Abstract
We previously discovered that a fat-metabolizing enzyme, 15-lipoxygenase-1 (15-LO-1), is high in human prostate cancer (PCa) and correlates with disease progression. The biologic link between the aberrant 15-LO-1/linoleic acid (LA) metabolism and fat (which is a rich source of growth factors) in PCa is unknown. Therefore, we tested the hypothesis that the metabolic product of the polyunsaturated fatty acid LA (i.e., 13-Shydroxyoctadecadienoic acid or 13-(S)-HODE) affects the proliferation status of PCa cells through one or more growth factors. We used parental prostate cancer cell line-3 (PC-3) and engineered PC-3 cell lines [PC3-Zeo (mock-transfected), PC3-15LOS (15-LO-1-overexpressing), and PC3-15LOAS (15-LO-1-blocked)] to test our hypothesis. Of the growth factors examined, only insulin-like growth factor-1 (IGF-1) exhibited a two-fold to three-fold increase in growth response on PC3-15LOS cells compared to PC3-Zeo (control) cell line (P < .01). Insulin-like growth factor-1 receptor (IGF-1R) immunohistochemical analyses of human normal and adenocarcinoma prostate tissues, as well as levels in tumors derived from nude mice injected with PC-3 cells, demonstrated that elevated IGF-1R expression correlated with 15-LO-1 levels. Radioligand binding assays demonstrated two-fold higher IGF-1 binding sites in PC3-15LOS cells (P < .05 vs PC3-Zeo cells). IGF-1R promoter reporter assay and affinity-purified IGF-1R receptor levels demonstrated a four-fold higher activity in PC3-15LOS cells (P < .01 vs PC3-Zeo cells). IGF-1R promoter activation is 13-(S)-HODE-dependent. IGF-1R blockade with a dominant-negative adenovirus caused significant growth inhibition in PC-3 cells (P < .0001; PC3-15LOAS versus PC3-15LOS cells), as well as affected the IGF-1-stimulated mitogen-activated protein (MAP) kinase (Erk1/2) and Akt activation levels. Our study suggests that overexpression of 15-LO-1 in PCa contributes to the cancer progression by regulating IGF-1R expression and activation.
Keywords: 15-lipoxygenase-1 (15-LO-1), hydroxyoctadecadienoic acid (HODE), insulin-like growth factor-1 (IGF-1), insulin-like growth factor-1 receptor (IGF-1R), linoleic acid (LA)
Introduction
In 2003, 220,900 men were diagnosed with prostate cancer (PCa) in the United States. Approximately 28,900 died of the disease in 2003 (American Cancer Society—Facts and Figures, 2003). Current therapies [1] for PCa are limited, and there is no cure for metastatic disease. Oncogenic signals involve activation of membrane receptors, kinases, and transcription factors. These can be either a primary event, when they are directly mutated in a tumor cell, or a secondary event, as recipients and mediators of oncogenic signals. Of note is that epigenetic factors such as diets high in fat seem to be associated with an increased risk of PCa, although the molecular mechanism is still unknown.
Dietary lipids are metabolized by cyclooxygenases (COX-1 and/or COX-2 [2]) or lipoxygenases (5-LO [3], 12-LO [4], 15-LO-1 [5,6], and 15-LO-2 [7]), but the precise role of these enzymes (or enzymatic products) in PCa is an area of active investigation. As principal macronutrients in today's diet, the n-6 series of dietary fatty acids is known to play a prominent role in cancers. An important member of this group is linoleic acid [LA; n-6 polyunsaturated fatty acid (PUFA), C18:2n-6], which is an essential PUFA found in sunflower and peanut oils. 15-Lipoxygenase-1 (15-LO-1) metabolizes LA to 13-S-hydroxyoctadecadienoic acid [13-(S)-HODE], which can regulate cell growth and differentiation and vascular homeostasis [8–24]. Our previously published findings [6,17] and other reports strongly suggest that 13-(S)-HODE enhances cellular proliferation [8–18,21–24]. While investigating the cellular and molecular biology of the “anti-inflammatory” properties of 15-LO-1, we discovered that 15-LO-1 gene expression is upregulated by a mutant form of p53, although no effect was seen with wild-type p53 [18]. The p53 protein is a tumor suppressor and mutations in it are often associated with the development of cancer.
Furthermore, we observed higher 15-LO-1 expression and enzyme activity in the epithelium of human PCa compared to the normal surrounding tissues; the higher level of 15-LO-1 also correlated with the degree of malignancy, as assessed by Gleason grades. Interestingly, the level of mutant p53 is also correlated with the level of 15-LO-1 [6]. Recently, we developed a human PCa cell line, prostate cancer cell line-3 (PC-3), that either overexpresses 15-LO-1 (PC3-15LOS cells) or expresses 15LOAS (antisense) mRNA to block endogenous 15-LO-1 (PC3-15LOAS cells), and shows that only PC3-15LOS cells, when injected subcutaneously in athymic mice, produce aggressive tumors exhibiting augmented angiogenesis [17]. Although our experimental observations suggest a correlation between 15-LO-1 activity and tumorigenesis [6,17], the mechanism of 15-LO-1 overexpression linking tumorigenesis is unknown. Based on our previous observations, we hypothesize that “mitogenic” 13-(S)-HODE may be acting as a signaling molecule to support both prolonged survival and increased proliferation of prostate epithelial cells. The overexpression of 15-LO-1 specifically in the normal prostate epithelium could be one of the contributory factors facilitating the progression to high-grade intraepithelial neoplasia (HGPIN; Kelavkar et al., unpublished results) and eventually to prostate adenocarcinoma. It has been recently shown that the insulin-like growth factor (IGF) signaling pathway is linked to PCa and that 13-(S)-HODE (the LA metabolite) upregulated—and 15-(S)-hydroxyeicosatetraenoic acid (15-(S)-HETE; the arachidonic acid metabolite) downregulated—both the mitogen-activated protein kinase (MAPK) and Akt pathways after activation with insulin-like growth factor-1 (IGF-1) [24]. Therefore, in a likely scenario, the epithelial cells of primary prostate tumor overexpressing 15-LO-1 may support interactions with stromal elements (e.g., growth factors) and facilitate invasion.
This manuscript describes experiments designed to show that the overexpression of a lipid-metabolizing enzyme, 15-LO-1, regulates Insulin-like growth factor-1 receptor (IGF-1R) expression and activation and thereby affects the proliferation status of PCa cells (PC-3 cell line) in vitro and in vivo. Our goal is to provide a mechanism that explains the observation that diets high in fat appear to be associated with an increased risk of PCa. The series of experiments utilizes our previously developed PC-3 cell line that either overexpresses 15LOS (sense) or expresses 15LOAS (antisense) mRNA, and our objectives were as follows: 1) to test whether 13-(S)-HODE, which is a metabolic product of the fatty acid LA (metabolized by 15-LO-1), reverses the growth inhibition of PC cells by PD146176; 2) compare the expression of IGF-1R in 10 normal and 16 PCa tissues by image cytometry; 3) measure differences in IGF-1R levels in tumors from four nude mice, injected with different types of PC-3 cells, by immunohistochemistry; 4) evaluate whether 15-LO-1 expression causes IGF-l R activation (phosphorylation) by performing Western blot analyses; 5) evaluate whether 15-LO-1 affects IGF-1R promoter activity by measuring the relative luciferase activity in the different PC-3 cell lines; 6) explore the impact of 13-(S)-HODE on IGF-1R promoter (reporter activity) by treating PC3-Zeo (control) cells with increasing doses of 13-(S)-HODE; 7) determine whether targeting IGF-1R can block PC-3 proliferation and whether growth inhibition differs for PC-3 cells overexpressing 15-LO-1; and 8) evaluate whether 15-LO-1-mediated IGF-1R expression affects MAPK activation.
Materials and Methods
Cell Culture
PC-3 parental PCa epithelial cells (CRL-1435) were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and cultured in RPMI medium (Gibco-BRL, Carlsbad, CA) without phenol red, containing 10% fetal bovine serum (FBS) (complete medium) per milliliter in 5% CO2 at 37°C. The cells were split after every 3 days. The stable PC-3 transfectants used in this study are as described previously [17]. PC3-15LOS (15-LO-1-overexpressing) and PC3-Zeo (mock-transfected) were grown in a medium containing 50 µg/ml Zeocin (Invitrogen, Carlsbad, CA).
