Abstract
This study demonstrates that a CD34–, vascular endothelial cadherin– (VE-cadherin–), AC133+, and fetal liver kinase+ (Flk1+) multipotent adult progenitor cell (MAPC) that copurifies with mesenchymal stem cells from postnatal human bone marrow (BM) is a progenitor for angioblasts. In vitro, MAPCs cultured with VEGF differentiate into CD34+, VE-cadherin+, Flk1+ cells — a phenotype that would be expected for angioblasts. They subsequently differentiate into cells that express endothelial markers, function in vitro as mature endothelial cells, and contribute to neoangiogenesis in vivo during tumor angiogenesis and wound healing. This in vitro model of preangioblast-to-endothelium differentiation should prove very useful in studying commitment to the angioblast and beyond. In vivo, MAPCs can differentiate in response to local cues into endothelial cells that contribute to neoangiogenesis in tumors. Because MAPCs can be expanded in culture without obvious senescence for more than 80 population doublings, they may be an important source of endothelial cells for cellular pro- or anti-angiogenic therapies.
Introduction
Vasculogenesis, the in situ differentiation of the primitive endothelial progenitors known as angioblasts into endothelial cells that aggregate into a primary capillary plexus, is responsible for the development of the vascular system during embryogenesis (1). In contrast, angiogenesis, defined as the formation of new blood vessels by a process of sprouting from preexisting vessels, occurs both during development and in postnatal life (1–3). Until recently, it was thought that blood vessel formation in postnatal life was mediated by sprouting of endothelial cells from existing vessels. However, recent studies have suggested that endothelial stem cells may persist into adult life, where they contribute to the formation of new blood vessels (4–7). This in turn suggests that, as during development, neoangiogenesis in the adult may depend at least in part on a process of vasculogenesis. Precursors of endothelial cells have been isolated from bone marrow (BM) and peripheral blood (1, 2). The ontogeny of these endothelial progenitors is unknown.
We describe for the first time the in vitro generation of vast numbers of endothelial cells, that engraft in vivo and contribute to neoangiogenesis, from a nonendothelial BM stem cell, which also differentiates into other mesodermal cell types and neuroectodermal cell types. We have termed this cell a multipotent adult progenitor cell (MAPC). MAPCs can be expanded in culture for more than 80 population doublings, and endothelial cells generated from MAPCs can be expanded for at least an additional 20 population doublings. MAPCs may therefore be an ideal source of endothelial cells for clinical therapies. In addition, because MAPCs are ontogenically less mature than the angioblast, this model should be useful for the characterization of endothelial commitment and differentiation.
Methods
MAPC cultures.
BM was obtained from 55 healthy volunteer donors (2–45 years of age) after obtaining informed consent per the guidelines of the University of Minnesota Committee on the Use of Human Subjects in Research. MAPCs were generated as previously described (3). Briefly, BM mononuclear cells were depleted of CD45+ and glycophorin A+ cells using micromagnetic beads (Miltenyi Biotec, Sunnyvale, California, USA). Cells (5 × 103) that were negative for CD45 and glycophorin A were diluted in 200 μl expansion medium consisting of 58% low-glucose DMEM (Invitrogen Corp., Grand Island, New York, USA) and 40% MCDB-201 (Sigma Chemical Co., St. Louis, Missouri, USA), supplemented with 1× insulin-transferrin-selenium, 1× linoleic acid–BSA, 10–8 M dexamethasone, 10–4 M ascorbic acid 2-phosphate (all from Sigma Chemical Co.), 100 U penicillin, and 1,000 U streptomycin (Invitrogen Corp.); along with 0–10% FCS (HyClone Laboratories, Logan, Utah, USA), 10 ng/ml EGF (Sigma Chemical Co.), and 10 ng/ml PDGF-BB (R&D Systems Inc., Minneapolis, Minnesota, USA). Cells were then plated in wells of 96-well plates that had been coated with 5 ng/ml of fibronectin (Sigma Chemical Co.). Cells were fed every 4–6 days. Once cells were more than 40–50% confluent, adherent cells were detached with 0.25% trypsin-EDTA (Sigma Chemical Co.) and replated at 1:4 dilution, in bigger culture vessels coated with 5 ng/ml fibronectin and MAPC expansion medium, to maintain cell densities between 2 × 103 and 8 × 103 cells/cm2.
Differentiation conditions and characterization.
To induce differentiation into endothelial cells, MAPCs were replated at 1 × 104 to 2 × 104 cells/cm2 in fibronectin-coated culture vessels or chamber slides, in 60% low-glucose DMEM (Invitrogen Corp.) and 40% MCDB-201 (Sigma Chemical Co.), supplemented with 1× insulin-transferrin-selenium, 1× linoleic acid–BSA, 10–8 M dexamethasone, 10–4 M ascorbic acid 2-phosphate (all from Sigma Chemical Co.), 100 U penicillin, and 1,000 U streptomycin (Invitrogen Corp.), plus 10 ng/ml VEGF (a kind gift from S. Ramakrishna, University of Minnesota). In some instances, FCS (HyClone Laboratories) was added. Cultures were maintained by media exchange every 4–5 days. In some instances, cells were subcultured after day 9 at a 1:4 dilution under the same culture conditions for more than 20 population doublings.
Recipient mice.
A breeding colony of NOD/SCID mice was established using mice obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). Mice were kept in specific pathogen–free conditions, and maintained on acidified water and autoclaved food. Trimethoprim (60 mg/ml water) and sulfamethoxazole (300 mg/ml water) (Hoffmann–La Roche Inc., Nutley, New Jersey, USA) were given twice weekly.
Transplantation of MAPC-derived endothelial cells to an animal model for tumor and wound neoangiogenesis.
