Abstract
Antigen administration via oral and other mucosal routes can suppress systemic immunity to the antigen and has been used to prevent experimental autoimmune disease. This approach may prove ineffective or even harmful if it leads to a concomitant induction of cytotoxic T lymphocytes (CTLs), and indeed, mucosal administration of the model antigen ovalbumin (OVA) has been shown to elicit CTL activation while simultaneously inducing oral tolerance. Here we show that induction by oral OVA of CTLs in wild-type mice, and of diabetes in mice expressing OVA transgenically in pancreatic β cells, can be prevented by transiently blocking the CD40 ligand (CD40L). However, CD40L blockade did not diminish oral tolerance, as measured by suppression of systemic OVA-primed T cell proliferation, IFN-γ secretion, and Ab production. Consistent with these findings, mice lacking CD40 expression could be orally tolerized to OVA. Transient CD40L blockade therefore dissociates pathogenic from protective immunity and should enhance the efficacy and safety of oral tolerance for preventing autoimmune disease.
Introduction
Mucosal administration of protein antigen suppresses the ability of the antigen to subsequently prime immune responses (induces oral tolerance), and has been used to prevent or suppress autoimmune disease in rodents (1, 2). However, oral autoantigen has also been shown to exacerbate disease (3–6). Human trials of oral myelin basic protein in multiple sclerosis (7), oral type II collagen in rheumatoid arthritis (8, 9), and oral insulin in type 1 diabetes (10, 11) have not demonstrated clinical benefit. Besides inducing tolerance, antigens containing peptides that bind to MHC class I molecules could also induce CD8+ cytotoxic T lymphocytes (CTLs). CTLs may potentially undermine the efficacy and safety of mucosal tolerance, which may explain its therapeutic failure in some cases. This important issue has received relatively little attention. Previously, a single oral dose of the model antigen ovalbumin (OVA) was shown to prime OVA-specific CTLs in C57BL/6 (B6) mice, and to trigger autoimmune diabetes in B6 mice expressing OVA transgenically in pancreatic β cells (12). Recently, we reported that pathogenic OVA-specific CTLs are induced in B6 mice by a range of doses and schedules of oral, aerosol, or intranasal OVA that have been reported to induce mucosal tolerance (13). Paradoxically, we also found, as have others (14, 15), that oral OVA induced tolerance of CTLs to subsequent systemic priming.
In the absence of potent inflammatory stimuli, activation of CD8+ CTLs by dendritic cells (DCs) requires help from CD4+ T cells, usually provided by the interaction of CD40 ligand (CD40L) with CD40 on DCs (16–18). Blockade of CD40L with mAb prevents systemic priming of CTLs (16). In addition, CD8+ cells themselves express functional CD40L (19), which is required for their activation after recruitment to the gut in response to priming with intraperitoneal OVA (20). However, the role of CD40L-CD40 interactions in the CTL response to oral antigen has not been reported, although CD40L gene knockout (CD40–/–) mice were reported to be resistant to induction of oral tolerance (21). In the present study, we aimed to determine whether CTL induction by oral OVA could be prevented by targeting CD40L, without limiting oral tolerance, in order to improve the clinical efficacy and safety of mucosal tolerance induction.
Methods
Mice.
Mice were bred and housed at the Walter and Eliza Hall Institute of Medical Research. Immunity and tolerance following administration of oral OVA were examined in female B6 mice aged 6–8 weeks. OT-I/recombination activating gene knockout mice (Rag–/– mice) (22), subsequently referred to as OT-I mice, bear a transgenic T cell receptor from CD8+ T cells specific for the MHC class I–restricted OVA257-264 peptide. OT-II mice (23) bear a transgenic T cell receptor from CD4+ T cells specific for the MHC class II–restricted OVA323-339 peptide. Both OT-I and OT-II mice were used at 6–12 weeks of age as donors of OVA-reactive T cells. OT-I T cells were transferred into B6 mice (made congenic for Ly5.1 to facilitate detection of transferred OT-I T cells, which are Ly5.2). OT-I and OT-II cells were transferred into rat insulin promoter–OVAlo (RIP-OVAlo) transgenic mice expressing a low level of OVA on their β cells (12, 24).
To generate bone marrow chimeras, 6- to 8-week-old B6 mice were lethally irradiated with two doses of 550 cGy, 3 hours apart, and reconstituted intravenously with 5 × 106 T cell–depleted bone marrow cells from B6 or CD40 knockout mice. The CD40 knockout mice (25), originally on the 129 background, were backcrossed three times onto the B6 background. T cells were depleted with a cocktail of anti-CD4+ (RL172), anti-CD8+ (3.168), and anti-Thy1 (J1j) mAb’s and rabbit complement. One day after reconstitution, mice were given 0.1 ml of another anti-Thy1 (T24) mAb intraperitoneally to eliminate any radioresistant host T cells. Mice were then maintained for 8–10 weeks before use.
Administration of mucosal antigen.