Antibodies
The isotypes and specificities of monoclonal and polyclonal antibodies are as follows: polyclonal CheY antibody IgG1 is specific for 15-LO-1 (obtained from Dr. Elliott Sigal), monoclonal antibody IgG1 is specific for human IGF-1Rα (N-20) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and a general anti-phosphotyrosine antibody PY-7E1 (Zymed Laboratories, Inc., San Francisco, CA) is used. For Akt, an antibody specific for phospho-Akt (Ser 473) is used, and for Erk1/2, phospho-p44/42 MAP kinase (Thr 202/Tyr 204) is used (New England Biolaboratories, Beverly, MA). Similarly, antibodies recognizing total IGF-1R, ERK, and Akt proteins are also used individually (New England Biolaboratories).
Cell Growth Assays
PC-3 cells and their genetically engineered 15-LO-1 derivatives were tested for proliferation using the Boehringer Mannheim Cell Proliferation Kit (MTT) (Boehringer Mannheim, Ridgefield, CT). PC-3 cells were plated on 24-well plates (50,000–80,000 cells/well) and maintained overnight in complete medium. Cells were then changed to serum-free medium in the absence or presence of IGF-1 (10 ng/ml medium) and treated with different adenoviruses as described in the text. At the indicated time periods, the medium was aspirated from the wells, and 200 µl of MTT reagent (1 mg/ml) was added to each well. The cells were then incubated for 1 hour at 37°C and lysed by addition of 200 µl of isoamyl alcohol and shaking for 20 minutes. A 200-µl aliquot of each sample was then translated to 96-well plates and read in an enzyme-linked immunosorbent assay reader at 570 to 690 nm. The percentage increase (corrected absorbance) in cell proliferation was measured. Cells grown in parallel were also harvested and survival was estimated from those that excluded 0.2% trypan blue [17].
Immunohistochemical and Image Cytometric Quantitation Analyses of Human Prostate Tissues and Tumor Samples from Athymic Mice
Experiments performed with tumor tissues from mouse and human prostate tissues were carried out according to the guidelines of the Committees on Experimental Animals and Human Tissue Use of the University of Pittsburgh.
Sections of formalin-fixed, paraffin-embedded tissues (5 µm) were tested for the presence of IGF-1R (1:200), using an avidin biotin complex technique, steam heat-induced antigen retrieval, and diaminobenzidine (DAB) staining brown, quantitated by image cytometry as described previously [6].
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis
Western blot analysis of IGF-1R,MAP kinase (Erk1/2), and Akt was performed similarly as previously described [6]. Protein was measured by the method of Bradford [25]. Briefly, 25 µg of protein whole cell extracts or immunoprecipitated samples was separated on SDS gels and transferred onto individual PVDF membranes by electroblotting. Ponceau S staining of the blots was conducted to ensure equivalent protein loading. The nitrocellulose membraneswere incubated with their respective antibodies (1:10,000 dilution) for 1 hour at room temperature. Following incubation with appropriate IgG peroxidase second antibody (1:8000), proteins were visualized by using the Luminol/Enhancer (ECL) solutions as described by the manufacturer. The intensity of phosphorylation value is quantified by densitometric intensity values, normalized by dividing the intensity value of the total protein band.
Transient Transfection and Dual-Luciferase Assay
Transient transfections were carried out using luciferase reporter construct (a plasmid containing the luciferase gene driven by base pairs -2350 to +640 of the rat IGF-1R promoter region) [26], along with a Renilla expression vector as a control for transfection efficiency. The luciferase activity, as a measure of IGF-1R activation, was normalized to Renilla in each case. Cells were plated at 40% confluence in 12-well plates. After 24 hours, the plasmid DNA (1 µg) was individually cotransfected with the Renilla coreporter, Rluc (Promega, Madison, WI), into PC-3 cells by FuGENE 6 Transfection reagent (Boehringer Mannheim). The cells were grown for 24 hours before being harvested for assays. For assays, the cells were trypsinized and washed thrice with phosphatebuffered saline (PBS). The cell extracts were prepared with 400 µl of reporter lysis buffer (Promega) and clarified by centrifugation at 20,000g at 4 °C for 2 minutes, and the supernatant was transferred to a new tube. The clarified supernatants were individually analyzed for dual-luciferase reporter assays (100 µl) using a luminometer with autoinjector (Turner design), as described by the manufacturer (Promega).
Dominant-Negative IGF-1R Adenoviral Vector (AdTrackCMV-DNIGF-1R-GFP)
For IGF-1R blocking, recombinant adenovirus (Ad-TrackCMV-DNIGF-1R-GFP) that contains a dominant negative α chain of the receptor was used. There is high homology of IGF-1R among humans and rats (98% at the protein level) that allows the use of dominant-negative IGF-1R from rats in studies with human cells [26,27]. It encodes amino acids 1 to 952 of the rat IGF-1 receptor, has an influenza hemagglutinin epitope (HA1) tag, and a TGA (stop codon). The basic pAdTrackCMV-GFP vector (a gift of Dr. Vogelstein, Baltimore, MD) is used to produce green fluorescent protein (GFP)-trackable viruses containing transgenes. Briefly, the dominant-negative IGF-1R was subcloned into a pAdTrackCMV-GFP vector as described [28] to produce AdTrackCMV-DNIGF-1R-GFP. The efficiency of both the control pAdTrackCMV-GFP as well as the dominant-negative AdTrackCMV-DNIGF-1R-GFP adenovirus-infected PC-3 parental cells was found to be 90% by GFP expression (data not shown). To generate higher titer viral stocks of both vectors, 293 cells are reinfected until a total of 5 x 108 packaging cells and generally a range of 1.16 to 2.2 x 1011 plaque-forming units (pfu) are obtained, and we used 1.16 x 1011 pfu for our experiments. The viruses are purified by cesium chloride (CsCl) gradient centrifugation and stored in liquid nitrogen. Just before conducting the actual experiments, both adenoviruses are individually dialyzed against water and used immediately.
Binding Assays and Scatchard Analysis
Binding assay for IGF-1 was performed with PC-3 cells using tracer amounts of [125I]IGF-1 (2000 Ci/mmol specific activity; Amersham Life Science, Inc., Arlington Heights, IL). Cells were plated in 24-well culture dishes and grown to 80% confluence. A total of 100,000 cells/well was then washed four times with Hepes buffer containing 10 mM Hepes, 135 mM NaCl, 4.8 mM KCl, 1.7 mM MgSO4, 2.5 mM CaCl2, and 1.0 mM NaH2PO4, pH 7.4, followed by incubation of the cells for 30 minutes at 37°C with [125I]IGF-1, in the presence or absence of different concentrations (5–100 nM) of IGF-1. After incubation, the cells were washed with ice-cold Hepes buffer and lysed in 0.1 M NaOH, and radioactivity was counted by γ-counter. The binding was analyzed by Scatchard analysis as described [29].
Statistical Analyses
All experimental data are representative of experiments performed at least in triplicates. Data are expressed as mean ± standard error (SE) or SD. The criterion for statistical significance was taken as P < .05.
Results
Survival of PC-3 Cells Depends on the LA Product of 15-LO-1: 13-(S)-HODE
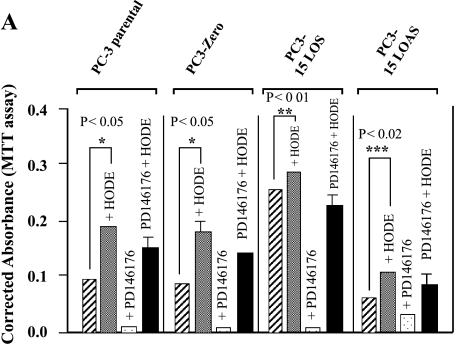
We previously showed that PD146176, a specific 15-LO-1 enzyme inhibitor [30], inhibited the proliferation of cultured PC-3 cells (i.e., parental PC-3, mock-transfected PC3-Zeo, and 15-LO-1-overexpressing PC3-15LOS) in a concentration-dependent manner (complete inhibition at 1 M concentration) [17]. However, PD146176 did not completely inhibit the growth of PC3-15LOAS cells that do not express 15-LO-1 enzyme [17]. This result suggested that the growth inhibition of PC-3 cells by PD146176 was dependent on 15-LO-1 expression and enzyme activity. PC3-15LOS cells produced increased 13-(S)-HODE levels, and grew faster compared to PC3-Zeo, PC3 parental, and PC3-15LOAS cells, respectively [17]. Although 15-LO-1 preferentially metabolizes LA to 13-(S)-HODE, it also can substantially convert arachidonic acid to 15-(S)-HETE. Therefore, in order to rule out the 15-(S)-HETE effects attributed by the arachidonic acid metabolism, we initially performed experiments using 10 µM each of 15-(S)-HETE as well as with an inactive enantiomer of 13-(S)-HODE, called 13-(R)-HODE, as a control, respectively. We did not observe any significant growth differences in any of the PC-3 cell lines with 15-(S)-HETE and 13-(R)-HODE, respectively (data not shown).