Three Lewis lung carcinoma spheroids were implanted subcutaneously in the shoulder of each mouse. Three days and 5 days after tumor implantation, mice were injected with 0.25 × 106 human MAPC–derived endothelial cells or human foreskin fibroblasts via tail vein injection. After 14 days, animals were sacrificed, and tumors were removed and cryopreserved using OCT compound (Sakura Finetek USA Inc., Torrance, California, USA) at –80°C. In addition, we removed the ears that had been clipped to tag each mouse; these were cryopreserved using OC compound at –80°C. Sections of the tissues (5 μm thick) were mounted on glass slides, and were fixed and stained as described below.
Undifferentiated MAPCs (106) were injected intravenously into 6-week-old NOD-SCID mice. Animals were maintained for 12 weeks and then sacrificed. In one animal, a thymic tumor was found. The thymus was removed and cryopreserved in OCT compound at –80°C. Tissue sections (10 μm thick) were mounted on glass slides, and were fixed and stained as described below.
FACS analysis.
MAPCs, or MAPCs induced to differentiate into endothelial cells for 2–21 days, were detached using 0.25% trypsin-EDTA (Sigma Chemical Co.). Cells were stained with Ab’s against Tie, Tek, Flk1, Flt1, vWF (all from Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), CD13, CD31, CD34, CD36, CD44, CD49b, AC133, HLA class I, HLA class II, β2-microglobulin, cKit (all from Becton Dickinson Immunocytometry Systems, Mountain View, California, USA), VCAM, ICAM-1, VE-cadherin, CD62E, CD62L, CD62P, or MUC18, recognized by the Ab H1P12, (all from Chemicon International USA Inc., Temecula, California) at saturating concentrations. Cells were washed and labeled with FITC, phycoerythrin (PE), or APC-conjugated secondary goat anti-mouse, goat anti-rabbit, or sheep anti-goat Ab’s (Sigma Chemical Co.), then washed and analyzed using a FACSCalibur flow cytometry system (Becton Dickinson Immunocytometry Systems).
Immunofluorescence.
For in vitro cultures, undifferentiated MAPCs, or MAPCs induced to differentiate into endothelium by plating in fibronectin-coated chamber slides with VEGF for 2–18 days, were fixed with 4% paraformaldehyde (Sigma Chemical Co.) for 4 minutes at room temperature and stained for surface markers. Alternatively, for cytoskeleton staining, chamber slides were fixed with methanol for 2 minutes at –20°C. For nuclear ligands, cells were further permeabilized with 0.1% Triton X-100 (Sigma Chemical Co.) for 10 minutes. Slides were incubated for 30 minutes with primary Ab, then incubated with FITC-, PE-, or Cy5-coupled anti-mouse IgG, anti-goat IgG, or anti-rabbit IgG Ab. After each incubation step, slides were washed with PBS containing 1% BSA. Primary Ab’s against CD31, CD34, CD36, CD44, HLA class I, HLA class II, and β2-microglobulin were used at a 1:50 dilution. Primary Ab’s against VCAM, ICAM-1, VE-cadherin, H1P12, ZO-1, MUC18, αvβ3, αvβ5, β-catenin, γ-catenin (all from Chemicon International USA Inc.), Tek, Tie, and vWF (all from Santa Cruz Biotechnology Inc.) were used at a 1:50 dilution. Stress fibers were stained with Ab’s (used at 1:200 dilution) against the 20 k-Da myosin light chain (Sigma Chemical Co., clone no. MY-21). Secondary Ab’s were purchased from Sigma Chemical Co., and were used at the following dilutions: anti-goat IgG–Cy3 (1:40), anti-goat IgG–FITC (1:160), anti-mouse IgG–Cy3 (1:150), anti-mouse IgG–FITC (1:320), anti-rabbit FITC (1:160), and anti-rabbit Cy3 (1:30). TOPRO-3 was purchased from Sigma Chemical Co. Cells were examined by fluorescence microscopy using a Zeiss Axiovert scope (Carl Zeiss Inc., Thornwood, New York, USA) and by confocal fluorescence microscopy using a confocal microscope (Olympus AX70; Olympus Optical Co. Ltd., Tokyo, Japan).
Samples of normal tissue or tumor were sectioned using a cryostat, into sections 5–10 μm thick. Sections were fixed with acetone for 10 minutes at room temperature and permeabilized with 0.1% Triton X-100 for 5 minutes. Slides to be analyzed for vWF, Tie, or Tek were incubated overnight, followed by secondary incubation with FITC-, PE-, or Cy5-coupled anti-mouse IgG, anti-goat IgG, or anti-rabbit IgG Ab’s, and sequential incubation with Ab’s against mouse CD45–PE, human CD45–FITC, human β2-microglobulin–FITC, mouse CD31–FITC, and TOPRO-3 for 60 minutes. After each step, slides were washed with PBS containing 1% BSA. Slides were examined by fluorescence microscopy using a Zeiss Axiovert scope, and by confocal fluorescence microscopy using a confocal microscope. Three-dimensional reconstruction consisted of the acquisition of sequential 0.5-μm confocal photos from 35 slides of 5-μm-thick sections to a total of 350 photos. Slides were stained with vWF-Cy3, and alternately double-stained with human β2-microglobulin–FITC or mouse CD31–FITC Ab’s. The photos from each slide were aligned with the next slide in MetaMorph software (Universal Imaging Corp., West Chester, Pennsylvania, USA), and the 3D reconstruction was made in 3D Doctor software (Able Software Corp., Lexington, Massachusetts, USA). The volume of the relative contributions of human (green) and murine endothelial cells (false colored as blue) to the 3D vessel were calculated as cubic pixels of each color. The area of each color was also calculated as square pixels in four tumor vessels appearing in each of the 35 slides to obtain an accurate percentage of contribution. The area of each color was also calculated in alternate slides of four different tumors.
Hypoxia studies.