Female B6 mice were given oral OVA (grade VII; Sigma Chemical Co., St. Louis, Missouri, USA) in sterile, endotoxin-free mouse-tonicity PBS, or PBS alone. The endotoxin concentration of the OVA solution used (10 mg/ml) measured in the Limulus lysate assay (BioWhittaker Inc., Walkersville, Maryland, USA) was ≤ 0.5 ng/ml. Oral OVA was administered by high- or low-dose schedules that have been reported to induce oral tolerance (1, 13, 26, 27): either 20 mg on three alternating days (every other day, with the third dosage given on day 5) (high dose) or 0.5 mg on five alternating days (low dose) via intragastric intubation under light methoxyflurane (Penthrane) anesthesia. Two hours prior to the first OVA treatment, mice were injected with a single intraperitoneal dose of 250 μg hamster IgG1 anti-mouse CD40L mAb (MR1; American Type Culture Collection, Rockville, Maryland, USA) or the control hamster IgG1 mAb 6C8, which is specific for human Bcl-2 (28). Both mAbs were purified from hybridoma cell culture medium by affinity chromatography on protein G–Sepharose (Pharmacia Biotech AB, Uppsala, Sweden).
Priming and assay of CTLs.
Mice were challenged intravenously with 2 × 107 OVA-coated H-2Kbm-1 splenocytes, a form of priming that is dependent on CD4+ T cell help (29). In some experiments, mice were primed subcutaneously with 0.1 mg OVA in CFA, a form of priming that is independent of CD4+ T cell help (29). After 7 days, mice were killed and their spleen cells were stimulated in vitro for another 6 days with irradiated syngeneic spleen cells coated with epitope peptide (OVA257-264), before being used as effector cells in a standard 51Cr release assay (12, 29). Lytic units were calculated by dividing the total number of effector cells generated from each spleen by the number of effector cells required for 30% OVA-specific lysis.
Effect of oral OVA treatment, with and without anti-CD40L, on OT-I CTL precursors.
To determine the effect on OVA-specific CTLs of oral OVA in the presence and absence of anti-CD40L Ab, 3 × 106 OT-I cells were injected intravenously into female B6 mice (made congenic for Ly5.1), and the mice were then injected with either MR1 or control mAb 6C8 (250 μg, administered intraperitoneally). The next day, mice from each treatment group were divided into two groups and fed either PBS or 20 mg OVA in PBS on three alternating days. Ab treatment was repeated just before the third feeding. After 14 days, mice were killed and the numbers of splenic OT-I cells and their expression of activation markers CD44 and L-selectin (CD62L) were analyzed by FACScan using Lysys II software (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). Cells were incubated with FITC-conjugated anti-CD44 and CD62L mAbs (PharMingen, San Jose, California, USA), together with biotinylated anti-Ly5.2 mAb (PharMingen) and phycoerythrin-conjugated anti-CD8+ mAb (Sigma Chemical Co.). This incubation was followed by a second-step incubation with streptavidin-conjugated peridin chlorophyll protein (PerCP; PharMingen) to detect Ly5.2. In addition, intracellular IFN-γ expression was measured according to the recommended protocol (PharMingen). In brief, splenocytes dispensed into 24-well plates were incubated for 6 hours in “complete medium” (RPMI 1640 medium containing 2 mM glutamine, 50 μM 2-mercaptoethanol, and 5% FCS), with either 100 μg/ml OVA257-264, or 25 ng/ml PMA and 500 ng/ml ionomycin (Sigma Chemical Co.). Brefeldin A (10 μg/ml; Sigma Chemical Co.) was added for the last two hours of the incubation period. Cells were then stained for surface markers and for intracellular IFN-γ. For the detection of OT-I cells, splenocytes were surface-stained with FITC-conjugated rat anti-mouse CD8+ and biotinylated rat anti-mouse Ly5.2 Ab’s, followed by streptavidin-conjugated PerCP. After paraformaldehyde fixation and permeabilization with saponin, cells were stained intracellularly with Ab against IFN-γ or with isotype control Ab, each conjugated to phycoerythrin (PharMingen). To analyze OT-I cells, cells that were positive for the markers CD8+ and Ly5.2 were collected in the live gate.
Effect of oral OVA with and without anti-CD40L treatment on diabetes induction in RIP-OVAlo mice.
To examine the effect of anti-CD40L treatment on activation of CTLs by oral OVA in vivo, RIP-OVAlo mice expressing OVA in pancreatic β cells were adoptively transferred with 3 × 105 OT-II and 2 × 105 OT-I cells, followed by intraperitoneal injection with 250 μg control mAb 6C8 or anti-CD40L mAb MR1. Starting the next day, oral OVA (0.5 mg) was given on five alternating days. Blood glucose was measured in a drop of retroorbital venous blood with a glucometer on days 12 and 20; confirmed values above 12 mmol/l were considered to be diagnostic for diabetes.
Evaluation of mucosal tolerance.