Although 15-(S)-HETE and 13-(R)-HODE did not cause differences in growth of PC-3 cells, we further investigated the importance and specificity of 15-LO-1 to determine whether 13-(S)-HODE, 13-(R)-HODE (inactive), and 15-(S)-HETE (arachidonic acid metabolite) could reverse the growth inhibition caused by PD146176. We added 10-µM concentrations of 13-(S)-HODE, 13-(R)-HODE, and 15-(S)-HETE (replaced by fresh media every 12 hours) in vitro to the PC-3 cells treated with 1 M PD146176. Our results illustrate that the growth inhibition of PC-3 cells by PD146176 is rescued or reversed only by 13-(S)-HODE (Figure 1A), and not by 13-(R)-HODE or 15-(S)-HETE (data not shown), respectively. These data suggest that 15-LO-1 [13-(S)-HODE] specifically supports proliferation, as well as survival, of PC-3 cells. Therefore, the effects of 15-(S)-HETE and 13-(R)-HODE were not evaluated in further studies.
Figure 1.
(A). 13-(S)-HODE rescues inhibition of 15-LO-1 enzyme caused by 1 µM PD146176. PC-3 cells were grown in (1) medium alone ( ); (2) medium + 10 µM 13-(S)-HODE (
); (2) medium + 10 µM 13-(S)-HODE (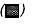 ); (3) medium + inhibitor (
); (3) medium + inhibitor (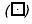 ); and (4) medium + inhibitor + 10 µM 13-(S)-HODE (▪), for 72 hours (after every 12 hours, replaced appropriately by fresh media). Cell death is assessed by Trypan blue exclusion assay, versus survival, by MTT assay. The data shown are mean ± SE of six determinations. *P < .05, **P < .01, ***P < .02. (B). Effect of growth factors on PC-3 cell proliferation. PC-3 cells were treated with growth factors in growth medium. Medium — FBS (A) (
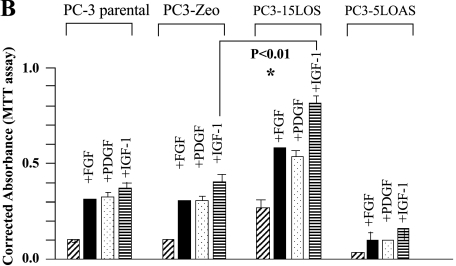
); and (4) medium + inhibitor + 10 µM 13-(S)-HODE (▪), for 72 hours (after every 12 hours, replaced appropriately by fresh media). Cell death is assessed by Trypan blue exclusion assay, versus survival, by MTT assay. The data shown are mean ± SE of six determinations. *P < .05, **P < .01, ***P < .02. (B). Effect of growth factors on PC-3 cell proliferation. PC-3 cells were treated with growth factors in growth medium. Medium — FBS (A) (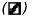 ); A + FGF (5 ng/ml) (▪); A + PDGF (10 ng/ml) (
); A + FGF (5 ng/ml) (▪); A + PDGF (10 ng/ml) (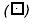 ); A + IGF-1 (10 ng/ml) (
); A + IGF-1 (10 ng/ml) ( ). Proliferation was assessed by MTT assay. The data shown are mean ± SE of six determinations. *P < .01.
). Proliferation was assessed by MTT assay. The data shown are mean ± SE of six determinations. *P < .01.
There Is a Link Between 15-LO-1 Overexpression and Stromal Element (IGF-1), Which Together Support Prostate Epithelial Cell Proliferation
While further characterizing the phenotypes of PC-3 cell lines, we observed that the growth factors such as platelet-derived growth factor (PDGF), IGF-1, and basic fibroblast growth factor (bFGF), when added exogenously in serumstarved growth medium, also participated in cell growth. However, there is a potential limitation when comparing the growth induction in these cells because of the differences in growth and phenotypes. Therefore, it seems that the proliferation (by MTT assay) is similar (i.e., three-fold to four-fold) comparing PC3-Zeo and PC3-Zeo treated with IGF-1, and then comparing PC3-15LOS and PC3-15LOS treated with IGF-1) and that there is no clear margin to show that IGF-1 is significantly more growth-promoting than FGF and PDGF (Figure 1B).
Interestingly, only the PC3-15LOS (15-LO-1-overexpressing) cells exhibited a two-fold to three-fold greater response (n = 6, P < 0.01, if compared with PC3-Zeo cells) to IGF-1 in 72 hours (Figure 1B). These results suggested that the proliferation of 15-LO-1-overexpressing PC3-15LOS cells may be modulated by IGF-1 consequently via the IGF-1 receptor (IGF-1R). Thus, we hypothesized that the differences observed in the IGF-1 effects on growth capability of PC-3 cells may be because of the differences in IGF-1R levels that are responsive to 13-(S)-HODE concentration.
Similarly, we have previously found that 15-LO-1 enzyme is present in high levels in the epithelium of prostate adenocarcinoma compared to normal surrounding tissues. The level of 15-LO-1 expression is strongly correlated with the degree of malignancy, as assessed by Gleason grades [6]. In order to study if the IGF-1R levels also correlated with the degree of malignancy, we immunohistochemically analyzed (n = 26) 10 (benign prostatic hyperplasia or BPH) and 16 adenocarcinoma tissues (Gleason grade = 8–10) of human prostate from different patients for IGF-1R localization. Therefore, we did not compare IGF-1R localization between simple prostatectomy-derived BPH tissues and the high-grade tumor specimens in the same patients. Nevertheless, 15-LO-1 expression is low in BPH and normal tissues, and high in the high-grade tumors of Gleason grades 8 to 10 [6].
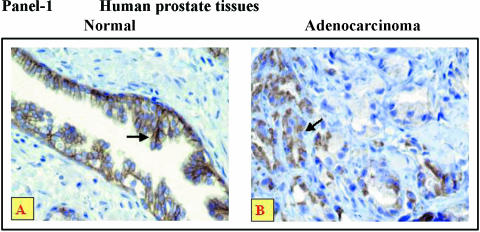
Therefore, this study may have limitations, and there may be differences in the immunophenotypic (histologic) alterations in IGF-1R levels in benign and tumor tissues from different patients versus data compared in the same patients. The prostate tumor tissues we analyzed display a higher expression of IGF-1R in cancerous foci (highly proliferating cells) compared to BPH tissues (representatives shown in Figure 2, Panel 1A; some foci negative for IGF-1R versus Panel 1B), further quantitatively assessed by image cytometry analyses (Table 1). The most striking differences in the IGF-1R localizations were observed in BPH prostate compared to cancer tissues. There is uniform immunostaining in the stratified luminal epithelium in the benign gland in Figure 2, Panel 1A versus an extensive immunostaining in the infiltrative tumor as shown in Figure 2, Panel 1B. The IGF-1R is predominantly localized in the glandular epithelium of benign tissues, whereas in adenocarcinomas, it is mainly localized and upregulated in highly proliferating epithelial cells (Table 1), and this is proportional to 15-LO-1 expression we have previously observed [6,17]. These results demonstrate that although specific binding sites for IGF-1 are present, both in the benign as well as adenocarcinoma prostatic tissues, higher IGF-1R levels are associated with high-grade prostate tumor epithelial cells.
Figure 2.
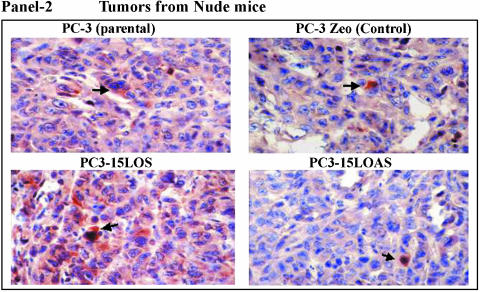
Representative immunohistochemistry of human prostate tissues with IGF-1 receptor antibody. Panel 1: Immunostaining (brown, shown by arrows) of (A) normal and (B) adenocarcinoma tissues of human prostate (n = 26); 10 normal and 16 adenocarcinoma tissues of Gleason grade = 8 to 10, with polyclonal antibody for IGF-1R (original magnification, x200). The values are reported as assessed by image cytometric quantitation (Table 1). Panel 2: IGF-IR Immunohistochemistry ot tumors in nude mice. Immunostaining (brown, indicated by arrows) of tumors, from nude mice caused by PC3-15LOS, PC3-15LOAS, PC-3 parental and mocktransfected PC3-Zeo, with IGF-1R polyclonal antibody (original magnification, x200). The values are reported as assessed by image cytometric quantitation (Table 1).
Table 1.
