Abstract
Aberrant activation of the Wingless-type (Wnt)/β-catenin signaling pathway is associated with a variety of human cancers. Little is known regarding the role that Wnt ligands play in human carcinogenesis. To test whether a Wnt-1 signal is a survival factor in human cancer cells and thus may serve as a potential cancer therapeutic target, we investigated the effect of inhibition of Wnt-1 signaling in a variety of human cancer cell lines, including non small cell lung cancer, breast cancer, mesothelioma, and sarcoma. Both monoclonal antibody and RNA interference (RNAi) were used to inhibit Wnt-1 signaling. We found that incubation of a monoclonal anti-Wnt-1 antibody induced apoptosis and caused downstream protein changes in cancer cells overexpressing Wnt-1. In contrast, apoptosis was not detected in cells lacking or having minimal Wnt-1 expression after the antibody incubation. RNAi targeting of Wnt-1 in cancer cells overexpressing Wnt-1 demonstrated similar downstream protein changes and induction of apoptosis. The antibody also suppressed tumor growth in vivo. Our results indicate that both monoclonal anti-Wnt-1 antibody and Wnt-1 siRNA inhibit Wnt-1 signaling and can induce apoptosis in human cancer cells. These findings hold promise as a novel therapeutic strategy for cancer.
Keywords: Wnt-1, monoclonal antibody, apoptosis, cancer, human
Introduction
The Wingless-type (Wnt)/Frizzled (Fz) protein receptor pathway involves important regulatory genes that carry polymorphisms associated with primary carcinomas. In the absence of Wnt signals, free cytosolic β-catenin is incorporated into a complex consisting of Axin, the adenomatous polyposis coli (APC) gene product, and glycogen synthase kinase (GSK)-3β. Conjunctional phosphorylation of Axin, APC, and β-catenin by GSK-3β designates β-catenin for the ubiquitin pathway, where ubiquitination of β-catenin targets it for degradation by proteasomes [1–4].
Binding of Wnt ligands to their Fz receptors leads to phosphorylation and increased activity of Dishevelled (Dvl). Phosphorylated Dvl inhibits the phosphorylating activity of GSK-3β. The regulation of GSK-3β is mediated through casein kinase-1 (CK-1), which phosphorylates Dvl [5]. This, in turn, prevents GSK-3β from phosphorylating its substrates, critically decreasing the binding affinities of the negative regulators Axin and APC for β-catenin [6]. Unphosphorylated β-catenin escapes recognition by a β-transducing repeat-containing protein, a component of an E3 ubiquitin ligase [7]. As β-catenin accumulates, it translocates into the nucleus, where it binds to T-cell (Tcf) and lymphoid-enhancing (Lef) transcription factors to form a complex that activates transcription of downstream target genes.
Increasing evidence indicates that aberrant activation of the Wnt signaling pathway is associated with tumor development and/or progression [2,8–10], suggesting that Wnt signaling functions in oncogenesis, possibly through antiapoptotic mechanisms [11]. Upregulation of Wnt-1 in different human cancers has also been reported [12,13]. In the present study, we found that blocking Wnt-1 signaling by using a monoclonal anti-Wnt-1 antibody can induce rapid and significant apoptosis in numerous cancer cell lines including lung, breast, mesothelioma, and sarcoma that overexpress Wnt-1. In contrast, antibody incubation with cells that lack or have little or no Wnt-1 expression has minimal effect. Antibody blocking of Wnt-1 signaling causes downregulation of several key downstream components, including Dvl and β-catenin. Antibody-induced apoptosis appears mediated, in part, through the release of cytochrome c to the cytosol, inactivation of Survivin, and subsequent caspase activation. Similar apoptotic effects by antibodies were demonstrated by Wnt-1 silencing using RNA interference (RNAi). Finally, we show that the monoclonal anti-Wnt-1 antibody suppresses tumor growth in vivo. Our results demonstrate that a monoclonal antibody against the Wnt-1 ligand of the Wnt-1 signaling pathway can induce specifically selective apoptosis in cancer cells, and may be relevant as a therapeutic strategy for the treatment of cancer.
Materials and Methods
Cell Lines and Tissue Samples
Human non small cell lung cancer (NSCLC) cell lines (NCI-H1703, NCI-H460, NCI-H838, and NCI-A549), a normal lung cell line (CCL-75, fibroblast), human breast cancer cell lines (MCF-7, HuL100, and SKBR-3), and human mesothelioma cancer cell lines (H28 and H513) were from the American Type Culture Collection (ATCC; Manassas, VA). Other human mesothelioma cancer cell lines 290 and MS-1 were from NIH (Bethesda, MD), and REN was kindly provided by Dr. Steven Albelda's laboratory at the University of Pennsylvania (Philadelphia, PA). The normal mesothelial cell line LP-9 was from the Cell Culture Core Facility at Harvard University (Boston, MA). Human sarcoma cancer cell lines Saos-2 and MES-SA were obtained from the Cell Culture Facility at University of California (San Francisco, CA). These cells, except CCL-75, LP-9, and Saos-2, were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU/ml), and streptomycin (100 µg/ml). CCL-75 was cultured in minimum essential medium (MEM) with Earle's BSS containing 2 mM l-glutamine, 1.0 mM sodium pyruvate, 0.1 mM nonessential amino acids, 1.5 g/l sodium bicarbonate, and 10% FBS. LP-9 was cultured in M199 containing 15% CS plus 10 ng/ml EGF plus 0.4 µg/ml HC. Saos-2 was cultured in McCoy's 5a medium supplemented with 2 mM l-glutamine and 15% FBS. Normal human small airway epithelial cells (SAEC) and normal human bronchial epithelial (NHBE) cells were obtained from Clonetics (Walkersville, MD) and cultured in Clonetics SAGM Bullet Kit. All cells were cultured at 37°C in a humid incubator with 5% CO2.
