Abstract
Several oncogene and tumor-suppressor gene products are known substrates for the calpain family of cysteine proteases, and calpain is required for transformation by v-src and tumor invasion. Thus, we have now addressed whether calpain is generally associated with transformation and how calpain contributes to oncogene function. Our results demonstrate that calpain activity is enhanced upon transformation induced by the v-Src, v-Jun, v-Myc, k-Ras, and v-Fos oncoproteins. Furthermore, elevated calpain activity commonly promotes focal adhesion remodelling, disruption of actin cytoskeleton, morphological transformation, and cell migration, although proteolysis of target substrates (such as focal adhesion kinase, talin, and spectrin) is differently specified by individual oncoproteins. Interestingly, v-Fos differs from other common oncoproteins in not requiring calpain activity for actin/adhesion remodelling or migration of v-Fos transformed cells. However, anchorage-independent growth of all transformed cells is sensitive to calpain inhibition. In addition, elevated calpain activity contributes to oncogene-induced apoptosis associated with transformation by v-Myc. Taken together, these studies demonstrate that calpain activity is necessary for full cellular transformation induced by common oncoproteins, but has distinct roles in oncogenic events induced by individual transforming proteins. Thus, targeting calpain activity may represent a useful general strategy for interfering with activated protooncogenes in cancer cells.
Keywords: Calpain, oncogene, focal adhesion, migration, apoptosis
Introduction
The calpains represent a highly conserved family of intracellular, nonlysosomal, calcium-dependent cysteine proteases. Calpain activity in vivo is tightly regulated by its ubiquitously expressed endogenous inhibitor, calpastatin [1–3]. The calpains cleave a broad spectrum of cellular proteins. Thus, the calpain-calpastatin proteolytic system represents a major mediator of posttranslational modification in cells that influences many aspects of cell physiology, including apoptosis, cell migration, and cell proliferation [4–8]. The mammalian calpains comprise 14 family members, of which calpains 1 and 2 are the best-characterized calpain isoforms. Calpains 1 and 2 function as heterodimeric enzymes composed of a unique large catalytic subunit (calpains 1 and 2) associated with a common small regulatory subunit (calpain 4) [9]. Several studies indicate that calpain 2 can localize to integrin-associated adhesive structures [10,11]. Studies utilizing pharmacological inhibitors and calpain knockout cells indicate that calpain plays an important role in mediating the dynamic regulation of focal adhesions required for cell motility [4,5,12,13]. Further studies demonstrating calpain cleavage of the actin regulator, RhoA, and the actin-binding proteins cortactin, spectrin, and EZRIN suggest other mechanisms whereby calpain may influence the actin cytoskeleton [14–17].
Oncogenic cell transformation is characterized by morphological changes often arising from adhesion loss and disruption of the actin cytoskeleton, as well as deregulated growth control and loss of anchorage dependence for cell proliferation. A role for calpain activity during oncogenesis was first inferred from studies indicating that calpains degrade several oncogene products such as platelet-derived growth factor receptor, c-Jun, c-Fos, c-Src, c-Mos, and epidermal growth factor receptor (EGF-R) [18–23]. These studies led to the suggestion that calpain may play a general suppressive role in malignant transformation. This antitumorigenic role is supported by studies indicating that calpain-mediated cleavage of protein kinase C (PKC), a downstream effector for tumor-promoting phorbol esters, inhibits malignant transformation [24]. Similarly, one study has suggested a role for calpain 9 (nCL-4) in the suppression of cell transformation [25].