MAPCs and MAPC-derived endothelial cells were incubated at 37°C in 20% or 10% O2 for 24 hours. Cells were stained with Ab’s against Flk1, Flt1, Tek, and IgG control, fixed in 2% paraformaldehyde, and analyzed by flow cytometry. In addition, VEGF concentrations in the culture supernatants were measured using an ELISA kit (Amersham Biotech, Piscataway, New Jersey, USA).
Histamine-mediated release of vWF.
MAPCs and MAPC-derived endothelial cells were incubated with 10 μM histamine (Sigma Chemical Co.) in serum-free medium for 25 minutes. Untreated and treated cells were fixed with methanol at –20°C for 2 minutes, stained with Ab’s against vWF and myosin, and analyzed using fluorescence and/or confocal microscopy.
IL-1α treatment.
MAPCs (106) and MAPC-derived endothelial cells (106) were incubated with 75 ng/ml IL-1α (R&D Systems Inc.) in serum-free medium for 24 hours. Cells were fixed in 2% paraformaldehyde and stained with Ab’s against HLA class I, HLA class II, β2-microglobulin, vWF, CD31, VCAM, CD62E, and CD62P, or with control Ab’s, and analyzed using FACS.
In vitro vascular tube formation: Extracellular matrix (Sigma Chemical Co.) was added to a 24-well plate at 0.5 ml per well, and incubated for 3 hours at 37°C. MAPCs and MAPC-derived endothelial cells (104 per well) were added in 0.5 ml of serum-free medium containing 10 ng/ml VEGF and incubated at 37°C.
a-LDL uptake.
The DiI-Ac-LDL staining kit was purchased from Biomedical Technologies (Stoughton, Massachusetts, USA). The assay was performed per the manufacturer’s recommendations.
Results
Human MAPCs differentiate into cells with phenotypic characteristics of endothelium.
MAPCs were generated as described from BM of donors 2–45 years old (3). Undifferentiated MAPCs did not express CD31, CD34, CD36, CD44, CD45, CD62E, CD62L, CD62P, HLA class I, HLA-DR, cKit, Tie, Tek, αvβ3, VE-cadherin, VCAM, or ICAM-1. MAPCs expressed low or very low levels of β2-microglobulin, αvβ5, AC133, Flk1, and Flt1, and high levels of CD13 and CD49b (Figure 1a).
Figure 1.
FACS analysis of undifferentiated MAPCs and MAPCs cultured with VEGF. MAPCs (after 40 population doublings; donor age, 28 years) were replated at 2 × 104 cells/cm2 in fibronectin-coated wells in serum-free defined medium without EGF or PDGF-BB, but with 10 ng/ml VEGF. Undifferentiated MAPCs at day 0, or VEGF-induced cells recovered after short trypsinization after 3, 9, or 14 days of culture, were stained with Ab’s against β2-microglobulin, HLA class I, MUC18, Flk1, Flt1, VCAM, CD62P, CD62E, vWF, CD31, CD34, CD36, AC133, VE-cadherin, or control IgG. Cells were analyzed by FACS. Plots show isotype control IgG staining profile (thin line) versus specific Ab staining profile (thick line). Each analysis shown is one representative example from a total of three donors. Values on x axes indicate intensity log. (a) Phenotype of undifferentiated MAPCs. MAPCs express low levels of β2-microglobulin, Flk1, Flt1, and AC133, but do not stain with any of the other anti-endothelial markers. (b) Phenotype of MAPCs cultured for 14 days with 10 ng/ml VEGF. MAPCs express high levels of most endothelial markers associated with endothelial cells, but lose expression of AC133. (c) Phenotype of MAPCs cultured for 3–9 days with 10 ng/ml VEGF. MAPCs lose expression of AC133 by day 3 of culture with VEGF, and acquire expression of Tek by day 3, and vWF, CD34, and MUC18 by day 9. β2-mic, β2-microglobulin; VE-cad, VE-cadherin.
To induce endothelial differentiation, MAPCs obtained after 20–80 population doublings were replated at 2 × 104 cells/cm2 in fibronectin-coated wells in serum-free defined medium without EGF or PDGF-BB, but with 10 ng/ml VEGF. We have previously reported that these conditions allow differentiation of MAPCs into CD31–, CD34–, and vWF+ endothelial cells (3). Here we defined endothelial differentiation from MAPCs more extensively by FACS and immunohistochemical analysis of cells after 3–18 days. Expression of Flk1 and Flt1 on undifferentiated MAPCs was low (Figure 1a). Expression was maximal after day 9, and persisted after day 14 (Figure 1, b and c). VE-cadherin, present on endothelial progenitors found in BM and blood (1, 4), was not expressed on undifferentiated MAPCs, but was expressed after 3 days of culture with VEGF, and persisted after day 14 (Figure 1b). MAPCs expressed low levels of AC133 (Figure 1a), which is found on endothelial progenitors as well as hematopoietic progenitors (1, 5), but AC133 was no longer detectable after day 3 (Figure 1, b and c). CD34, also present on endothelial progenitors and hematopoietic progenitors (1, 6, 7), was not present on undifferentiated MAPCs (Figure 1a), but was expressed from day 9 until day 18 following addition of VEGF (Figure 1b). MUC18 has been used to purify endothelial progenitors from blood (8). Although undifferentiated MAPCs did not stain with H1P12 (Figure 1a), MAPCs treated with VEGF for 9 days were MUC18+; however, expression of MUC18 was lost by day 18 (not shown).
