Abstract
Sulindac sulfide, a metabolite of the nonsteroidal antiinflammatory drug (NSAID) sulindac sulfoxide, is effective at reducing tumor burden in both familial adenomatous polyposis patients and in animals with colorectal cancer. Another sulindac sulfoxide metabolite, sulindac sulfone, has been reported to have antitumor properties without inhibiting cyclooxygenase activity. Here we report the effect of sulindac sulfone treatment on the growth of colorectal carcinoma cells. We observed that sulindac sulfide or sulfone treatment of HCA-7 cells led to inhibition of prostaglandin E2 production. Both sulindac sulfide and sulfone inhibited HCA-7 and HCT-116 cell growth in vitro. Sulindac sulfone had no effect on the growth of either HCA-7 or HCT-116 xenografts, whereas the sulfide derivative inhibited HCA-7 growth in vivo. Both sulindac sulfide and sulfone inhibited colon carcinoma cell growth and prostaglandin production in vitro, but sulindac sulfone had no effect on the growth of colon cancer cell xenografts in nude mice.
Keywords: sulindac, sulindac sulfide, sulindac sulfone, chemoprevention, colorectal cancer
Introduction
Colorectal cancer remains a significant health concern for much of the industrialized world. Diagnosis often occurs at a late stage in the progression of this disease, which reduces the likelihood of effective treatment. Current treatment strategies often involve a combination of surgical resection and adjuvant therapy. Because of the unsatisfactory outcome of existing treatment methods, much emphasis has been placed on developing new treatment and prevention strategies.
Numerous epidemiologic studies indicate that chronic use of low-dose nonsteroidal antiinflammatory drugs (NSAIDs) are chemopreventive for colorectal cancer [1,2]. NSAIDs cause regression of existing polyps in familial adenomatous polyposis (FAP) patients and reduce the tumor burden in animal models of colorectal cancer [3–6]. NSAIDs inhibit the activity of the cyclooxygenases (COX), which are key enzymes in prostaglandin (PG) production. Unfortunately, prolonged NSAID use can result in side effects such as gastrointestinal ulceration and bleeding. It is widely believed that this ulcerogenic activity is due to chronic inhibition of PG production via COX-1 inhibition in the gastric mucosa. Accordingly, researchers have tried to identify NSAID derivatives that retain antitumor activity but do not affect PG production in gastric mucosa.
Sulindac sulfoxide is a prodrug that is reduced in vivo to sulindac sulfide or oxidized to sulindac sulfone. The sulfide derivative effectively inhibits the COX enzymes. Sulindac sulfone does not interfere with COX activity when applied to the recombinant enzyme in vitro (data not shown [7]. Studies in which colorectal cancer cell lines were treated with high doses of either sulfide or sulfone indicated that both derivatives can induce apoptosis and alter proliferation rates [7]. There is evidence that sulindac sulfone can induce apoptosis in cell lines that do not express either COX-1 or COX-2 [7]. Accordingly, the mechanism of action of this compound, at the effective doses, is independent of any possible interaction with COX in these cells. Because sulindac sulfone does not appear to inhibit the COX activity and can induce apoptosis in colorectal cancer cell lines independent of COX expression, it held some promise as a potential chemopreventive agent for colorectal cancer with improved safety over NSAIDS.
It has previously been reported that sulindac sulfone is capable of reducing the incidence, multiplicity and tumor burden in the azoxymethane (AOM) rat model of colorectal cancer [8]. In another animal model, the Min mouse, sulindac sulfone had no effect on polyp number or size whereas sulindac sulfide was quite effective in causing polyp regression [4]. In both studies, drug treatment was initiated before a lesion had developed. In this study we tested the hypothesis that sulindac sulfone can inhibit the growth of highly malignant colorectal carcinoma cells in both in vitro and in vivo tumor models. These studies may help to clarify the potential in vivo effectiveness of sulindac sulfone in colorectal cancer treatment.
Materials and Methods
Reagents
Sulindac sulfide and sulindac sulfone were obtained from Merck and Co, Rahway, NJ, and dimethyl sulfoxide (DMSO) (EM Science, Gibbstown, NJ) was used as the solvent. Concentrated drug stocks were diluted in Dulbecco's modified Eagle′s medium (DMEM) (GIBCO-BRL, Grand Island, NY) before addition to cell cultures. The DMSO concentration in cultures was kept at 0.1%, and the purity of sulindac sulfone was verified by NMR.
