Abstract
Activation of NF-κB by bacterial LPS promotes the upregulation of proinflammatory cytokines that contribute to the pathogenesis of Gram-negative septic shock. LPS activation of NF-κB is dependent upon the interaction of two death domain–containing (DD-containing) proteins, MyD88 and IL-1 receptor–associated kinase IRAK. Another DD-containing protein, Fas-associated death domain (FADD), also binds MyD88 through respective DD-DD interactions. Although FADD has been classically described as a proapoptotic signaling molecule, several reports have implicated a role for FADD in mediating NF-κB activation. In the present report, we investigated whether FADD could mediate LPS activation of NF-κB. Overexpression of FADD blocked LPS-induced NF-κB activation, whereas absence of FADD enhanced activation of NF-κB by LPS. Further, LPS-induced expression of two NF-κB–dependent gene products, IL-6 and KC, was enhanced in FADD–/– mouse embryo fibroblasts (MEFs) compared with wild-type. This increase in NF-κB activity correlated with enhanced IκB degradation. FADD–/– MEFs were also resistant to NF-κB activation induced by IL-1β. Finally, reconstitution of full-length FADD in the FADD–/– MEFs completely reversed the enhanced activation of NF-κB elicited by either LPS or IL-1β. Together, these data indicate that FADD negatively regulates LPS- and IL-1β–induced NF-κB activation and that this regulation occurs upstream of IκB degradation.
Introduction
The death rate due to septicemia continues to increase despite improvements in therapeutic care. Overwhelming infection by Gram-negative organisms and the subsequent development of septic shock is estimated to result in 20,000 deaths annually in the US alone (1). LPS, a potent inflammatory molecule residing in the outer membrane of Gram-negative bacteria, contributes to the pathogenesis of Gram-negative septic shock (2). One mechanism by which LPS is thought to promote the development of septic shock is through its ability to induce excessive host cell production of proinflammatory cytokines.
The upregulation of several proinflammatory cytokines by LPS, including IL-1β, IL-6, IL-8, and TNF-α, is dependent upon the activation of NF-κB. Prior to the identification of a transmembrane receptor for LPS, it was unclear how LPS could activate NF-κB signaling. It was originally reported that LPS activation of cells of monocytic lineage was dependent upon LPS recognition by membrane-associated CD14 (mCD14) (3). mCD14, however, is a glycosyl phosphatidylinositol-anchored protein that lacks an intracellular cytoplasmic domain, rendering it incapable of transducing a signal across the cell membrane. Further, several cell types, including endothelial cells, are sensitive to LPS despite lacking mCD14. Recently, Toll-like receptor-4 (Tlr-4) has been identified in both cells of monocytic lineage and non–mCD14-bearing endothelial cells as an LPS transmembrane receptor capable of activating NF-κB signaling (4, 5). The extracellular domain of Tlr-4 contains repeating leucine-rich motifs characteristic of innate immune response pattern recognition receptors (6). The cytoplasmic domain contains regions that are homologous to the intracellular signaling domain of the type 1 IL-1 receptor. Although the exact mechanism of LPS recognition by Tlr-4 remains unclear, cell activation is dependent upon the cell surface assembly of a multiprotein recognition complex consisting of mCD14, MD-2, and Tlr-4 (7). Following recognition of LPS, the adapter protein MyD88 is recruited to the cytoplasmic domain of Tlr-4. MyD88 contains a highly conserved death domain (DD) that facilitates its interaction with another DD-containing signaling molecule, IL-1 receptor–associated kinase (IRAK) (6). Following recruitment to MyD88, IRAK undergoes rapid autophosphorylation and dissociation from the signaling complex. Phosphorylated IRAK subsequently interacts with TNF receptor–associated factor-6 (TRAF-6), initiating the activation of a kinase cascade involving NF-κB–inducing kinase (NIK) and IκB kinase (IKK). Activation of this cascade culminates in the phosphorylation and degradation of the NF-κB inhibitor IκB, enabling NF-κB to translocate to the nucleus and promote new gene expression.
In addition to the NF-κB pathway, multiple other signaling cascades are activated by LPS, including a proapoptotic pathway dependent upon Fas-associated death domain (FADD) (8). FADD is an adapter protein that couples death receptors to initiator caspases. Activation of these upstream caspases following recruitment to FADD initiates a proteolytic cascade leading to the activation of downstream effector caspases and the onset of apoptosis. Similar to MyD88 and IRAK, FADD contains a highly homologous DD that is responsible for promoting protein-protein interactions. Reportedly, MyD88 and FADD interact with each other through respective binding of their DD regions, suggesting cross-talk between Tlr-initiated pathways leading to NF-κB signaling and apoptosis (9). In addition to structural similarities, FADD has been demonstrated to mediate NF-κB activation, a functional role shared by MyD88 and IRAK as well (10–13). We, therefore, decided to investigate whether FADD regulates LPS-induced NF-κB activation. In the present report, we demonstrate that FADD downregulates LPS-induced NF-κB–dependent gene expression and that FADD exerts this effect upstream of IκB degradation. We also identify a negative regulatory role for FADD in mediating NF-κB activation elicited by IL-1β, a proinflammatory cytokine that activates NF-κB through the same pathway as LPS.