Histologic Analyses of IGF-1R Immunostaining in Human Prostate Tissues and Tumors from Nude Mice.
| Human prostate tissues | Tumors from Nude mice | ||||
| Normal | Prostate tumors | PC3-parental | PC3-Zeo | PC3-15LOS | PC3-15LOS |
| (BPH) | [Gleason 8–10] | (n = 4) | (n = 4) | (n = 8) | (n = 8) |
| (n = 10) | (n = 16) | ||||
| ++ | ++++ | ++ | ++ | ++++ | + |
| (predominantly glandular epithelium) | (focal expression in highly proliferating epithelium) | ||||
The results were analyzed by image cytometry and semiquantitatively measured as 0 to 4+: 0—no staining; 1+—weak intensity staining; 2+—moderate intensity staining in less than one-half of the cells, but less than strong uniform staining; 3+— intense staining in all cells, but less than strong/robust uniform staining; 4+— strong/robust uniform staining in all tissue/cells.
15-LO-1 Expression Caused Concomitant Increase in IGF-1R Levels as Well as IGF-1R Activation
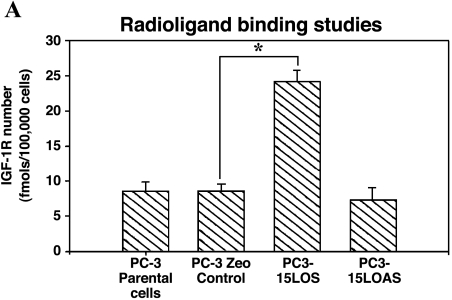
We further measured differences in IGF-1R levels by immunohistochemistry (DAB staining brown) in tumors from an experiment with nude mice injected with individual PC-3 cells (same PC-3 cells as previously described) [17]. We found the levels of IGF-1R in the primary tumors to be: PC3-15LOS > PC3 = PC3-Zeo > PC3-15LOAS (representatives shown in Figure 2, Panel 2). These observations further supported the possible relationship between IGF-1R and 15-LO-1, and their roles in PC-3 cell growth. Based on these results, we studied whether 15-LO-1 overexpression caused an increase in the number of available receptors for ligand (i.e., IGF-1) binding or not. We examined the IGF-1R levels by [125I]IGF-1 ligand binding assay, in the presence or absence of cold IGF-1 (5–100 nM). Our results (n = 4), as shown in Figure 3A, suggest that PC3-15LOS (15-LO-1-overexpressing) cells have greater than two-fold to three-fold number (P < .05 vs PC3-Zeo) of IGF-1 binding sites (25 ± 4 fmol/105 cells) versus those in both PC3-Zeo (7 ± 3 fmol/105 cells) and parental PC-3 (8 ± 2 fmol/105 cells), respectively. However, although not significantly, the binding sites in PC3-15LOAS cells (6 ± 3 fmol/105 cells) were reduced (Figure 3A). This experiment suggests that 15-LO-1 overexpression caused an upregulation of IGF-1R.
Figure 3.
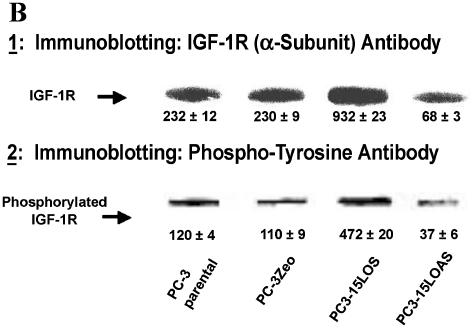
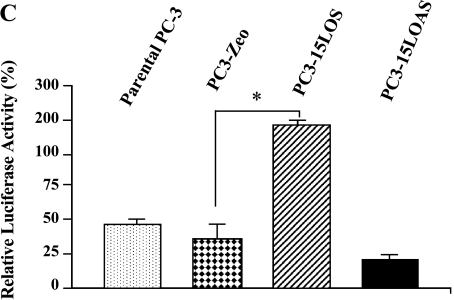
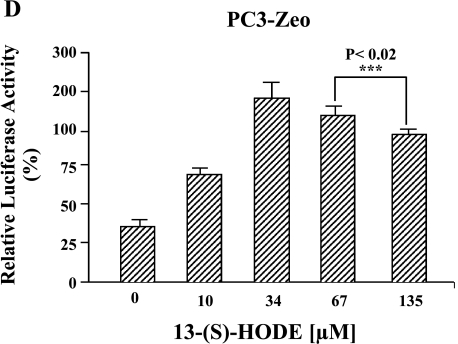
(A) IGF-1R expression levels in PC-3 cells. Radiolabeled IGF-1 binding to PC-3 cells was performed. Cells (100,000 cells/ml) were incubated for 30 minutes at 37 °C with [125I]IGF-1, in the presence or absence of different concentrations (5–100 nM) of cold IGF-1. After incubation, the cells were washed with icecold Hepes buffer, lysed in 0.1 M NaOH, and counted by γ-counter. Data are analyzed by ligand program. Results shown are the mean ± SE of four separate experiments. *P < .01. (B) Western blot analyses of immunoprecipitated IGF-1R from PC-3 cells. Equal protein (1 mg, total protein) from each PC-3 cell line was immunoprecipitated by an antibody specific for IGF-1P (N-20), resolved on 10% SDS-PAGE, and immunoblotted by the N-20 antibody (shown in B1), and the same blot by anti-phosphotyrosine antibody PY-7E1 for tyrosine-phosphorylated proteins, respectively (shown in B2). Ponceau S staining of the blots was conducted to ensure equivalent loading. Protein levels and phosphorylation were measured by densitometry. The data shown are representative of three independent experiments. The values ± standard deviation are reported in the brackets below the gels. (C) 15-LO-1-dependent activation of the IGF-1R promoter. The indicated cells were transiently transfected with a plasmid containing the luciferase gene driven by base pairs -2350 to +640 of the rat IGF-1R promoter region, along with a Renilla expression vector as a control for transfection efficiency. The luciferase activity, as a measure of IGF-1R activation, was normalized to Renilla in each case. Results shown comparing the IGF-1R promoter activity in parental PC-3, PC3-Zeo, PC3-15LOS, and PC3-15LOAS cells are the mean ± SE of six separate experiments. *P < .01. (D) 13-(S)-HODE-dependent activation of the IGF-1R promoter. The PC3-Zeo cells were transiently transfected with rat IGF-1R promoter-reporter plasmid as described for (C), and after 24 hours posttransfection, they were individually treated (exogenously added) with increasing concentrations (10, 34, 67, and 135 µM) of 13-(S)-HODE respectively, and allowed to grow further until 36 hours. The luciferase activity, as a measure of IGF-1R activation, was normalized to Renilla in each case. Results shown comparing the IGF-1R promoter activity in parental PC-3, PC3-Zeo, PC3-15LOS, and PC3-15LOAS cells are the mean ± SE of six separate experiments. ***P < .02.
One of the primary steps in signaling pathway(s) is the phosphorylation status of receptor(s) required for activation of its receptor complex. To evaluate whether 15-LO-1 overexpression is also causal to IGF-1R activation (phosphorylation status), we first immunoprecipitated IGF-1R from the total proteins extracted from PC-3 cells (n = 4) using IGF-1P, and then performed Western blot analyses using IGF-1P antibody for measuring the IGF-1R levels. Similarly, we also determined the phosphorylation status, using a phosphotyrosine antibody (Figure 3B). As assessed by densitometry scanning, the IGF-1R receptor levels were three-fold higher in PC3-15LOS (15-LO-1-overexpressing) cells versus the PC3-15LOAS, PC-3 parental or PC3-Zeo (control) cells (Figure 3B1). Also the IGF-1R receptors from PC3-15LOS (15-LO-1-overexpressing) cells were phosphorylated fourfold compared to the PC3-Zeo or PC-3 cells (Figure 3B2). Thus, 15-LO-1 overexpression in PC-3 (PC3-15LOS) cells increases IGF-1R expression and therefore phosphorylation.
15-LO-1 Expression Caused an Increase in IGF-1R Promoter Activation
To elucidate the mechanism for the increased IGF-1R expression, we measured IGF-1R promoter activation in PC-3, PC3-Zeo, PC315LOS, and PC3-15LOAS cells using a luciferase assay (Figure 3C). PC3-15LOS (15-LO-1-overexpressing) cells displayed four-fold higher levels of IGF-1R promoter activation (n = 6, P < .01 vs the PC3-Zeo), whereas PC315LOAS (in which 15-LO-1 is blocked) displayed decreased IGF-1R promoter activation (Figure 3C).
In order to further test whether the observed IGF-1R promoter activation is 13-(S)-HODE-specific, the PC3-Zeo cells were similarly transfected as described above. Twentyfour hours after IGF-1R reporter vector transfection, the PC3-Zeo cells were individually treated with increasing concentrations (10, 34, 67, and 135 µM) of 13-(S)-HODE and allowed to grow over a 36-hour time period. Luciferase activity assays were performed as described above. We observed a concentration-dependent increase in luciferase activity from 10 to 34 µM 13-(S)-HODE-treated cells versus untreated control (Figure 3D). These results further supported our observation as depicted in Figure 3, A–C, respectively.