Prostaglandin Measurement
Subconfluent cell cultures were treated with the indicated compound for 24 hours. One hour before harvesting, arachidonate was added to the media to a final concentration of 10 µmol/L along with fresh drug. Eicosanoids were quantified in media from cell incubations by using stable isotope dilution techniques with gas chromatography negative ion chemical ionization-mass spectrometry, as previously described [9,10]. The limits of sensitivity for detection of either PGE2, PGD2, PGF2, thromboxane B2, or 6-ketoPGF1 is 4 pg/mL. PG levels were standardized to the number of viable cells.
In Vitro Growth Assays
Either 2.5 x 104 cells/well for HCT-116, or 5 x 104 cells per well for HCA-7 cells were suspended in 0.5 mL of 1:2 diluted Matrigel (Collaborative Biomedical Products, Bedford, MA). The cell/Matrigel mixture was plated into 24-well plates and treated with the indicated dose of sulindac sulfide or sulfone. Media was replaced every other day with media containing the appropriate amount of drug.
Western Blotting
Immunoblot analysis of cell protein lysates were performed as previously described [11]. Briefly, cells were lysed for 30 minutes in radio imunoprecipitation buffer (RIPA) (1 x phosphate-buffered saline [PBS], 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) and then clarified cell lysates (50 µg) were denatured and fractionated by 10% SDS-polyacrylamide gel electrophoresis (PAGE). The proteins were transferred to polyvinylidene flouride (PVDF) membranes after electrophoresis. The filters were blocked 3 hours in BLOTTO (0.15 mol/L NaCl; 10 mmol/L Tris-HCl, pH 7.4: 0.1% Tween-20; 5% nonfat dry milk), and they were then blotted overnight with either COX-1 (1:500) or COX-2 (1:500) specific antibodies (COX-1, cat #1753; COX-2, cat #1746; Santa Cruz Biotechnology Inc, Santa Cruz, CA). The membranes were washed in BLOTTO before a 1-hour incubation with donkey anti-goat horseradish peroxidase (HRP) conjugated secondary antibody. The membranes were washed in TBST (0.15 mol/L NaCl; 10 mmol/L Tris-HCl, pH 7.4; 0.1% Tween-20) and developed using the Enhanced Chemiluminescence (ECL) plus chemiluminescence system (Amersham, Arlington Heights, IL), and then exposed to Hyperfilm (Amersham).
Xenograft Model of Tumor Biology
HCA-7 and HCT-116 (ATCC #CCL 247) cells were grown on plastic culture dishes according to standard cell culture techniques [12]. The cells were trypsinized and resuspended in sterile phosphate buffered saline (PBS) and then pelleted by brief centrifugation at 1500 rpm. The supernant was aspirated and cells were resuspended in PBS and counted with a hemocytometer. A final concentration of 5 x 107 cells/mL was made and 100 µL of cell suspension was injected subcutaneously with a tuberculin syringe and a 27-gauge needle. For both HCA-7 and HCT-116 cell lines animals were randomly divided into treatment and control arms, with five animals in each group. In the sulindac sulfide experiments seven animals were included in each group. Treatment was initiated at the time the tumors were implanted with the exception of the sulindac sulfide treatment group in which treatment was delayed until the tumor was established (7 weeks). When no effect was observed on tumor growth with sulindac sulfone treatment, the amount of sulfone was increased until a maximal dose of 100 mg/kg was achieved by week 5. The size of the tumor was determined at 1-week intervals by direct measurement. The volume was calculated according to the equation (V = [L x W2] x 0.5), where V = volume, L = length, and W = width [13].
Statistical Methods
Student's t-test was used to determine significant differences in in vitro assays. In the xenograft experiments, ANOVA with repeated measures was employed to evaluate the significance of drug treatment on tumor growth.
