Abstract
Pneumococcus is the most common and aggressive cause of bacterial meningitis and induces a novel apoptosis-inducing factor–dependent (AIF–dependent) form of brain cell apoptosis. Loss of production of two pneumococcal toxins, pneumolysin and H2O2, eliminated mitochondrial damage and apoptosis. Purified pneumolysin or H2O2 induced microglial and neuronal apoptosis in vitro. Both toxins induced increases of intracellular Ca2+ and triggered the release of AIF from mitochondria. Chelating Ca2+ effectively blocked AIF release and cell death. In experimental pneumococcal meningitis, pneumolysin colocalized with apoptotic neurons of the hippocampus, and infection with pneumococci unable to produce pneumolysin and H2O2 significantly reduced damage. Two bacterial toxins, pneumolysin and, to a lesser extent, H2O2, induce apoptosis by translocation of AIF, suggesting new neuroprotective strategies for pneumococcal meningitis.
Introduction
Bacteria-induced apoptosis plays an important role in tissue damage in infectious diseases (1) and may play an essential role in neuronal cell death in meningitis. Survivors of bacterial meningitis suffer from a broad spectrum of neurologic sequelae that arise from neuronal cell damage. Pneumococcus is the most common and most aggressive human meningeal pathogen, causing death in up to 30% of cases and neurologic sequelae in 30–50% of survivors (2–4). Permanent loss of neurons by the induction of apoptosis in the hippocampus (5–8) likely contributes to this poor outcome. One trigger of this damage is the host inflammatory response, as blocking leukocyte invasion into the cerebrospinal fluid (CSF) is partially neuroprotective (7). In addition, pneumococcus directly induces the rapid apoptosis of primary rat hippocampal and cortical neurons, and of human microglial and neuronal cell lines (9). As is true for most disease models including meningitis, the bacterial toxins that trigger the host cell apoptotic response have not been identified.
Apoptosis of brain cells induced by direct exposure to pneumococci is not dependent on caspases but rather involves rapid and massive damage to mitochondria. This results in the release of apoptosis-inducing factor (AIF), and blocking AIF function effectively blocks pneumococcus-induced apoptosis (9). Pneumococcus produces a wide range of potentially toxic factors that could contribute to this apoptosis, including cell wall components and surface-associated and secreted toxins (10–13). Important pathogenicity factors unique to Streptococcus pneumoniae include the exotoxins H2O2 and the pore-forming molecule pneumolysin. Pneumococcus is unusual in that it is the only invasive human pathogen that lacks catalase (14), and the release of H2O2 is important in models of pneumococcal pneumonia (15). Pneumolysin is a thiol-activated pore-forming molecule that has potent cytotoxic activity (16), and pneumolysin also plays an important pathogenic role in pneumococcal-induced pneumonia and otitis media (17, 18).
During pneumococcal meningitis, the major site of apoptotic damage is the dentate gyrus of the hippocampus (5, 7). Pneumococci do not penetrate the hippocampus but rather accumulate in high numbers in the CSF of the cerebral ventricles (19). Since the lateral ventricles are in close proximity to the hippocampus, neurons could be directly exposed to secreted bacterial toxins. In particular, the extracellular fluid around brain cells is contiguous with the CSF (20), and the diffusion between the CSF and the cerebral extracellular fluid could deliver soluble, secreted bacterial toxins. The uniquely high morbidity of pneumococcal meningitis versus other meningeal pathogens suggests that pneumococcus-specific toxins induce the apoptotic response. Soluble and secreted factors like pneumolysin and H2O2 fit well to these criteria.
Here we report that two secreted toxins, pneumolysin and H2O2, mediate pneumococcal-induced apoptosis. The mechanism of this apoptosis involves translocation of intracellular calcium and AIF. Either pneumolysin or H2O2 is sufficient to induce mitochondrial damage and apoptosis in vitro. Inactivation of these toxins effectively prevents damage to neurons of the dentate gyrus in experimental pneumococcal meningitis.
Methods
Brain cell culture.
A human microglial cell line (HMC) (C.A. Colton, Georgetown University, Washington, DC, USA) that expresses most markers common to primary human microglial cells was cultured as previously described (9, 21). Primary rat hippocampal neurons were isolated from fetal rats at embryonic day 18 as described (22). Briefly, hippocampus was dissected, incubated in trypsin-EDTA (Biochrom, Berlin, Germany), dissociated with a Pasteur pipette, and plated in starter medium (neurobasal medium and supplement B27; Invitrogen, Karlsruhe, Germany). The status of cultures was assessed by light microscopy, and viability was tested by trypan blue dye exclusion. TNF-α, IL-1β, and RANTES were measured with ELISA kits (BioSource International, Rattlingen, Germany). Nitrite was detected by Griess reaction. A monoclonal antibody against TNF-α (IP-140; Genzyme GmbH, Konstanz, Germany) was used.
Bacterial cell culture.
To determine which pneumococcal factors contribute to the induction of host cell apoptosis, we tested a panel of pneumococcal mutants for their ability to induce apoptosis in vitro. Because of their acute susceptibility to pneumococcal-induced apoptosis, human microglial cells were chosen as the most permissive system to screen these mutants. The following pneumococci and isogenic mutants were used: D39 capsular type 2 and R6, its nonencapsulated derivative (Rockefeller University, New York, New York, USA); the pneumolysin-negative mutant plnA– (provided by D. Briles, University of Alabama, Birmingham, Alabama, USA) (23); the pyruvate oxidase mutant spxB–, deficient in H2O2 production (12); the plnA–/spxB– double mutant; the choline-binding protein A mutant cbpA–, deficient in adhesion (24); the competence-defective mutant comA– (25); the permease mutant psaA– (26), deficient in import of manganese; the IgA1 protease mutant igA– (27); the mutant zmpB– (13), deficient in a zinc metalloprotease; and lytA– (28), which is defective in autolysin.