However, although the reporter activity increased threefold from 10 to 34 µM, and was then slightly higher in 135 µM compared to 10 µM 13-(S)-HODE-treated PC3-Zeo cells, the reporter activity significantly decreased from 34 to 135 µM (Figure 3D; P < .02) respectively. No obvious cell death or apoptosis was observed in any of these cells (data not shown). These data suggest that 15-LO-1 expression and, consequently, 13-(S)-HODE can affect IGF-1R transcription. At lower physiological levels (up to 34 µM), 13-(S)-HODE increases transcription of IGF-1R, whereas at high nonphysiological levels (greater than 34 µM), 13-(S)-HODE causes downregulation of IGF-1R. Therefore, it is intriguing to speculate that there could be a central role for 13-(S)-HODE-dependent regulation of IGF-1R, and therefore a fundamental role of 15-LO-1 in prostate cell proliferation.
Blocking 15-LO-1-mediated IGF-1R Levels Inhibited the Proliferation of PC-3 Cells
From the results of our experiments, it was imperative to determine whether targeting IGF-1R can potentially block PC-3 cell proliferation. In order to address this, we used a recombinant adenovirus, AdTrackCMV-DNIGF-1RGFP, which contains a dominant-negative α chain of the IGF-1 receptor to block IGF-1R expression in PC-3 cells. Vector alone (pAdTrackCMV-GFP) is used as the control. Fortunately, the high degree of homology of IGF-1R in humans and rats [26] allows a dominant-negative IGF-1R from rats to be used in studies of human cells [27,28]. IGF-1 at 10 ng/ml was the mitogenic stimulant. Serumstarved PCa cell lines (i.e., parental PC-3, PC3-Zeo, PC315LOS, and PC3-15LOAS cells) were treated in vitro (n = 6), in the presence of IGF-1 and appropriately with AdTrackCMV-DNIGF-1R-GFP and pAdTrackCMV-GFP adenoviruses, each at a concentration of 1.16 x 1011 pfu, respectively.
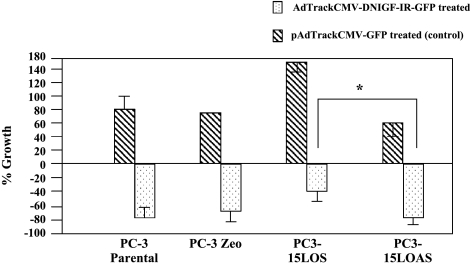
The objective was also to determine whether inhibition of IGF-1R blocked growth of PC-3 cells even though 15-LO-1 is active. After treatment of PC-3 cells with AdTrackCMVDNIGF-1R-GFP (expressing the dominant-negative IGF-1R), there was significant growth inhibition of PC3-15LOAS cells (P < .0001 vs PC3-15LOS cells similarly treated) compared to cells similarly treated with the empty adenoviral vector, pAdTrackCMV-GFP, as control. Thus, inhibition of growth was more pronounced in PC-3 cells expressing inactive 15-LO-1 (PC3-15LOAS), PC-3 parental, and PC3-Zeo cells compared to the PC-3 cells overexpressing 15-LO-1 (PC3-15LOS) (Figure 4). Overall, these results supported our hypothesis and suggest that the efficacy of growth inhibition by IGF-1R blockade was dependent on 15-LO-1 levels.
Figure 4.
Effect of IGF-1R blocking on growth of PC-3 cells. PC-3 cells were grown for 48 hours in 1 ml of medium + FBS, washed, and then grown further in serum-starved medium + IGF-1 (10 ng/ml) and treated with adenoviral vectors pAdTrackCMV-GFP (control) and AdTrackCMV-DNIGF-1R-GFP (both at a concentration of 1.16 x 1011 pfu), and grown further for 48 hours in medium (FBS). Percentage increase in growth (treated with pAd-TrackCMV-GFP alone) = (OD after 96 hours - OD after 48 hours) / OD after 48 hours. Percentage decrease in growth (treated with AdTrackCMVDNIGF-1R-GFP) = (OD after 96 hours - OD after 48 hours) / OD after 48 hours. The data shown are mean ± SE of six independent determinations of MTT assays. *P < .0001.
Blocking 15-LO-1-dependent IGF-1R Levels Inhibited the IGF-1/MAP Kinase Signaling and Proliferation of PC-3 Cells
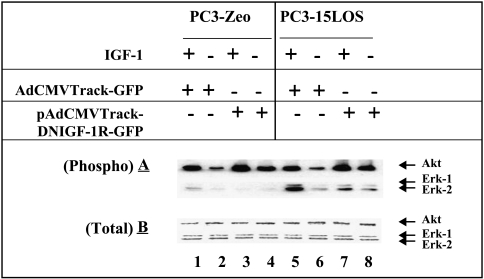
Furthermore, 15-LO-1-dependent IGF-1R activation could lead to stimulation of the downstream signaling pathway(s). In order to study this, we compared the activation levels of mitogen-activated protein kinase (MAPK or Erk1/2) and Akt enzymes in PC3-Zeo cells (control) with those in 15-LO-1-overexpressing PC3-15LOS cells (Figure 5). We used the same adenovirus strategy in order to block IGF-1R. Specifically, serum-starved PCa cell lines (i.e., PC3-Zeo and PC3-15LOS) were individually grown with or without 10 ng/ml IGF-1, in the presence and absence of Ad-TrackCMV-DNIGF-1R-GFP and pAdTrackCMV-GFP (control) adenoviruses, and assayed for their ability to proliferate, as depicted in Figure 5. Total Erk1/2 (MAP kinase) and Akt protein levels as well as their corresponding activation (phosphorylation) status using phospho-Erk and phospho-Akt were determined in these cells by immunoblotting and quantitated by densitometry scanning.
Figure 5.
15-LO-1 overexpression modulates IGF-1R levels that cause an increase in MAP kinase activation. Western blot analysis of protein (25 µg/well) from PC3-Zeo (mock-transfected) compared to PC3-15LOS cell line, treated with (+) and without (-) IGF-1 (10 ng/ml) in the presence of adenovirus vector alone (pAdTrackCMV-GFP) and the IGF-1R blocking adenovirus (AdTrackCMV-DNIGF-1R-GFP) (1.16 x 1011 pfu) by (A) an antibody specific for phospho-Akt (Ser 473) and for Erk-1/2 (i.e., phospho-p44/42 MAP kinase) (Thr 202/Tyr 204) and (B) an antibody for total Akt and Erk-1/2. Ponceau S staining of the blots was conducted to ensure equivalent protein loading. The data shown are representative of three independent experiments.
Our results demonstrate that IGF stimulation and subsequent treatment with the adenovirus, pAdTrackCMV-GFP, as control dramatically increased (8- to 10-fold) Erk1/2 activation (Figure 5, lane 5) in PC3-15LOS (15-LO-1-overexpressing) cells versus PC3-Zeo cells (Figure 5, lane 1), whereas no difference was seen in Akt activation. Furthermore, the activation of Erk1/2 by IGF-1 is partially blocked by AdTrackCMV-DNIGF-1R-GFP, whereas an increase in Akt activation is detected in both the cell lines (Figure 5, lane 3 compared to lane 7). In both the unstimulated PC-3 cells (but treated with adenovirus pAdTrackCMV-GFP as control), activated Erk1/2 and Akt at low levels were detected, but only Erk1/2 were higher in PC3-15LOS compared to PC3-Zeo cells (Figure 5, lane 2 compared to lane 6). However, in a similar comparison of unstimulated PC-3 cells in which IGF-1R is blocked by AdTrackCMV-DNIGF-1R-GFP, the activation of endogenous Erk1/2 is not completely blocked, but interestingly, an increase in Akt activation is detected in both cell lines (Figure 5, lane 4 compared to lane 8). These results suggest that blocking of 15-LO-1-induced increase in IGF-1R expression leads to inhibition of MAP kinase activation in the presence of IGF-1. Thus, these results support our hypothesis that IGF-1R levels and the downstream signaling pathway(s) are affected by 15-LO-1 expression status, and by blocking IGF-1R or possibly inhibiting 15-LO-1 activity, we can alter the proliferation status of PCa cells.
Discussion
The 15-LO-1 metabolic product of LA, 13-(S)-HODE, has been shown to participate in signaling processes [8–24]; a direct role of this lipid metabolite in prostate cellular proliferation is unknown. De Vries and van Noorden [33] reported that long-chain PUFAs of the n-3 family [e.g., eicosapentaenoic acids (C20:5n3) and docosahexaenoic acids (C22:5n-3)] inhibit prostate tumor growth whereas metastasis is promoted by long-chain n-6 PUFAs [e.g., LA (C18:2n-6)]. Others have reported that LA supports the growth of both the androgen-unresponsive PC-3 and the androgen-responsive DU145 human PCa cell lines in vitro and in athymic mice [31–34].