Results
Effect of Sulindac Sulfone on Prostaglandin E2 Production in HCA-7 Cells
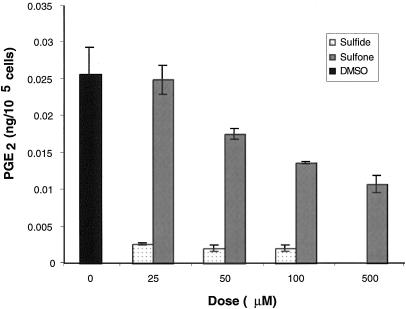
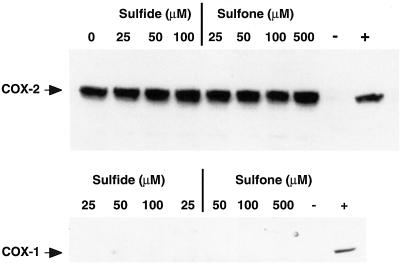
Sulindac sulfide, the active metabolite of sulindac, inhibits the enzymatic activity of recombinant COX-1 and COX-2. It has been reported that sulindac sulfone does not inhibit the activity of either COX isoform using recombinant enzyme assay [7]. We confirmed this by testing the effect of sulfone on recombinant COX activity and saw no change in enzyme activity even when millimolar amounts of drug were added (data not shown). However, it is possible that in vitro the sulfone derivative may affect other steps in the eicosanoid biosynthetic pathway. Therefore, we tested whether or not sulfone treatment altered PGE2 levels in a colorectal cancer cell line, HCA-7, which expresses high levels of COX-2. As expected, 25 µmol/L sulfide treatment reduced PGE2 levels by 95%. However, to our surprise, sulindac sulfone treatment also inhibited PGE2 production by HCA-7 cells (IC50 = .360 µmol/L; Figure 1). To evaluate whether or not the decrease in PG synthesis was limited to PGE2 we tested the effect of sulfone treatment on PG production in rat intestinal epithelial (RIE) cells overexpressing COX-2, which produce mainly prostacyclin. Levels of 6-ketoPGF1α, the stable metabolite of prostacyclin, were measured after treatment with either sulindac sulfide or sulfone. Sulindac sulfide at 25 µmol/L reduced 6-ketoPGF1α levels by 73.5% and sulindac sulfone at 100 µmol/L reduced 6-ketoPGF1α by 29.2%. Therefore, inhibition of PG production by sulindac sulfone in vitro is not specific to one class of PGs. There was no detectable COX-1 in HCA-7 cells and neither sulindac sulfide nor sulfone affected COX-2 expression (Figure 2). Therefore, the decrease in PG production did not result from alterations in steady state levels of COX-2.
Figure 1.
Sulindac sulfide and sulfone decrease PG levels in HCA-7 cells. HCA-7 cells were plated in 100mm dishes and grown to 70% confluence, then treated for 23 hours with sulindac sulfide, sulindac sulfone, or vehicle (DMSO). One hour before harvesting media, the cells were washed with PBS and media containing 10 µmol/L arachidonic acid and the indicated dose of drug was added. PGE2 levels were determined by using gas chromatography negative ion chemical ionization-mass spectrometry and standardized to cell number. Error bars represent the standard error of the mean.
Figure 2.
Sulindac sulfide and sulfone do not affect the expression of COX-1 or COX-2 in HCA-7 cells. Whole cell lysates (50 µg) from HCA-7 cells treated for 24 hours with either sulindac sulfide or sulfone were resolved by SDS-PAGE. Proteins were transferred to PVDF membranes and blotted with either COX-2 (top panel) or COX-1 (bottom panel) specific antisera, then imaged by ECL, followed by autoradiography. For COX-2, 50 µg of HCA-7 or HCT-116 cell lysates served as COX-2 positive (+) and negative (-) controls, respectively. For COX-1, 50 µg of MC-26 or HCT-116 cell lysates served as COX-1 positive (+) and negative (-) controls, respectively.