The plnA–/spxB– double mutant was constructed as follows: A 690-bp fragment, generated by PCR using primers that flank Sau3A sites in pneumolysin (5′-TAATCCCACTCTTCTTGCGG-3′ and 5′-ATAGGAAATCGGCAAGCCTG-3′), was ligated into the vector pWG5 carrying chloramphenicol resistance (29). The recombinant plasmid was amplified and used to transform spxB– to create a double knockout mutant (23). Homologous recombination was confirmed by PCR. Pneumococci were grown in C+Y medium (30) without (wild-type) or with (mutants) 1 μg/ml erythromycin and, for the double mutant, 2 μg/ml chloramphenicol.
Pneumolysin was purified as previously described (31) and used in concentrations of 0.1–10 μg/ml. H2O2 was purchased from Merck KGaA (Darmstadt, Germany) and used in concentrations of 2–2000 μM. The concentrations of the toxins used were within physiological levels: 1 μg pneumolysin is equivalent to 108 CFU pneumococci (32), and the production of up to 410 μM H2O2 per gram of pneumococcal protein per minute has been reported (15). S. pneumoniae has been shown to produce H2O2 in liquid culture in ranges between 1 and 10 mM (46). Point mutants of pneumolysin were from T.J. Mitchell as described (16).
Assays for differentiation of live, apoptotic, and necrotic cells.
Ethidium bromide (EB) (Sigma Chemical Co., St. Louis, Missouri, USA) and acridine orange (AO) (Sigma Chemical Co.) are fluorescent intercalating DNA dyes that allow for differentiation of live, apoptotic, and necrotic cells (9, 33). The in situ cell death TUNEL detection kit was purchased from Roche Molecular Biochemicals (Grenzach-Wylen, Germany), and the annexin V–FITC propidium iodide kit was from Beckman Coulter Inc. (Miami, Florida, USA). Both were used as described by the manufacturers. Cell death was also assessed by the release of lactate dehydrogenase (LDH) into the culture medium (34) as determined by a colorimetric assay (Sigma Chemical Co.).
Immunohistochemistry and fluorescence stainings.
Brain sections were incubated with an anti-pneumolysin specific antibody (Novocastra Laboratories Ltd., Newcastle upon Tyne, United Kingdom), and Vectastain Elite ABC kit (Vector Laboratories Inc., Burlingame, California, USA) was used to visualize primary antibody binding. Following fixation (4% paraformaldehyde) and permeabilization (0.1% Triton X-100), neurons were incubated with an anti-AIF antibody (35) diluted 1:500 for 1 hour (control was with PBS). A fluorescent Cy3 antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) visualized binding of the primary anti-AIF antibody. Nuclei were stained with HOECHST 33258 dye (Molecular Probes Inc., Eugene, Oregon, USA). Increases of intracellular reactive oxygen species (ROS) were monitored with the ROS-specific dye dihydrorhodamine 123 (DHR123) (36) with a multiwell fluorescence plate reader (PerSeptive Biosystems, Framingham, Massachusetts, USA) according to the manufacturer’s instructions and blocked with N-acetyl cysteine (NAC) (10 mM; Sigma Chemical Co.) or catalase (1,250 U/ml; Sigma Chemical Co., C3155, 50,000 units/mg, <0.01 mg Thymol/ml, <0.05 endotoxin units LPS; E-Toxate test, Sigma Chemical Co.). Cells were preincubated with 10 μM DHR123 (Molecular Probes Inc.) for 1 hour and then incubated with pneumococci. DHR123 fluoresces when oxidized to rhodamine 123. Increases of intracellular Ca2+ were monitored by Fluo-4 (37). After incubation with pneumococci, cells were incubated with Fluo-4 (10 μM, for 45 minutes) (Molecular Probes Inc.) and intracellular Ca2+ was determined by fluorescence. BAPTA-AM was used as an intracellular and extracellular Ca2+ chelator (38). Cells were preincubated with BAPTA-AM (5 μM) (Molecular Probes Inc.) for 1 hour and then incubated with pneumococci in the presence and absence of BAPTA-AM. Fluo-4 was measured with a multiwell fluorescence plate reader (excitation 485 nm, emission 520 nm). For electron microscopy, after pneumococcal challenge, microglial cells were fixed in 2.5% glutaraldehyde in 100 mM cacodylate buffer overnight at 4°C, postfixed, dehydrated, and embedded as described previously (9).
Rabbit model of pneumococcal meningitis.
Thirty-two 2-kg male New Zealand white rabbits (Myrtle’s Rabbitry Inc., Thompson Station, Tennessee, USA) were anesthetized with a mixture of ketamine (35 mg/kg; Fort Dodge Laboratories, Fort Dodge, Iowa, USA) and xylazine (5 mg/kg; Miles Laboratories, Shawnee Mission, Kansas, USA). The bacterial inoculum (200 μl, 104 CFU, D39 or plnA– or plnA–/spxB–) was introduced into the cisterna magna (39). NAC (300 mg/kg at 0, 8, and 16 hours) was given intravenously and catalase was given intravenously (at 0, 3, 6, 12, 15, 17, 18, and 19 hours) and intracisternally (at 0, 12, and 15 hours) in 12 animals. Animals were anesthetized again at 20 hours and CSF was sampled to confirm bacterial growth. Thereafter, phenobarbital (Abbott Laboratories, Abbott Park, Illinois, USA; 25 mg/kg) was administered to the animals and they were perfused transcardially with 2.5% paraformaldehyde and 0.1% glutaraldehyde (Sigma Chemical Co.) in 0.1 M cacodylate buffer (Ted Pella Inc., Redding, California, USA), pH 7.4, adjusted with sucrose to 300 mOsm. After 10 minutes’ perfusion-fixation, brains were removed and postfixed. Neuronal damage was assessed using hematoxylin and eosin (H&E) and TUNEL staining (7). All experiments were performed in compliance with NIH and institutional guidelines.