Fresh lung cancer tissues and adjacent normal lung tissues from patients undergoing curative primary resection of their tumors were collected at the time of surgery, and immediately snap-frozen in liquid nitrogen. These tissue samples were kept at -170°C in a liquid nitrogen freezer before use.
Immunoprecipitation
The anti-Wnt-1 mouse monoclonal antibody (IgG1) was custom-made at Rockland, Inc. (Gilbertsville, PA). The antigen used to raise this monoclonal antibody was a synthetic peptide corresponding to amino acids 201 to 212 of the human Wnt-1 (Ac-HNNEAGRTTVFS-amide). The monoclonal antibody was affinity-purified by using Protein A and kept at -20°C. Seize X mammalian Immunoprecipitation Kit (Pierce Biotechnology, Rockford, IL) was used to precipitate Wnt-1 protein from cell line extracts according to the manufacturer's protocol and followed by Western blot analysis. The same monoclonal antibody was used to detect the Wnt-1 protein on the Western blots.
Antibody Incubation with Cells
Cells were plated in six-well plates or 10-cm dishes 1 day before the experiments. Then normal medium was replaced by media containing antibodies (all antibodies used in our experiments were the same subtype, IgG1) at various concentrations and the cells were incubated at 37°C in a humid incubator with 5% CO2. At various time points, the cells were collected using standard protocols for further analysis.
RNA Interference
Cells were plated into a six-well plate with fresh media without antibiotics 24 hours before testing. The ion exchange high-performance liquid chromatography-purified siRNA (Wnt-1 siRNA and nonsilencing siRNA control, >97% pure) were purchased from Qiagen-Xeragon (Germantown, MD). The lyophilized siRNA were dissolved in annealing buffer and reheated to 95°C for 1 minute followed by 1 hour at 37°C incubation. We followed the protocol described by Elbashir et al. [14] with some modifications. After siRNA transfection, we incubated plates for 3 to 5 days at 37°C before further analysis.
Western Blot Analysis
Standard protocol was used. Anti-Dvl3, anti-cyclin D1, and anti-Survivin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-caspase-3 antibody was from Oncogene (Cambridge, MA). Anti-β-actin antibody was obtained from Cell Signaling Technology, Inc. (Beverly, MA). Anti-β-catenin antibody was purchased from Transduction Laboratories (Lexington, KY). Anti-cytochrome c antibody was purchased from BD Biosciences (San Diego, CA). For detecting alterations of β-catenin, cytosolic extracts were prepared and examined as described previously [15].
Semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and cDNA Expression Array
Total RNA from cancer cell lines was isolated using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's protocol. RT-PCR was performed in a GeneAmp PCR system 9700 using One-Step RT-PCR Kit from Life Technologies, Inc. (Rockville, MD), according to the manufacturer's protocol. Primers for RT-PCR were obtained from Operon Technologies, Inc. (Alameda, CA). Primer sequences for a 282-bp fragment of the human Dvl-3 cDNA were: 5′-ATCTACATTGGCTCTATCATG-3′ (forward) and 5′-GGTCATGGCTGCAGTGTGGG-3′ (reverse). A 395-bp fragment of a gene encoding the L19 ribosomal protein was used as an internal control and their primer sequences were: 5′-GAAATCGCCAATGCCAACT-3′ (forward) and 5′-TCTTAGACCTGCGAGCCTCA-3′ (reverse). For analyzing different gene expressions after the anti-Wnt-1 monoclonal antibody treatment in cancer cells, Atlas human cancer pathwayfinder II gene array (Clontech Laboratories, Inc., Palo Alto, CA) was used. The materials provided with the kit were used, and the recommended protocol was followed in all steps. Five micrograms of total RNA was converted into 33P-labeled cDNA for hybridization. The hybridized membranes were exposed to X-ray film for 3 days.
TOPFLASH Assay
Cells were plated in six-well plates. After incubation with control or anti-Wnt-1 monoclonal antibody (8.0 µg/ml) for 48 hours, the TOPFLASH or FOPFLASH reporter plasmid was transfected transiently into cells as described previously [16]. Tcf-mediated gene transcription was determined by the ratio of pTOPFLASH/pFOPFLASH luciferase activity, each normalized to luciferase activities of the pRL-TK reporter (cotransfected internal control). All experiments were performed in triplicate.
Apoptosis Analysis
Cells were harvested by trypsinization and stained using an Annexin V FITC Apoptosis Detection Kit (Oncogene), according to the manufacturer's protocol. Stained cells were immediately analyzed by flow cytometry (FACScan; Becton Dickinson, Franklin Lake, NJ). Early apoptotic cells with exposed phosphatidylserine but intact cell membranes bound to Annexin V-FITC but excluded propidium iodide (PI). Cells in necrotic or late apoptotic stages were labeled with both Annexin V-FITC and PI. TDT-mediated dUTPbiotin nick end-labeling (TUNEL) staining of the tumor tissue samples harvested from in vivo experiments was performed using the ApopTag Peroxidase In Situ Oligo Ligation Apoptosis Detection Kit (Chemicon International, Temecula, CA) according to the manufacturer's protocol.
