Survivin is a structurally unique member of the inhibitor of apoptosis (IAP) family of proteins that is potentially involved in both control of cell division and inhibition of apoptosis (1). Specifically, its antiapoptotic function is related to the ability to directly or indirectly inhibit caspases (2). The notion that survivin is overexpressed in most of the common human tumors (3, 4) but absent in normal adult tissues with only a few exceptions (5, 6) has led to the proposal of survivin as a promising therapeutic target for novel anticancer therapies (7). Indeed, in October 2001, Mesri et al. (8) reported in the JCI that infection with a replication-deficient adenovirus encoding a Thr34→Ala mutant of survivin caused apoptosis in human tumor cell lines of different histology and reduced tumor growth in xenograft breast cancer models. Moreover, inhibition of survivin expression enhanced taxol-induced cell death in tumor cells.
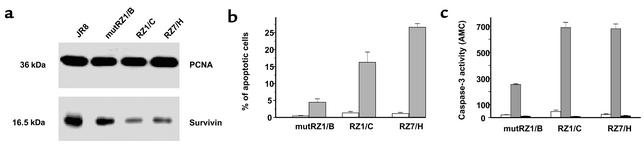
As an alternative strategy for survivin inhibition we developed hammerhead ribozymes targeting the 3′ end of the CUA110 (RZ1) and the GUC294 (RZ7) triplets in the survivin mRNA. In a cell-free system, both ribozymes induced cleavage of a synthetic RNA substrate obtained by cloning a portion of survivin mRNA, with cleavage products being detectable starting from a ribozyme/substrate ratio of 1:0.5. Conversely, the catalytically inactive mutRZ1 (which was produced by introducing a mutation in the catalytic core of the active ribozyme RZ1 and was used as control throughout the study) did not show any cleavage activity. RZ1, RZ7, and mutRZ1 sequences were inserted into the pRC expression vector under the control of the cytomega-lovirus promoter and transfected into the human metastatic melanoma cell line JR8 overexpressing survivin. For the present study we selected three stably transfected clones proven to endogenously express RZ1 (clone RZ1/C), RZ7 (clone RZ7/H), or mutRZ1 (clone mutRZ1/B). RZ1/C and RZ7/H cells were characterized by a markedly lower survivin protein level (68% and 60% lower, respectively) than JR8 parental cells, whereas a negligible reduction (13%) in survivin expression was observed in mutRZ1/B cells (Figure 1a). To evaluate the effect of survivin inhibition on the susceptibility of melanoma cells to undergo cisplatin-induced apoptosis, we treated the different clones with 10 μg/ml of the drug for 1 hour and determined the presence of apoptotic nuclei in cells stained with propidium iodide under fluorescence micro-scopy at 72 hours after treatment. A very modest apoptotic response was ob-served in the mutRZ1/B cells, whereas a significant increase in the percentage of apoptotic cells was observed in RZ1/C (P = 0.01) and RZ7/H (P = 0.005) cells (Figure 1b). Processing of caspase-3 to its active subunits of approximately 17 and 19 kDa was observed in all three drug-treated clones. However, the caspase-3 catalytic activity as assessed by hydrolysis of the fluorogenic substrate N-Acetyl-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-AMC) was about threefold higher in RZ1/C and RZ7/H clones than in the mutRZ1/B clone (Figure 1c).
Figure 1.