Results
Identification of possible toxic factors from pneumococci.
To assess whether bacterial toxins were secreted, or were cell wall– or cell surface–associated, we initially evaluated the ability of heat-killed pneumococci or purified pneumococcal cell walls, a potent proinflammatory stimulus in vivo (40), to kill microglial cells. Neither induced apoptosis (data not shown). Further, there was no difference in the proapoptotic activity of encapsulated and unencapsulated pneumococcal strains. Therefore, the pneumococcal toxins that mediate brain cell apoptosis appeared to be factors secreted by the bacterium. In support of this notion, supernatants of bacterial cultures induced rapid apoptosis of human microglial cells even at dilutions of 1:10 (72.4% ± 6.3% apoptotic cells by 9 hours).
To identify which secreted factor(s) mediates brain cell apoptosis, we assessed the toxicity of pneumococcal mutants defective in production of potential pathogenicity factors. Human microglial cells were exposed to wild-type pneumococcus (D39) or pneumococcal strains bearing the following loss-of-function mutations in the following genes: adherence to host cells (cbpA–), autolysis (lytA–), DNA transformation (comA–), manganese transport (psaA–), surface IgA-protease (igA–), and zinc metalloprotease (zmpB–). All of these mutants were as potent as their parental strain in inducing microglial apoptosis (data not shown).
S. pneumoniae R6 and pneumolysin did not induce IL-1β, RANTES, or nitric oxide as possible toxic factors in primary rat hippocampal neurons. There was a slight increase of TNF-α by R6 (71.5 ± 62.5 pg/ml) and pneumolysin (12.9 ± 17.2 pg/ml) compared with controls (2.4 ± 4.2 pg/ml). After addition of an anti-TNF antibody, TNF-α was no longer detectable, but no effect on LDH release (181.7 ± 126.5 for R6 vs. 188.7 ± 26.7 for R6 + anti-TNF antibody; 83.2 ± 26.4 for controls vs. 81.7 ± 18.4 for controls + antibody) nor on the number of apoptotic cells (data not shown) was observed.
Pneumolysin and H2O2 initiate pneumococcal-induced apoptosis.
To assess the role of released H2O2 in apoptosis, we reduced H2O2 concentrations in the pneumococcal supernatant by adding the antioxidant NAC or with catalase prior to exposure to microglia. NAC or catalase alone had no effect on microglia cells (data not shown). Apoptosis was verified by typical changes of TUNEL, electron microscopy, and annexin V/propidium iodide and AO/EB staining. We also evaluated the ability of the pneumococcal mutant spxB–, which produces less than 5% of wild-type levels of H2O2 (12), to induce apoptosis. Either pharmacological or genetic inactivation of H2O2 failed to prevent microglial apoptosis (Figure 1). Similarly, the pneumolysin-deficient mutant plnA– (23) was fully active in inducing apoptosis of either microglia or neurons (Figure 1 and data not shown). Therefore, inactivation of either of these pathogenicity factors alone did not block pneumococcal-induced apoptosis.
Figure 1.
Inactivation of pneumolysin and blocking H2O2 inhibits apoptosis induced by live pneumococci. (a and b) Human microglia incubated with wild-type pneumococci D39 (107 CFU/ml, 6 hours) in the absence or presence of NAC (10 mM) underwent massive shrinkage and condensation of their nuclei by AO/EB staining, whereas NAC effectively impaired changes in nuclear morphology and cell membrane damage in cells exposed to plnA– (107 CFU/ml, 6 hours). Bars = 10 μm. (b) Quantification of apoptotic microglia after exposure to wild-type (D39) or mutant pneumococci defective in pneumolysin (plnA–) or H2O2 production (spxB–) or both (plnA–/spxB–). Shown are the results (means + SD) of three independent experiments after 6 hours of incubation. Addition of the antioxidant NAC prevented apoptosis induced by plnA–, but not that induced by D39 or spxB– (b). plnA– in the presence of NAC or catalase (Cat) (1,250 U/ml) and plnA–/spxB–were significantly different compared with all others (P < 0.05). plnA– combined with NAC or catalase was more efficient to prevent apoptosis than was the double mutant plnA–/spxB– (P < 0.05). ANOVA and Student-Newman-Keuls test were used. WT, wild-type.
We next assessed the possibility that more than one factor secreted by pneumococcus possessed apoptosis-inducing activity. Initially we assessed whether incubation of brain cells with NAC or catalase (Figure 1) blocked apoptosis induced by the plnA–pneumococcus. Although exposure of microglia to wild-type or plnA– pneumococci caused early and massive damage to mitochondria (Figure 2) and subsequent apoptosis, treatment of cells exposed to the plnA– strain with NAC or catalase effectively blocked mitochondrial damage and apoptosis (Figures 1 and 2). Based on more than 50 electron micrographs, the percentage of cells with swollen mitochondria was estimated as follows [mean ± SD (range); n > 4]. Control: 10% ± 10% (0–26%); D39: 83% ± 9% (79–95%); D39 + NAC: 78% ± 12% (70–93%); plnA–: 86% ± 17% (62–100%); plnA– + NAC: 19% ± 5% (13–27%); plnA–/spxB–: 26% ± 12% (6–43%).