In Vivo Tumor Suppression Study
Female nude mice, 5 to 10 weeks old, were injected subcutaneously with 4 x 106 tumor cells (the NSCLC cell line H460) in the dorsal area in a volume of 100 µl. Animals were then injected intraperitioneally with monoclonal anti-Wnt-1 antibody, a control monoclonal antibody, or a phosphate-buffered saline (PBS) buffer in a volume of 100 µl as well. Both the monoclonal anti-Wnt-1 and the control antibodies were injected at a dosage of 50 µg. Each injection was done once weekly. Each group consisted of six mice. Tumor size was determined at weekly intervals, and tumor volumes were calculated using width (x) and length (y) (x2y / 2, where x < y) [17]. All animals were cared for in accord with University of California San Francisco (UCSF) guidelines.
Statistical Analysis
Data shown represent mean values (± SD). Student's t-test was used for comparing different treatments and cell lines.
Results
Detection of Wnt-1 Expression in Different Human Cancer Cell Lines and Primary Lung Cancer Tissue Samples
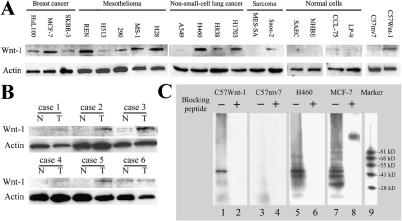
We developed a monoclonal antibody against a synthetic peptide corresponding to amino acids 201 to 212 of the human Wnt-1 (Ac-HNNEAGRTTVFS-amide). The monoclonal antibody was affinity-purified by using Protein A. We then examined Wnt-1 expression using this monoclonal antibody in numerous human cell lines, including three breast cancer cell lines (HuL100, MCF-7, and SKBR-3), fivemalignant pleural mesothelioma cell lines (REN, H513, H290, MS-1, and H28), four NSCLC cell lines (A549, H460, H838, and H1703), two sarcoma cell lines (MES-SA and Saos-2), and four normal cell lines (SAEC, NHBE cells, LP-9, and CCL-75). We found high levels of Wnt-1 expression in the majority of these cancer cell lines. Several cell lines (A549, MES-SA, H513, and SKBR-3) had minimal or no Wnt-1 expression (Figure 1A). No Wnt-1 expression was observed in the two primary normal lung cell lines (SAEC and NHBE). We only detected trace Wnt-1 expression in the normal lung fibroblast CCL-75 and in a normal mesothelial cell line (LP-9). As a positive control experiment, we found Wnt-1 expression using the same monoclonal antibody in Wnt-1-transfected mousemammary cells (C57Wnt-1), but not in empty vector-transfected cells (C57mv7). In addition, we found that four of six fresh lung cancer tissues had increased expression of Wnt-1 protein compared with autologous matched normal lung tissue controls (Figure 1B).
Figure 1.
Detection and immunoprecipitation of Wnt-1 protein in cell lines with a monoclonal anti-Wnt-1 antibody. (A) Western blot analysis of Wnt-1 expression in various cancer cell lines, including breast cancer (HuL100, MCF-7, and SKBR-3), malignant pleural mesothelioma (REN, H513, H290, MS-1, and H28), NSCLC (A549, H460, H838, and H1703), sarcoma (MES-SA and Saos-2), and normal cells (primary cultured SAEC, primary cultured NHBE cells, lung fibroblast cell line CCL-75, and mesothelial cell line LP-9). Mouse mammary cell lines C57mv7 (empty vector-transfected) and C57Wnt-1 (Wnt-1-transfected) served as negative and positive controls. Whole cell extract from each cell type was prepared. Protein A-purified mouse monoclonal anti-Wnt-1 antibody (IgG1) was used to hybridize the Western blots. (B) Western blot analysis of Wnt-1 expression in primary NSCLC tissue samples. Whole cell extracts were prepared from freshly resected tumors and autologous matched normal lung tissues. (C) Immunoprecipitation of Wnt-1 protein with the monoclonal anti-Wnt-1 antibody. Lane 9 is protein molecular weight marker. Immunoprecipitation was performed with the following: lanes 1 and 2, monoclonal antibody alone and monoclonal antibody blocked by preincubation with blocking peptide (30-fold over the antibody) with C57Wnt-1 cell extracts; lanes 3 and 4, monoclonal antibody alone and monoclonal antibody blocked by preincubation with blocking peptide with C57mv7 cell extracts; lanes 5 and 6, monoclonal antibody alone and monoclonal antibody blocked by preincubation with blocking peptide with H460 cell extracts; lanes 7 and 8, monoclonal antibody alone and monoclonal antibody blocked by preincubation with blocking peptide with MCF-7 cell extracts.