To evaluate CTL tolerance to systemic priming, 14 or 21 days after the last dose of oral OVA, mice were injected intravenously with 2 × 107 OVA-coated H-2Kbm-1 spleen cells, or subcutaneously with 0.1 mg OVA in CFA. Subsequently, splenic CTL activity was measured as described above. To evaluate conventional indices of mucosal tolerance, 7 days after the last dose of oral OVA, mice were immunized by subcutaneous injection with 0.1 mg OVA in CFA at the base of the tail. Ten days later, they were anesthetized, bled from the retroorbital venous plexus with a glass capillary tube, and killed by CO2 asphyxiation. Spleens and inguinal lymph nodes were removed. Serum was harvested, and was stored at –20°C for assay of OVA Ab’s. Cell suspensions were prepared from spleens and nodes by mechanical disruption by passing them through a stainless steel mesh. Cells were then washed, counted, and resuspended in complete medium to assay proliferative and cytokine responses to OVA.
Proliferative responses of splenocytes (1 × 106) or inguinal lymph node cells (5 × 105) in 200 μl complete medium were measured in replicates of eight in round-bottomed wells of 96-well Linbro plates (Flow Laboratories Inc., McLean, Virginia, USA), after incubation with or without 0.1 mg/ml OVA at 37°C in 5% CO2/air for 96 hours. 3H-thymidine (1 μCi) was added to each well for the last 10–16 hours of the incubation period, and the cells were harvested, washed, and counted using a TopCount Scintillation Counter (Packard BioScience B.V., Groningen, The Netherlands). Splenocyte or inguinal lymph node cell IFN-γ and IL-4 responses to OVA were measured by ELIspot assay. Cells (5 × 105 per 200 μl) were added to wells of MultiScreen Immobilon-P membrane 96-well plates (MAIPS 4510; Millipore Australia Ltd., North Ryde, Australia) that had been precoated with monoclonal rat anti-mouse IFN-γ (clone R4-6A2) or IL-4 (clone 11-B11) Ab at 5 μg/ml PBS overnight, and incubated with or without 0.1 mg OVA at 37°C in 5% CO2 /air for 24 hours. After washing to remove cells, membrane-bound cytokine was reacted with 4 μg/ml biotin-conjugated monoclonal rat anti-mouse IFN-γ (clone XMG1.2) or IL-4 (clone BVD6-24G2) overnight at 4°C. After washing the membrane, color was developed with streptavidin-peroxidase followed by 3-amino-9-ethylcarbazole (DAKO Corp., Carpinteria, California, USA). All mAb’s were from PharMingen.
Serum IgG subclass Ab’s to OVA were measured by ELISA using peroxidase-conjugated anti-mouse IgG1, IgG2a, IgG2b, or IgG3 Ab’s (Southern Biotechnology Associates, Birmingham, Alabama, USA) as previously described (30). IgG1 was measured at a serum dilution of 1:1,000 and IgG2 and IgG3 at 1:100, which gave no background and OD values in the range of 0.1–1.0.
Statistics.
Differences between treatment groups were analyzed by Fisher’s exact test or the unpaired Student t test.
Results
CTL induction by oral OVA requires CD40L signaling.
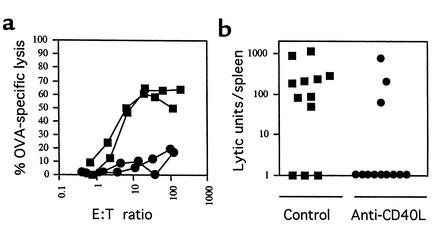
Initially, we determined that systemic priming of CTLs by intravenous OVA-coated splenocytes, which is dependent on CD4+ T cells (29), could be blocked by a single 250-μg intraperitoneal dose of the anti-CD40L mAb MR1 (Figure 1a). The same dose of MR1 or control mAb 6C8 was then given to B6 mice that had been fed a high dose (20 mg) (26, 27) of OVA on three alternating days. A CTL response to oral OVA was detected in 25% (3/12) of the mice given anti-CD40L treatment, compared with 75% (9/12) of controls (P = 0.04) (Figure 1b). A single dose of MR1 given before low-dose oral OVA (0.5 mg on five alternating days) reduced CTL responses by a similar degree (see also Figure 3 and Figure 4, b and d).
Figure 1.
CTL priming by systemic or oral OVA requires CD40L signaling. (a) A single intraperitoneal injection of 250 μg of control mAb 6C8 (squares) or anti-CD40L mAb MR1 (circles) was given to B6 mice (n = 4 in each group), 1 day before challenge with 2 × 107 intravenous OVA-coated H-2Kbm-1 splenocytes to prime CTLs. After 14 days, mice were killed and their splenocytes were tested for CTL activity, expressed as OVA-specific lysis for representative individual mice. Primed splenocytes as effectors (E) were tested against 51Cr-loaded cells as targets (T). (b) The same doses of 6C8 or MR1 were given to mice (n = 12 in each group) that were then fed 20 mg OVA on three alternating days. After 14 days, without further priming, mice were killed and their splenocytes were tested for CTL activity, this time expressed as lytic units per spleen for individual mice.
