandin synthase 2 gene both in Swiss 3T3 cells and in murine embryo fibroblasts. J Cell Physiol. 1994;159:67–75. doi: 10.1002/jcp.1041590110. [DOI] [PubMed] [Google Scholar]
- 33.Berchuck A, MacDonald PC, Milewich L, Casey ML. Transforming growth factor-beta inhibits prostaglandin production in amnion and A431 cells. Prostaglandins. 1989;38:453–464. doi: 10.1016/0090-6980(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 34.Reddy ST, Gilbert RS, Xie W, Luner S, Herschman HR. TGF-beta 1 inhibits both endotoxin-induced prostaglandin synthesis and expression of the TIS10/prostaglandin synthase 2 gene in murine macrophages. J Leukocyte Biol. 1994;55:192–200. doi: 10.1002/jlb.55.2.192. [DOI] [PubMed] [Google Scholar]
- 35.Xie W, Herschman HR. v-src induces prostaglandin synthase 2 gene expression by activation of the c-Jun N-terminal kinase and the c-Jun transcriptional factor. J Biol Chem. 1995;270:27622–27628. doi: 10.1074/jbc.270.46.27622. [DOI] [PubMed] [Google Scholar]
- 36.Xie W, Herschman HR. Transcriptional regulation of prostaglandin synthase 2 gene expression by platelet-derived growth factor and serum. J Biol Chem. 1996;271:31742–31748. doi: 10.1074/jbc.271.49.31742. [DOI] [PubMed] [Google Scholar]
- 37.Guan Z, Buckman SY, Pentland AP, Templeton DJ, Morrison AR. Induction of cyclooxygenase-2 by the activated MEKK1/SEK1/MKK4 p38 mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:12901–12908. doi: 10.1074/jbc.273.21.12901. [DOI] [PubMed] [Google Scholar]
- 38.Subbaramaiah K, Chung WJ, Danenberg AL. Ceramide regulates the transcription of cyclooxygenase-2. J Biol Chem. 1998;273:32943–32949. doi: 10.1074/jbc.273.49.32943. [DOI] [PubMed] [Google Scholar]
- 39.Sheng HM, Williums CS, Shao J, Peng L, DuBois RN, Beauchamp RD. Induction of cyclooxygenase-2 by activated Ha-Ras oncogene in rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:22120–22127. doi: 10.1074/jbc.273.34.22120. [DOI] [PubMed] [Google Scholar]
- 40.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberhammer F, Wilson LW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warna JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 43.Dudley DT, Pang L, Decker ST, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kramer RM, Roberts EF, Um LS, Borsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J Biol Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- 45.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 46.Herrmann M, Lorenz H-M, Voll R, Grunke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elias JA, Gustilo K, Baeder W, Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987;135:3812–3816. [PubMed] [Google Scholar]
- 48.Topley N, Floege J, Wessel K, Hass R, Radeke H, Kaever V, Resch K. Prostaglandin E2 production is synergistically increased in cultured human glomerular mesangial cells by combinations of IL-1 and tumor necrosis factor-alpha-1. J Immunol. 1989;143:1989–1995. [PubMed] [Google Scholar]
- 49.Berenbaum F, Jacques C, Thomas G, Corvol MT, Bereziat G, Masliah Synergistic effect of Interleukin-1β and tumor necrosis factor α on PGE2 production by articular chondrocytes does not involve PLA2 stimulation. J Exp Cell Res. 1996;222:379–384. doi: 10.1006/excr.1996.0047. [DOI] [PubMed] [Google Scholar]