As well as a potential antitumorigenic role, calpain also degrades several tumor-suppressor proteins such as p53, NF2, IKBα, and p107 [26–29]. Our recent work demonstrated that in response to conditional mutants of v-Src, calpain induces proteolytic cleavage of focal adhesion kinase (FAK), contributing to focal adhesion disassembly, morphological transformation, and cell motility [4]. We further demonstrated that calpain activity also contributes to cell cycle progression and anchorage-independent growth of v-Src transformed cells [30,31]. A recent study has also indicated that calpain activity acquires unique regulatory roles in cells transformed by SV40 large T antigen, promoting invasive behavior [32]. In addition, the proto-oncogene EGF-R, which is upregulated in several tumor types in association with enhanced malignancy, promotes cell migration by extracellular regulated kinase/mitogen-activated protein kinase (ERK/MAPK)-induced activation of calpain [33]. Furthermore, the Her-2/neu oncogene, a member of the EGF-R family, promotes cell survival and the transformed phenotype by activation of the transcription factor, NFkB. Her-2/neu-induced activation of NFkB is mediated by casein kinase II (CK2)-induced phosphorylation of the NFkB-binding partner and repressor IkB-α; this phosphorylation event subsequently targets IkB-α for degradation by calpain, thus leading to activation of NFkB [34]. Taken together, these studies present indirect evidence suggesting that calpain may have a general role in facilitating cell transformation and tumorigenesis.
A recent in vivo study directly implicates a role for calpain in tumor invasion, antisense-mediated suppression of calpain 2 suppressed invasion of prostate carcinoma cells [35]. Previous in vivo studies also suggest a role for calpain during the development of other tumor types. For example, increased calpain 1 mRNA expression levels in renal cell carcinoma correlate with increased malignancy [36], and calpain-mediated cleavage of the tumor-suppressor protein, NF2, is associated with the development of some schwannomas and meningiomas [27]. Also, elevated calpain activity and calpain-mediated cleavage of cyclin E occur in cells and tissues derived from breast tumors [37,38], and calpain 1 activity is significantly elevated in chronic lymphocytic leukemia (B-CLL) cells when compared with nonmalignant cells [39]. In contrast to these protumorigenic effects, the expression of calpain 9 is downregulated in gastric cancer tissues and cell lines [40]. This, together with evidence that depletion of calpain 9 by antisense RNA results in cell transformation and tumorigenesis, implies that at least one calpain isoform (calpain 9) may function as a tumor suppressor [25].
The aim of the present study was to definitively determine the role of calpain activity during oncogenic transformation. Our studies indicate that total cellular levels of calpain activity are elevated during transformation induced by many oncogenes, including v-Src, v-Jun, v-Myc, k-Ras and v-Fos, with the greatest increase observed in v-Myc transformed cells. We found that calpain-mediated proteolysis of focal adhesion proteins was a common oncogenic event, although precise cleavage events varied. We further demonstrate that calpain inhibitors suppressed remodelling of focal adhesions, and cell migration of cells transformed by v-Myc but not the v-Fos oncoprotein. In addition, inhibition of calpain activity suppressed anchorage-independent growth in cells transformed by all the oncoproteins under study. These findings indicate that calpain activity is generally required for full cellular transformation induced by a variety of common oncoproteins.
Materials and Methods
Cell Culture
Primary chicken embryo fibroblasts (CEFs) were subcultured as previously described [41]. Low-density cultures were tranysfected with replication-competent avian retroviral SFCV-v-src construct, encoding the temperature-sensitive ts LA29 v-Src mutant [42]. Transfected CEFs were cultured at the permissive temperature of 35°C until cells were uniformly infected and expressed the ts v-Src protein. For analysis of v-Src-induced transformation, cells were cultured at the restrictive temperature (41°C) and then examined following shift to the permissive temperature (35°C). ASV17 (v-Jun) and MC29 (v-Myc) transformed CEFs were provided by D. Gillespie. 208F fibroblasts are a subclone of the Rat-1 fibroblast cell line. v-Fos transformed cells were generated by infection of 208F fibroblasts with v-Fos FBR-MuSv and provided by B. Ozanne. Rat-1 fibroblasts were transformed by expression of constructs of k-Ras that contained an activating point mutation at codon 12 provided by W. Kolch. v12K-ras and v-FosFBR transformed fibroblasts and parental controls were cultured in 1 x DMEM supplemented with 10% fetal calf serum (FCS) and 2 mM L-glutamine.