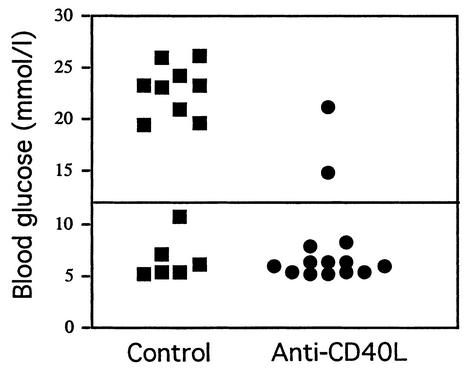
Figure 3.
Anti-CD40L treatment prevents induction of diabetes by oral OVA in RIP-OVAlo mice. RIP-OVAlo mice bearing OT-I and OT-II cells were injected intraperitoneally with 250 μg control mAb 6C8 (squares) or anti-CD40L mAb MR1 (circles). To mimic low-dose oral tolerance regimens, mice were then fed 0.5 mg OVA on five alternating days. Blood glucose was measured 12 days after the start of feeding; values above 12 mmol/l were considered diagnostic of diabetes. Data on individual mice are pooled from two experiments.
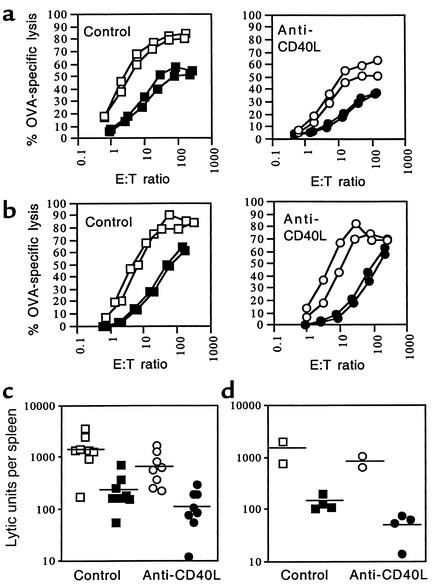
Figure 4.
Anti-CD40L treatment does not prevent oral tolerance as measured by suppression of systemic priming of CTLs. CTL activity in response to intravenous priming with OVA-coated splenocytes (a) or to subcutaneous priming with OVA in CFA (b) is similarly suppressed after oral OVA given to mice treated with control mAb 6C8 (squares) and those given anti-CD40L mAb MR1 (circles). Mice were injected with 6C8 or MR1 (250 μg intraperitoneally), then fed either PBS (open symbols) or OVA in PBS (filled symbols). After 14 days, mice were primed systemically. Seven days later they were killed and their splenocytes were recovered for a standard in vitro 51Cr release assay. CTL activity was expressed as percent OVA-specific lysis; the percent of total radioactivity, corrected for background, released from 51Cr-loaded, OVA257-264 peptide-coated target cells (T) by primed effector spleen cells (E). CTL activity plots for individual mice (shown in a and b) were converted into lytic units per spleen from four experiments (c) in which mice received either PBS or 20 mg oral OVA in PBS on three alternating days and were primed as in a, and from two experiments (d) in which mice received either PBS or 0.5 mg oral OVA in PBS on five alternating days and were primed as in b. Horizontal bars are mean values.
Activation and expansion of CTLs by oral OVA requires CD40L.
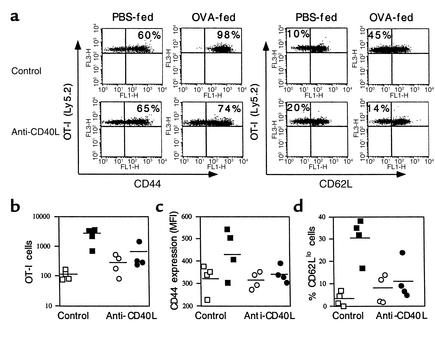
To demonstrate that CTL induction by oral OVA is associated with activation and expansion of CTL precursors, we adoptively transferred OVA-specific OT-I cells into naive Ly5.1 congenic B6 mice and fed them OVA. The role of CD40L in the response of OT-I cells to oral OVA was examined by pretreating recipient mice with either control mAb 6C8 or anti-CD40L mAb MR1. OT-I cells in the spleen were analyzed 14 days after the last dose of oral OVA, to allow time for activation, proliferation, recirculation, and possible cell death. The protocol was the same as that used to measure the OVA-induced CTLs shown in Figure 1. In response to oral OVA given after control mAb, OT-I cells in the spleen (mean ± SD) expanded significantly (2,398 ± 1,273 compared with 108 ± 40 for oral PBS, P = 0.01) (Figure 2b). However, in the presence of anti-CD40L mAb, the number of OT-I cells after oral OVA was not significantly different from the number measured after treatment with oral PBS (564 ± 578 compared with 280 ± 191, P = 0.31) (Figure 2b). Oral OVA increased CD44 and decreased L-selectin (CD62L) expression on OT-I cells (example shown in Figure 2a), indicating the acquisition of an activated/memory phenotype. The oral OVA–induced increase in CD44 expression for the group (Figure 2c) failed to reach significance because of one unresponsive outlier, but the increase in the percentage of cells expressing a low level of CD62L was significant (30.5 ± 9.3 compared with 3.3 ± 3.1, P = 0.03) (Figure 2d). As with the expansion of OT-I cells, anti-CD40L treatment blocked the effect of oral OVA on CD44 and CD62L expression (Figure 2, c and d).