Results presented in this study demonstrate for the first time that 13-(S)-HODE can cause an increase in the expression of IGF-1R in prostate carcinoma cells. This, in turn, can trigger prostate cell proliferation and/or migration. This could explain why PCa cells express IGF-1R and often metastasize to bone stroma, an environment rich in IGFs. In short, 15-LO-1 overexpression can augment IGF-1 to exert a paracrine action in PCa cell proliferation and migration, and may have a profound impact in prostate carcinogenesis. In the prostate, as is the case with other glandular and nonglandular tissues, epithelial-stromal interactions are an essential feature of normal morphogenesis and cytodifferentiation [35,36]. Furthermore, there is growing evidence that stromal factors contribute to the evolution of PCa [37]. Although 15-LO-1 preferentially was able to rescue the PC-3 cells' growth inhibition caused by the 15-LO-1 enzyme inhibitor, PD146176, added in vitro, it can be inferred that 13-(S)-HODE can also act as a survival factor for PC-3 cells, possibly via modifying the levels of the anti-apoptotic gene, BCL2 [23]. Although it is unknown whether 13-(S)-HODE is also present in all stromal cells or not, Rao et al. [38] have reported that LA and its product, HODE, in smooth muscle cells induced DNA synthesis and c-fos, c-jun, and c-myc mRNA expression, and caused MAPK activation, leading to proliferation. Regardless, it is intriguing to speculate that 13-(S)-HODE secreted by proliferating prostate epithelial cells could also interact with stromal cells and play an important role in the evolution of PCa progression.
Furthermore, when PC-3 cells were exposed to growth factors in serum-free medium, namely PDGF, IGF-1, and bFGF, PC3-15LOS (15-LO-1-overexpressing) cells exhibited a two-fold to three-fold greater response to IGF-1 in 72 hours (Figure 1B). These results suggested that the proliferation in 15-LO-1-overexpressing cells is possibly modulated by IGF-1 through IGF-1R. There is also substantial evidence that IGF-1R signaling is involved in antiapoptosis [39–41]. Previously published reports [42,43] indicate that cells lacking IGF-1R fail to grow in serum-free medium supplemented with growth factors (i.e., PDGF, TGF, EGF, insulin, IGF-1, or bFGF, separately or in a combination). Baserga et al. [42] reported that IGF-1R is required for optimal growth throughout the phases of cell cycle, and that the expression of IGF-1 and IGF-1R is regulated by tumorsuppressor genes WT1 and RB [42] and wild-type p53 [43]. These findings suggest that activation of the IGF-1R is an absolute requirement for growth factors to exert their mitogenic effects.
Immunohistochemical analyses of human benign and adenocarcinoma prostate tissues (Figure 2, Panel 1) and tumors derived from nude mice subcutaneously injected with PC-3 cells (Figure 2, Panel 2) suggest that: 1) IGF-1R expression in tumors correlated with 15-LO-1 levels; and 2) although IGF-1R is present in benign and adenocarcinoma prostatic tissues, in high-grade tumor cells, their epithelial localization seems to be specifically modified (i.e., from glandular epithelium in benign versus highly proliferating epithelial cells in adenocarcinoma) [44,45]. The growth hormone (GH)/IGF-1 axis has a clearly established role in somatic growth regulation, and there is evidence suggesting that it can play a role in neoplastic tissue growth [46]. Epidemiologic studies suggest an association between increased serum levels of IGF-1 and an increased risk of PCa [47,48].
Ligand binding (Figure 4), Western blot analysis (Figure 3B), and IGF-1R promoter activation assays (Figure 3C) in PC-3 cells demonstrated the levels of IGF-1R and their corresponding activation (phosphorylation) status as: PC315LOS > PC-3 = PC3-Zeo > PC3-15LOAS. Importantly, PC3-15LOS displayed greater than two-fold number of receptors that were four-fold activated. Further study to determine the specificity of 13-(S)-HODE for IGF-1R upregulation revealed a concentration-dependent increase in the reporter activity by 10 and 34 µM 13-(S)-HODE, respectively (Figure 3D). However, gradual but significant reduction of reporter activity is noted with exogenously added 13-(S)-HODE at 67 and 135 µM concentration, suggesting that a 34-µM concentration was optimum to support cellular proliferation and higher concentrations in this property. Even though we did not observe cell death or apoptosis in these cells after 12 hours of exposure to higher concentrations of 13-(S)-HODE, we suspect that the cells may have undergone growth arrest and therefore reflect decreased reporter activity. Although it remains to be seen how much endogenous 13-(S)-HODE concentration is actually produced by cultured PC-3 cells in vitro or in PCa tissues (in vivo) to impart a biologic effect, these results support our hypothesis and further demonstrate a direct correlation between 15-LO-1 expression and IGF-1R levels in PCa cells. Human PCa cell lines, PC-3, and DU-145 express IGF-1 receptors [49] and are inhibited by an IGF-1 receptor-specific monoclonal antibody [50]. Similarly, IGFs are paracrine growth stimulators in both the normal and the hyperplastic PCa cells [51,52]. Recently, a more direct study of transgenic mice expressing human IGF-1 in basal epithelial cells of prostate led to activation of IGF-1R and spontaneous tumorigenesis in prostate epithelium [53,54]. This phenomenon may be dependent on the PI3 kinase and/or MAP/Erk kinase pathway [55–58].
It is conceivable that there could be a central role for 13-(S)-HODE-dependent regulation of IGF-1R levels and, consequently, its role in the transformation of normal prostate epithelial cells to a dysregulated, mitogenic, proliferative state. When the IGF-1R is blocked, there is inhibition in growth of all PC-3 cells, except that the overexpressing 15-LO-1 (PC3-15LOS) requires a higher concentration of blockade for complete growth/proliferation inhibition. Thus, there is a positive association between 13-(S)-HODE-dependent activation of the IGF-1R pathway and growth. This phenomenon may occur by: 1) activating the 13-(S)-HODE-dependent downstream signaling pathway; and/or 2) preventing apoptosis.
Recently, Hsi et al. compared the effects of 13-(S)-HODE, a 15-LO-1/LA metabolite, to the effects of 15-(S)-HETE, a 15-LO-2/arachidonic acid metabolite, on MAP kinase signaling and PPARγ levels in our PCa cell line PC-3, and showed that 13-(S)-HODE upregulated whereas 15-(S)-HETE downregulated MAP kinase [24]. Thus, depending on the available substrate (i.e., LA versus arachidonic acid), the 15-LO metabolites have opposing effects on the regulation of the MAP kinase signaling pathway that affects PPARγ, which is the downstream target of MAP kinase pathway.
Comparing PC3-15LOS cells versus PC3-Zeo, the robust stimulation of Erk1/2/MAPK by IGF-1 and subsequent reduction in MAP kinase activation by IGF-1R blockade suggest a 15-LO-1-dependent IGF-1R activation (Figure 4). However, it seems evident that Erk1/2 activation was not solely dependent on IGF-1 or IGF-1R activation, but also by 15-LO-1-dependent mechanism(s) (Figure 5). These observations suggest that the basal Erk1/2 activation is 15-LO-1-dependent and can also occur through a mechanism(s) or pathway(s) independent of IGF-1R. What is also interesting is that increased activation levels of the “survival kinase” Akt by IGF-1 (from the basally activated levels; i.e., without IGF-1) are observed in both PC3-Zeo and PC3-15LOS cells, suggesting that Akt activation does not seem to be solely dependent on 15-LO-1 expression, but is IGF-1-dependent.
It is unclear and remains to be demonstrated as to how and why IGF-1R blockade alone, as well as the IGF-1R blockade in concert with IGF-1, activate the survival protein, Akt. Consequently, it also remains to be seen whether the MAPK/Akt and IGF-1 interactions are governed by an as yet unidentified 13-(S)-HODE receptor, or whether it is a consequence of 13-(S)-HODE binding directly to the IGF-1R in the membranes. Although one or both possibilities could exist, it is conceivable that 13-(S)-HODE could interact with cis- or trans-acting elements that regulate IGF-1R expression because we observed that 13-(S)-HODE caused an increase in IGF-1R promoter-reporter activity (Figure 3D). Interestingly, a recent study by Kiely et al. [59] has shown that the receptor for activated C kinases (RACK1) is an IGF-1R-interacting protein that can modulate receptor signaling and that RACK1 has a role in regulating Akt activation and cell survival. RACK1 interacts with the IGF-1R to negatively regulate activation of the PI3-K pathway but has a positive effect on activation of the MAP kinase and JNK pathways. It is possible that RACK1 and its associated proteins may interact with 13-(S)-HODE to regulate MAPK signaling through IGF-1R.