Antibodies and Reagents
ERK inhibition studies were performed using the MEK inhibitor, UO126 (Promega, Madison, WI). CEFs expressing ts v-Src were preincubated with UO126 (50 µM) for 1 hour prior to shift to 35°C and subsequently incubated at 35°C in the presence of UO126 (50 µM). All other cells were incubated with UO126 (50 µM) for the indicated time period. Calpain inhibitor studies were performed using calpain inhibitor 1 (N-acetyl-Leu-Leu-norleucinal, ALLN, 10 µM; Calbiochem-Novabiochem Corp., San Diego, CA) and calpain inhibitor 2 (ALLM, 10 µM; Calbiochem-Novabiochem Corp.). Caspase inhibitor studies were carried out with caspase inhibitor 1 (ZVAD-FMK, 100 µM; Calbiochem-Novabiochem Corp.). Proteasome inhibition was performed with the specific inhibitor, lactacystin (10 µM). Lysosomal cathepsins were inhibited by ammonium chloride (NH4Cl, 10 mM). Lysosomal and nonlysosomal cathepsin B activities were inhibited by the cell-permeable cathepsin B inhibitor, CA-074-Me (10 µM; Calbiochem-Novabiochem Corp.). Antibodies for Western blot detection and immunocytochemistry included FAK and paxillin (BD Pharmingen, San Diego, CA), talin (Sigma, Poole, England), α-II spectrin (Santa Cruz, Santa Cruz, CA), and anti-α-tubulin (Sigma). Anti-mouse and anti-rabbit peroxidase-conjugated secondary antibodies were purchased from New England Biolabs, Inc. (Beverly, MA). Anti-goat peroxidase antibodies were from Sigma.
Calpain Activity Assays
Analysis of calpain activity in total cell lysates was performed using a calpain activity assay kit from BIOvision, Inc., (Mountain View, CA) according to the manufacturer's instructions. The calpain activity kit contains a fluorogenic peptide calpain substrate (Ac-LLY-AFC), lysis buffer, and reaction buffer. Briefly, cells are lysed in lysis buffer for 20 minutes at 4°C. Clarified cell lysates are then incubated with substrate and reaction buffer for 1 hour at 37°C in the dark. Upon cleavage of substrate, the fluorogenic portion (AFC) releases yellow-green fluorescence at a wavelength of 505 nm following excitation at 400 nm. Fluorescence emission was measured by a standard fluorimeter. For each sample, control reactions were performed in the presence of 5 µg of recombinant calpastatin (Calbiochem-Novabiochem Corp.) to monitor any calpain-independent proteolysis of fluorogenic peptide. Values from control reactions were subtracted from total activity values to specifically determine calpain activity for each sample. Results are expressed as relative fluorescence units per microgram of protein lysate. Analysis of calpain activity in live cells was performed by incubation with the cell-permeable fluorogenic calpain substrate, BOC-LM-CMAC (Molecular Probes, Inc., Eugene, OR). BOC-LM-CMAC contains a fluorogenic subunit and a quencher subunit. Upon cleavage by calpain, the fluorogenic portion separates from the quencher, resulting in an increase in fluorescence emission. Cells were incubated with BOC-LM-CMAC (50 µM) for 20 minutes. Calpain-mediated cleavage of BOC-LM-CMAC resulting in increased fluorescence was visualized by confocal microscopy (magnification, x 400).
Protein Immunoblotting
Cells were washed twice with phosphate-buffered saline (PBS) and lysed in lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 0.5% NP40, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM NaF, 10 mM β-glycerophosphate, 10 mM Na4P2O7, 100 µM NaVO4, with protease inhibitors, 1 mM PMSF, 10 µg/ml leupeptin, and 10 µg/ml aprotinin). Lysates were clarified by high-speed centrifugation at 4°C, supplemented with sodium dodecyl sulfate (SDS) sample buffer and separated by 10% SDS polyacrylamide gel electrophoresis (PAGE), and immunoblotted with specific antibodies.
Immunocytochemistry
Cells were cultured on permanox plastic chamber slides (Nalge Nunc International, Naperville, IL). Cells were fixed in 3.7% formaldehyde for 10 minutes at room temperature, permeabilized in 0.5% NP40 in PBS for 10 minutes at room temperature, and washed serially in PBS, 0.15 M glycine/PBS + 0.02% NaN3, and PBS. Cells were blocked in 10% FCS/PBS prior to 1-hour incubation at room temperature with the primary antibody, anti-paxillin (BD Pharmingen). Primary antibody incubation was followed by several washes in PBS and subsequent incubation with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Jackson Laboratories, West Grove, PA). Actin cytoskeleton was monitored by staining with FITC-conjugated phalloidin (Sigma). Immunostaining of cells was analyzed by confocal microscopy (magnification, x 1000).