Figure 2.
Activation and expansion of CTLs by oral OVA requires CD40L. B6 recipient mice congenic for Ly5.1 were adoptively transferred with 3 × 106 transgenic OT-I cells (Ly5.2) and then given control mAb 6C8 or anti-CD40L mAb MR1 (250 μg, intraperitoneally). Mice from each treatment group were then divided into two groups and fed either PBS or 20 mg OVA in PBS on three alternating days. mAb treatment was repeated before the third feeding. Mice were killed 14 days from the start of feeding, and the numbers and phenotype of OT-I cells in their spleens were analyzed by flow cytometry. (a) Dot plots of individual mice show CD44 (left) and CD62L (L-selectin) (right) expression on OT-I cells. The percentage of cells expressing a high level of CD44 or a low level of CD62L is shown in the corresponding quadrant. The number of OT-I cells per spleen (b), CD44 expression as mean fluorescence intensity (MFI) (c), and % CD62Llo OT-I cells (d) in individual recipient mice treated with 6C8 (squares) or MR1 (circles) and then fed PBS (open symbols) or OVA (filled symbols). Data on individual mice are pooled from two experiments.
Anti-CD40L treatment does not alter IFN-γ expression by oral OVA–activated OT-I cells.
Loss of IFN-γ expression has been shown to reflect functional inactivation or anergy of virus-specific CTLs (31). We measured intracellular IFN-γ in splenic T cells from B6 mice after treatment with control or with anti-CD40L mAb and oral OVA, according to the protocol used to obtain the data shown in Figure 2, following restimulation ex vivo. Splenocytes were analyzed by three-color fluorescence, in which OT-I (Ly5.2) cells were gated from host CD8+ (Ly5.1) cells. Host CD8+ T cells and OT-I cells both produced IFN-γ in response to stimulation with PMA and ionomycin. In addition, OT-I cells produced IFN-γ in response to OVA257-264, but this was not altered by anti-CD40L treatment (data not shown).
Induction of diabetes by oral OVA in RIP-OVAlo mice is blocked by anti-CD40L treatment.
Feeding small doses of OVA (0.5 mg, five alternating days) to mimic low-dose oral tolerance regimens induces diabetes in RIP-OVAlo mice bearing adoptively transferred OVA-specific transgenic CD4+ (OT-II) and CD8+ (OT-I) T cells (13). The transfer of transgenic T cells is required because RIP-OVAlo mice also express OVA in the thymus, which deletes their OVA-specific T cell repertoire. In separate experiments, we found that the minimum numbers of transferred OT-II and OT-I cells required to induce diabetes after priming RIP-OVAlo mice systemically with OVA-coated splenocytes were 3 × 105 and 2 × 105, respectively. These numbers of OT-II and OT-I cells were transferred into RIP-OVAlo mice. The following day, mice were given control mAb 6C8 or anti-CD40L mAb MR1, and then fed 0.5 mg OVA on five alternating days. In response to oral OVA, 60% (9/15) of the control mice developed diabetes, compared with only 14% (2/14) of mice treated with anti-CD40L mAb (P = 0.02) (Figure 3).
Induction of oral tolerance is not blocked by anti-CD40L treatment.
Although experiments in CD40L–/– mice indicated that CD40L signaling is required for induction of oral tolerance (21), it was necessary to determine whether oral tolerance could still be induced in genetically unmanipulated mice treated short-term with anti-CD40L mAb. To determine the effect of anti-CD40L treatment on the tolerogenic effect of oral OVA to suppress systemic priming of CTLs (12–15), B6 mice were first given control mAb 6C8 or anti-CD40L mAb MR1 (250 μg, intraperitoneally), and then fed PBS or OVA in PBS. After 14 or 21 days, and prior to measurement of OVA-specific CTL activity ex vivo, mice were primed in a CD4+ T cell–dependent manner with intravenous OVA-coated splenocytes (Figure 4a), or in a CD4+ T cell–independent manner with 100 μg subcutaneous OVA in CFA (Figure 4b). CTL activity plots (examples shown for individual mice in Figure 4, a and b) were converted into lytic units per spleen (Figure 4, c and d). In all cases, oral OVA suppressed the CTL response to systemic priming, but this was not altered by anti-CD40L treatment. For example, lytic units per spleen (mean ± SD) for treatment with oral PBS versus OVA were 1,540 ± 981 and 254 ± 202 (P = 0.002) after control Ab, and 633 ± 498 and 116 ± 92 after anti-CD40L Ab.