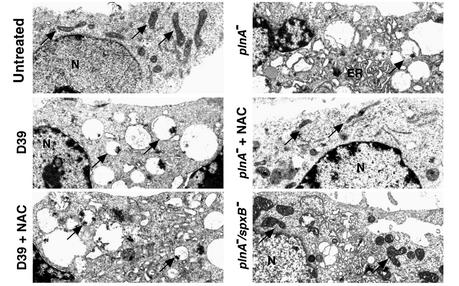
Figure 2.
Inactivation of pneumolysin and blocking H2O2 inhibits mitochondrial damage induced by live pneumococci. Changes of ultrastructure of microglial cells exposed to pneumococci (107 CFU/ml) for 3 hours were monitored by transmission electron microscopy. D39 and plnA– caused massive swelling of the mitochondria (arrows) and endoplasmic reticulum (ER). Treatment of D39-exposed cells with NAC (10 mM) failed to prevent that damage. By contrast, when cells exposed to plnA– were treated with NAC (10 mM), swelling of mitochondria and the endoplasmic reticulum was markedly attenuated. The double mutant plnA–/spxB– also showed attenuated mitochondrial damage. ×7,500. N, nucleus.
To confirm that the effects of antioxidants were specific, we generated a pneumococcal mutant defective in both H2O2 and pneumolysin (spxB–/plnA–). Although this mutant grew at rates comparable to those of wild-type pneumococci (data not shown), it was significantly impaired in its ability to induce mitochondrial damage and apoptosis of microglial cells or neurons (Figures 1 and 2, and data not shown). Therefore, both pneumolysin and bacteria-derived H2O2 contribute to pneumococcal-induced apoptosis.
Purified pneumolysin or H2O2 are sufficient to induce brain cell apoptosis.
To confirm that pneumolysin and H2O2 were each sufficient to induce apoptosis, microglial cells or primary hippocampal neurons were incubated with purified recombinant pneumolysin or H2O2 (Figure 3). Pneumolysin (0.1–10 μg/ml) or H2O2 (2–2000 μM) induced apoptosis of microglial cells (Figure 3a) as well as primary rat hippocampal neurons (Figure 3, b and c), with kinetics and changes in nuclear morphology indistinguishable from apoptosis induced by live pneumococci (Figure 1), including margination and condensation of chromatin, and cell and nuclear shrinkage. Cell death was confirmed by release of LDH (data not shown).
Figure 3.
Pneumolysin and H2O2 are each sufficient to induce apoptosis in microglia and neurons. (a) Microglia cells were incubated for 8 hours with purified pneumolysin (0.1 μg/ml) or H2O2 (0.2 mM) and stained with AO and EB. Either treatment resulted in shrinkage and condensation typical of apoptosis induced by wild-type pneumococci. The apoptosis-inducing activity of pneumolysin correlates with its cytolytic (Lys) but not its complement-activating (Compl) function. Microglial cells were incubated with wild-type or mutant pneumolysin (0.1 μg/ml) for 12 hours and stained with AO and EB. A point mutant in the domain required for cytolytic activity of pneumolysin (W433F) failed to induce apoptosis, whereas a point mutant in the domain required for complement activation (D385N) retained full apoptosis-inducing capacity. Bars = 10 μm. (b) Quantification of the effects of wild-type or mutant pneumolysin on primary rat hippocampal neurons. Results shown (means + SD) are representative for three independent experiments performed in triplicate. (c) Neurotoxicity (primary rat hippocampal neurons) of various concentrations of H2O2.
Pneumolysin activities associated with different domains of the protein include complement activation and cytolysis (16). We incubated microglial cells and primary rat hippocampal neurons with wild-type pneumolysin or with pneumolysin point mutants defective in complement (D385N) or cytolytic (W433F or W433F/C428G) activity (41). Pneumolysin proteins defective in cytolytic activity failed to induce apoptosis, whereas pneumolysin defective in complement activation induced apoptosis to the same extent as did wild-type pneumolysin (Figure 3, a and b). Therefore, the pore-forming activity of pneumolysin was required for its proapoptotic function.
Pneumococci, pneumolysin, and H2O2 initiate apoptosis by inducing Ca2+ flux.
Influx of Ca2+ or the release of Ca2+ from intracellular sources precedes morphologic changes of brain cell apoptosis induced by several agents (42), and overloading of mitochondria with Ca2+ induces permeability transition pores (43). We therefore evaluated whether Ca2+ flux played a role in pneumococcal-induced apoptosis. To assess Ca2+ levels we used Fluo-4, a dye which fluoresces upon binding to Ca2+ (44). Within 45 minutes of exposure to pneumococci, there was a profound increase in intracellular Ca2+ over 3 hours. This effect was blocked after incubation with the extra- and intracellular Ca2+ chelator BAPTA-AM (Figure 4b). Similarly, the pneumococcal toxins pneumolysin and H2O2 induced a rapid increase of Ca2+ concentrations in primary rat hippocampal neurons (Figure 5, top row).
Figure 4.
Pneumococcus triggers early increases of intracellular ROS and Ca2+. Microglial cells were untreated (Control) or incubated with pneumococcus D39 (107 CFU/ml) for 45 minutes to 3 hours. ROS (a) and Ca2+ (b) levels were visualized by fluorescence of the dyes DHR123 (10 μM) and Fluo-4 (10 μM), respectively, and quantified with a multiwell fluorescence plate reader. Results shown (mean + SD) are representative for three independent experiments performed in triplicate. (b and c) The effects of the Ca2+ chelator BAPTA-AM (5 μM) were investigated with the AO/EB assay after exposure of cells to D39 in the presence and absence of BAPTA-AM. Bars = 10 μm.