Wound Healing Migration Assay
A total of 2 x 105 CEFs, nontransformed or expressing ts v-Src, v-Jun, or v-Myc, was cultured at 41°C in 60-mm dishes until 70% to 80% confluent. The cell monolayers were wounded by scoring with a sterile micropipette tip and cultures were incubated at 41°C (or 35°C in the case of CEF expressing ts v-Src) for a further 12 hours in the absence or presence of a panel of protease inhibitors at concentrations described above. A total of 2 x 105 v12k-Ras and v-Fos transformed cells and nontransformed rat-1 and 208F parental cells was cultured at 37°C in 60-mm dishes until 70% to 80% confluent prior to scoring with a sterile micropipette tip and subsequent culture at 37°C in the absence or presence of protease inhibitors. Cells were fixed and stained with Coomassie blue and analyzed by microscopy (magnification, x 50). For quantification of wound assays, three defined areas along a wound were monitored 4, 8, and 12 hours following wound generation. The width of the denuded wound area remaining at these time points was measured and mean values of the three wound areas for each culture condition were calculated as percentage of wound closure.
Anchorage-Independent Growth Assay
Briefly, 60-mm bacterial culture dishes were coated with 0.5% base agar supplemented with normal CEF or rat fibroblast culture medium. Transformed cells were preincubated with or without calpain inhibitors ALLN or ALLM (10µM) for 1 hour in suspension prior to the addition of an equal volume of top layer agar consisting of 0.6% agar, double-concentrated CEF or rat growth medium, and ALLN or ALLM, where required. Following several days in culture, top layer agar was overlaid with base agar supplemented with culture media + ALLN or ALLM, where required. Anchorage-independent growth was quantified as the number of cell colonies formed per high-power field.
Apoptosis Detection
Cell apoptosis of v-Myc transformed cultures was monitored using the Annexin V FITC apoptosis detection kit I (BD Pharmingen) as per manufacturer's instructions. Briefly, v-Myc transformed cells were cultured under low serum conditions either untreated or incubated with calpain inhibitors (ALLN and ALLM), proteasome inhibitor (lactacystin), cathepsin inhibitors (ammonium chloride and CA-074-Me), or caspase inhibitor (ZVAD) for 18 hours at concentrations indicated above. Cells were washed twice with cold PBS and resuspended in binding buffer. Cells were then incubated with FITC-conjugated Annexin V and propidium iodide prior to analysis by flow cytometry.
Results
Total Cellular Calpain Activity Is Elevated during Oncogenic Transformation
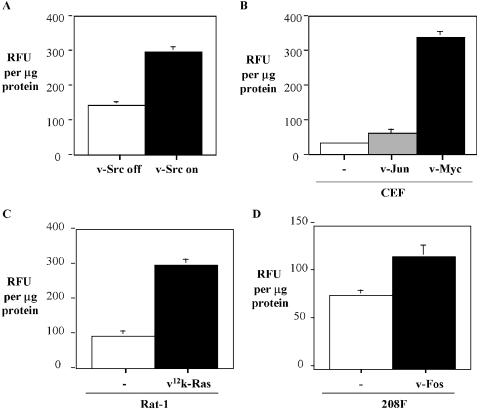
Recent studies demonstrate that calpain acquires novel regulatory roles in the presence of SV40 large T antigen [32] and contributes to transformation mediated by the Her-2/neu oncogene [34]. Our studies have shown that calpainmediated cleavage of FAK and, consequently, focal adhesion turnover contribute to v-Src-induced morphological transformation and anchorage-independent growth [4,30]. To determine whether calpain protease is generally modulated during oncogenic transformation, we have employed a calpain activity assay that utilizes a synthetic fluorogenic substrate to monitor total levels of calpain activity in cell extracts. To monitor and examine the role of calpain activity in v-Src transformed cells, we have used CEFs that express a conditional temperature-sensitive v-Src mutant (ts v-Src). When ts v-Src CEFs are cultured at the restrictive temperature (41°C), v-Src is inactive and the cells are not transformed; upon switch of ts v-Src CEFs to the permissive temperature (35°C), v-Src is activated and the cells undergo transformation [42]. We consistently found that total levels of calpain activity in CEFs expressing ts v-Src are increased during v-Src transformation (Figure 1A). On average, calpain activity is 2.2-fold greater in v-Src transformed cells