The endothelium-specific integrin αvβ3 (9) was not present on undifferentiated MAPCs, whereas αvβ5 was expressed at very low levels (not shown). Expression of both integrins increased progressively during differentiation, and was maximal by day 14 (Figure 2). The tyrosine kinase receptors Tie1 and Tek, which are important for angiogenesis but not endothelial cell differentiation (10), were not expressed on MAPCs (not shown). Expression of Tek could be seen after day 3, and Tie1 after day 7 (Figure 1c). MAPCs also do not express vWF, but vWF was expressed from day 9 on (11, 12). Markers of more mature endothelial cells, including CD31, CD36, CD62P (13) (Figure 1, a and b), and the adhesion junction proteins ZO-1, β-catenin, and γ-catenin (Figure 2) were detected after day 14 (14–16). Neither VCAM nor CD62E was expressed at high levels at any timepoint during differentiation (Figure 1b), unless endothelium was activated with IL-1α, as described below. Differentiation to endothelium was associated with acquisition of β2-microglobulin and low levels of HLA-class I antigens, but not HLA-class II antigens (Figure 1b). Differentiation to endothelial cells was similar when MAPCs from donors 2–45 years old were used. In addition, differentiation of MAPCs to endothelium occurred in a similar fashion in both MAPCs used after 20 (n = 30) population doublings, and in MAPCs used after more than 50 (n = 25) population doublings.
Figure 2.
Immunohistochemical evaluation of MAPC-derived endothelial cells. (a) MAPCs (after 65 population doublings; donor age, 22 years) were replated at 2 × 104 cells/cm2 in fibronectin-coated wells in serum-free defined medium with 10 ng/ml VEGF. After 14 days, cells were fixed with paraformaldehyde, permeabilized with Triton X-100, and stained with Ab’s against αvβ5 (scale bar = 50 μm), ZO-1, β-catenin, and γ-catenin. Cells were then evaluated by confocal fluorescence microscopy. Typical membrane staining is seen for the adhesion, αvβ5, and for the adhesion junction proteins ZO-1, β-catenin, and γ-catenin. Representative example from one of three total donors. Scale bar = 50 μm. (b) Morphology of MAPCs at day 0 (upper panel) and day 21 (lower panel) after VEGF treatment in bright-field microscopy. Scale bar = 25 μm.
We have reported previously that endothelial differentiation can be obtained only from MAPCs expanded with 2% FCS or less, not from those expanded with 10% FCS (3), because higher concentrations of FCS support growth of classical mesenchymal stem cells that differentiate only into osteoblasts, chondroblasts, and adipocytes (3, 17). We now also demonstrate that when FCS was present during the initial 9 days of differentiation, endothelial differentiation could not be induced (Figure 3a). Further, when nonconfluent MAPCs (104 or fewer cells/cm2) were treated with VEGF, no endothelial differentiation was seen (Figure 3b). When MAPCs were subcultured 9 days after exposure to VEGF using serum-free medium with 10 ng/ml VEGF, cells could undergo at least an additional 12 population doublings. When 10% FCS and 10 ng/ml VEGF were added to the medium for subculturing, MAPC-derived endothelial cells could undergo an additional 20+ population doublings, regardless of the number of population doublings that undifferentiated MAPCs had undergone (Figure 3c).
Figure 3.
Differentiation into endothelial cells requires absence of serum and high density of MAPCs. (a) MAPCs (after 65 population doublings; donor age, 22 years) were replated at 2 × 104 cells/cm2 in fibronectin-coated wells in serum-free medium with 10 ng/ml VEGF in the presence or absence of serum (2% FCS). After 9 days, cells were fixed with paraformaldehyde, permeabilized with Triton X-100, and stained with Ab’s against vWF and CD34. Representative example of three experiments, one from each of three different donors. (b) MAPCs (after 65 population doublings; donor age, 22 years) were replated at 2 × 104 cells/cm2 or ≤ 1 × 104 cells/cm2 in fibronectin-coated wells in serum-free defined medium with 10 ng/ml VEGF. After 9 days, cells were fixed with paraformaldehyde, permeabilized with Triton X-100, and stained with Ab’s against vWF and CD34. Representative example of three experiments from three donors. (c) MAPCs (after 45 population doublings; donor age, 28 years) were replated at 2 × 104 cells/cm2 in fibronectin-coated wells in serum-free defined medium with 10 ng/ml VEGF. After 9 days, endothelial cells were further expanded in 10% FCS with 10 ng/ml VEGF for more than 20 population doublings. Representative example of six experiments from six donors.
Functional characteristics of MAPC-derived endothelium.
We next tested whether progeny of MAPCs that had been induced to differentiate with VEGF had functional characteristics of endothelial cells. Functional characteristics were indistinguishable between MAPC-derived endothelial cells from donors 2–45 years old and endothelial cells derived from MAPCs maintained for 20–65 population doublings.
Endothelial cells contain vWF, stored in Weibel-Palade bodies, that is released in vivo when endothelium is activated (12). This can be induced in vitro by stimulating cells with histamine (11), which also results in activation of the cell cytoskeleton (18). MAPC-derived endothelial cells were plated at high confluence (2 × 104 cells/cm2) in fibronectin-coated chamber slides. After 24 hours, cells were treated with histamine and stained with Ab’s against vWF and myosin. vWF was present throughout the cytoplasm of untreated endothelial cells. Cytoplasm of endothelial cells treated with histamine contained significantly less vWF, and vWF was detectable only in the perinuclear region, likely representing vWF present in the endoplasmic reticulum (Figure 4a). Histamine treatment caused widening of gap junctions, and cytoskeletal changes with increased numbers of myosin stress fibers (Figure 4a).
Figure 4.