The Monoclonal Anti-Wnt-1 Antibody Precipitates Wnt-1 Proteins in Cell Extracts
To test if the monoclonal anti-Wnt-1 antibody can specifically bind to the native form of Wnt-1 protein in cultured cells, we performed immunoprecipitation by using monoclonal antibody alone or monoclonal antibody blocked by preincubation with blocking peptide (30-fold over the antibody) in cell extracts from several cell lines (Figure 1C). C57Wnt-1 and C57mv7 cells served as positive and negative controls. NSCLC (H460) and breast cancer (MCF-7) cell lines were also tested. In C57Wnt-1, H460, and MCF-7 cells, Wnt-1 protein was precipitated by the monoclonal anti-Wnt-1 antibody (Figure 1C, lanes 1, 5, and 7). In contrast, when the monoclonal anti-Wnt-1 antibody was preincubated with blocking peptide, its ability to precipitate Wnt-1 protein was blocked in these cells (Figure 1C, lanes 2, 6, and 8). In the negative control C57mv7 cell extract, no Wnt-1 protein was precipitated by either monoclonal anti-Wnt-1 antibody alone or monoclonal antibody preincubated with blocking peptide (Figure 1C, lanes 3 and 4). These data indicate that the anti-Wnt-1 monoclonal antibody can specifically bind to the native form of Wnt-1 protein.
The Monoclonal Anti-Wnt-1 Antibody Induces Apoptosis in Human Cancer Cells, Which Correlates with Wnt-1 Expression
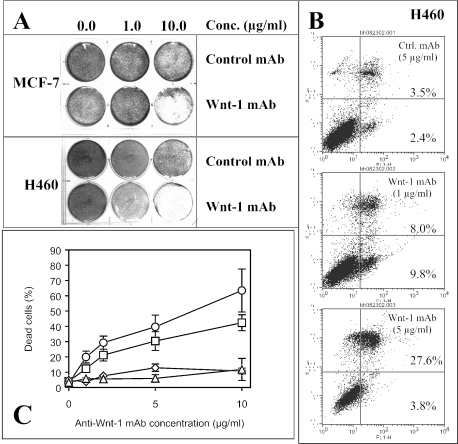
Next, we treated the NSCLC cell line H460 and the breast cancer cell line MCF-7 with this monoclonal antibody. After about 48 to 72 hours of incubation, we found significant cell death in both cell lines (over 60% cell death at 10 µ/ml antibody, P < .005) (Figure 2A). We saw no noticeable effect, however, in both cell lines after control monoclonal antibody treatment. Cell killing was largely due to induction of apoptosis (Figure 2B). Induction of apoptosis by this monoclonal antibody was dosage- and time-dependent (over 60% cell death in H460 at 10 µg/ml antibody after 72 hours of incubation and over 40% cell death in MCF-7 at 10 µg/ml antibody after 48 hours of incubation) (Figure 2C).
Figure 2.
The monoclonal anti-Wnt-1 antibody induces apoptosis in different human cancer cell lines in vitro. (A) Crystal violet (0.5%) staining of cancer cells MCF-7 (upper two rows) at about 48 hours and H460 (bottom two rows) at about 72 hours after control or the monoclonal anti-Wnt-1 antibody treatment. Concentrations of the control or anti-Wnt-1 antibodies used from left to right are 0.0, 1.0, and 10.0 µg/ml, respectively. (B) Example of apoptosis analysis by flow cytometry. From top to bottom, H460 cancer cells were treated with 5.0 µg/ml control antibody, and 1.0 and 5.0 µg/ml anti-Wnt-1 antibody, respectively, for about 72 hours. FL1-H represents Annexin V-FITC staining and FL3-H represents propidium iodide (PI) staining. (C) Dose responses of H460 and MCF-7 cancer cells to monoclonal antibody treatment. Measurements were taken after 72 hours of incubation for H460 and 48 hours of incubation for MCF-7. Squares (□) and circles (◯) represent fraction of cell death in MCF-7 and H460 cells treated with anti-Wnt-1 antibody, respectively. Diamonds (◊) and triangles (▴) represent fraction of cell death in MCF-7 and H460 cells treated with control antibody, respectively. Results are mean ± SD (error bars). The control antibody that we used in all experiments was the same subtype as the anti-Wnt-1 antibody (IgG1).
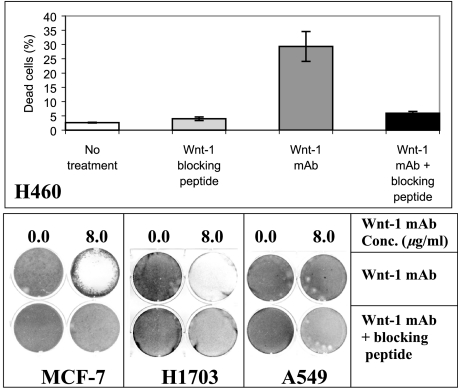
As a specificity control, we examined induction of apoptosis by using monoclonal antibody blocked by overnight preincubation with blocking peptide (30-fold over the antibody) in H460, MCF-7, and H1703 (Figure 3). After 48 hours of incubation, we found that anti-Wnt-1 antibody-induced apoptosis could be inhibited significantly by its blocking peptide (P < .003). The same dose-blocking peptide alone did not affect the viability of these cells (8.0 µg/ml for 48 hours). As a negative control, we used A549 cells that lack significant Wnt-1 expression (Figure 1A). After about 48 hours of treatment with either monoclonal antibody alone (8.0 µg/ml) or with monoclonal antibody blocked by preincubation with blocking peptide (30-fold over the antibody), no significant apoptosis induction was detected. This result is consistent with the Wnt-1 expression status of A549 cells.
Figure 3.