when compared to their nontransformed counterparts. In addition, we monitored calpain activity in CEFs expressing constitutively active v-Jun or v-Myc compared to normal primary CEFs. On average, calpain activity is increased 1.8-fold in v-Jun transformed CEFs and around 11-fold in v-Myc transformed CEFs (Figure 1B). We also monitored calpain activity in Rat-1 fibroblasts transformed with the activating valine 12 k-Ras mutant (v12k-Ras) (Figure 1C) and v-Fos (FBR) transformed 208F rat fibroblasts (Figure 1D), and compared values with parental nontransformed counterparts. Calpain activity was elevated 3.2-fold and 1.5-fold, respectively, in v12k-Ras (Figure 1C) and v-Fos (Figure 1D) transformed cells. These data demonstrate for the first time that the total cellular levels of calpain proteolytic activity are modulated in cells transformed by, v-src, v-jun, v-myc, k-ras, and v-fos oncogenes. In comparison to v-Myc transformed cells, total levels of calpain activity in other transformed cells are more modestly increased. However, it is possible that calpain activity localized at specific subcellular sites is elevated to a greater extent than can be detected in total cell lysates. The calpain activity assay used in this study does not distinguish between different calpain isoforms. Thus, the specific calpain isoforms responsible for increased calpain activity during oncogenic transformation remain to be determined.
Figure 1.
Total cellular calpain activity is elevated during oncogenic transformation. Total cellular levels of calpain activity in cell extracts were determined by monitoring fluorescence emission induced by cleavage of a specific fluorescent peptide substrate. Calpain activity levels were measured in (A). CEFs expressing ts v-Src following culture at the restrictive temperature (v-Src off) and 24 hours at the permissive temperature (v-Src on) for v-Src activation. (B) Nontransformed (-), and v-Jun and v-Myc transformed CEFs. (C) Nontransformed (-) and v12k-Ras transformed Rat-1 fibroblasts. (D) Nontransformed (-) and v-Fos transformed 208F fibroblasts. Calpain activity was quantified by fluorimeter and results expressed as relative fluorescence units (RFU) per microgram of cell lysate. Data represent the average values from three separate experiments.
Oncogene-Induced Calpain Activation Is Not Exclusively Dependent on Upstream Regulation by ERK/MAPK
Activation of the ERK/MAPK signalling pathway occurs as a consequence of v-Src and k-Ras activation [43,44]. Previous studies have demonstrated that EGF-R-induced activation of calpain is dependent upon ERK/MAPK activity [33,45]. To determine whether oncogene-induced activation of calpain is also mediated by the ERK/MAPK pathway, we have monitored levels of calpain activity in transformed cells following treatment with the MEK inhibitor, UO126. UO126 treatment effectively suppressed p42 and p44 ERK/MAPK activity in all transformed cells as determined by immunoblotting with an antibody that specifically recognizes the active phosphorylated forms of p44 and p42 ERK (results not shown). Inhibition of MEK suppressed both basal levels of calpain activity in nontransformed cells and elevated calpain activity in transformed cells. Our data indicate that UO126 reduces calpain activity levels in v-Src, v-Jun, and v-Fos transformed cells to levels found in their nontransformed counterparts (Figure 2, A, B, and E). Thus, in response to these oncogenes, ERK/MAPK plays an important role in calpain activation. However, the MEK inhibitor only partially suppressed calpain activity in v-Myc and v12k-Ras transformed cells, indicating that a substantial proportion of calpain activation in response to these oncogenes appears to be independent of ERK/MAPK activity (Figure 2, C and D). This result is surprising as the major effector pathway activated in k-Ras transformed cells is ERK/MAPK, which, as mentioned, is now recognized as a major calpain activator [33,45].