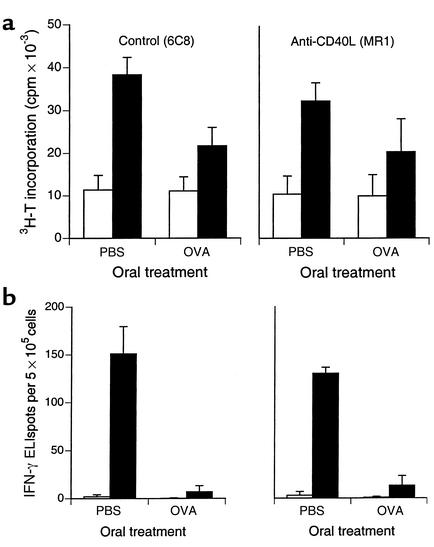
We then determined the effect of anti-CD40L treatment on more conventional parameters of oral tolerance. Anti-CD40L mAb MR1 given before the first dose of oral OVA (20 mg on three alternating days) had no effect on oral OVA–induced suppression of systemic OVA-primed T cell proliferation (3H-thymidine incorporation) (Figure 5a), IFN-γ production measured as ELIspots (Figure 5b), or serum anti-OVA Ab responses (not shown). Mean ± SD stimulation indices for proliferation (ratio of 3H-thymidine incorporation in the presence and absence of OVA) decreased from 3.56 ± 1.07 (oral PBS) to 1.96 ± 0.199 (oral OVA) (P = 0.03) after treatment with control mAb 6C8, and from 3.21 ± 0.199 to 2.01 ± 0.091 (P = 0.001) after anti-CD40L mAb MR1 (Figure 5a). Suppression of primed IFN-γ ELIspot responses following oral OVA was more dramatic, and was not affected by anti-CD40L treatment (Figure 5b). Very few IL-4 ELIspots (≤ 4/well) were detected under any conditions. Mean ± SD ELISA absorbance values (nm) for IgG1 anti-OVA Ab’s decreased from 0.71 ± 0.07 (oral PBS) to 0.15 ± 0.10 (oral OVA) (P = 0.04) after control mAb, and from 0.60 ± 0.11 to 0.11 ± 0.09 (P = 0.02) after anti-CD40L mAb. Values for IgG2a, IgG2b, and IgG3 showed the same directional changes and, except in the case of IgG2a, were below the level of detection after oral OVA.
Figure 5.
Anti-CD40L treatment does not prevent oral tolerance measured as suppression of systemic priming of (a) T cell proliferation measured as 3H-thymidine (T) incorporation, or (b) IFN-γ production measured as ELIspots. Mice (n = 3 in each group) were injected with control mAb 6C8 or anti-CD40L mAb MR1 (250 μg intraperitoneally), and then fed either PBS or 20 mg OVA in PBS on three alternating days. After 7 days, they were immunized subcutaneously with OVA (0.1 mg) in CFA in the base of tail. Ten days later, spleens and inguinal lymph nodes were harvested for measurement of T cell proliferation (a) and cytokine production (b) in the absence (white bars) or presence (black bars) of 0.1 mg/ml OVA (mean and SD shown for spleen), and sera obtained for measurement of anti-OVA Ab’s (not shown, see text), as described in Methods. 3H-T, 3H-thymidine.
Oral tolerance can be induced in the absence of CD40.
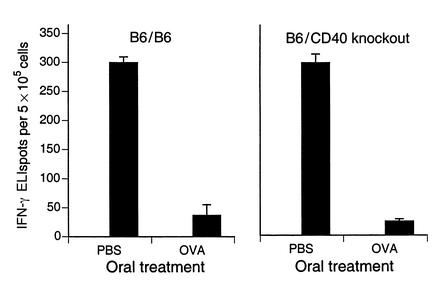
To confirm that induction of oral tolerance did not require CD40-CD40L signaling, we compared oral tolerance in B6 mice chimeric for bone marrow from either CD40–/– mice or control B6 mice. After oral OVA (20 mg administered on three alternating days), systemic OVA-primed T cell responses were suppressed to a similar degree in both wild-type B6 and CD40 knockout chimeras. This is shown for IFN-γ production (Figure 6). The effect on T cell proliferation (not shown) was similar but less dramatic. Compared with oral PBS, mean ± SD stimulation indices for T cell proliferation after oral OVA decreased from 4.53 ± 0.730 to 2.64 ± 0.221 (P = 0.02) in wild-type chimeras, and from 4.67 ± 1.11 to 2.93 ± 0.848 (P = 0.05) in CD40 knockout chimeras.
Figure 6.
Oral tolerance can be induced in CD40-deficient mice. B6 mice were irradiated and reconstituted with bone marrow from either wild-type B6 or CD40 knockout mice. Mice were fed PBS or 20 mg OVA in PBS on three alternating days. As described in the legend to Figure 5, mice (n = 3 in each group) were then immunized subcutaneously with OVA in CFA. Ten days later, their spleens were harvested for measurement of T cell proliferation (not shown, see text) and IFN-γ ELIspots in the absence or presence (black bars) of 0.1 mg/ml OVA (mean and SD are shown). No ELIspots were detected in the absence of OVA.