Figure 5.
Pneumolysin and H2O2 induce increase of intracellular Ca2+ and release of mitochondrial AIF. Primary rat neurons were exposed to either pneumolysin (0.1 μg/ml) or H2O2 (0.2 mM). Intracellular Ca2+ was visualized by Fluo-4 (10 μM) after 1.5 hours. Release of mitochondrial AIF, its translocation to the nucleus, and the effects of BAPTA-AM (5 μM) were assessed immuncytochemically after 6 hours’ incubation. Insets show colocalization of HOECHST 33258 (blue fluorescence) and AIF (red fluorescence). Bar = 10 μm.
Within 45 minutes of exposure of microglial cells to wild-type pneumococcus, we found marked increases of intracellular ROS (Figure 4a), and this was effectively reduced by treatment with NAC (DHR fluorescence units at 0 minutes: 38 ± 13; 90 minutes: 55 ± 11 vs. 123 ± 11 without NAC; 180 minutes: 53 ± 1 vs. 149 ± 26 without NAC) or catalase (0 minutes: 41 ± 13; 90 minutes: 72 ± 7 vs. 126 ± 9 without catalase). Exposure to the spxB– strain caused a delayed and — as expected — an attenuated increase of ROS (0 minutes: 52 ± 8; 45 minutes: 58 ± 3; 90 minutes: 62 ± 12; 180 minutes: 116 ± 14 DHR fluorescence units). This attenuated response was accompanied by a delayed and decreased influx of Ca2+. The pneumolysin-defective plnA– strain was not deficient in inducing rapid increases of intracellular ROS and Ca2+ (data not shown).
To assess whether this increase in Ca2+ contributed to the apoptotic response, we pretreated microglia with the extra- and intracellular Ca2+ chelator BAPTA-AM and then exposed them to pneumococcus. For control, appropriate chelation of calcium by BAPTA-AM was shown by blocking of Ca2+-induced increase of Fluo-4 fluorescence. Strikingly, treatment of infected microglia with BAPTA-AM blocked the apoptotic response (Figure 4c), demonstrating that the increases in Ca2+ were essential for the pneumococcal-induced cell death program. These data therefore suggest that pneumolysin and bacteria-derived H2O2 independently mediate increases in intracellular Ca2+, which is essential for apoptosis.
Pneumolysin and pneumococcal H2O2 induce mitochondrial damage and the release of AIF.
Pneumolysin and bacterial H2O2 recapitulated the changes in Ca2+ that are associated with pneumococcal-induced apoptosis. To assess whether purified pneumolysin and H2O2 were also capable of inducing mitochondrial damage and the release of AIF, the final steps in pneumococcal-induced brain cell apoptosis (9), primary rat neurons were exposed to the toxins and mitochondrial AIF was monitored by immunocytochemistry. In untreated neurons, AIF colocalized with mitochondria (9). Wild-type pneumococci (9) as well as the supernatant of wild-type pneumococci induced a massive release of mitochondrial AIF into the cytoplasm. In contrast, supernatant of the plnA–/spxB– mutant induced only a slight release of AIF (data not shown). H2O2 and pneumolysin induced the release of mitochondrial AIF and translocation to the nucleus (Figure 5, middle row). Pneumolysin defective in complement activation but not pneumolysin defective in pore forming activity was still able to release AIF (data not shown). Furthermore, BAPTA-AM inhibited the release of AIF (Figure 5, bottom row), suggesting that AIF release caused by pneumolysin or H2O2 was mediated by increases in intracellular Ca2+. To address the ability of pneumococcal supernatant–derived pneumolysin and H2O2 to induce the release of AIF, neurons were exposed to culture supernatants from wild-type and double mutant spxB–/plnA– bacteria.
Pneumolysin and H2O2 contribute to neuronal damage in the rabbit model of pneumococcal meningitis.
To address the role of pneumolysin and H2O2 in hippocampal neuronal apoptosis in experimental pneumococcal meningitis, we used a rabbit model in which bacteria (104 CFU per animal) are directly inoculated into the CSF of the animals. Within 24 hours of exposure there is a massive host inflammatory response and damage to neurons of the dentate gyrus of the hippocampus (7). We compared the disease course in rabbits challenged with wild-type (D39) or pneumolysin-negative (plnA–) pneumococci in the presence or absence of NAC (Figure 6a). CSF was analyzed for bacterial and leukocyte numbers at 26 hours, and the brains of the animals were assessed for hippocampal damage. NAC or catalase treatment did not inhibit bacterial growth in vivo and did not block the influx of leukocytes into the CSF compartment (data not shown). The degree of damage to the dentate gyrus was reduced in animals infected with plnA– (Figure 6a). However, NAC treatment of plnA–-infected animals, but not wild-type infected rabbits, drastically reduced neuronal damage (Figure 6a). Catalase treatment also greatly reduced neuronal damage of plnA–-infected animals (Figure 6a). As NAC or catalase can block both bacteria- and host-derived H2O2, the double mutant plnA–/spxB–, which fails to produce bacterial H2O2, was tested in vivo. plnA–/spxB– induced less neuronal damage than did plnA– (51.1 ± 6.7 vs. 39.5 ± 0.9 apoptotic cells per mm2; P = 0.04, t test), supporting a partial role for bacterial H2O2. However, as catalase further decreased neuronal damage in plnA–/spxB––infected versus plnA–-infected animals (22.2 ± 4.0 vs. 12.6 ± 1.3 apoptotic cells per mm2; P = 0.004, t test), this finding suggests that host-derived H2O2 also mediates toxic effects in vivo. We have systematically screened other areas of the brain including Purkinje cells in the cerebellum and cortical areas. We could not detect neuronal damage in other areas in our rabbit model of bacterial meningitis after 24 hours.