Figure 2.
Oncogene-induced calpain activation is not exclusively dependent on upstream regulation by ERK/MAPK. The influence treatment with the MEK 1 inhibitor, UO126 (50 µM, 6 hours), upon oncogene-induced calpain activation was monitored in (A). CEFs expressing ts v-Src following culture at the restrictive temperature (v-Src off) and 6 hours at the permissive temperature (v-Src on) for v-Src activation. (B) Nontransformed (-) and v-Jun transformed CEFs. (C) Nontransformed (-) and v-Myc transformed CEFs. (D) Nontransformed (-) and v12k-Ras transformed Rat-1 fibroblasts. (E) Nontransformed (-) and v-Fos transformed 208F fibroblasts. Calpain activity was monitored by incubating cell lysates with fluorogenic calpain substrate and quantified by fluorimeter. Results are expressed as relative fluorescence units (RFU) per microgram of cell lysate. Data represent the average values from three separate experiments.
Our previous studies demonstrated that in response to v-Src activation, calpain 2 protein levels are upregulated in parallel with calpain-mediated degradation of calpastatin [30]. These results indicated that in v-Src transformed cells, a positive feedback loop mechanism operates, whereby calpain degrades its own inhibitor, calpastatin. In contrast to v-Src transformed cells, calpain 2 protein levels are not significantly elevated in other transformed cells when compared with normal counterparts, and no evidence of calpainmediated cleavage of calpastatin can be detected in v-Jun, v-Myc, v12k-Ras, or v-Fos transformed cells (results not shown). These data indicate that in contrast to v-Src transformed cells, elevated calpain activity in v-Jun, v-Myc, v12k-Ras, and v-Fos cells is not the result of calpain-mediated degradation of calpastatin, demonstrating that although calpain activity is enhanced by all the oncoproteins, mechanisms of activation are distinct and may be oncogene-specific.
Calpain Activity in Live Cells Is Repressed by Treatment with Calpain Inhibitors but Not Other Protease Inhibitors
To begin to understand the functional significance of elevated calpain activity in different transformed cells, we have applied the use of a panel of protease inhibitors. ALLN and ALLM both selectively inhibit calpain protease activity [46–48]. ALLN has also previously been demonstrated to inhibit the proteolytic activity of cathepsins B and L and the proteasome, whereas ALLM can also inhibit cathepsin B and L activity. To ensure that phenotypic effects resulting from treatment with ALLN and ALLM are specifically due to calpain inhibition, we have also treated cells with the specific proteasome inhibitor, lactacystin [49]; an inhibitor of all lysosomal cathepsins, ammonium chloride [50,51]; and a cell-permeable inhibitor of cathepsin, B (CA-074-Me) [52]. To demonstrate that only the calpain inhibitors, but not inhibitors of the other proteases, suppress calpain activity, we have monitored calpain activity in live cells following treatment with the panel of protease inhibitors. Cells were pretreated for 3 hours with the calpain inhibitors, ALLN and ALLM (10 µM); the proteasome inhibitor, Lactacystin (Lacta, 10 µM); the cathepsin inhibitors, ammonium chloride (NH4Cl, 10 mM) and CA-074-Me (10 µM); and the broad caspase inhibitor, ZVAD (100 µM), prior to loading with the cell-permeable fluorogenic calpain substrate, BOC-LM-CMAC. Upon cleavage by calpain inside the cell, BOC-LM-CMAC emits fluorescence. Fluorescence intensity of cells relating to calpain activity can be visualized by confocal microscopy. Our results demonstrated that only the calpain inhibitors, ALLN and ALLM, but not the other protease inhibitors suppressed calpain activity in nontransformed and v-Myc transformed CEFs and also nontransformed 208F and v-Fos transformed 208F cells (Figure 3). Similar results were also obtained for v-Src and v-Jun transformed CEFs and nontransformed and v12k-Ras transformed Rat-1 cells (results not shown).