Functional characterization of MAPC-derived endothelial cells. MAPCs (after 45 population doublings; donor age, 28 years) were replated at 2 × 104 cells/cm2 in fibronectin-coated wells in serum-free defined medium with 10 ng/ml VEGF for 14 days. (a) Histamine-mediated release of vWF from MAPC-derived endothelium. MAPC-derived endothelial cells were plated at 104 cells/cm2 in fibronectin-coated chamber slides. After 24 hours, cells were treated with 10 μM histamine in serum-free medium with VEGF for 25 minutes. Cells were then stained with Ab’s against vWF (FITC) and myosin (Cy3). vWF staining is found throughout the cytoplasm in untreated cells, but decreased and was detectable only in the perinuclear region following treatment with histamine. Staining with Ab’s against myosin shows cytoskeletal changes with increased numbers of myosin stress fibers, and widening of gap junctions. Representative example of three experiments from three donors. Scale bar = 60 μm. (b) MAPC-derived endothelium takes up a-LDL. MAPCs induced to differentiate with VEGF for 3, 7, and 9 days with VEGF were incubated with DiI-Ac-LDL. Cells were colabeled with Ab’s against Tek, Tie1, or vWF, and analyzed by confocal microscopy. After 3 days, we detected expression of Tek, but no uptake of a-LDL. After 7 days, cells expressed Tie1, but again did not take up a-LDL. However, acquisition of expression of vWF on day 9 was associated with uptake of a-LDL. Representative example of ten experiments from six donors. Scale bar = 100 μm. (c) Vascular tube formation by MAPC-derived endothelium. MAPC-derived endothelial cells were replated in Matrigel with VEGF. After 6 hours, typical vascular tubes could be seen. Representative example of six experiments from six donors. Scale bar = 200 μm.
Another characteristic of endothelial cells is that they take up LDL (19). This was tested by incubating MAPCs induced to differentiate with VEGF for 2, 3, 5, 7, 9, 12, 5, and 21 days with DiI-Ac-LDL. Cells were colabeled with either anti-Tek, anti-Tie1, or anti-vWF Ab’s. After 3 days, we detected expression of Tek, but no uptake of a-LDL. After 7 days, cells expressed Tie1, but did not take up significant amounts of a-LDL. However, acquisition of expression of vWF on day 9 was associated with uptake of a-LDL (Figure 4b).
We next tested whether endothelial cells would form “vascular tubes” when plated on Matrigel (Becton Dickinson and Co., Franklin Lakes, New Jersey, USA) or extracellular matrix (20). As shown in Figure 4c, culture of MAPC-derived endothelial cells on extracellular matrix resulted in vascular tube formation within 6 hours.
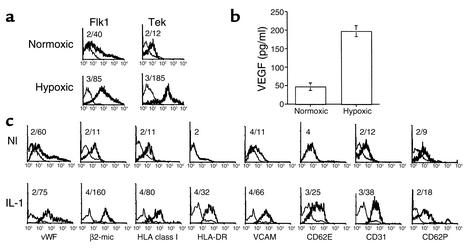
Endothelial cells respond to hypoxia by upregulating expression of VEGF, as well as the VEGF receptor Flk1 and the angiogenesis receptor Tek (21). MAPC-derived endothelial cells and undifferentiated MAPCs were exposed to hypoxic conditions for 24 hours compared with normally oxygenated cultures. Expression of Flk1 and Tek was significantly increased on MAPC-derived endothelial cells exposed to hypoxia (Figure 5a), whereas the levels of these receptors remained unchanged on undifferentiated MAPCs (not shown). In addition, levels of VEGF in culture supernatants of hypoxic endothelial cultures were increased by fourfold (Figure 5b), compared with normoxic cultures, whereas VEGF levels in MAPC cultures exposed to hypoxia remained unchanged (not shown) compared with normoxic cultures.
Figure 5.
Further functional characterization of MAPC-derived endothelial cells. (a) Hypoxia upregulates Flk1 and Tek expression on MAPC-derived endothelial cells. MAPC-derived endothelial cells (after 30 population doublings; donor age, 33 years) were incubated at 37°C in 20% or 10% O2 for 24 hours. Cells were recovered by trypsinization and stained with Ab’s against Flk 1, Tek, and IgG control, and were analyzed by flow cytometry. Plots show isotype control IgG staining profile (thin line) versus specific Ab staining profile (thick line). Representative example of more than three experiments from three donors. Number above plots shows mean fluorescence intensity (MFI) for the control IgG staining and the specific Ab staining. (b) Hypoxia upregulates VEGF production by MAPC-derived endothelial cells. MAPCs (after 30 population doublings; donor age, 45 years) and MAPC-derived endothelial cells were incubated at 37°C in 20% or 10% O2 for 24 hours. Medium was collected, and VEGF levels were measured by ELISA. Results are shown as mean ± SEM of six experiments from three donors. (c) IL-1α induces expression of HLA-DR, a type of HLAclass II antigens and increases expression of adhesion receptors. MAPC-derived endothelial cells (after 40 population doublings, donor age = 31 years) were incubated with 75 ng/ml IL-1α in serum-free medium for 24 hours. Cells were stained with Ab’s against HLA-class I, HLA class II, β2-microglobulin, vWF, CD31, VCAM, CD62E, CD62P, or control Ab’s, and analyzed using FACS. Plots show isotype control IgG staining profile (thin line) versus specific Ab staining profile (thick line). Representative example of three experiments from three donors. Numbers above each plot show MFI for the control IgG staining and the specific Ab staining. Nl, normal; IL-1 induced populations.
Finally, we tested whether MAPC-derived endothelial cells would upregulate expression of HLA antigens and cell adhesion ligands in response to inflammatory cytokines such as IL-1α (22, 23). When undifferentiated MAPCs or MAPC-derived endothelial cells were exposed to 75 ng/ml IL-1α for 24 hours, significantly increased levels of HLA class I, HLA class II, β2-microglobulin, VCAM, vWF, CD31, CD62E, and CD62P were seen by FACS analysis of endothelial cells (Figure 5c). In contrast, on undifferentiated MAPCs, upregulation of only Flk1 was seen (not shown).
MAPC-derived endothelial cells contribute to tumor angiogenesis in vivo.