Effect of Sulindac Sulfone or Sulfide on Colorectal Cancer Cell Colony Growth in Matrigel
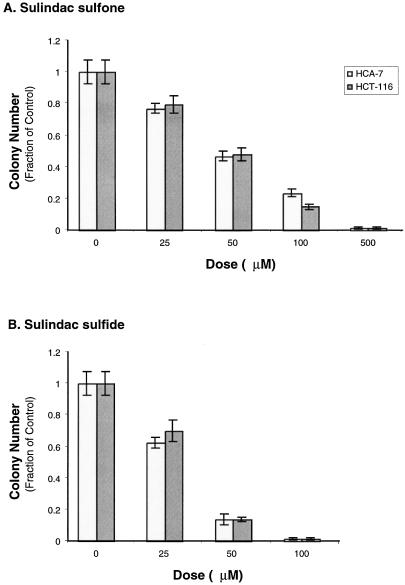
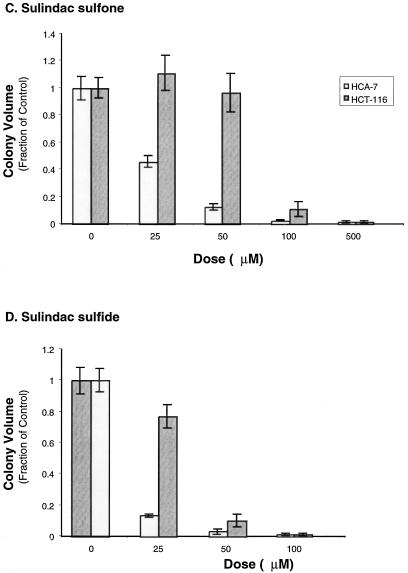
Because sulindac sulfone decreased PG levels in cultured HCA-7 cells we tested whether sulfone impaired the ability of HCA-7 or HCT-116 cells to form discrete colonies in Matrigel. HCA-7 cells express high levels of COX-2, whereas there is no detectable COX-1 or COX-2 protein in HCT-116 cells [12]. COX-2 selective inhibitors reduce the growth of HCA-7 cells in Matrigel, whereas there is no effect on the growth of HCT-116 cells. Each cell line was plated in triplicate in Matrigel mixed with the indicated amount of either sulindac sulfide, sulfone, or vehicle (DMSO). Both HCA-7 and HCT-116 cells showed equivalent reductions in colony number with sulfone or sulfide treatment (sulfone EC50 ≈ 50 µmol/L, sulfide EC50 < 50 µmol/L (Figure 3A), however, HCA-7 colony size was much more sensitive than HCT-116 to treatment with either sulfone or sulfide (Student t-test at 25 µmol/L: sulfone, P < .01; sulfide, P < .0001; Figure 3C).
Figure 3.
HCA-7 and HCT-116 cell growth in an artificial matrix is attenuated by treatment with sulindac sulfone. HCT-116 (2.5 x 104 cells/well) and HCA-7 (5 x 104 cells/well) cells were grown in Matrigel containing the indicated dose of sulindac sulfide (B and D), sulindac sulfone (A and C), or the solvent DMSO in triplicate. The media was replaced every other day with media containing fresh drug. After 14 days, colony counts were obtained by examining 10 random fields per well (A and B). Each well was photographed three times, and measurements of 10 random fields were obtained and volumes calculated (C and D). Error bars represent standard error of the mean.