Figure 6.
Pneumolysin and H2O2 contribute to neuronal damage in the rabbit model of pneumococcal meningitis. (a) Neurotoxicity in the dentate gyrus was quantified by neuronal loss or cell shrinkage assessed in H&E-stained sections. Bars represent means + SD of five brain sections per rabbit. Compared with control animals challenged with PBS, wild-type pneumococci D39 induced significant neurotoxicity (*P < 0.05). Damage in D39-challenged animals was also statistically significant compared with all other tested groups except D39 + NAC (P < 0.05; ANOVA, Student-Newman-Keuls test). #Significant difference between plnA–/spxB– and plnA–, P = 0.04. ##Significant difference between plnA–/spxB– with and without catalase, P = 0.004; t test. (b) Immunohistochemical staining with anti-pneumolysin antibody followed by peroxidase-conjugated secondary antibody and diaminobenzidine (dark color) detected pneumolysin in dentate gyrus neurons of D39- but not plnA–- or PBS-treated animals. Bar = 10 μm.
To test the hypothesis that the neurons of the hippocampus are actually exposed to pneumolysin in vivo, immunohistochemical analyses were performed. Immunostaining with a pneumolysin-specific antibody detected high levels of pneumolysin protein in dying neurons of the dentate gyrus of the hippocampus (Figure 6b). Therefore, released pneumolysin indeed contacts hippocampal neurons, which are susceptible to apoptosis during meningitis.
Discussion
Mechanisms of neuronal damage in pneumococcal meningitis are still largely unknown. Recently, apoptosis has been reported as a major mechanism for damage to the hippocampus (5, 7). We have demonstrated that pneumococci may contribute significantly to neuronal cell death since the bacteria are able to directly induce apoptosis (9). Here we report that the pneumococcal toxins pneumolysin and H2O2 induce massive and rapid apoptosis. Blocking these proapoptotic factors markedly attenuates mitochondrial damage and apoptosis in vitro and in experimental meningitis, suggesting that strategies targeting these toxins may offer therapeutic benefit.
H2O2 rapidly diffuses through eukaryotic cell membranes to damage intracellular targets (e.g., mitochondria and DNA) (15, 45) and to trigger apoptosis. Pneumococcus is the only meningeal pathogen that lacks catalase and therefore produces excessive amounts of H2O2 (14, 46). Pneumococcal-derived H2O2 appears to be an additional bacterial factor contributing to increases of intracellular ROS and Ca2+ and release of AIF. Chelating Ca2+ blocked this AIF release. Increased intracellular Ca2+ is clearly important for inducing mitochondrial damage and apoptosis, as H2O2 is sufficient to induce apoptosis as well as AIF release and blocking intracellular Ca2+ prevents mitochondrial damage and cell death.
Despite the proapoptotic effects of H2O2, it is clearly not the only trigger of pneumococcal-induced apoptosis. A mutant defective in H2O2 production is similar to wild-type pneumococci in its ability to induce death, and blocking ROS with antioxidants does not prevent death induced by wild-type pneumococci. However, this strategy effectively disables the apoptotic potential of the pneumolysin-deficient strain plnA–, suggesting that the other toxin that contributes to the response is pneumolysin. In support of this notion, the H2O2/pneumolysin–deficient double mutant has little apoptotic potential.
Pneumolysin is a multifunctional protein toxin that induces cytolysis, complement activation, and cytokine and nitric oxide production (16, 31, 47). Here we have shown that pneumolysin is also a potent neurotoxin that triggers apoptosis in primary hippocampal neurons by inducing mitochondrial damage and the release of the proapoptotic factor AIF. Pneumolysin-induced increases of intracellular Ca2+ and AIF release could be blocked by chelating Ca2+, indicating a central role for Ca2+ in the pathophysiological effects of both pneumococcal toxins. This is consistent with the finding that Ca2+ is one known factor initiating the release of AIF from mitochondria (35). Specific domains of pneumolysin independently cause proinflammatory and pore-forming activities (16). Following binding to cholesterol, pneumolysin monomers insert into the lipid bilayer, oligomerize, and create pores 35–45 nm in diameter (48). The pore-forming activity of pneumolysin was required for inducing AIF release and apoptosis in vitro since the cytolysis- but not the complement-deficient mutant of pneumolysin protein was unable to induce AIF release and death. Pneumolysin is related to other bacterial hemolysins that have also been noted to induce apoptosis, including listeriolysin of Listeria monocytogenes (49), α toxin of Staphylococcus aureus (50), and Escherichia coli hemolysin (51). It will therefore be interesting to determine whether this hemolysin family shares a common mechanism in inducing mitochondrial damage and AIF-dependent apoptosis.
A central issue for the in vivo relevance of pneumococcal-induced cell death is the ability of the toxins to invade the site of damage. Pneumolysin was found in the hippocampus colocalized to damaged neurons. The extracellular fluid around brain cells is contiguous with the CSF (20), and pneumolysin and other soluble, secreted bacterial toxins may diffuse into the parenchyma. Meningitis caused by the H2O2/pneumolysin–deficient double mutant reduced hippocampal apoptosis by 50%, clearly demonstrating the in vivo role of these pneumococcal toxins.