Specific cell killing by the monoclonal anti-Wnt-1 antibody in cancer cell lines. Top panel shows percentage of dead cells in the H460 cell line after about 48 hours of treatment with monoclonal antibody alone (8.0 µg/ml) and with monoclonal antibody blocked by preincubation with blocking peptide (30-fold over the antibody). As another control, H460 cells were also treated with same dose-blocking peptide (8.0 µg/ml for 48 hours). After 48 hours of incubation, cells were collected for flow cytometry analysis. Results are mean ± SD (error bars). Bottom panel shows 0.5% crystal violet staining of three other cancer cell lines MCF-7, H1703, and A549 after about 48 hours of treatment with monoclonal antibody alone (8.0 µg/ml) and with monoclonal antibody blocked by preincubation with blocking peptide (30-fold over the antibody). A549 has no detectable Wnt-1 expression (see Figure 1A) and was used as a negative control in this experiment.
The Monoclonal Anti-Wnt-1 Antibody Inhibits Wnt/β-Catenin Signaling Pathway and Induces Apoptosis through Release of Cytochrome c, Downregulation of Survivin, and Subsequent Activation of Caspase-3
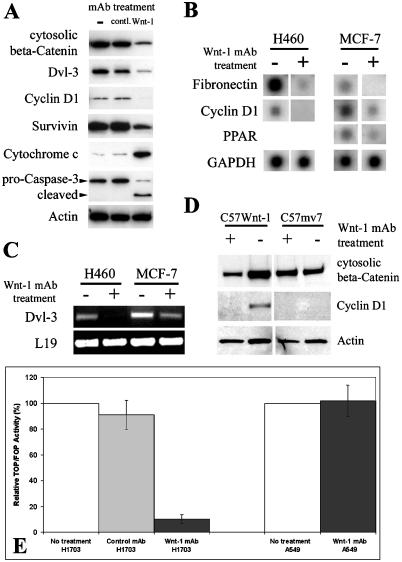
Recent work in our laboratory has shown that Dvl-3—not Dvl-1 nor Dvl-2—is overexpressed in NSCLC and mesothelioma [ 16,18]. We found that cytosolic β-catenin, Dvl-3, and cyclin D1 were downregulated after monoclonal anti-Wnt-1 antibody treatment in the cancer cells examined (Figure 4A shows the example in the NSCLC cell line H460). Microarray analysis also confirmed downregulation of β-catenin downstream target gene expression, including cyclin D1, fibronectin, and peroxisome proliferative activated receptor (PPAR) in both H460 and MCF-7 cells (Figure 4B). Interestingly, Dvl-3 was found to be downregulated after the treatment (Figure 4A). To confirm this observation, we examined the level of Dvl-3 mRNA upon treatment with the monoclonal anti-Wnt-1 antibody and found a decrease of the Dvl-3 transcription (Figure 4C). To our knowledge, this is the first report of Dvl-3 downregulation due to inhibition of Wnt-1 signaling. Others have shown that Wnt-1 signaling is on in C57Wnt-1 cells, but off in C57mv7 cells [19]. As a control, we found that both cytosolic β-catenin and cyclin D1 were downregulated after the antibody treatment (8.0 µg/ml for 48 hours) in C57Wnt-1 cells, but no cyclin D1 expression was detected in C57mv7 cells. Cytosolic β-catenin levels in C57mv7 cells also remained unchanged after the antibody treatment (Figure 4D). In addition, we found significant reduction in Tcf-dependent transcriptional activity (TOPFLASH assay) (P < .01) in those cells expressing Wnt-1after the monoclonal anti-Wnt-1 antibody treatment (Figure 4E). In contrast, no change of Tcf-dependent transcriptional activity (Figure 4E) or cytosolic β-catenin level (data not shown) was found in cells lacking Wnt-1 expression after the antibody treatment. Taken together, these results suggest that the monoclonal anti-Wnt-1 antibody-induced apoptosis is mediated, at least in part, through inhibition of the Dvl/β-catenin-dependent transcription.
Figure 4.
Western blot analysis after the monoclonal anti-Wnt-1 antibody treatment. (A) Western blot analysis on NSCLC cell line H460 cancer cells before treatment, and after the control and monoclonal anti-Wnt-1 antibody treatments [same subtype (IgG1); 6.0 µg/ml for 48 hours]. Cytosolic proteins were prepared and used in these Western blots. Actin served as loading control for each lane. (B) Microarray analysis after the monoclonal anti-Wnt-1 antibody treatment (8.0 µg/ml for 48 hours) in NSCLC cell line H460 and breast cancer cell line MCF-7. Total RNA was used for hybridization. (C) Semiquantitative RT-PCR analysis of the Dvl-3 expression after the monoclonal anti-Wnt-1 antibody treatment (8.0 µg/ml for 48 hours) in NSCLC cell line H460 and breast cancer cell line MCF-7. The Dvl-3 cDNA fragment amplified is 282 bp. A 395-bp fragment of the L19 ribosomal protein gene is used as a positive control for RNA quality and loading. (D) Western blot analysis on C57mv7 or C57Wnt-1 cells before and after the monoclonal anti - Wnt-1 antibody treatment (8.0 µg/ml for 48 hours). Cytosolic proteins were prepared and used in the Western blots. (E) TOPFLASH assay of Tcfdependent transcriptional activity in NSCLC cell lines H1703 (expressing Wnt-1) and A549 (lacking Wnt-1 expression). The results are mean ± SD (error bars). Experiments were performed in triplicate.