Treatment of COX-2-Positive and Negative Colorectal Cancer Cell Xenografts with Sulindac Sulfone
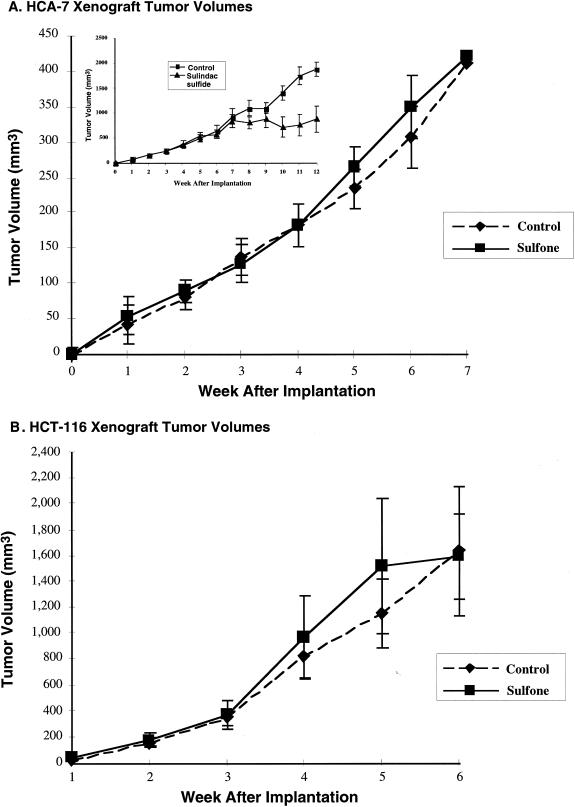
Because sulindac sulfone inhibited the in vitro growth of HCA-7 and HCT-116 cells, we sought to test whether sulindac sulfone altered the in vivo growth of either cell line by using a xenograft tumor model. HCA-7 or HCT-116 cells were implanted into the dorsal flank of nude mice. We have found that in this model NSAIDs reduce tumor growth when given every other day at doses between 5 to 10 mg/kg. Therefore, immediately preceding tumor cell implantation an initial dose of 10 mg/kg of sulfone was injected intraperitoneally. This dose was repeated every other day, and tumor dimensions were measured at weekly intervals as shown in Figure 4. No effect was observed on either HCA-7 or HCT-116 tumor growth after 2 weeks of sulfone treatment at this dosing regimen. Previously, we have observed a significant difference in tumor size after 2 weeks of treatment of HCA-7 xenografts with selective inhibitors of COX-2 [12,14]. Therefore, the amount of sulfone was increased until a maximal dose of 100 mg/kg was achieved by week 5. Throughout the duration of this experiment no differences in tumor volume were observed with sulfone treatment in either HCA-7 or HCT-116 xenografts.
Figure 4.
Sulindac sulfone does not alter the growth of HCA-7 or HCT-116 xenografts. HCA-7 (A) or HCT-116 (B) cells were grown to 70% confluence and then trypsinized; 5 x 106 cells were injected subcutaneously into the dorsal flank of athymic mice. The mice were divided into treatment and control groups. The initial dose of sulindac sulfone was 10 mg/kg and then increased to 100 mg/kg by week 5. The volume of the tumor was calculated according to the equation (V = [L x W2] x 0.5), where V = volume, L = length, and W = width (insert). HCA-7 cells (5 x 106 cells) were xenograft into nude mice. After 7 weeks of tumor growth intraperitoneal injections with sulindac sulfide were initiated at a dose of 10 mg/kg every other day. Tumor measurements were obtained as described.
A more rigorous test of the chemotherapeutic potential of a drug is to test its effect on the growth of established xenografts. This removes the possibility of the drug interfering with tumor implantation and directly measures the ability of a compound to influence the growth of an established tumor. Sulindac sulfide was used at a dose of 10 mg/kg every other day to treat HCA-7 xenografts that had been implanted 7 weeks earlier. In this experiment, a significant reduction in tumor growth was observed within 2 weeks of treatment initiation (Figure 4A, insert).
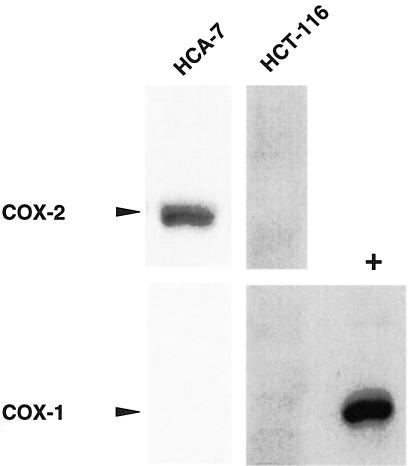
To measure the COX expression profile of HCA-7 and HCT-116 xenografts, tumor lysates were made and Western blotting analysis was done with COX-1 or COX-2 selective antibodies. In agreement with previous reports, HCA-7 xenografts expressed COX-2 but not COX-1 protein, and HCT-116 tumors contained neither COX-1 nor COX-2 protein (Figure 5) [12].
Figure 5.
Colorectal cancer xenograft COX-1 and COX-2 expression. Tumor lysates from HCA-7 or HCT-116 xenografts were resolved via SDS-PAGE. Proteins were transferred to membranes and then blotted with either COX-1- or COX-2-specific antisera. Fifty micrograms of MC-26 (+) cell lysates served as COX-1-positive controls.