Since both NAC and catalase scavenge host and bacterial H2O2, one way to determine whether the source of the H2O2 is the host or the bacteria was to test the plnA–/spxB– mutant. Removing the ability of the bacteria to make H2O2 decreased apoptosis compared with plnA– alone. However, since this effect was not as strong as the benefit of NAC or catalase, the bacteria can be said to contribute to damage by producing H2O2, but the host also represents a major source of damaging H2O2.
Overall the combined data (refs.7, 9, and this report) suggest that hippocampal apoptosis in S. pneumoniae–induced meningitis is a two-pronged problem (Figure 7). On the one hand, there is caspase-dependent apoptosis of neurons in the dentate gyrus that is due to the host inflammatory response (Figure 7). Thus, strategies to inhibit the host inflammatory response by inhibiting migration, adhesion, or function of leukocytes, or the production of reactive oxygen intermediates, matrix metalloproteinases (52), and/or caspases, are needed to decrease apoptosis and subsequent morbidity. The relevance of the complex host response has been demonstrated by the fact that in a model of experimental meningitis using group B streptococcus, a pathogen that does not produce the candidate factors of pneumococcus caused a comparable apoptotic injury in the dentate gyrus (53).
Figure 7.
Schematic model of pneumococcal-induced apoptosis of neurons. Neuronal apoptosis is triggered in part by the host inflammation and is mediated in part by caspase activation. Pneumolysin and H2O2 are direct triggers of S. pneumoniae, inducing apoptosis by increasing intracellular Ca2+, damaging mitochondria, and causing the release and translocation of mitochondrial AIF.
Hippocampal neurons have been shown to be susceptible to various noxious stimuli, e.g., ischemia, inflammation, or irradiation. Unlike other neurons in the brain, hippocampal dentate gyrus neurons have the ability to undergo proliferation and neurogenesis (54). In the specific case of bacterial meningitis, the proximity to the ventricle with high bacterial concentrations and diffusable factors like pneumolysin has to be considered. Both factors may contribute to this specific vulnerability.
On the other hand, the direct exposure of these neurons to pneumococcal toxins induces AIF-dependent apoptosis (9). As shown here, the toxins released by pneumococcus include pneumolysin and H2O2, and to eliminate neuronal damage during meningitis these also need to be targeted, for example by treating with antioxidants and neutralizing pneumolysin. Pneumolysin and, to a lesser extent, pneumococcal-derived H2O2 initiate the AIF death cascade by increasing intracellular Ca2+, and agents that can block the release of or sequester Ca2+ may also be of therapeutic benefit, as blocking Ca2+ dramatically impairs the pneumococcal toxin–induced apoptotic response. The multiplicity of mechanisms by which pneumococci kill CNS cells may explain the exceptionally devastating course of this infection. Using pneumococci as a bioprobe also allows the study of the diversity of apoptotic mechanisms in the CNS.
Acknowledgments
We thank Donna Davis and K. Gopal Murti for assistance with the electron microscopy, and Daimin Zhao and Miriam Schickhaus for technical support. This work was supported by NIH grants AI-27913 (to E.I. Tuomanen), CA-76379 (to J.L. Cleveland), and DK-44158 (to J.L. Cleveland); Cancer Center CORE grant CA-21765; SFB 507/B6 Deutsche Forschungsgemeinschaft (to J.R. Weber) and Wilhelm Sander-Stiftung; the Meningitis Research Foundation (to J.S. Braun and J.R. Weber); and the American Lebanese and Syrian Associated Charities of St. Jude Children’s Research Hospital.
References
- 1.Zychlinsky A, Sansonetti P. Apoptosis in bacterial pathogenesis. J Clin Invest. 1997; 100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohr V, Paulson OB, Rasmussen N. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch Neurol. 1984; 41:1045–1049. doi: 10.1001/archneur.1984.04050210043012. [DOI] [PubMed] [Google Scholar]
- 3.Durand ML, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993; 328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 4.Pfister HW, Feiden W, Einhäupl KM. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch Neurol. 1993; 50:575–581. doi: 10.1001/archneur.1993.00540060015010. [DOI] [PubMed] [Google Scholar]
- 5.Zysk G, et al. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol. 1996; 55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Täuber, M.G., Kim, Y.S., and Leib, S.L. 1997. Neuronal injury in meningitis. In In defense of the brain: current concepts in the immunopathogenesis and clinical aspects of CNS infections. P.K. Peterson and J.S. Remington, editors. Blackwell Science. Malden, Massachusetts, USA. 124–143.