MAPC-derived endothelial cells or human foreskin fibroblasts (0.25 × 106) were injected intravenously into NOD-SCID mice 3 days and 5 days after implantation of murine Lewis lung carcinoma spheroids near the shoulder blades (n = 5). Computer-aided analysis of the length and number of branches counted on five sections of each tumor showed that tumors in mice that received human MAPC–derived endothelial cells had a 1.45 ± 0.04–fold greater vascular mass than tumors in control mice that did not receive endothelial cells, or that received fibroblasts. All tumors were stained with anti-human β2-microglobulin or HLA class I Ab’s, combined with anti-mouse CD31. Ab’s and anti-vWF, anti-Tek, or anti-Tie1 Ab’s, which recognize both human and mouse endothelial cells. These initial studies showed that some blood vessels in the tumors contained anti-human β2-microglobulin or HLA class I–positive cells that colabeled for either vWF, Tie, or Tek, but not for mouse CD31, indicating that human MAPC–derived endothelial cells contributed to tumor neoangiogenesis in vivo. To better address the degree of contribution, we obtained 35 sequential 5-μm slides that were stained in an alternate fashion with either anti-human β2-microglobulin–FITC or anti-mouse CD31–Cy5 and anti–vWF-Cy3. All slides were examined by confocal microscopy. The different images were then assembled in 3D to determine the relative contribution of human and murine endothelial cells to the tumor vessels. When we analyzed tumors obtained from animals injected with human MAPC–derived endothelial cells, approximately 35% of the tumor vessels were positive for anti-human β2-microglobulin as well as vWF, whereas approximately 40% of endothelial cells stained positive with anti-mouse CD31 Ab’s (Figure 6, a–g). Tumors in animals that did not receive endothelial cells, or received human fibroblasts, did not contain endothelial cells that stained positive with the anti–β2-microglobulin or anti–HLA class I Ab’s (not shown).
Figure 6.
Contribution of human MAPC-derived endothelial cells to neoangiogenesis in tumors and wound healing. (a–g) MAPC-derived endothelial cells (0.25 × 106) (after 30–65 population doublings before differentiation) were injected intravenously into NOD-SCID mice after implantation of murine Lewis lung carcinoma spheroids (n = 5) from three donors, aged 19, 28, and 31 years). After 2 weeks, animals were sacrificed, and tumors were removed, sectioned, and stained with either anti-human β2-microglobulin–FITC or anti-mouse CD31–FITC and anti–vWF-Cy3. Shown are the 3D reconstructed figures of 350 images for either anti-human β2-microglobulin–FITC (c) or anti-mouse CD31–FITC (false colored as blue) (d), and merging of the two (e); anti–vWF-Cy3 (f); and merging of the three staining patterns (g). (a and b) Scale bar = 100 μm. (h) Wound healing. Ears of NOD-SCID mice used in the studies described in a were punched 3 and 5 days prior to intravenous injection of human MAPC-derived endothelial cells (a–c) or human foreskin fibroblasts (d–f). After 14 days, animals were sacrificed and ears were obtained and cryopreserved. Five-micrometer slides were stained with anti-human β2-microglobulin–FITC and anti–vWF-Cy3. Scale bar = 20 μm. C, cartilage; D, dermis. (i) Tumor angiogenesis is derived from endothelial cells generated in vivo from MAPCs. MAPCs (106) (after 45 population doublings; donor age, 28 years) were injected intravenously into a NOD-SCID mouse (n = 1). After 12 weeks, the animal was sacrificed, at which time a thymic tumor was detected. Ten-micrometer slides were stained with anti-human β2-microglobulin–FITC and anti–vWF-Cy3. Shown is a highly vascularized area in the tumor stained with Ab’s against β2-microglobulin–FITC, vWF, and TOPRO-3 (not shown). Scale bar = 20 μm.
We also performed analysis to determine whether MAPC-derived endothelial cells contribute to wound-healing angiogenesis. We examined the area of the ear that had been clipped to tag the mouse. Neoangiogenesis in the ear wounds was in part derived from the MAPC-derived endothelial cells. Similar to blood vessels in the analyzed tumor sections, human endothelial cells made up 30–45% of the healed skin wound (Figure 6h).
Undifferentiated human MAPCs differentiate into endothelial cells in vivo.
Undifferentiated MAPCs (106) were infused intravenously into 6-week-old nonirradiated NOD-SCID mice. Animals were maintained for 12 weeks and then sacrificed. In one animal, a host-cell thymic lymphoma developed, which is commonly seen in aging NOD-SCID mice (24). All hematopoietic cells stained positive for mouse CD45 but not human CD45, indicating that they were murine in origin. We then stained the tumor with an anti-human β2-microglobulin–FITC Ab and an anti–vWF-Cy3 Ab that recognizes both human and mouse endothelial cells. Approximately 12% of the vasculature was derived from human MAPCs (Figure 6i). These studies further confirmed that the hematopoietic elements were not of human origin, as no human β2-microglobulin was detected outside the vascular structures.
Discussion
The central finding of this study is that cells that copurify with mesenchymal stem cells from BM have the ability to differentiate into endothelial cells that have in vitro functional characteristics that are indistinguishable from those of mature endothelial cells. We also show that such endothelial cells contribute to neoangiogenesis in vivo in the setting of wound healing and tumorigenesis, and that undifferentiated MAPCs can respond to local cues in vivo to differentiate into endothelial cells, contributing to tumor angiogenesis. Since the same cell that differentiates into endothelium also differentiates into other mesodermal cell types (3), as well as into cells of nonmesodermal origin (Reyes et al., unpublished observations), the cell defined here precedes the angioblast, and even the hemangioblast, in ontogeny.