Prostaglandin Measurements in HCA-7 Xenografts
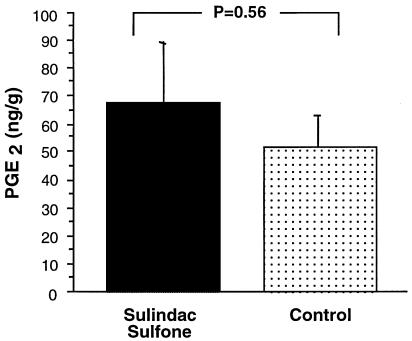
Although a significant change in tumor size was not observed with sulfone treatment, we assessed the effect of the sulfone on PGE2 production in the HCA-7 xenografts. PGE2 is the major PG produced by HCA-7 cells and is reported to be elevated in colorectal cancers [15]. At necropsy the tumors were assayed for PGE2 levels. Sulindac sulfone treatment had no effect on the PGE2 levels in either HCA-7 (Figure 6) or HCT-116 xenografts (data not shown).
Figure 6.
HCA-7 xenograft PGE2 levels. HCA-7 tumor PGE2 levels with and without sulindac sulfone treatment as determined by stable isotope dilution techniques with gas chromatography negative ion chemical ionization-mass spectrometry (see methods).
Discussion
Considerable excitement has been generated about the chemopreventative effects of NSAIDs on colorectal cancer since Waddell and Loughry first observed that sulindac reduced the number of rectal polyps in a patient with Gardner′s syndrome [16]. Sulindac, in a controlled clinical trial, was found to decrease the size and number of polyps in FAP patients [17]. Unfortunately, sulindac, like most NSAIDs, can exhibit undesirable gastrointestinal side effects.
Sulindac is a prodrug, which is reduced in vivo to sulindac sulfide. Sulindac sulfide can inhibit both COX enzymes and has been shown to have antiproliferative and proapoptotic properties in colorectal carcinoma cells [18]. Sulindac can also be oxidized to a sulfone derivative which also has been reported to induce apoptosis and growth arrest in colorectal cancer cell lines [7]. Piazza and colleagues reported that sulindac sulfone treatment reduced the number of adenomas and carcinomas in the AOM-treated rat [8]. However, in another animal model of colorectal cancer, the APC mutant Min mouse, sulindac sulfone was ineffective at reducing tumor multiplicity [4]. Both AOM rat and Min mouse studies were designed to test the ability of the sulfone derivative to prevent tumor growth in a setting in which normal epithelium is induced to a transformed state by either carcinogen exposure or by the presence of a preexisting genetic mutation. In this study, we tested the effect of sulfone treatment on the growth of colorectal cancer cells by using both in vitro and in vivo growth assays. We have previously found that selective COX-2 inhibitors block tumor growth in this model.
We found that sulindac sulfone did not modify the growth of colorectal cancer cells xenografted into nude mice. In order to control the dose given to individual animals and to increase the likelihood that sulfone would enter the circulation and thus reach the tumor site, we chose to deliver the drug by intraperitoneal injection. There was no effect on tumor growth observed in either HCA-7 or HCT-116 xenografts, even when doses of 100 mg/kg were given. In humans, sulindac sulfide has a serum half-life of 13 to 14 hours, and sulindac sulfone has a longer serum half-life of 20 to 22 hours [19,20], suggesting that differences in the chemotherapeutic potential of these compounds is not due to rapid clearance of sulindac sulfone. Furthermore, similar studies with selective inhibitors of COX-2 such as SC-58125 or meloxicam at 7.5 mg/kg and 40 mg/kg, respectively, inhibited tumor growth within 2 weeks of starting treatment [12,14]. Additionally, sulindac sulfide effectively inhibited tumor growth within 2 weeks of initiating treatment.
An alternative explanation for sulindac sulfone's lack of an effect on tumor growth may be a requirement for gut specific cofactors in order for an antineoplastic effect in vivo. However, sulindac sulfone is able to induce apoptosis and inhibit the in vitro growth of colorectal cancer cells [7,21] in the absence of gut-derived cofactors and sulindac sulfone is effective at inhibiting the in vitro and in vivo growth of nongastrointestinal-derived cancers [22]. Furthermore, sulindac sulfone has no effect on tumor growth in the Min mouse. In this model the tumors arise in the gastrointestinal tract and are exposed to luminal contents during drug treatment.