- 7.Braun JS, et al. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med. 1999; 5:298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- 8.Nau R, Soto A, Bruck W. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J Neuropathol Exp Neurol. 1999; 58:265–274. doi: 10.1097/00005072-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Braun JS, et al. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J Infect Dis. 2000; 184:1300–1309. doi: 10.1086/324013. [DOI] [PubMed] [Google Scholar]
- 10.AlonsoDeVelasco E, Verheul AFM, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995; 59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paton JC, Andrew PW, Boulnois GJ, Mitchell TJ. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993; 47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 12.Spellerberg B, et al. Pyruvate oxidase as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996; 19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 13.Novak R, et al. Extracellular targeting of choline-binding proteins in Streptococcus pneumoniaeby a zinc metalloprotease. Mol Microbiol. 2000; 36:366–376. doi: 10.1046/j.1365-2958.2000.01854.x. [DOI] [PubMed] [Google Scholar]
- 14.Avery OT, Morgan HJ. The occurrence of peroxide in cultures of pneumococcus. J Exp Med. 1924; 39:275–288. doi: 10.1084/jem.39.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duane PG, Rubins JB, Weisel HR, Janoff EN. Identification of hydrogen peroxide as a Streptococcus pneumoniaetoxin for rat alveolar epithelial cells. Infect Immun. 1993; 61:4392–4397. doi: 10.1128/iai.61.10.4392-4397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell TJ, Andrew PW. Biological properties of pneumolysin. Microb Drug Resist. 1997; 3:19–26. doi: 10.1089/mdr.1997.3.19. [DOI] [PubMed] [Google Scholar]
- 17.Canvin JR, et al. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis. 1995; 172:119–123. doi: 10.1093/infdis/172.1.119. [DOI] [PubMed] [Google Scholar]
- 18.Winter AJ, et al. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect Immun. 1997; 65:4411–4418. doi: 10.1128/iai.65.11.4411-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daum RS, Scheifele DW, Syriopoulou VP, Averill D, Smith AL. Ventricular involvement in experimental Hemophilus influenzaemeningitis. J Pediatr. 1978; 93:927–930. doi: 10.1016/s0022-3476(78)81213-x. [DOI] [PubMed] [Google Scholar]
- 20.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985; 326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 21.Janabi N, Peudenier S, Héron B, Ng KH, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett. 1995; 195:105–108. doi: 10.1016/0304-3940(94)11792-h. [DOI] [PubMed] [Google Scholar]
- 22.Lautenschlager M, et al. Role of nitric oxide in the ethylcholine aziridinium model of delayed apoptotic neurodegeneration in vivo and in vitro. Neuroscience. 2000; 97:383–393. doi: 10.1016/s0306-4522(99)00599-0. [DOI] [PubMed] [Google Scholar]
- 23.Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989; 57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenow C, et al. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997; 25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Q, Campbell EA, Naughton AM, Johnson S, Masure HR. The com locus controls genetic transformation in Streptococcus pneumoniae. Mol Microbiol. 1997; 23:683–692. doi: 10.1046/j.1365-2958.1997.2481617.x. [DOI] [PubMed] [Google Scholar]
- 26.Novak R, Braun JS, Charpentier E, Tuomanen E. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol Microbiol. 1998; 29:1285–1296. doi: 10.1046/j.1365-2958.1998.01016.x. [DOI] [PubMed] [Google Scholar]
- 27.Wani JH, Gilbert JV, Plaut AG, Weiser JN. Identification, cloning, and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect Immun. 1996; 64:3967–3974. doi: 10.1128/iai.64.10.3967-3974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasz A, Albino A, Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970; 227:138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- 29.Lacks SA, Greenberg B. Sequential cloning by a vector walking along the chromosome. Gene. 1991; 104:11–17. doi: 10.1016/0378-1119(91)90458-n. [DOI] [PubMed] [Google Scholar]
- 30.Lacks SA, Hotchkiss RD. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim Biophys Acta. 1960; 39:508–517. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 31.Braun JS, Novak R, Gao G, Murray PJ, Shenep JL. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect Immun. 1999; 67:3750–3756. doi: 10.1128/iai.67.8.3750-3756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houldsworth S, Andrew PW, Mitchell TJ. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect Immun. 1994; 62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitrak DL, Tsai HC, Mullane KM, Sutton SH, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J Clin Invest. 1996; 98:2714–2719. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987; 20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 35.Susin SA, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999; 397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 36.Royall JA, Ischiropoulos H. Evaluation of 2’,7’-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2in cultured endothelial cells. Arch Biochem Biophys. 1993; 302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 37.Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989; 264:8171–8178. [PubMed] [Google Scholar]
- 38.Kruman II, Mattson MP. Pivotal role of mitochondrial calcium uptake in neuronal cell apoptosis and necrosis. J Neurochem. 1999; 72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- 39.Dacey RG, Sande MA. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974; 6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985; 151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 41.Andrew PW, Mitchell TJ, Morgan PJ. Relationship of structure to function in pneumolysin. Microb Drug Resist. 1997; 3:11–17. doi: 10.1089/mdr.1997.3.11. [DOI] [PubMed] [Google Scholar]
- 42.Lipton SA, Nicotera P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium. 1998; 23:165–171. doi: 10.1016/s0143-4160(98)90115-4. [DOI] [PubMed] [Google Scholar]
- 43.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999; 341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers J, et al. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999; 400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- 45.Cochrane CG. Cellular injury by oxidants. Am J Med. 1991; 91:23S–30S. doi: 10.1016/0002-9343(91)90280-b. [DOI] [PubMed] [Google Scholar]
- 46.Pericone CD, Overweg K, Hermans PWM, Weiser JN. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniaeon other inhabitants of the upper respiratory tract. Infect Immun. 2000; 68:3990–3997. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paton JC. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996; 4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert RJC, et al. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell. 1999; 97:647–655. doi: 10.1016/s0092-8674(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 49.Guzmán CA, et al. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996; 20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 50.Jonas D, Schultheis B, Klas C, Krammer PH, Bhakdi S. Cytocidal effects of Escherichia colihemolysin on human T lymphocytes. Infect Immun. 1993; 61:1715–1721. doi: 10.1128/iai.61.5.1715-1721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonas D, et al. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994; 62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leib SL, Leppert D, Clements J, Täuber MG. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun. 2000; 68:615–620. doi: 10.1128/iai.68.2.615-620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leib SL, Kim YS, Chow LL, Sheldon RA, Täuber MG. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Invest. 1996; 98:2632–2639. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999; 2:203–205. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]