Methods
Materials.
LPS from Escherichia coli serotype 0111:B4 was purchased from Sigma Chemical Co. (St. Louis, Missouri, USA). Recombinant human and murine IL-1β were purchased from R&D Systems Inc. (Minneapolis, Minnesota, USA).
Cell culture.
The human dermal microvascular endothelial cell line (HMEC-1) (developed and generously provided by F.J. Candal and E. Ades, Centers for Disease Control, Atlanta, Georgia, USA; and T. Lawley, Emory University, Atlanta, Georgia, USA) (14) was cultured in RPMI medium (BioWhittaker Inc., Walkersville, Maryland, USA) enriched with 10% FBS (HyClone Laboratories, Logan, Utah, USA), endothelial cell growth factor prepared from bovine hypothalamus, L-glutamine (2 mM), sodium pyruvate (1 mM), and nonessential amino acids, in the presence of penicillin (100 U/ml) and streptomycin (100 μg/ml) (all purchased from BioWhittaker Inc.). FADD+/+ and FADD–/– mouse embryo fibroblasts (MEFs) (generous gift of Wen-Chen Yeh, Amgen Institute, Toronto, Canada) were generated as previously described (15) and cultured in DMEM medium (BioWhittaker Inc.) enriched with 10% FBS, L-glutamine (2 mM), sodium pyruvate (1 mM), and nonessential amino acids, in the presence of penicillin (100 U/ml) and streptomycin (100 μg/ml).
Cloning and stable expression of cDNA constructs.
cDNA encoding either the DD of FADD or full-length FADD (generous gifts of Vishva Dixit, Genentech Inc., South San Francisco, California, USA) was cloned into the EcoRI/XhoI sites of the bicistronic retroviral expression plasmid, pBMN-IRES–enhanced green fluorescent protein (EGFP) (kindly provided by Gary Nolan, Stanford University, Stanford, California, USA) (16). High-titer retrovirus was prepared from the Phoenix amphotropic packaging cell line (American Type Culture Collection, Manassas, Virginia, USA) transfected with 24 μg of the expression plasmid by calcium phosphate precipitation. Recombinant retroviral supernatants were collected 48 hours after transfection and filtered through a Millex-HV 0.45-μM filter (Millipore Corp., Bedford, Massachusetts, USA). For infection, 2 × 105 HMEC-1 cells or MEFs were seeded per well of a six-well plate for 24 hours to achieve approximately 80% confluence. The growth medium was replaced with 2.5 ml of retroviral supernatant supplemented with 32 μg/ml polybrene and 10 mM HEPES, and the plate was centrifuged for 2 hours (1430 g, 32°C). The cells were then incubated for 10 hours (5% CO2, 37°C), after which the retroviral supernatant was replaced with normal growth medium. Cells were analyzed and sorted on the basis of EGFP expression using a FACVantage SE cell sorter (Becton, Dickinson and Co., Franklin Lakes, New Jersey, USA). High overexpression of full-length FADD resulted in cell death. To select for cells overexpressing FADD at a level compatible with viability, FADD-transfected cells were cultured for another 2 weeks. Viable transfectants were then sorted on the basis of EGFP expression and used in subsequent experiments.
Immunoblotting.
Cell monolayers were washed once with PBS, lysed with ice-cold modified radioimmunoprecipitation assay lysis buffer (50 mM Tris-HCl [pH 7.4], 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, protease inhibitor cocktail tablet [Roche Molecular Biochemicals, Indianapolis, Indiana, USA], 1 mM vanadate, 50 mM NaF), scraped, transferred to microcentrifuge tubes, and centrifuged (16,000 g, 10 minutes, 4°C). Total protein was determined using the BCA protein assay (Pierce Chemical Co., Rockford, Illinois, USA). The supernatants were combined with 5× sample buffer (Genomic Solutions Inc., Chelmsford, Massachusetts, USA) and boiled for 3 minutes, and 20 μg of protein per lane were resolved by SDS-PAGE on a 4–20% Tris-glycine gradient gel (Invitrogen Corp., Carlsbad, California, USA). Protein was subsequently transferred for 1 hour at 100 V to polyvinylidene fluoride membrane (Millipore Corp.). Blots were blocked with 5% dry milk and then incubated with anti-murine FADD (1.0 μg/ml; Calbiochem-Novabiochem Corp., San Diego, California, USA), anti-human FADD (0.5 μg/ml; Transduction Laboratories, Lexington, Kentucky, USA), anti-AU1 (1.0 μg/ml; Berkeley Antibody Co., Richmond, California, USA), anti–IκB-α (1:5000 dilution; Becton, Dickinson and Co.), or anti–IκB-β (0.04 μg/ml; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) antibodies for 1 hour at room temperature. The blots were incubated with horseradish peroxidase–conjugated anti-mouse (0.2 μg/ml; Santa Cruz Biotechnology Inc.) or anti-rabbit IgG (0.13 μg/ml; Becton, Dickinson and Co.), developed with enhanced chemiluminescence (Amersham Life Sciences Inc., Arlington Heights, Illinois, USA), and exposed to Kodak X-Omat Blue film (NEN Life Science Products Inc., Boston, Massachusetts, USA).