- 50.Modeer T, Yucel-Lindberg T, Lerner UH, Anderson G. Epidermal growth factor potentiates interleukin 1 and tumor necrosis factor-induced prostaglandin biosynthesis in human gingival fibroblasts. Cytokine. 1993;5:198–204. doi: 10.1016/1043-4666(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 51.Song JH, Sirois J, Houde A, Murphy BD. Cloning and developmental expression, and immunohistochemistry of cyclooxygenase 2 in the endometrium during embryo implantation and gestation in the mink (mustela vision) Endocrinology. 1998;139:3629–3636. doi: 10.1210/endo.139.8.6142. [DOI] [PubMed] [Google Scholar]
- 52.Duffy DC, Calcaterra TC, Lichtenstein AK. Regulation of transforming growth factor-beta 1-mediated apoptosis in head and neck squamous cell carcinoma. Laryngoscope. 1996;106:889–894. doi: 10.1097/00005537-199607000-00020. [DOI] [PubMed] [Google Scholar]
- 53.Oberhammer F, Fritish G, Pavelka M, Froschel G, Tiefenbacher R, Puricho T, Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in the regressing liver by transforming growth factor-beta 1 occurs without activation of an endonuclease. Toxicol Lett. 1992;64–65:701–770. doi: 10.1016/0378-4274(92)90250-n. [DOI] [PubMed] [Google Scholar]
- 54.Zushi S, Shinomura Y, Kiyohara T, Miyazaki Y, Kondo S, Sugimachi M, Higashimoto T, Kanayama S, Matsuzawa Y. STAT3 mediates the survival signal in oncogenic ras transfected intestinal epithelial cells. Int J Cancer. 1998;78:326–330. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 55.McClellan M, Keivit P, Auersperg N, Rodland K. Regulation of proliferation and apoptosis by epidermal growth factor and protein kinase C in human ovarian surface epithelial cells. Exp Cell Res. 1999;246:471–479. doi: 10.1006/excr.1998.4328. [DOI] [PubMed] [Google Scholar]
- 56.Fabregat I, Sanchez A, Alverez AM, Nakamura T, Benito M. Epidermal growth factor, but not hepatocyte growth factor, suppresses the apoptosis induced by transforming growth factor-beta in fetal hepatocytes in primary culture. FEBS Lett. 1996;384:14–18. doi: 10.1016/0014-5793(96)00266-9. [DOI] [PubMed] [Google Scholar]
- 57.Fox JC, Shanley JR. Antisense inhibition of basic fibroblast growth factor induces apoptosis in vascular smooth muscle cells. J Biol Chem. 1996;271:12578–12584. doi: 10.1074/jbc.271.21.12578. [DOI] [PubMed] [Google Scholar]
- 58.Gardner AM, Johnson GL. Fibroblast growth factor-2 suppress of tumor necrosis factor α-mediated apoptosis requires ras and the activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- 59.Ottonello L, Gonella R, Dapino P, Sacchetti C, Dallegri F. Prostaglandin E2 inhibits apoptosis in human neutrophilic polymorphonuclear leukocytes: role of intracellular cyclic AMP levels. Exp Hematol. 1998;26:895–902. [PubMed] [Google Scholar]
- 60.Sheng HM, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 61.Liu X-H, Yao S, Krischenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and downregulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 62.Ballif B, Mincek N, Barret J, Wilson M, Simmons D. Interaction of cyclooxygenases with an apoptosis- and autoimmunity-associated protein. Proc Natl Acad Sci USA. 1996;93:5544–5549. doi: 10.1073/pnas.93.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 64.Terada Y, Nakashima O, Inoshita S, Kuwahara M, Sasaki S, Marumo F. Mitogen-activated protein kinase cascade and transcription factors: the opposite role of MKK3/6-p38K and MKK1-MAPK. Nephrol Dial Transplant. 1999;14:45–47. doi: 10.1093/ndt/14.suppl_1.45. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Zhou G, Hu MCT, Yao Z, Tan TH. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor beta (TGF-beta)-activated kinase (TAK1), a kinase mediator of TGF beta signal transduction. J Biol Chem. 1997;272:22771–22775. doi: 10.1074/jbc.272.36.22771. [DOI] [PubMed] [Google Scholar]