In H460 cells in which the monoclonal anti-Wnt-1 antibody induces apoptosis, we found that the cleaved (active) form of caspase-3 was upregulated (Figure 4A). Consistent with the caspase-3 activity, we detected increased levels of cytochrome c in the cytosol of H460 cells after the monoclonal anti-Wnt-1 antibody treatment (Figure 4A). In addition, we found that Survivin was downregulated in these H460 cells after the antibody treatment (Figure 4A).
RNA Interference
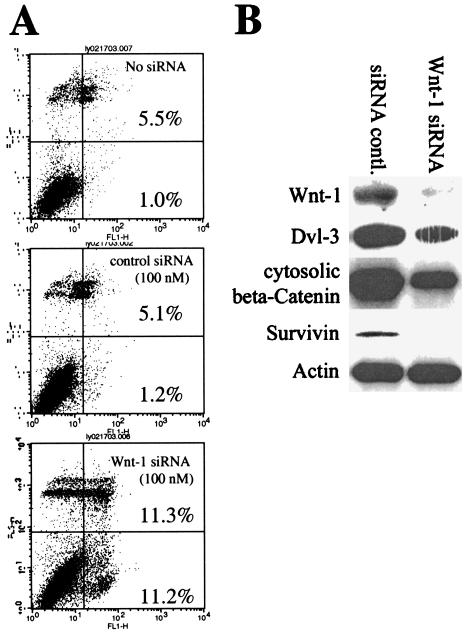
We followed the protocol described by Elbashir et al. [14] to investigate the effects after the silencing of Wnt-1 expression by using RNAi. Similar to the monoclonal anti-Wnt-1 antibody, treatment with Wnt-1 siRNA for 3 to 5 days induced apoptosis in cancer cell lines that express Wnt-1 (Figure 5 shows the example in MCF-7 cells). Significant apoptosis was induced at 100 nM Wnt-1 siRNA, but no apoptosis was induced by either nonsilencicing siRNA control (100 nM) or transfection reagents alone (P < .01) (Figure 5A). We confirmed the silencing of Wnt-1 expression after Wnt-1 siRNA treatments (100 nM for 72 hours) by Western blot analysis (Figure 5B) (nonsilencing siRNA served as control, 100 nM for 72 hours). To determine whether the apoptotic effects correlated with the inhibition of Wnt-1 signaling, we have shown that expression levels of Dvl-3, cytosolic β-catenin, and Survivin were downregulated after Wnt-1 siRNA treatment (Figure 5B).
Figure 5.
Wnt-1 siRNA induces apoptosis. (A) Annexin V analysis of apoptosis induced by Wnt-1 siRNA. From top to bottom: MCF-7 cancer cells were treated without siRNA (vehicle only), 100 nM nonsilencing control siRNA, and 100 nM Wnt-1 siRNA, respectively. FL1-H represents Annexin V-FITC staining and FL3-H represents PI staining. (B) Western blot analysis after Wnt-1 siRNA treatments (100 nM for 72 hours); nonsilencing siRNA served as control. MCF-7 cells were treated with either Wnt-1 siRNA or nonsilencing siRNA control. β-Actin served as loading control.
The Monoclonal Anti-Wnt-1 Antibody Suppresses Tumor Growth In Vivo
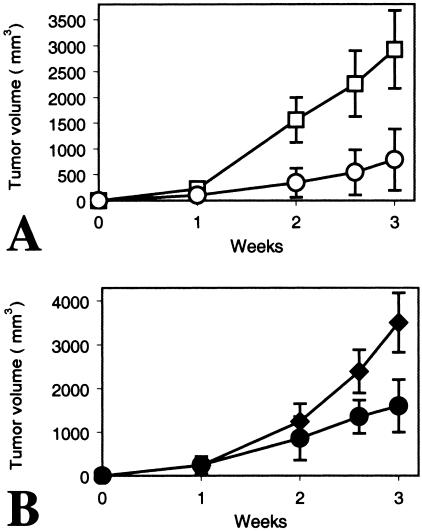
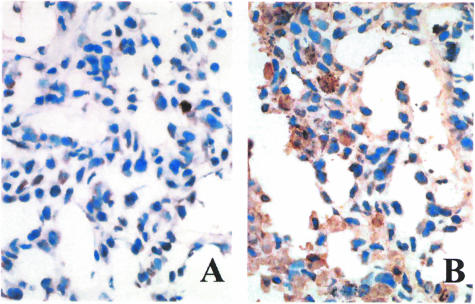
Finally, we injected NSCLC H460 cells subcutaneously into nude mice. Animals were then treated with 50 µg of the monoclonal anti-Wnt-1 antibody, a control monoclonal antibody, or PBS by intraperitoneal injection once weekly. We found that the monoclonal anti -Wnt-1 antibody inhibited significantly tumor growth versus controls (Figure 6). Suppression of tumor growth was seen not only when the anti-Wnt-1 monoclonal antibody injection was started immediately after tumor cell inoculation (P < .003; Figure 6A), but also when the treatment was initiated after the tumors were already established (1 week after tumor cell inoculation; P < .005; Figure 6B). After sacrificing mice, the tumor tissues were analyzed by TUNEL staining (Figure 7). We saw apoptotic cells in those tumor tissues upon treatment with the anti-Wnt-1 monoclonal antibody. Furthermore, we examined, at gross macroscopic level, several organs including the lung, liver, and heart of those mice treated with the antibody. No noticeable toxicities or abnormalities were detected (data not shown).
Figure 6.