Luciferase assay.
A recombinant adenovirus (KZ142) system was used to transfect cells with a luciferase reporter construct. The adenoviral construct was created as follows: an oligonucleotide encoding a consensus NF-κB binding site, the tandem NF-κB binding sites of the HIV-1 long terminal repeat (17), two copies of the collagenase AP-1 element, and a single copy of the c-jun TRE (18) were ligated into a luciferase reporter cassette, and then placed in the pACCMV.pLpA adenoviral shuttle vector for construction of recombinant adenovirus as described (19). For transfection of the luciferase reporter construct, HMEC-1 cells or MEFs were seeded into 96-well black view plates (Corning Inc., Corning, New York, USA) at a density of 50,000 or 25,000 cells per well, respectively, for 24 hours and subsequently incubated for 16 hours at an moi of 2000 in RPMI supplemented with 1% FBS. Following infection, MEFs were exposed to experimental treatment in Ham’s F12 medium supplemented with 2.5% FBS, 20 mM HEPES, and 0.5% BSA for 4 hours at 37°C. Luciferase activity was determined using an assay kit and a TopCount NXT luminescence counter (both from Packard Instrument Co., Meriden, Connecticut, USA).
ELISA.
MEFs were seeded into 96-well plates at a density of 25,000 cells per well and cultured for 24 hours. Following treatment, plates were centrifuged (220 g, 10 minutes) and the supernatants analyzed using commercially available kits for murine IL-6 (Pierce Chemical Co.) or murine KC (R&D Systems Inc.). The OD at 450 nm and a correction wavelength of 550 nm were measured on a microplate reader (Bio-Tec Instruments Inc., Winooski, Vermont, USA). Values expressed in pg/ml were extrapolated from a standard curve using linear regression.
Statistical methods.
ANOVA was used to compare the mean responses among experimental and control groups. The Tukey post hoc comparison test was used to determine between which groups significant differences existed. All statistical analyses were performed using GraphPad Prism version 3.00 for Macintosh (GraphPad Software Inc., San Diego, California, USA). A P value of less than 0.05 was considered significant.
Results
Overexpression of FADD inhibits LPS- and IL-1β–induced NF-κB activity in HMEC-1 cells.
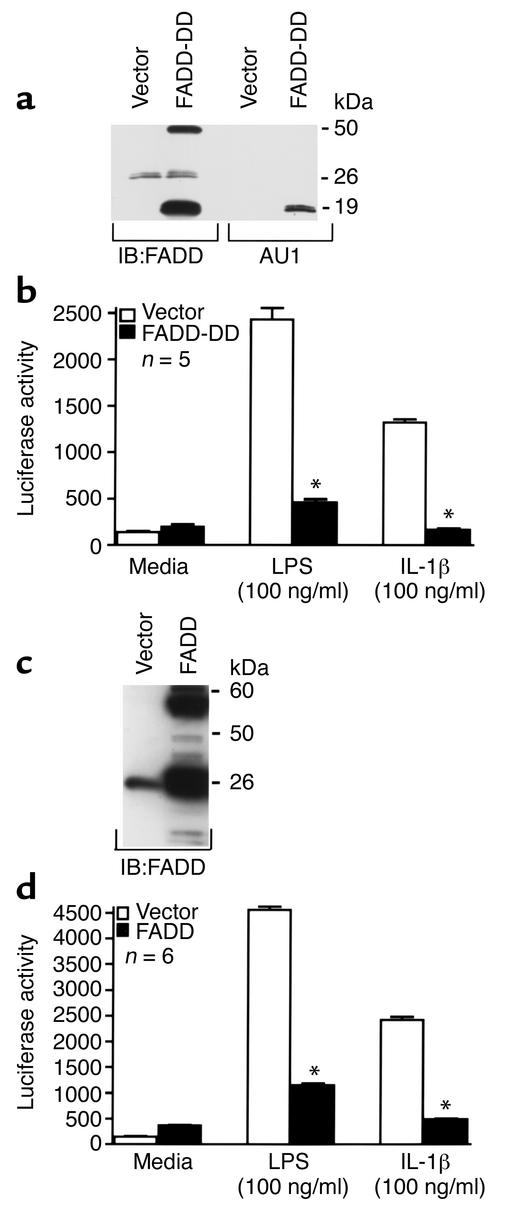
To determine whether FADD mediates LPS-induced activation of NF-κB, HMEC-1 cells were stably transfected with the DD of FADD (Figure 1, a and b). This domain has previously been reported to function as a dominant negative protein capable of inhibiting LPS-induced proapoptotic signaling (8). Expression of the DD of FADD was confirmed by Western blot analysis (Figure 1a). To assay for NF-κB activity, a quantitative luciferase reporter assay was used. Expression of the luciferase construct is under the control of an NF-κB–driven promoter. To confirm the specificity of this assay to detect NF-κB activation, we expressed an IκB-α mutant construct that is resistant to degradation (20). Expression of this NF-κB super-repressor, which we have previously shown to inhibit NF-κB activation by electrophoretic gel mobility shift assay (21) and NF-κB–dependent expression of VCAM-1 (22), completely blocks