(a) Survivin protein expression in JR8 parental cells and melanoma cell clones transfected with the active ribozymes RZ1 (RZ1/C clone) and RZ7 (RZ7/H clone) or with the mutant ribozyme mutRZ1 (mutRZ1/B clone). Western blots were probed with a polyclonal antibody for survivin. Proliferating cell nuclear antigen (PCNA) was used as a control for loading. (b and c) Induction of apoptosis in melanoma cell clones exposed to 10 μg/ml cisplatin for 1 hour. Seventy-two hours after treatment, cells were collected and the occurrence of apoptosis was determined. (b) The percentage of cells with an apoptotic morphology with respect to the overall population was assessed by fluorescence microscopy after cell staining with propidium iodide. White bars, control (no drug) cells; gray bars, cisplatin-treated cells. Data represent mean values ± SD of three experiments. (c) The caspase-3 catalytic activity was determined by hydrolysis of the fluorogenic substrate N-Acetyl-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-AMC) in the presence or absence of the caspase-3 inhibitor Ac-DEVD-CHO. White bars, control (no drug) cells; gray bars, cisplatin-treated cells; black bars, cisplatin-treated cells + CHO. Data represent mean values ± SD of three experiments.
These results are in agreement with the previous finding of Grossman et al. (9), who observed enhancement of cisplatin-induced apoptosis by expression of the survivin Thr34→Ala mutant in YUSAC2 melanoma cells. Unlike what was reported by these authors, attenuation of survivin expression in our melanoma cell system was not sufficient to appreciably trigger apoptosis in the absence of other stimuli. Other antiapoptotic factors besides survivin, such as bcl-2 and bcl-xL, are strongly expressed in JR8 cells and may contribute to preventing programmed cell death in this tumor model. However, it should be stressed that in JR8 cells survivin expression was attenuated but not completely abrogated. It may be that inhibition below a certain threshold is insufficient to determine a proapoptotic effect. Interestingly, and in accordance with such a hypothesis, when we transduced the human prostate cancer cells DU145 with a Moloney-based retroviral vector carrying the catalytic sequence of the ribozyme RZ7, we were able to select a ribozyme-expressing clone characterized by an almost complete abrogation of survivin expression (99.5% lower compared with control cells, as assessed by Western blotting, and lack of detectable protein expression by confocal microscopy). This ribozyme-expressing clone also showed a markedly higher percentage of apoptotic cells than control culture (20% and 3% of cells on the overall cell population, respectively).
In conclusion, the present results obtained with the ribozyme-mediated approach in melanoma cells extend and corroborate earlier evidence indicating that attenuating survivin expression renders these cells more susceptible to cisplatin-induced apoptosis. These data also suggest a possible strategy to enhance the chemosensitivity profile of such a drug-refractory human malignancy.
Footnotes
Marzia Pennati and Gennaro Colella contributed equally to this work.
References
- 1.Altieri DC, Marchisio PC. Survivin apoptosis: an interloper between cell death and cell proliferation in cancer. Lab Invest. 1999;79:1327–1333. [PubMed] [Google Scholar]
- 2.Reed JC. The survivin saga goes in vivo. J Clin Invest. 2001;108:965–969. DOI:10.1172/JCI200114123. doi: 10.1172/JCI14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosini G, Adida C, Altieri D. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 4.Velculescu VE, et al. Analysis of human transcriptomes. Nat Genet. 1999;23:387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 5.Konno R, et al. Expression of survivin and Bcl-2 in the normal human endometrium. Mol Hum Reprod. 2000;6:529–534. doi: 10.1093/molehr/6.6.529. [DOI] [PubMed] [Google Scholar]
- 6.Chiodino C, et al. Expression of the novel inhibitor of apoptosis survivin in normal and neoplastic skin. J Invest Dermatol. 1999;113:415–418. doi: 10.1046/j.1523-1747.1999.00711.x. [DOI] [PubMed] [Google Scholar]
- 7.Grossman D, McNiff JM, Li F, Altieri DC. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999;113:1076–1081. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 8.Mesri M, Wall NR, Li J, Kim RW, Altieri DC. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108:981–990. DOI:10.1172/JCI200112983. doi: 10.1172/JCI12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman D, Kim PJ, Schechner JS, Altieri DC. Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci USA. 2001;98:635–640. doi: 10.1073/pnas.230450097. [DOI] [PMC free article] [PubMed] [Google Scholar]