During development, endothelial cells are derived from mesoderm. Flk1 (also known as VEGF receptor 2) characterizes the hemangioblast, a bipotent stem cell found in the aorto-gonad-mesonephros region (25–27) and in fetal liver (26); and commitment of embryoid bodies to hemangioblasts is accompanied by expression of Flk1 (28, 29). Whether hemangioblasts persist in adult life is not known, and only hematopoietic stem cells (HSC) and endothelial progenitors have been identified in adult life. Like hemangioblasts, endothelial progenitors express Flk1 (1), and one report has suggested that HSC in postnatal life express Flk1 (30). During embryology, commitment of the hemangioblast to the endothelial lineage is characterized by the sequential expression of VE-cadherin, CD31, and (shortly afterward) CD34 (31, 32). In postnatal life, endothelial progenitors have been selected from BM and blood using Ab’s against AC133, Flk1, CD34, and the MUC18 (1, 5, 8). AC133 has also been found on other cells, including neural stem cells (33) and gastrointestinal epithelial cells (34). Upon differentiation to mature endothelium, the AC133 receptor is quickly lost (1, 5). Another receptor found on circulating endothelial progenitor cells is MUC18 (8). MUC18 is lost upon differentiation of endothelial progenitors to endothelium. CD34 is expressed on endothelial progenitors, as well as on hematopoietic progenitors (1, 35) and hepatic oval cells (36). This antigen is also lost upon differentiation of endothelial progenitors to endothelium. Most mature endothelial cells, except for microvascular endothelial cells, no longer express CD34.
Like hemangioblasts and endothelial progenitors, undifferentiated MAPCs express low levels of Flk1 and AC133. In contrast to endothelial progenitors, MAPCs do not express VE-cadherin, CD31, cKit, or CD34. When cultured with VEGF, MAPCs differentiate into endothelial cells, and after 14–21 days, more than 90% of cells express markers of mature endothelial cells. The low level of expression of Flk1 and AC133 seen by FACS analysis may indicate that the starting population is not homogeneous, and that endothelial cells are derived from the Flk1+/AC133+ endothelial-committed mesodermal cells. However, there is no obvious cell loss during differentiation, suggesting that even Flk1–/AC133– cells may differentiate into endothelium. However, we cannot exclude the possibility that a fraction of MAPCs undergo apoptosis during differentiation, whereas the Flk1+/AC133+ cells proliferate. The other possibility is that all cells express low levels of Flk1 and AC133, which is consistent with what we observed by immunohistochemistry analysis (not shown). This observation, together with our observation that the same single cell that differentiates into endothelium also differentiates into other mesodermal cell types (3) as well as neuroectodermal cells (Reyes et al., unpublished observations), leads us to speculate that the multipotent cell present in MAPC cultures expresses low levels of Flk1 and AC133. We further speculate that this cell is the predecessor of the Flk1+, AC133+, CD34+, PECAM+, and cKit+ endothelial progenitor described by others (1, 5, 8).
As we have shown for other lineages (3), differentiation from MAPCs to endothelium required that differentiation be induced in high-density cultures. This may be needed to inhibit cell proliferation and allow cell differentiation. This may explain not only why differentiation could not be induced when cells were plated at low density, but also why differentiation was not seen when FCS, which is known to contain a number of mitogens (37), was added to the differentiation cultures. Although VEGF plays an important role in vasculogenesis and angiogenesis, other factors are required for these processes, including signaling via bFGF (38) and TGF-β (39). Because MAPCs themselves produce bFGF as well as TGF-β (data not shown), it is possible that these cytokines serve as paracrine factors in high-density cultures. Additional receptors, including Notch-4 (40) and ephrins (41) are important regulators of vasculogenesis and angiogenesis. Whether these receptors are present on MAPCs is currently under investigation.
We not only showed that MAPCs differentiate into cells that express markers of endothelial cells, but proved that VEGF induced MAPCs to function like endothelial cells. Endothelial cells modify lipoproteins during transport in the artery wall (41), and they maintain a permeability barrier through intercellular junctions that widen when exposed to hemodynamic forces or vasoactive agents, such as histamine (11, 14, 15, 22). Endothelial cells release prothrombotic molecules such as vWF, tissue factor, and plasminogen activator inhibitor to prevent bleeding (22), and regulate egress of leukocytes by changing expression levels of adhesion molecules in response to inflammation (19, 20). Endothelium also reacts to hypoxia by producing VEGF and expressing VEGF receptors to increase vascular density (18). We demonstrated that endothelial cells generated from MAPCs can perform all of these tasks when tested in vitro.
Finally, we found that in vitro–generated MAPC-derived endothelial cells respond to angiogenic stimuli by migrating to tumor sites and contributing to tumor vascularization, and by participating in wound healing in vivo. This finding confirms that endothelial cells generated from MAPCs have all the functional characteristics of mature endothelium. The degree of contribution of endothelial cells to tumor angiogenesis and to neoangiogenesis was 35–45%, levels similar to those described for other sources of endothelial cells (42, 43). In addition, we found that angiogenic stimuli in vivo provided in a tumor microenvironment are sufficient to recruit MAPCs to the tumor bed and induce their differentiation into endothelial cells that contribute to the tumor vasculature. These studies therefore extend studies reported by other groups demonstrating that cells present in marrow can contribute to new blood vessel formation (1, 5, 7, 8) in a process similar to vasculogenesis, since we have identified the precursor responsible for this process.
As MAPCs can be expanded in culture without obvious senescence for more than 80 population doublings, they may be a very useful source of endothelial cells for cellular pro- or anti-angiogenic therapies. Furthermore, MAPCs precede the angioblast and even the hemangioblast in ontogeny. Therefore the model of CD34–, VE-cadherin–, AC133+, and Flk1+ MAPC differentiation to a CD34+, VE-cadherin+, AC133–, and Flk1+ “angioblast,” and subsequently to a mature endothelial cell, should prove very useful in the study of cellular commitment to the angioblast and beyond.