LPS-induced luciferase activity (data not shown). HMEC-1 cells overexpressing the FADD-DD displayed a marked reduction in NF-κB activity following LPS stimulation compared with HMEC-1 cells transfected with vector alone (Figure 1b). Since LPS and IL-1β use the same signaling pathway leading to activation of NF-κB, it was conceivable that a similar effect would be observed in response to IL-1β. Consistent with this notion, overexpression of the DD of FADD completely blocked IL-1β–induced NF-κB activity (Figure 1b). The finding that overexpression of the FADD-DD inhibits LPS- and IL-1β–induced NF-κB activation suggests one of two possibilities: (a) FADD contributes to LPS- and IL-1β–induced NF-κB signaling, and overexpression of the DD functions as a dominant negative protein to inhibit the ability of full-length FADD to convey NF-κB signaling, or (b) FADD acts as a repressor of LPS- and IL-1β–induced NF-κB signaling, and its ability to interfere with this signaling is localized to its DD. To explore these possibilities, HMEC-1 cells overexpressing full-length FADD (Figure 1c) were exposed to either LPS or IL-1β, and NF-κB activity was assayed (Figure 1d). Similar to HMEC-1 cells expressing the DD of FADD, overexpression of full-length FADD inhibited LPS- and IL-1β–induced NF-κB activation. These data suggest that FADD downregulates NF-κB signaling elicited by either of these agents and that its negative regulatory function is conferred by the DD region of the molecule.
Figure 1.
Overexpression of FADD or the DD of FADD inhibits LPS- and IL-1β–induced NF-κB activation. HMEC-1 cells were stably transfected with GFP vector alone (a–d), the DD of FADD (a and b), or full-length FADD (c and d), and expression was confirmed by Western blot analysis (a and c). In separate studies, these cells were treated for 4.5 hours with medium, LPS (100 ng/ml), or hIL-1β (100 ng/ml), lysed, and assayed for luciferase activity (b and d). Vertical bars represent mean (± SE) luciferase activity in arbitrary units. *Significantly decreased compared with HMEC-1 cells transfected with vector alone that were exposed to identical treatment.
Absence of FADD enhances LPS- and IL-1β–induced NF-κB activity and NF-κB–dependent cytokine production in MEFs.
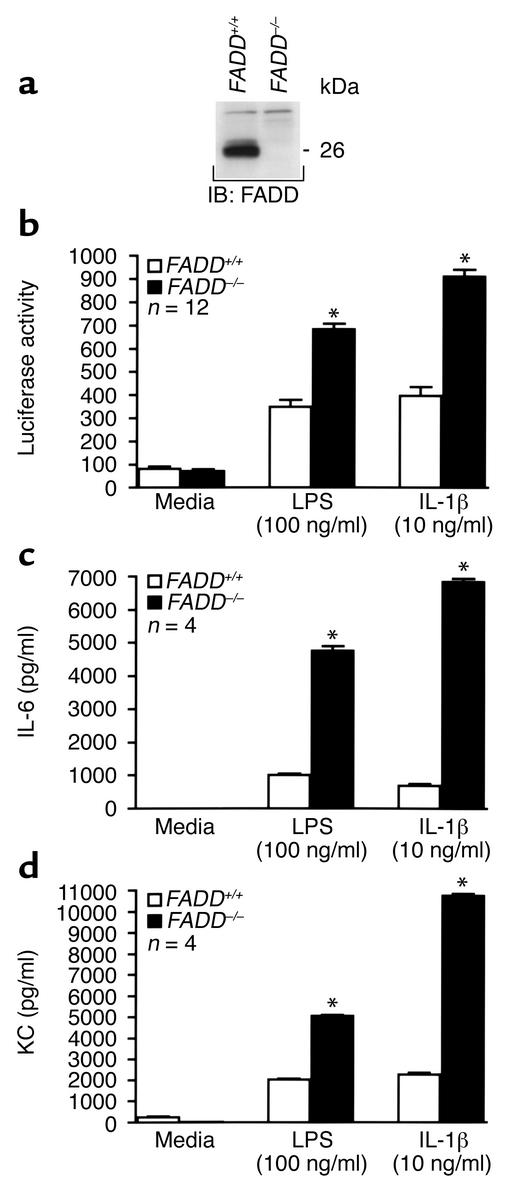
Since overexpression of FADD represses LPS- and IL-1β–induced NF-κB activation, we hypothesized that the absence of FADD would enhance NF-κB signaling elicited by either of these two inflammatory mediators. FADD–/– or wild-type MEFs were exposed to LPS or IL-1β and assayed for NF-κB activity (Figure 2b). FADD–/– MEFs demonstrated a ≥ 100% increase in NF-κB activity over FADD+/+ MEFs following stimulation with either agonist. That loss of FADD in MEFs enhanced NF-κB activation agreed with the finding that overexpression of FADD in HMEC-1 cells repressed NF-κB activation induced by either LPS or IL-1β. In contrast to stimulation with either LPS or IL-1β, FADD+/+ and FADD–/– MEFs demonstrated equivalent NF-κB activation following exposure to TNF-α (data not shown). This finding is consistent with previous reports that TNF-α–induced NF-κB signaling occurs independent of FADD (23, 24).