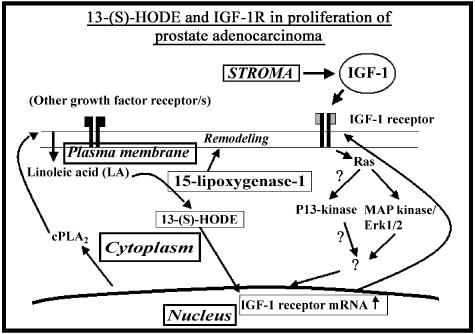
Overall, these experiments confirm that increased levels and therefore phosphorylation of IGF-1R by 13-(S)-HODE transmitted signals downstream through the MAP kinase signaling pathway. Thus, our present study suggests that the ability of the transforming agent, IGF-1, to exert growthpromoting/proliferative effects in PCa cells is exacerbated by the 15-LO-1 metabolic product, 13-(S)-HODE. The proposed model for interaction of 13-(S)-HODE with the IGF-1R and the signaling pathway is summarized in Figure 6. Further experiments are required to study the downstream signaling pathway(s) and the proliferation status mediated through 15-LO-1 metabolites and IGF-1R in PCa cells. Ultimately, a drug/blocker targeted to directly inhibit 15-LO-1 activity may have therapeutic benefit against PCa, which becomes largely refractory to conventional chemotherapeutic strategies.
Figure 6.
Proposed model for the role of 15-LO-1 enzyme metabolite, 13-(S)-HODE interaction with the IGF-1R, and downstream signaling pathway in proliferation of the prostate cancer cells. Cytoplasmic phospholipase A2 (cPLA2) activity releases plasma membrane-bound LA into the cytoplasm (or LA is available by high-lipid diet). Increased 15-LO-1 activity releases 13-(S)-HODE. 13-HODE (15-LO-1/LA activity) upregulates IGF-1R. Stromal growth factor IGF-1 interacts with IGF-1R that in turn activates MAP kinase (Erk1/2/MAPK) signaling. Further MAPK-dependent IGF-1R phosphorylation is increased, leading to another round of an increased activation of MAPK signaling; this process ultimately contributes to increased cellular proliferation. Thus, overexpression of 15-LO-1 leads a normal prostate epithelial cell to undergo neoplastic progression to a tumor cell.
Acknowledgements
We thank Haim Werner and Renatto Baserga for the rat IGF-1R promoter reporter plasmid, and Leland W. K. Chung and Thomas E. Eling for reading the manuscript. We also thank Jie Du for the DNIGF-1R adenoviral vector, and Kamal F. Badr for moral support. We are thankful to the histopathology laboratory at Emory University, Atlanta, for tissue sectioning, and especially to Diane Lawson and Debbie Sexton for immunohistochemistry of tissues.
Abbreviations
- 15-LO-1
15-lipoxygenase-1
- AA
arachionic acid
- COX
cyclooxygenase
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxyoctadecadienoic acid
- IGFBP
insulinlike growth factor binding protein
- IGF-1
insulin-like growth factor-1
- IGF-1R
insulin-like growth factor-1 receptor
- LO
lipoxygenase
- LA
linoleic acid
- PBS
phosphate-buffered saline
- PC-3
prostate cancer cell line-3
- VEGF
vascular endothelial growth factor
Footnotes
This work was supported by American Cancer Society grant no. RSG-03-022-01 and NIH grant no. 1-R21-CA098657-01 (to U.P.K).
References
- 1.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 2.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases 533 (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh J, Myers CE. Arachidonic acid stimulates prostate cancer cell growth critical role of 5-lipoxygenase. Biochem Biophys Res Commun. 1997;235:418–423. doi: 10.1006/bbrc.1997.6799. [DOI] [PubMed] [Google Scholar]
- 4.Gao X, Grignon DJ, Chbihi T, Zacharek A, Chen YQ, Sakr W, Porter AT, Crissman JD, Pontes JE, Powell IJ. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–237. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 5.Spindler SA, Sarkar FH, Sakr WA, Blackburn ML, Bull AW, La Gattuta M, Reddy RG. Production of 13-hydroxyoctadecadienoic acid (13-HODE) by prostate tumors and cell lines. Biochem Biophys Res Commun. 1997;239:775–781. doi: 10.1006/bbrc.1997.7471. [DOI] [PubMed] [Google Scholar]
- 6.Kelavkar UP, Cohen C, Kamitani H, Eling TE, Badr KF. Concordant induction of 15-lipoxygenase-1 and mutant p53 expression in human prostate adenocarcinoma: correlation with Gleason staging. Carcinogenesis. 2000;21:1777–1787. doi: 10.1093/carcin/21.10.1777. [DOI] [PubMed] [Google Scholar]
- 7.Shappell SB, Boeglin WE, Olson SJ, Kasper S, Brash AR. 15-Lipoxygenase-2 (15-LOX-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. Am J Pathol. 1999;547:235–245. doi: 10.1016/S0002-9440(10)65117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy N, Everhart A, Eling T, Glasgow W. Characterization of a 15-lipoxygenase in human breast carcinoma BT-20 cells stimulation of 13-HODE formation by TGF alpha/EGF. Biochem Biophys Res Commun. 1997;231:111–116. doi: 10.1006/bbrc.1997.6048. [DOI] [PubMed] [Google Scholar]
- 9.Bertomeu MC, Gallo S, Lauri D, Haas TA, Orr FW, Bastida E, Buchanan MR. Interleukin 1-induced cancer cell/endothelial cell adhesion in vitro and its relationship to metastasis in vivo role of vessel wall 13-HODE synthesis and integrin expression. Clin Exp Metastasis. 1993;11:243–250. doi: 10.1007/BF00121167. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan MR, Horsewood P, Brister SJ. Regulation of endothelial cell and platelet receptor-ligand binding by the 12- and 15-lipoxygenase monohydroxides: 12. 15-HETE and 13-HODE. Prostaglandins Leukot Essent Fat Acids. 1998;58:339–346. doi: 10.1016/s0952-3278(98)90069-2. [DOI] [PubMed] [Google Scholar]
- 11.Ikawa H, Kamitani H, Calvo BF, Foley JF, Eling TE. Expression of 15-lipoxygenase-1 in human colorectal cancer. Cancer Res. 1999;59:360–366. [PubMed] [Google Scholar]
- 12.Kamitani H, Geller M, Eling T. Expression of 15-lipoxygenase by human colorectal carcinoma Caco-2 cells during apoptosis and cell differentiation. J Biol Chem. 1998;273:21569–21577. doi: 10.1074/jbc.273.34.21569. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan R, Nadler J. Role of lipoxygenases in breast cancer. Front Biosci. 1998;3:E81–E88. doi: 10.2741/a369. [DOI] [PubMed] [Google Scholar]
- 14.Cesano A, Visonneau S, Scimeca JA, Kritchevsky D, Santoli D. Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res. 1998;18:1429–1434. [PubMed] [Google Scholar]
- 15.Zock PL, Katan MB. Linoleic acid intake and cancer risk a review and meta-analysis. Am J Clin Nutr. 1998;68:142–153. doi: 10.1093/ajcn/68.1.142. [DOI] [PubMed] [Google Scholar]
- 16.Taub M, Wang Y, Szczesny TM, Kleinman HK. Epidermal growth factor or transforming growth factor alpha is required for kidney tubulogenesis in Matrigel cultures in serum-free medium. Proc Natl Acad Sci USA. 1990;87:4002–4006. doi: 10.1073/pnas.87.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelavkar UP, Nixon J, Cohen C, Dillehay D, Badr Eling KF. Overexpression of 15-lipoxygenase-1 (15-LO-1) in PC-3 human prostate cancer cells causes aggressive tumorigenesis. Carcinogenesis. 2001;22:1765–1773. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- 18.Kelavkar U, Badr K. Effects of mutant p53 expression on human 15-lipoxygenase promoter activity and murine 12/15-lipoxygenase gene expression: evidence that 15-lipoxygenase is a mutator gene. Proc Natl Acad Sci USA. 1999;96:4378–4783. doi: 10.1073/pnas.96.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shureiqi I, Wojno KJ, Poore JA, Reddy RG, Moussalli MJ, Spindler SA, Greenson JK, Normolle D, Hasan AK, Lawrence TS, Brenner DE. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20:1985–1995. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- 20.Shureiqi I, Chen D, Lee JJ, Yang P, Newman RA, Lotan Brenner R, Fischer SM, Lippman SM. 15-LOX-1 a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. J Natl Cancer Inst. 2000;92:1136–1142. doi: 10.1093/jnci/92.14.1136. [DOI] [PubMed] [Google Scholar]
- 21.Hsi LC, Wilson L, Nixon J, Eling TE. 15-Lipoxygenase-1 metabolites down-regulate peroxisome proliferator-activated receptor gamma via the MAPK signaling pathway. J Biol Chem. 2001;276:34545–34552. doi: 10.1074/jbc.M100280200. [DOI] [PubMed] [Google Scholar]