Figure 3.
Calpain activity in transformed cells is repressed by treatment with calpain inhibitors but not other protease inhibitors. Nontransformed and v-Myc transformed CEFs and nontransformed and v-Fos transformed 208F rat fibroblasts were preincubated with calpain inhibitors (ALLN and ALLM), proteasome inhibitor lactacystin (Lacta.), lysosomal cathepsin inhibitor (NH4Cl), cathepsin B inhibitor (CA-074-Me), and caspase inhibitor (ZVAD). Cells were preincubated with protease inhibitors for 3 hours prior to incubation with the cell-permeable calpain substrate, BOC-LM-CMAC. Fluorescence intensity corresponding to calpain activity was visualized by confocal microscopy (magnification, x400).
These results demonstrate that the calpain inhibitors, ALLN and ALLM, effectively impair calpain activity in all nontransformed and transformed cells, whereas inhibition of the proteasomes, cathepsins, or caspases has no significant effect on calpain activity. The BOC assay also demonstrates a visible increase in fluorescence intensity relating to calpain activity in v-Myc transformed cells compared with nontransformed CEFs (Figure 3). This result confirms our previous observation demonstrating a significant upregulation of calpain activity in cell extracts from v-Myc transformed cells (Figure 1B).
Calpain-Mediated Regulation of Focal Adhesions and the Actin Cytoskeleton during Oncogene-Induced Cell Transformation
Our previous studies demonstrate that calpain activity promotes focal adhesion turnover and loss of actin stress fibers in v-Src transformed cells, thereby contributing to morphological transformation and migration [4]. To determine whether oncogene-induced calpain activity also modulates cell structural changes (i.e., morphological transformation) induced by other oncoproteins, we examined cell morphology, focal adhesion, and actin stress fibers in v-Jun, v-Myc, v12k-Ras, and v-Fos transformed cells with and without treatment with the calpain inhibitor, ALLN. The most striking effect with ALLN treatment was observed in v-Myc transformed cells where ALLN promoted cell attachment and spreading (Figure 4A). ALLN treatment also suppresses the high levels of cell detachment of v-Myc transformed cells, thus v-Myc transformed cell cultures treated with ALLN shown in Figure 4 appear more dense. In contrast, ALLN treatment did not revert the morphology of v-Fos transformed cells (Figure 4B). ALLN treatment reverted the v-Myc transformed cells from their small round phenotype with punctate focal adhesions to a more spread morphology with significant enlargement of focal adhesions (Figure 5A) more similar in appearance to that of normal nontransformed CEFs (not shown). ALLN treatment also enhanced the appearance of actin stress fibers in v-Myc transformed cells (Figure 5A). ALLN treatment had no influence on the loss of focal adhesion structures and disruption of the actin cytoskeleton in v-Fos transformed cells (Figure 5B).
Figure 4.
Calpain inhibitors repress morphological transformation of cells transformed by v-Myc but not v-Fos. Phase contrast images (magnification, x200) of (A) nontransformed CEFs and v-Myc transformed CEFs treated with calpain inhibitor (ALLN) or proteasome inhibitor (lactacystin). (B) Phase contrast images of nontransformed 208F rat fibroblast and v-Fos transformed 208F cells treated with calpain inhibitor (ALLN) or proteasome inhibitor (lactacystin).
Figure 5.
Calpain-mediated regulation of focal adhesions and the actin cytoskeleton during transformation induced by v-Myc and v-Fos. (A) v-Myc transformed CEFs or (B) v-Fos transformed rat fibroblasts were treated with the calpain inhibitor (ALLN, 10 µM) for 18 hours. Focal adhesion structures were analyzed by immunostaining with anti-paxillin antibody. The actin cytoskeleton was monitored by incubation with FITC-labeled phalloidin. Anti-paxillin- and phalloidin-stained cells were analyzed by confocal microscopy. Scale bar = 25 µm.