Figure 2.
Deletion of FADD enhances LPS- and IL-1β–induced NF-κB activity and NF-κB–dependent gene expression. FADD+/+ and FADD–/– MEF lysates were immunoblotted with anti-murine FADD antibody to confirm the genetic phenotype of these cells (a). Molecular mass (in kDa) is indicated. In other experiments, FADD+/+ and FADD–/– MEFs were treated for 4.5 hours with medium, LPS (100 ng/ml), or mIL-1β (10 ng/ml), lysed, and assayed for luciferase activity (b). Alternatively, MEFs were treated for 12 hours and the culture supernatants analyzed for IL-6 (c) or KC (d). Vertical bars represent mean (± SE) luciferase activity in arbitrary units (b) or pg/ml (c and d). *Significantly increased compared with FADD+/+ MEFs exposed to the same treatment.
To rule out that inhibition of LPS- and IL-1β–induced NF-κB activation was simply due to FADD interference with the exogenous gene product used to assay for NF-κB activity, luciferase, or the result of artifact introduced from adenoviral infection, FADD+/+ and FADD–/– MEFs were assayed for IL-6 and KC production (Figure 2, c and d). IL-6 and KC, the latter of which is a murine CXC homologue of human GRO, are two endogenously expressed LPS- and IL-1β–inducible gene products, the expression of which is dependent upon NF-κB activation (25, 26). Similar to luciferase activity, IL-6 production (Figure 2c) and KC production (Figure 2d) were both enhanced in FADD–/– MEFs compared with FADD+/+ MEFs following LPS or IL-1β exposure. Together, these data demonstrate that NF-κB–dependent gene expression is enhanced in the absence of FADD.
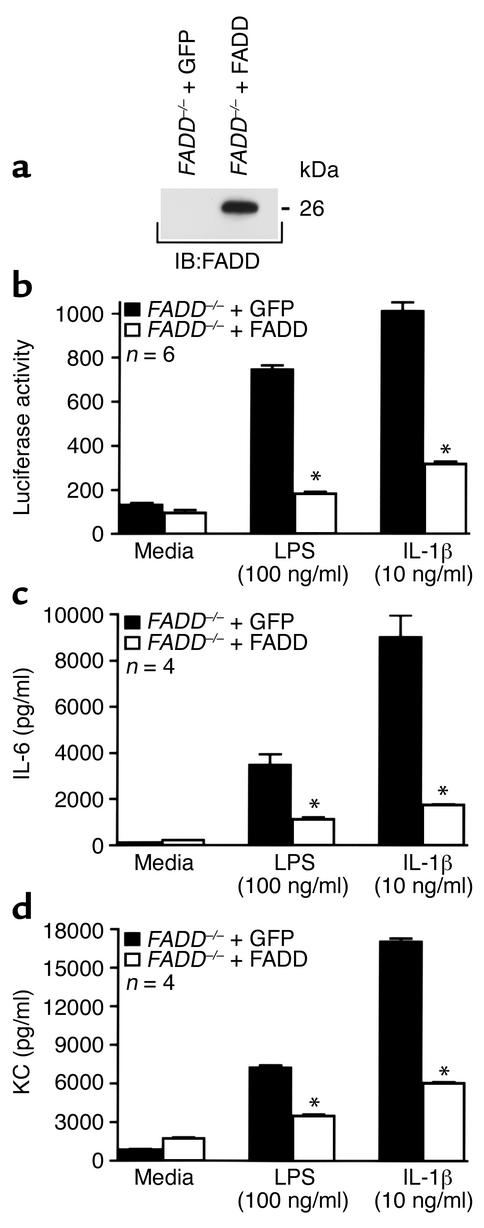
Reconstitution of FADD reverses the enhanced NF-κB activation in FADD–/– MEFs stimulated with either LPS or IL-1β.