The monoclonal anti-Wnt-1 antibody suppresses tumor growth in vivo. (A) Treatment was started immediately after NSCLC cell H460 inoculation. Squares (□) represent tumor volume (mm3) at various times with intraperitoneal injection of PBS (100 µl). Circles (◯) represent those with intraperitoneal injection of the monoclonal anti-Wnt-1 antibody (50 µg in 100 µl of PBS). Results are mean ± SD (error bars) for six animals. (B) Treatment was initiated 1 week after NSCLC cell H460 inoculation. Solid diamonds (◆) represent tumor volume (mm3) at various times with intraperitoneal injection of a control monoclonal antibody [same subtype (IgG1) as the anti-Wnt-1 antibody; 50 µg in 100 µl of PBS]. Solid circles (●) represent those with intraperitoneal injection of the monoclonal anti-Wnt-1 antibody (50 µg in 100 µl of PBS). Results are mean ± SD (error bars) for six animals.
Figure 7.
TUNEL staining of the tumor tissue samples after treatment with the monoclonal anti-Wnt-1 antibody in vivo. (A) Tumor treated with intraperitoneal injection of PBS control. (B) Tumor treated with intraperitoneal injection of the monoclonal anti-Wnt-1 antibody. Treatment was initiated 1 week after NSCLC cell H460 inoculation. Pictures were taken under a light microscope at x400 magnification.
Discussion
Wnt genes encode a family of at least 19 secreted, cysteinerich glycoproteins that play a critical role in development and oncogenesis [20,21]. Several Wnt proteins, including Wnt-1, have been shown to be overexpressed in a number of cancers. Although the connection has been made between the APC/β-catenin mutations and human cancer, little is known regarding the role that Wnt ligands play in human carcinogenesis. To test our hypothesis that Wnt-1 signal may be a survival factor in human cancer cells and thus may be a potential cancer therapeutic target, we have developed a monoclonal anti-Wnt-1 antibody and evaluated its effect in numerous human cancer cell lines.
The monoclonal anti-Wnt-1 antibody was raised against a synthetic peptide. First, we tested the ability of this monoclonal antibody to bind specifically native forms of Wnt-1 proteins in cultured cells by performing immunoprecipitation. We have demonstrated that in extracts of mouse mammary C57Wnt-1 cells, which express Wnt-1 cDNA, this monoclonal antibody precipitates Wnt-1 protein and the precipitation is blocked by preincubation with its blocking peptide. In contrast, in extracts of empty vector-infected C57mv7 cells, no protein is detected after precipitation by the monoclonal antibody. We have also shown that this monoclonal antibody precipitates Wnt-1 protein in extracts of human cancer cell lines that express Wnt-1 [e.g., NSCLC (H460) and breast cancer (MCF-7)] and that the precipitation is blocked by preincubation with its blocking peptide. Taken together, these results indicate that this monoclonal antibody can bind specifically to native forms of Wnt-1 proteins in various cell lines.
Next, our data demonstrate that incubation of the monoclonal anti-Wnt-1 antibody with human cancer cells induces apoptosis. The antibody-induced apoptotic cell death not only correlates with Wnt-1 expression, but also is consistent with decreased Dvl, cytosolic β-catenin, cyclin D1, and other target gene expression levels in the human tumor cells tested. It is interesting that we saw Dvl-3 downregulation upon inhibiting Wnt-1 signaling at both protein and mRNA levels. One possibility is that Dvl-3 expression is regulated by transcriptional factors other than β-catenin/Tcf, which are also under the control of Wnt-1 signaling. Recently, Rhee et al. [22] reported that a polyclonal anti-Wnt-1 antibody downregulated β-catenin/Tcf activity and induced apoptosis in head and neck squamous carcinoma cell (HNSCC) lines. Polyclonal antibodies, however, raise doubts due to their poor specificity and quality. To evaluate further the specificity of the monoclonal antibody that we used and to investigate the mechanisms of apoptosis induction, we treated Wnt-1-expressing cells with the monoclonal antibody after preincubation with its blocking peptide. In these cells, we did not observe apoptosis. In addition, the monoclonal antibody had minimal effects on cells lacking or having minimal Wnt-1 expression. These data indicate that the monoclonal anti-Wnt-1 antibody can specifically and selectively induce apoptosis in cancer cells that express Wnt-1. The monoclonal anti-Wnt-1 antibody also downregulates the Wnt-Dvl-β-catenin signaling pathway in human cancer cells and may have apoptosis-inducing effects through additional noncanonical pathways that are not completely understood. In addition, we found that blocking Wnt-1 signaling by using RNAi to silence Wnt-1 also induced apoptosis in those Wnt-1-expressing cells. Taken together, our results suggest a strong linkage between Wnt-1 signaling and tumor cell survival.
We examined several components of the apoptosis pathway. We observed an increased level of cytochrome c in the cytosol, as well as activation of caspase-3 in the cancer cells after treatment with the monoclonal anti-Wnt-1 antibody. Moreover, both monoclonal anti-Wnt-1 antibody and Wnt-1 siRNA treatments downregulate Survivin. These results provide more evidence that blocking Wnt-1 signaling, by two independent methods, can induce apoptosis in the cancer cells examined. Therefore, we postulate that Wnt-1 upregulation may function as a survival mechanism in cancer cells by inhibiting normal apoptotic machinery in the tumor cells studied.