Discussion
The concomitant induction of tolerance and CTLs potentially limits the efficacy and safety of mucosal administration of autoantigens for the prevention of autoimmune disease. Our results show that CD40L-CD40 signaling is required for the induction of CTL immunity, but not for induction of tolerance to oral antigen, and that CD40L blockade at the time of oral OVA administration dissociates CTLs from tolerance induction. This selective effect was associated with protection from diabetes development in mice bearing transgenic OVA-specific T cells and OVA in pancreatic β cells. In other studies (N.R. Martinez and L.C. Harrison, unpublished observations), we have found that anti-CD40L treatment can dissociate CTLs from tolerance induction after intranasal OVA in B6 and NOD mice.
Our results contrast with the apparent resistance of CD40L–/– mice to oral tolerance induction reported by Kweon et al. (21). They found that oral OVA did not diminish T cell proliferation or IL-2 secretion to subsequent priming with OVA. However, the level of T cell proliferation was low and IFN-γ secretion was suppressed in both CD40L–/– and wild-type mice. Developmental or secondary abnormalities in the CD40L–/– mice, rather than CD40L deficiency itself, could be responsible for these observations. Our findings both in mice treated with anti-CD40L Ab and in CD40-deficient chimeric mice argue strongly that CD40-CD40L interactions are not required for the induction of oral tolerance.
The suppression of oral OVA–induced CTLs by anti-CD40L treatment may be explained by more than one mechanism. First, because CD40L on CD4+ T cells activates DCs to prime CTL precursors, CD40L blockade should directly impair the ability of DCs to prime CTLs. At the same time, if the CD4+ T cells that provide help for CTL priming fail to receive reciprocal signals, such as IL-12 from activated DCs, they themselves may become tolerant (32). Alternatively, DCs that fail to be activated by CD40L could interact with CTL precursors to render them tolerant. In addition, activated CD8+ T cells themselves express low levels of CD40L (19), and anti-CD40L blockade may directly influence CD8+ T cells (20). When we gave oral OVA and then measured OVA-specific CTLs following either CD4+ T cell–dependent or –independent means of systemic priming, OVA-specific CTL responses were suppressed in both cases, indicating that the effect of oral OVA is exerted at least at the level of CTLs.
Using tetramer reagents to identify CTLs specific for the two major immunodominant epitopes of lymphocytic choriomeningitis virus (LCMV), Zajac et al. (31) showed that in the absence of CD4+ T cell help, viral challenge induced both deletion and functional inactivation of CTL with loss of IFN-γ expression. Activation of DCs in this LCMV model is mediated by CD40L (33), and to a lesser extent by TRANCE, another member of the TNF family (34). OVA-specific CTLs could respond analogously under CD40L blockade, assuming that CD40L-CD40 is the major pathway of CD4+ T cell help for CTL induction by oral OVA. To elucidate the fate of CTL precursors that respond to oral OVA during CD40L blockade, we determined the numbers and phenotype of adoptively transferred OT-I cells in Ly5.1 congenic B6 mice fed OVA following anti-CD40L treatment. We also measured intracellular IFN-γ in OT-I cells, because loss of IFN-γ expression has been reported to reflect CTL anergy (31). The expansion of OT-I cells, and the appearance of OT-I cells with activated/memory phenotype, was greatly diminished in response to oral OVA after CD40L-blockade, but there was no change in IFN-γ expression by OT-I cells, which might be expected if CTLs had been anergized. OT-I cell numbers were analyzed in the spleen at a relatively late time (14 days) after oral OVA treatment. It is possible that OT-I cells were only transiently activated in the mucosal compartment, and lacked appropriate stimulation for a sustained response. This would be consistent with evidence that accumulation but not differentiation of CTLs in intestinal mucosa is dependent on CD40L (20), and with a requirement for CD40L in order to sustain Th1 responses (35) and maintain CTL memory (33).
If these studies with OVA can be extrapolated to other proteins containing candidate CTL epitopes, then the outcome of mucosal antigen administration may well reflect the balance between protective tolerance and pathogenic CTL immunity. In the context of autoimmune disease prevention, induction of CTL immunity is a potential hazard that could nullify any benefit of oral tolerance induction, or could even exacerbate disease. Mucosal administration of unmodified protein antigens in rodent autoimmune disease models usually results in only partial clinical protection (1, 2), and the ability of oral autoantigen to exacerbate disease has been documented (3–6). Recognition that mucosal antigen can induce not only protective immunity but potentially pathogenic CTL immunity has important implications for clinical trials. Although CD40L is critical for humoral and cell-mediated immunity, its transient blockade during exposure to mucosal antigen is not likely to seriously compromise host immunity, but, as shown, may improve the efficacy and safety of mucosal tolerance induction in the prevention of CTL-dependent autoimmune diseases such as type 1 diabetes.
Acknowledgments
The authors thank Andrea Braakhuis for technical assistance, Tatiana Banjanin, Kate Johnstone, and Michelle Latimer for care of mice, and Catherine O’Shea for secretarial assistance. This work was supported by the Juvenile Diabetes Foundation International, The Sigrid Juselius Foundation (Finland), The Finnish Academy, the Finnish Cultural Foundation, and the National Health and Medical Research Council of Australia.
Footnotes
See the related Commentary beginning on page 171.