To confirm that the enhanced NF-κB activity in LPS- and IL-1β–stimulated FADD–/– MEFs could be ascribed solely to a loss of FADD and not to a developmental anomaly unique to the mice from which the MEFs were derived, FADD was reconstituted in the FADD–/– MEFs and NF-κB activation assayed. Western blot analysis confirmed expression of FADD in the FADD–/– MEFs transfected with FADD cDNA (Figure 3a). Reconstitution of FADD in the FADD-deficient MEFs reversed the enhancement in NF-κB activation following LPS or IL-1β exposure (Figure 3b). FADD–/– MEFs reconstituted with FADD, however, demonstrated NF-κB activation, after TNF-α stimulation, that was equivalent to that of FADD–/– MEFs expressing GFP vector alone (data not shown). Similar to NF-κB activation, the LPS- and IL-1β–elicited increases in IL-6 (Figure 3c) and KC (Figure 3d) production were markedly reduced in FADD–/– MEFs expressing FADD.
Figure 3.
Reconstitution of FADD reverses the enhanced NF-κB activation and NF-κB–dependent gene expression in FADD–/– MEFs exposed to LPS or IL-1β. Lysates derived from FADD–/– MEFs stably transfected with either GFP vector alone or FADD were immunoblotted with anti-human FADD antibody to confirm expression (a). Molecular mass (in kDa) is indicated. In other experiments, FADD–/– MEFs expressing either GFP or FADD were treated for 4.5 hours with medium, LPS (100 ng/ml), or mIL-1β (10 ng/ml), lysed, and assayed for luciferase activity (b). Alternatively, MEFs were treated for 12 hours and the culture supernatants analyzed for IL-6 (c) or KC (d). Vertical bars represent mean (± SE) luciferase activity in arbitrary units (b) or pg/ml (c and d). *Significantly decreased compared with GFP-expressing cells exposed to the same treatment.
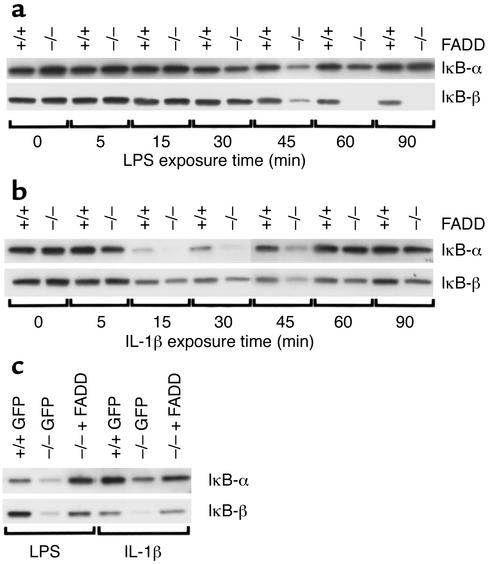
Absence of FADD enhances LPS- and IL-1β–induced IκB degradation.
LPS- and IL-1β–induced activation of NF-κB and expression of NF-κB–regulated gene products are dependent upon IκB degradation (21). Under basal conditions, IκB inhibits NF-κB activity by physically binding to and sequestering NF-κB in the cytosol. Following stimulation with either LPS or IL-1β, MyD88 binds to the respective receptor and recruits IRAK, which undergoes an autophosphorylation step (6). IRAK subsequently dissociates from MyD88 and interacts with TRAF-6, enabling TRAF-6 activation of the downstream serine/threonine kinase NIK. NIK subsequently forms a complex with two kinases involved in the phosphorylation of IκB, IKKα and IKKβ. Activation of these kinases results in the phosphorylation of two serine residues on IκB, targeting it for proteasome-mediated degradation. Although LPS- and IL-1β–induced NF-κB activation is dependent upon IκB degradation, an additional requisite role for phosphatidylinositol (PI) 3-kinase in mediating NF-κB activation by either of these agonists remains unclear. Studies have shown PI 3-kinase–dependent (27–29) and –independent (30, 31) involvement in LPS- and IL-1β–induced NF-κB activation. In those studies that have shown a requisite role, PI 3-kinase reportedly contributes to the activation of NF-κB downstream of IκB degradation (27–29). To determine the level at which FADD exerts a negative regulatory influence on NF-κB activation, FADD+/+ and FADD–/– MEFs were stimulated with either LPS or IL-1β for increasing exposure times and assayed for IκB degradation (Figure 4a). In wild-type MEFs, LPS exposure resulted in the preferential degradation of IκB-β whereas IκB-α levels remained relatively constant. FADD–/– MEFs demonstrated enhanced IκB-β degradation relative to FADD+/+ MEFs at ≥ 45 minutes of LPS exposure. Relative to FADD+/+ MEFs, there was also enhanced degradation of IκB-α in the FADD–/– MEFs following LPS exposures of 45 and 60 minutes. IL-1β treatment induced marked degradation of both IκB-α and IκB-β in FADD+/+ MEFs, and this degradation was enhanced in the FADD-deficient MEFs (Figure 4b). The transient decrease in IκB-α expression compared with the sustained degradation of IκB-β is consistent with previous studies (32, 33). Reconstitution of FADD reversed the enhanced degradation of IκB-α and IκB-β observed in FADD–/– MEFs treated with either LPS or IL-1β (Figure 4c). Together, these data suggest that FADD negatively regulates NF-κB upstream of IκB degradation.