In agreement with previous reports, by using in vitro growth assays, we found that sulindac sulfone could inhibit the growth of HCA-7 and HCT-116 cells. We observed a decrease in both the size and number of HCA-7 colonies grown in artificial matrix, suggesting that sulindac sulfone inhibits colony formation and alters the growth characteristics of surviving colonies, possibly by altering the balance between cell proliferation and programmed cell death.
If sulindac sulfone does not directly inhibit the activity of recombinant COX, then why is a reduction in PGs observed when HCA-7 cells are treated with sulindac sulfone? It is possible that sulfone treatment could affect the activity of PG synthases, but this seems unlikely because sulindac sulfone also inhibited the production of prostacyclin in RIE-COX-2 cells and PGE2 by RKO cells transiently transfected with rat COX-2 (data not shown). Decreasing the steady state levels of COX-2 in HCA-7 cells could result in decreased PG production. We determined by Western blotting that sulindac sulfone did not influence either COX-1 or COX-2 protein levels in HCA-7 cells. It has been reported that sulindac sulfone can inhibit phospholipase A2 (15% at 0.1 – 1000 µmol/L) [8]. In our experiments, we added exogenous arachidonate to the cell culture media 1 hour before obtaining samples for PG determination; it is unlikely that inhibition of PLA2 activity by sulindac sulfone could affect PG production under these circumstances.
Why does sulindac sulfone inhibit colorectal cancer cell growth in in vitro assays and yet have no effect on the growth of the cells in the tumor xenograft model? Tumor xenograft assays evaluate a compound's effectiveness in the setting of whole animal physiology. Clearance, bioavailability, and biotransformation may attenuate or amplify a compound's effect on the tumor's biological characteristics. Furthermore, in vivo assays test the ability of a compound to inhibit tumor growth in an environment where tumor/stromal interactions are occurring. It is possible that the pathway or pathway(s) by which sulfone inhibits the growth of HCA-7 and HCT-116 cells in vitro are compensated for by stromal-derived signals present in an in vivo system.
The effectiveness of sulindac sulfone treatment on in vivo tumor growth is questionable. Sulindac sulfone has been reported to be effective at reducing the tumor burden in the AOM rat model [8] and in the MNU-induced mammary tumor model [23]. However, in these reports, there were decreases in weight gain with sulindac sulfone treatment. It is possible that the decrease in weight gain during treatment may contribute to the reduction in tumor number in these models [24,25]. Furthermore, sulindac sulfone was ineffective at inhibiting polyp formation in the Min mouse model [4]. These studies were designed to test sulfone's effectiveness at preventing tumor formation. In this report, we determined that sulindac sulfone inhibited colorectal cancer cell growth in vitro with an associated reduction in PG production, but sulindac sulfone treatment did not alter the growth characteristics of colorectal cancer xenografts in vivo. Human clinical chemoprevention trials evaluating sulindac sulfone (FGN-1) are underway. These trials should provide definitive evidence about the usefulness of sulindac sulfone as a chemoprotective agent.
Acknowledgements
We thank Amit Kalgutkar and John Plastaras for their assistance with verifying the purity of the sulindac derivatives and for prostanoid assays. HCA-7 cells were a generous gift from Susan Kirkland.
This work was supported in part by United States Public Health Services Grants DK 47297-OIAI (R.N.D.), 5P030 ES-00267-29, CA68485-04 (R.N.D.), DK48831, and GM15431 (J.D.M.). R.N.D. is a recipient of a VA Research Merit Grant and the Mina C. Wallace Professor of Gastroenterology and Cancer Prevention. The T.J. Martell Foundation also provided support for this research project.