The link between Wnt-1 signaling and apoptosis has been studied by several groups [23–25]. Yet, the mechanism through which blocking Wnt-1 signaling induces apoptosis in human cancer cells remains unclear. Chen et al. [23] have demonstrated that Wnt-1 signaling can inhibit induced apoptosis in Rat-1 cells stably expressing Wnt-1. They have reported that cells expressing Wnt-1 are resistant to cancer therapy-mediated apoptosis and they have also shown that Wnt-1 signaling can inhibit cytochrome c release and caspase activation induced by the chemotherapeutic drugs or c-Myc [11,23]. These results are consistent with our current findings that the monoclonal anti-Wnt-1 antibody downregulates the Wnt-Dvl-β-catenin signaling pathway, and induces apoptosis through cytochrome c release and caspase- 3 activation in human cancer cells. Although their results suggest that the Wnt-1-mediated cell survival is dependent on the canonical pathway, no difference was found in the Bcl-2 and IAP family proteins in the cells expressing Wnt-1 in their experiments [23]. In this study, we report that both monoclonal anti-Wnt-1 antibody and Wnt-1 siRNA induce apoptosis, at least in part, by suppressing Survivin expression in human cancer cells. Previously, we have shown that antisense oligonucleotides to Survivin are sufficient to induce apoptosis in human mesothelioma cells [26], which also mimics the effect caused by either monoclonal anti-Wnt-1 antibody or Wnt-1 siRNA. To our knowledge, Survivin has not been known as one of the target genes of the Wnt-Dvl-β-catenin pathway. Therefore, it is possible that other unknown signaling pathway(s) may be involved in the apoptosis induced by the monoclonal anti-Wnt-1 antibody or Wnt-1 siRNA. These findings also suggest, in theory, that blocking Wnt-1 activity/expression may help abrogate chemotherapy resistance in tumors and/or potentiate chemotherapeutic effect.
In summary, our results indicate that the monoclonal anti-Wnt-1 antibody induces tumor-specific apoptosis in human cancer cells. The mechanism appears to signal through the canonical and/or still-to-be-determined pathway(s). Our data suggest that Wnt-1 may be a novel therapeutic target for cancer.
Abbreviations
- APC
adenomatous polyposis coli
- Dvl
Dishevelled
- GSK
glycogen synthase kinase
- NHBE
normal human bronchial epithelial
- NSCLC
non small cell lung cancer
- RNAi
RNA interference
- SAEC
small airway epithelial cell
- Wnt
Wingless type
Footnotes
This work was supported by the Larry Hall Memorial Trust and the Kazan, McClain, Edises, Abrams, Fernandez, Lyons, and Farrise Foundation.
Current address: The Fourth Department of Internal Medicine, Nippon Medical School, Tokyo 113-8602, Japan.
References
- 1.Uthoff SM, Eichenberger MR, McAuliffe TL, Hamilton CJ, Galandiuk S. Wingless-type frizzled protein receptor signaling and its putative role in human colon cancer. Mol Carcinog. 2001;31:56–62. doi: 10.1002/mc.1039. [DOI] [PubMed] [Google Scholar]
- 2.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 3.Sadot E, Geiger B, Oren M, Ben-Ze'ev A. Down-regulation of beta-catenin by activated p53. Mol Cell Biol. 2001;21:6768–6781. doi: 10.1128/MCB.21.20.6768-6781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Yuan H, Weaver CD, Mao J, Farr GH, III, Sussman DJ, Jonkers J, Kimelman D, Wu D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 7.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 8.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 9.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 10.Brown AM. Wnt signaling in breast cancer: have we come full circle? Breast Cancer Res. 2001;3:351–355. doi: 10.1186/bcr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh M. Expression and regulation of WNT1 in human cancer: up-regulation of WNT1 by beta-estradiol in MCF-7 cells. Int J Oncol. 2003;22:209–212. [PubMed] [Google Scholar]
- 13.Wong SC, Lo SF, Lee KC, Yam JW, Chan JK, Hsiao WL. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196:145–153. doi: 10.1002/path.1035. [DOI] [PubMed] [Google Scholar]
- 14.Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang CY, Guttridge DC, Mayo MW, Baldwin AS., Jr NF-kB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uematsu K, Kanazawa S, You L, He B, Xu Z, Peterlin M, McCormick F, Jablons DM. Wnt pathway activation in mesothelioma: evidence of Dishevelled overexpression and transcriptional activity of beta- catenin. Cancer Res. 2003;63:4547–4551. [PubMed] [Google Scholar]
- 17.Sonoda Y, Ozawa T, Hirose Y, Aldape KD, McMahon M, Berger MS, Pieper RO. Formation of intracranial tumor by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956–4960. [PubMed] [Google Scholar]
- 18.Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non-small-cell lung cancer: evidence of Dishevelled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 19.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112:1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- 20.Miller JR. The Wnts. Genome Biol. 2002;3:1–15. doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS 3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Gijn ME, Daemen MJ, Smits JF, Blankesteijn WM. The Wnt-frizzled cascade in cardiovascular disease. Cardiovasc Res. 2002;55:16–24. doi: 10.1016/s0008-6363(02)00221-3. [DOI] [PubMed] [Google Scholar]
- 22.Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–6605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating β-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem. 2002;277:38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- 25.Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem. 2002;277:44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- 26.Xia C, Xu Z, Yuan X, Uematsu K, You L, Li K, Li L, McCormick F, Jablons DM. Induction of apoptosis in mesothelioma cells by antisurvivin oligonucleotides. Mol Cancer Ther. 2002;1:687–694. [PubMed] [Google Scholar]