Figure 4.
Deletion of FADD enhances LPS- and IL-1β–induced degradation of IκB. FADD+/+ and FADD–/– MEFs were incubated with medium, LPS (100 ng/ml), or IL-1β (10 ng/ml) for increasing exposure times, and lysates derived from these cells were immunoblotted with antibodies raised against either IκB-α or IκB-β (a and b). In other experiments, FADD+/+ MEFs stably expressing GFP (+/+ GFP) or FADD–/– MEFs stably expressing either GFP (–/– GFP) or FADD (–/– + FADD) were treated with LPS (100 ng/ml) or IL-1β (10 ng/ml) for 45 minutes, and lysates were immunoblotted as above (c).
Discussion
The ability of FADD to mediate NF-κB signaling has previously been reported (10–12). In those studies, transient overexpression of FADD increased basal levels of NF-κB activity (10, 11) and induced the upregulation of two NF-κB–dependent gene products, monocyte chemotactic protein-1 and IL-8 (12). The present study has assessed the ability of FADD to mediate induced NF-κB activation. In one other study that examined the role of FADD in mediating induced NF-κB activity, FADD actually promoted NF-κB activation (13). Those authors report that TNF-α–, TRAIL-, and Fas ligand–induced NF-κB activity is dramatically reduced or completely abrogated in a FADD-deficient Jurkat cell line, suggesting that FADD contributes to NF-κB activation. Our data indicate that FADD downregulates NF-κB activation induced by either LPS or IL-1β, which share the same signaling pathway leading to NF-κB activation. Thus, the ability of FADD to either promote or inhibit inducible NF-κB activation appears to be stimulus- and/or signaling pathway–specific.
The mechanism by which FADD inhibits IκB degradation and NF-κB activation remains to be elucidated. Two reports have demonstrated FADD binding to MyD88, an upstream adapter protein involved in the LPS and IL-1β signaling pathway leading to NF-κB activation (9, 34). This interaction is mediated through a DD-DD interaction similar to the one reported for IRAK binding of MyD88. The possibility exists that IRAK and FADD compete for binding to the DD of MyD88. FADD occupation of the IRAK binding site could potentially preclude IRAK interaction with MyD88. Alternatively, FADD may bind directly to IRAK through a reciprocal DD-DD interaction, thus sequestering IRAK and preventing its recruitment to MyD88. In either scenario, inhibition of IRAK binding to MyD88 would be expected to block LPS- or IL-1β–induced NF-κB signaling. The current findings that FADD exerts its inhibitory effect upstream of IκB degradation and that FADD does not moderate TNF-α–induced NF-κB activation, the latter of which is a MyD88- and IRAK-1–independent event, are consistent with these proposed mechanisms of FADD inhibition.
Other signaling pathways and molecules distinct from the MyD88-IRAK–initiated signaling cascade have been implicated in the activation of NF-κB. Protein tyrosine kinase activation has been reported as a requisite event leading to LPS-induced degradation of IκB-α (33). The protein tyrosine phosphatase Shp-2 has been demonstrated to contribute to IL-1β–induced activation of NF-κB upstream of IκB degradation (35). Thus, in addition to the potential FADD interaction with MyD88 and/or IRAK through a DD-DD interaction, there are other signaling pathways that FADD may regulate to block LPS- and IL-1β–induced degradation of IκB. Further, although the data presented suggest that the influence of FADD on mediating NF-κB activation is upstream of IκB degradation, one cannot rule out an additional role for FADD in mediating signaling pathways (e.g., PI 3-kinase) downstream of IκB that may also contribute to NF-κB activation.
In summary, we have established that FADD downregulates NF-κB activation elicited by either LPS or IL-1β. Whereas stable overexpression of FADD inhibits LPS- or IL-1β–induced NF-κB activation, the absence of FADD enhances NF-κB activation. The increased NF-κB activity in FADD-deficient MEFs corresponded with enhanced IκB degradation, suggesting that FADD negatively regulates NF-κB activation upstream of IκB. Additional studies will be necessary to elucidate the mechanism by which FADD disrupts LPS- and IL-1β–induced NF-κB activation.