Abbreviations
- NSAID
nonsteroidal antiinflamatory drug
- FAP
familial adenomatous polyposis
- PGE2
prostaglandin E2
- COX
cyclooxygenases
- AOM
azoxymethane
- DMSO
dimethyl sulfoxide
- FGN-1
sulindac sulfone
References
- 1.Thun MJ, Namboodiri MM, Heath CWJ. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoud NN, Boolbol SK, Dannenberg AJ, Mestre JR, Bilinski RT, Martucci C, Newmark HL, Chadburn A, Bertagnolli MM. The sulfide metabolite of sulindac prevents tumors and restores enterocyte apoptosis in a murine model of familial adenomatous polyposis. Carcinogenesis. 1998;19:87–91. doi: 10.1093/carcin/19.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997;57:1301–1305. [PubMed] [Google Scholar]
- 6.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, Kinzler KW. Sulindac suppresses tumorigenesis in the min mouse. Carcinogenesis. 1996;17:1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 7.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 8.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, Bogert C, Guillen JM, Brendel K, Gross PH, Sperl G, Ritchie J, Burt RW, Ellsworth L, Ahnen DJ, Pamukcu R. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. [PubMed] [Google Scholar]
- 9.Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, DuBois RN, Jetton T, Morrow J. EGF receptor activation induces nuclear targeting of COX-2, basolateral release of prostaglandins and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci USA. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DuBois RN, Awad J, Morrow J, Roberts LJ, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-α and phorbol ester. J Clin Invest. 1994;93:493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuBois RN, Shao J, Sheng H, Tsujii M, Beauchamp RD. G1 delay in intestinal epithelial cells overexpressing prostaglandin endoperoxide synthase-2. Cancer Res. 1996;56:733–737. [PubMed] [Google Scholar]
- 12.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey R, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JL, Sun L, Myeroff X, Wang LE, Gentry J, Yang J, Liang E, Zborowska S, Markowitz JK, Willson, Brattain MG. Demonstration that mutation of the type II transforming growth factor beta receptor inactivates its tumor suppressor activity in replication error-positive colon carcinoma cells. J Biol Chem. 1995;270:22044–22049. doi: 10.1074/jbc.270.37.22044. [DOI] [PubMed] [Google Scholar]
- 14.Goldman AP, Williams CS, Sheng H, Lamps LW, Williams VP, Pairet M, Morrow JD, DuBois RN. Meloxicam inhibits the growth of colorectal cancer cells. Carcinogenesis. 1998 doi: 10.1093/carcin/19.12.2195. [DOI] [PubMed] [Google Scholar]
- 15.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 16.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 17.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 18.Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 19.Ravis WR, Diskin CJ, Campagna KD, Clark CR, McMillian CL. Pharmacokinetics and dialyzability of sulindac and metabolites in patients with end-stage renal failure. J Clin Pharmacol. 1993;33:527–534. doi: 10.1002/j.1552-4604.1993.tb04699.x. [DOI] [PubMed] [Google Scholar]
- 20.Strong HA, Warner NJ, Renwick AG, George CF. Sulindac metabolism: the importance of an intact colon. Clin Pharmacol Ther. 1985;38:387–393. doi: 10.1038/clpt.1985.192. [DOI] [PubMed] [Google Scholar]
- 21.Piazza GA, Rahm AL, Krutzsch M, Sperl G, Paranka NS, Gross PH, Brendel K, Burt RW, Alberts DS, Pamukcu R, et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55:3110–3116. [PubMed] [Google Scholar]
- 22.Thompson HJ, Jiang C, Lu JX, Mehta RG, Piazza GA, Paranka NS, Pamukcu R, Ahnen DJ. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer Res. 1997;57:267–271. [PubMed] [Google Scholar]
- 23.Thompson HJ, Jiang C, Lu J, Mehta RG, Piazza GA, Paranka NS, Pamukcu R, Ahnen DJ. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer Res. 1997;57:267–271. [PubMed] [Google Scholar]
- 24.Reddy BS, Wang CX, Maruyama H. Effect of restricted caloric intake on azoxymethane-induced colon tumor incidence in male F344 rats. Cancer Res. 1987;47:1226–1228. [PubMed] [Google Scholar]
- 25.Kumar SP, Roy SJ, Tokumo K, Reddy BS. Effect of different levels of calorie restriction on azoxymethane- induced colon carcinogenesis in male F344 rats. Cancer Res. 1990;50:5761–5766. [PubMed] [Google Scholar]