Arno Hänninen’s current address is: MediCity, University of Turku, Turku, Finland.
References
- 1.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 2.Harrison LC, Hafler DA. Antigen-specific therapy for autoimmune disease. Curr Opin Immunol. 2000;12:704–711. doi: 10.1016/s0952-7915(00)00166-7. [DOI] [PubMed] [Google Scholar]
- 3.Miller A, Lider O, Abramsky O, Weiner HL. Orally administered myelin basic protein in neonates primes for immune responses and enhances experimental autoimmune encephalomyelitis in adult animals. Eur J Immunol. 1994;24:1026–1032. doi: 10.1002/eji.1830240503. [DOI] [PubMed] [Google Scholar]
- 4.Terato K, Ye XJ, Miyahara H, Cremer MA, Griffiths MM. Induction by chronic autoimmune arthritis in DBA/1 mice by oral administration of type II collagen and Escherichia coli lipopolysaccharide. Br J Rheumatol. 1996;35:828–838. doi: 10.1093/rheumatology/35.9.828. [DOI] [PubMed] [Google Scholar]
- 5.Bellman K, Kolb H, Rastegar S, Jee P, Scott FW. Potential risk of oral insulin with adjuvant for the prevention of type 1 diabetes: a protocol effective in NOD mice may exacerbate disease in BB rats. Diabetologia. 1998;41:844–847. doi: 10.1007/s001250050997. [DOI] [PubMed] [Google Scholar]
- 6.Mordes JP. Oral insulin does not prevent insulin-dependent diabetes mellitus in BB rats. Ann NY Acad Sci. 1996;778:418–421. doi: 10.1111/j.1749-6632.1996.tb21161.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 8.Trentham DE, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 9.McKown KM, et al. Lack of efficacy of oral bovine type II collagen added to existing therapy in rheumatoid arthritis. Arthritis Rheum. 1999;42:1204–1208. doi: 10.1002/1529-0131(199906)42:6<1204::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Pozzilli P, et al. No effect of oral insulin on residual beta-cell function in recent-onset type 1 diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia. 2000;43:1000–1004. doi: 10.1007/s001250051482. [DOI] [PubMed] [Google Scholar]
- 11.Chaillous L, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabetes Insuline Orale group. Lancet. 2000;356:545–549. doi: 10.1016/s0140-6736(00)02579-4. [DOI] [PubMed] [Google Scholar]
- 12.Blanas E, Carbone FR, Allison J, Miller JF, Heath WR. Induction of autoimmune diabetes by oral administration of autoantigen. Science. 1996;274:1707–1709. doi: 10.1126/science.274.5293.1707. [DOI] [PubMed] [Google Scholar]
- 13.Hanninen A, Braakhuis A, Heath WR, Harrison LC. Mucosal antigen primes diabetogenic cytotoxic T lymphocytes regardless of dose or delivery route. Diabetes. 2001;50:771–775. doi: 10.2337/diabetes.50.4.771. [DOI] [PubMed] [Google Scholar]
- 14.Garside P, Steel M, Liew FY, Mowat AM. CD4+ but not CD8+ T cells are required for the induction of oral tolerance. Int Immunol. 1994;7:501–504. doi: 10.1093/intimm/7.3.501. [DOI] [PubMed] [Google Scholar]
- 15.Ke Y, Kapp JA. Oral antigen inhibits priming of CD8+ CTL, CD4+ T cells, and antibody responses while activating CD8+ suppressor T cells. J Immunol. 1996;156:916–921. [PubMed] [Google Scholar]
- 16.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 17.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 18.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 19.Hermann G, Beck FM, Sheridan JF. Stress-induced glucocorticoid response modulates mononuclear cell trafficking during an experimental influenza viral infection. J Neuroimmunol. 1995;56:179–186. doi: 10.1016/0165-5728(94)00145-e. [DOI] [PubMed] [Google Scholar]
- 20.Lefrancois L, Olsen S, Masopus DA. A critical role for CD40/CD40L interactions in amplification of the mucosal CD8 T cell response. J Exp Med. 1999;190:1275–1284. doi: 10.1084/jem.190.9.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kweon MN, et al. Mucosally induced systemic T cell unresponsiveness to ovalbumin requires CD40 ligand-CD40 interactions. J Immunol. 1999;162:1904–1909. [PubMed] [Google Scholar]
- 22.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 23.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 24.Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawabe T, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 26.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGF-beta secretion and T cell apoptosis. J Immunol. 1996;157:2348–2357. [PubMed] [Google Scholar]
- 27.Liu L, Kuchroo VK, Weiner HL. B7.2 (CD86) but not B7.1 (CD80) costimulation is required for the induction of low dose oral tolerance. J Immunol. 1999;163:2284–2290. [PubMed] [Google Scholar]
- 28.Hockenberry D, Nunuez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 29.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyle JS, Silva A, Brady JL, Lew AM. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc Natl Acad Sci USA. 1997;94:14626–14631. doi: 10.1073/pnas.94.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borrow P, et al. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmann MF, et al. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howland KC, Ausubel LJ, London CA, Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–4470. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]