Acknowledgments
This work was supported by NIH grants GM-07037, GM-42686, HL-18645, and HL-03174.
Footnotes
See the related Commentary beginning on page 579.
References
- 1.Pinner RW, et al. Trends in infectious diseases mortality in the United States. JAMA. 1996;275:189–193. [PubMed] [Google Scholar]
- 2.Danner RL, et al. Endotoxemia in human septic shock. Chest. 1991;99:169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- 3.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 4.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 5.Faure E, et al. Bacterial lipopolysaccharide activates NF-kappaB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of tlr-4 and tlr-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 6.Daun JM, Fenton MJ. Interleukin-1/Toll receptor family members: receptor structure and signal transduction pathways. J Interferon Cytokine Res. 2000;20:843–855. doi: 10.1089/10799900050163217. [DOI] [PubMed] [Google Scholar]
- 7.Akashi S, et al. Regulatory roles for CD14 and phosphatidylinositol in the signaling via toll-like receptor 4-MD-2. Biochem Biophys Res Commun. 2000;268:172–177. doi: 10.1006/bbrc.2000.2089. [DOI] [PubMed] [Google Scholar]
- 8.Choi KB, et al. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem. 1998;273:20185–20188. doi: 10.1074/jbc.273.32.20185. [DOI] [PubMed] [Google Scholar]
- 9.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhary PM, et al. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene. 2000;19:4451–4460. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 11.Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838–10844. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 12.Schaub FJ, et al. Fas/FADD-mediated activation of a specific program of inflammatory gene expression in vascular smooth muscle cells. Nat Med. 2000;6:790–796. doi: 10.1038/77521. [DOI] [PubMed] [Google Scholar]
- 13.Wajant H, et al. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J Biol Chem. 2000;275:24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- 14.Ades EW, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 15.Yeh WC, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita S, et al. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 17.Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 18.Ryseck RP, Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991;6:533–542. [PubMed] [Google Scholar]
- 19.Blumberg H, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 20.DiDonato J, et al. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zen K, et al. NF-kappaB activation is required for human endothelial survival during exposure to tumor necrosis factor-alpha but not to interleukin-1beta or lipopolysaccharide. J Biol Chem. 1999;274:28808–28815. doi: 10.1074/jbc.274.40.28808. [DOI] [PubMed] [Google Scholar]
- 22.Bannerman DD, et al. A constitutive cytoprotective pathway protects endothelial cells from lipopolysaccharide-induced apoptosis. J Biol Chem. 2001;276:14924–14932. doi: 10.1074/jbc.M100819200. [DOI] [PubMed] [Google Scholar]
- 23.Chinnaiyan AM, et al. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 24.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 25.Ouaaz F, Li M, Beg AA. A critical role for the RelA subunit of nuclear factor kappaB in regulation of multiple immune-response genes and in Fas-induced cell death. J Exp Med. 1999;189:999–1004. doi: 10.1084/jem.189.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanden Berghe W, et al. Signal transduction by tumor necrosis factor and gene regulation of the inflammatory cytokine interleukin-6. Biochem Pharmacol. 2000;60:1185–1195. doi: 10.1016/s0006-2952(00)00412-3. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Guerra MJ, Castrillo A, Martin-Sanz P, Bosca L. Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J Immunol. 1999;162:6184–6190. [PubMed] [Google Scholar]
- 28.Koul D, Yao Y, Abbruzzese JL, Yung WK, Reddy SA. Tumor suppressor MMAC/PTEN inhibits cytokine-induced NFkappaB activation without interfering with the IkappaB degradation pathway. J Biol Chem. 2001;276:11402–11408. doi: 10.1074/jbc.M007806200. [DOI] [PubMed] [Google Scholar]
- 29.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madge LA, Pober JS. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFkappa B in human endothelial cells. J Biol Chem. 2000;275:15458–15465. doi: 10.1074/jbc.M001237200. [DOI] [PubMed] [Google Scholar]
- 31.Pahan K, Raymond JR, Singh I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J Biol Chem. 1999;274:7528–7536. doi: 10.1074/jbc.274.11.7528. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JE, et al. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 33.Zen K, Karsan A, Eunson T, Yee E, Harlan JM. Lipopolysaccharide-induced NF-kappaB activation in human endothelial cells involves degradation of IkappaBalpha but not IkappaBbeta. Exp Cell Res. 1998;243:425–433. doi: 10.1006/excr.1998.4162. [DOI] [PubMed] [Google Scholar]
- 34.Horng T, Medzhitov R. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc Natl Acad Sci USA. 2001;98:12654–12658. doi: 10.1073/pnas.231471798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You M, Flick LM, Yu D, Feng GS. Modulation of the nuclear factor kappa B pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factor. J Exp Med. 2001;193:101–110. doi: 10.1084/jem.193.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]