- 22.Shappell SB, Gupta RA, Manning S, Whitehead R, Boeglin WE, Schneider C, Case T, Price J, Jack GS, Wheeler TM, Matusik RJ, Brash AR, Dubois RN. 15S-hydroxyeicosatetraenoic acid activates peroxisome proliferator-activated receptor gamma and inhibits proliferation in PC3 prostate carcinoma cells. Cancer Res. 2001;61:497–503. [PubMed] [Google Scholar]
- 23.Kelavkar UP, Glasgow W, Eling TE. The effect of 15-lipoxygenase-1 (15-LO-1) expression on cancer cells. Curr Urol Rep. 2002;3:207–214. doi: 10.1007/s11934-002-0066-8. [DOI] [PubMed] [Google Scholar]
- 24.Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate: alteration in PPARgamma. J Biol Chem. 2002;277:40549–40556. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Prager D, Yamasaki H, Weber MM, Gebremedhin S, Melmed S. Human insulin-like growth factor I receptor function in pituitary cells is suppressed by a dominant negative mutant. J Clin Invest. 1992;90:2117–2122. doi: 10.1172/JCI116096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster J, Prager D, Melmed S. Insulin-like growth factor-1 activation of extracellular signal-related kinase-1 and -2 in growth hormone-secreting cells. Mol Endocrinol. 1994;8:539–544. doi: 10.1210/mend.8.5.8058064. [DOI] [PubMed] [Google Scholar]
- 28.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du J, Peng T, Scheidegger KJ, Delafontaine P. Angiotensin II activation of insulin-like growth factor 1 receptor transcription is mediated by a tyrosine kinase-dependent redox-sensitive mechanism. Arterioscler Thromb Vasc Biol. 1999;9:2119–2126. doi: 10.1161/01.atv.19.9.2119. [DOI] [PubMed] [Google Scholar]
- 30.Bocan TM, Rosebury WS, Mueller SB, Kuchera S, Welch K, Daugherty A, Cornicelli JA. A specific 15-lipoxygenase inhibitor limits the progression and monocyte-macrophage enrichment of hypercholesterolemia-induced atherosclerosis in the rabbit. Atherosclerosis. 1998;136:203–216. doi: 10.1016/s0021-9150(97)00204-9. [DOI] [PubMed] [Google Scholar]
- 31.De Vries CE, van Noorden CJ. Effects of dietary fatty acid composition on tumor growth and metastasis. Anticancer Res. 1992;12:1513–1522. [PubMed] [Google Scholar]
- 32.Cesano A, Visonneau S, Scimeca JA, Kritchevsky D, Santoli D. Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res. 1998;18:1429–1434. [PubMed] [Google Scholar]
- 33.Rose DP, Connolly JM. Effects of fatty acids and eicosanoid synthesis inhibitors on the growth of two human prostate cancer cell lines. Prostate. 1991;18:243–254. doi: 10.1002/pros.2990180306. [DOI] [PubMed] [Google Scholar]
- 34.Connolly JM, Coleman M, Rose DP. Effects of dieta Prostatery fatty acids on DU145 human prostate cancer cell growth in athymic nude mice. Nutr Cancer. 1997;29:114–119. doi: 10.1080/01635589709514611. [DOI] [PubMed] [Google Scholar]
- 35.Chung LW. The role of stromal-epithelial interaction in normal and malignant growth. Cancer Surv. 1995;23:33–42. [PubMed] [Google Scholar]
- 36.Chung LW, Davies R. Prostate epithelial differentiation is dictated by its surrounding stroma. Mol Biol Rep. 1996;23:13–19. doi: 10.1007/BF00357069. [DOI] [PubMed] [Google Scholar]
- 37.Grossfeld GD, Hayward SW, Tlsty TD, Cunha GR. The role of stroma in prostatic carcinogenesis. Endocr-Relat Cancer. 1998;5:253–270. [Google Scholar]
- 38.Rao GN, Alexander RW, Runge MS. Linoleic acid and its metabolites hydroperoxyoctadecadienoic acids stimulate c-Fos c-Jun and c-Myc mRNA expression mitogen-activated protein kinase activation and growth in rat aortic smooth muscle cells. J Clin Invest. 1995;96:842–847. doi: 10.1172/JCI118130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baserga R, Resnicoff M, Dews M. The IGF-I receptor and cancer. Endocrine. 1997;1:99–102. doi: 10.1007/BF02778073. [DOI] [PubMed] [Google Scholar]
- 40.Parrizas M, Saltiel AR, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogenactivated protein kinase pathways. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 41.Resnicoff M. Antitumor effects elicited by antisense-mediated downregulation of the insulin-like growth factor I receptor. Int J Mol Med. 1998;1:883–888. doi: 10.3892/ijmm.1.5.883. [DOI] [PubMed] [Google Scholar]
- 42.Baserga R, Sell C, Porcu P, Rubini M. The role of the IGF-I receptor in the growth and transformation of mammalian cells. Cell Prolif. 1994;27:63–71. doi: 10.1111/j.1365-2184.1994.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 43.Werner H. Dysregulation of the type 1 IGF receptor as a paradigm in tumor progression. Mol Cell Endocrinol. 1998;141:1–5. doi: 10.1016/s0303-7207(98)00099-9. [DOI] [PubMed] [Google Scholar]
- 44.Fiorelli G, De Bellis A, Longo A, Giannini S, Vannelli GB, Serio M. Insulin-like growth factor-I receptors in human hyperplastic prostate tissue: characterization tissue localization and their modulation by chronic treatment with a gonadotropin-releasing hormone analog. J Clin Endocrinol Metab. 1991;72:740–746. doi: 10.1210/jcem-72-4-740. [DOI] [PubMed] [Google Scholar]
- 45.Hwa V, Tomasini-Sprenger C, Bermejo AL, Rosenfeld RG, Plymate SR. Characterization of insulin-like growth factor-binding protein-related protein-1 in prostate cells. J Clin Endocrinol Metab. 1998;83:4355–4362. doi: 10.1210/jcem.83.12.5341. [DOI] [PubMed] [Google Scholar]
- 46.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–224. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 47.Wolk A, Mantzoros CS, Andersson S-O, Bergstrom R, Signorello LB, Lagiou P. Insulin-like growth factor 1 and prostate cancer risk: a population-based case-control study. J Natl Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 48.Holly JMP, Gunnell DJ, Smith GD. Growth hormone IGF-1 and cancer: less intervention to avoid cancer? More intervention to prevent cancer? J Endocrinol. 1999;162:321–330. doi: 10.1677/joe.0.1620321. [DOI] [PubMed] [Google Scholar]
- 49.Iwamura M, Sluss PM, Casamento JB, Cockett AT. Insulinlike growth factor I action and receptor characterization in human prostate cancer cell lines. Prostate. 1993;22:243–252. doi: 10.1002/pros.2990220307. [DOI] [PubMed] [Google Scholar]
- 50.Kimura G, Kasuya J, Giannini S, Honda Y, Mohan S, Kawachi M, Akimoto M, Fujita-Yamaguchi Y. Insulin-like growth factor (IGF) system components in human prostatic cancer cell-lines LNCaP DU145 and PC-3 cells. Int J Urol. 1996;3:39–46. doi: 10.1111/j.1442-2042.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 51.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Hittmair A, Zhang J, Thurnher M, Bartsch G, Klocker H. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996;28:392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 52.Ruan W, Powell-Braxton L, Kopchick JJ, Kleinberg DL. Evidence that insulin-like growth factor I and growth hormone are required for prostate gland development. Endocrinology. 1999;140:1984–1989. doi: 10.1210/endo.140.5.6721. [DOI] [PubMed] [Google Scholar]
- 53.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltraán L, Moats S, Ramirez A, Jorcano J, Conti C. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA. 2000;97:3455–3460. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiGiovanni J, Bol DK, Wilker E, Beltran L, Carbajal S, Moats S, Ramirez A, Jorcano J, Kiguchi K. Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res. 2000;60:1561–1570. [PubMed] [Google Scholar]
- 55.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-34-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 57.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 58.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 59.Kiely PA, Sant A, O'Connor R. RACK1 is an insulin-like growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. J Biol Chem. 2002;277:22581–22589. doi: 10.1074/jbc.M201758200. [DOI] [PubMed] [Google Scholar]