Acknowledgments
We thank S. Ramakrishna for assistance with the in vitro vasculature formation assay, Sabita Roy for the VEGF ELISA, and the Biomedical Imaging Processing Lab assistants for help and expertise in developing the 3D reconstruction. This work was supported by NIH grants PO1-CA-65493 (to C.M. Verfaillie) and F31-AI-GM-10291 (to M. Reyes); the University of Minnesota Medical Scientist Training Program; the Children’s Cancer Research Fund, Minneapolis, Minnesota; the Tulloch Family Fund; the McKnight Foundation; and the University of Minnesota Bone Marrow Transplant Research Fund.
Footnotes
See the related Commentary beginning on page 313.
References
- 1.Peichev M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 2.Watt S, Gschmeissner S, Bates P. PECAM-1: its expression and function as a cell adhesion molecule on hemopoietic and endothelial cells. Leuk Lymphoma. 1995;17:229–235. doi: 10.3109/10428199509056827. [DOI] [PubMed] [Google Scholar]
- 3.Reyes M, et al. Purification and ex vivo expansion of post-natal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 5.Gehling UM, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 6.Rafii S, et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–18. [PubMed] [Google Scholar]
- 7.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliceiri BP, Cheresh DA. Role of alpha v integrins during angiogenesis. Cancer J Sci Am. 2000;6(Suppl. 3):S245–S249. [PubMed] [Google Scholar]
- 10.Partanen J, Dumont DJ. Functions of Tie1 and Tie2 receptor tyrosine kinases in vascular development. Curr Top Microbiol Immunol. 1999;237:159–172. doi: 10.1007/978-3-642-59953-8_8. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg JB, et al. Intracellular trafficking of factor VIII to von Willebrand factor storage granules. J Clin Invest. 1998;101:613–624. doi: 10.1172/JCI1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tedder T, Steeber D, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–871. [PubMed] [Google Scholar]
- 14.Li CX, Poznansky MJ. Characterization of the ZO-1 protein in endothelial and other cell lines. J Cell Sci. 1990;97:231–237. doi: 10.1242/jcs.97.2.231. [DOI] [PubMed] [Google Scholar]
- 15.Van Rijen H, et al. Gap junctions in human umbilical cord endothelial cells contain multiple connexins. Am J Physiol. 1997;272:C117–C130. doi: 10.1152/ajpcell.1997.272.1.C117. [DOI] [PubMed] [Google Scholar]
- 16.Petzelbauer P, Halama T, Groger M. Endothelial adherens junctions. J Investig Dermatol Symp Proc. 2000;5:10–13. doi: 10.1046/j.1087-0024.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Vischer UM, Barth H, Wollheim CB. Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodeling in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:883–891. doi: 10.1161/01.atv.20.3.883. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg D, Pittman RC, Carew TE. Mechanisms involved in the uptake and degradation of low density lipoprotein by the artery wall in vivo. Ann NY Acad Sci. 1985;454:195–206. doi: 10.1111/j.1749-6632.1985.tb11858.x. [DOI] [PubMed] [Google Scholar]
- 20.Haralabopoulos GC, Grant DS, Kleinman HK, Maragoudakis ME. Thrombin promotes endothelial cell alignment in Matrigel in vitro and angiogenesis in vivo. Am J Physiol. 1997;273:C239–C245. doi: 10.1152/ajpcell.1997.273.1.C239. [DOI] [PubMed] [Google Scholar]
- 21.Kourembanas S, et al. Hypoxic responses of vascular cells. Chest. 1998;11(Suppl. 1):25S–28S. doi: 10.1378/chest.114.1_supplement.25s-a. [DOI] [PubMed] [Google Scholar]
- 22.Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev. 1999;10:27–39. doi: 10.1016/s1359-6101(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 23.Steeber DA, Tedder TF. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res. 2001;22:299–317. doi: 10.1385/IR:22:2-3:299. [DOI] [PubMed] [Google Scholar]
- 24.Prochazka M, Gaskins HR, Shultz LD, Leiter EH. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci USA. 1992;89:3290–3294. doi: 10.1073/pnas.89.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–911. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 26.Fong G-H, Zhang L, Bryce D-M, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 27.Peault B. Hematopoietic stem cell emergence in embryonic life: developmental hematology revisited. J Hematother. 1996;5:369–378. doi: 10.1089/scd.1.1996.5.369. [DOI] [PubMed] [Google Scholar]
- 28.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 29.Choi K. Hemangioblast development and regulation. Biochem Cell Biol. 1998;76:947–956. [PubMed] [Google Scholar]
- 30.Ziegler B, et al. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999;285:1553–1558. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 31.Nishikawa SI, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita J, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 33.Uchida N, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbeil D, et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512–5530. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 35.Baumhueter S, Dybdal N, Kyle C, Lasky L. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood. 1994;84:2554–2562. [PubMed] [Google Scholar]
- 36.Crosby HA, Kelly DA, Strain AJ. Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology. 2001;120:534–544. doi: 10.1053/gast.2001.21175. [DOI] [PubMed] [Google Scholar]
- 37.Iyer VR, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 38.Faloon P, et al. Basic fibroblast growth factor positively regulates hematopoietic development. Development. 2000;127:1931–1941. doi: 10.1242/dev.127.9.1931. [DOI] [PubMed] [Google Scholar]
- 39.Larsson J, et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoneya T, et al. Molecular cloning of delta-4, a new mouse and human Notch ligand. J Biochem. 2001;129:27–34. doi: 10.1093/oxfordjournals.jbchem.a002832. [DOI] [PubMed] [Google Scholar]
- 41.Adams RH, Klein R. Eph receptors and ephrin ligands: essential mediators of vascular development. Trends Cardiovasc Med. 2000;10:183–188. doi: 10.1016/s1050-1738(00)00046-3. [DOI] [PubMed] [Google Scholar]
- 42.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 43.Ribatti D, Vacca A, Nico B, Roncali L, Dammacco F. Postnatal vasculogenesis. Mech Dev. 2001;100:157–163. doi: 10.1016/s0925-4773(00)00522-0. [DOI] [PubMed] [Google Scholar]