Treatment with the proteasome inhibitor, lacatacystin (Figure 4), or the cathepsin inhibitors, ammonium chloride and CA-074-Me (results not shown), did not revert the phenotype of any of the transformed cells, implying that the ability of ALLN treatment to suppress v-Myc-induced cell rounding, focal adhesion remodelling, and loss of actin stress fibers is mediated by the inhibition of calpain activity. Although focal adhesion loss and morphological transformation of v-Jun and v12k-Ras transformed cells are more subtle than that observed for v-Myc or v-Fos transformed cells, calpain inhibitors also promoted enhanced staining of paxillin at focal adhesions in v12k-Ras and v-Jun transformed fibroblasts (results not shown), suggesting that calpain inhibitors suppress the morphological effect of these oncoproteins, too.
Oncogene-Induced Calpain-Mediated Cleavage of FAK, Talin, and Spectrin
Our data above (Figures 4 and 5) suggest that elevated calpain activity plays a significant role in focal adhesion remodelling and morphological transformation induced by a variety of specific oncoproteins. To determine whether elevated calpain activity in transformed cells resulted in proteolytic cleavage of calpain substrates known to regulate focal adhesions and cell morphology, we examined the protein levels of three well-characterized calpain substrates, FAK, talin, and spectrin. FAK and talin are two components of focal adhesion complexes that influence the integrity and turnover of focal adhesions [53,54]. In addition, FAK plays a role in the transmission of signals by focal adhesions to downstream signalling pathways [55]. Spectrin is an actin-binding protein that plays a role in maintaining organization of the plasma membrane-proximal actin cytoskeleton [56]. Previous studies have shown that FAK is proteolytically cleaved by calpain, giving rise to a 95-kDa N-terminal fragment [57,58]. As previously reported [4], FAK is proteolytically cleaved extensively in v-Src transformed cells (Figure 6A). In v-Myc transformed cells, an anti-N-terminal FAK antibody detects a clear 95-kDa fragment of FAK; however, no significant loss in levels of native FAK is observed (Figure 6A), which suggests that proteolysis of FAK may be compensated for by increased resynthesis so that steady-state levels are not impaired. Appearance of the 95-kDa FAK fragment is also slightly upregulated in v12k-Ras transformed cells, but again no significant loss in levels of native FAK protein was observed. In contrast, proteolytic cleavage of FAK appears to be decreased below basal levels in v-Jun transformed cells and is not clearly observed in v-Fos transformed cells. Previous studies have shown that talin is proteolytically cleaved by calpain, giving rise to a 190-kDa N-terminal fragment [59], and spectrin is cleaved by calpain, giving rise to a 150-kDa fragment [60]. We demonstrate the appearance of the 190-kDa talin cleavage product and the 150-kDa spectrin cleavage product in v-Myc and v12k-Ras transformed cells. No significant cleavage of talin or spectrin could be observed in either v-Src, v-Jun, or v-Fos transformed cells (Figure 6A). These studies indicate that a different spectrum of calpain substrates is cleaved at focal adhesions during transformation by distinct oncogenes. In keeping with our data demonstrating a lack of morphological reversion upon treatment of v-Fos cells with calpain inhibitors, no significant cleavage of any of the above calpain substrates can be detected in v-Fos transformed fibroblasts.
Figure 6.
Oncogene-induced calpain-mediated cleavage of FAK, talin, and spectrin. (A) Total cell lysates were prepared from v-Src, v-Jun, v-Myc, v12k-Ras, and v-Fos transformed cells and their nontransformed counterparts. Lysates were separated by SDS-PAGE and immunoblotted with antibodies against the calpain substrates, FAK, talin, and α II spectrin. α-Tubulin immunoblots were performed as a control for protein loading. Molecular weight sizes of native protein and proteolytic fragments are indicated. (B) Total cell lysates were prepared from v-Myc transformed cells that were untreated (control, C) or treated for 18 hours with a calpain inhibitor (+ALLN), a proteasome inhibitor lactacystin (+Lacta), and a caspase inhibitor (+ZVAD). Lysates were separated by SDS-PAGE and immunoblotted with antibodies against FAK, talin, α II spectrin, and α-tubulin.
Consistent with our data (Figure 1) demonstrating that v-Myc transformed cells had the highest levels of calpain activity, proteolytic cleavage of all three calpain substrates examined (FAK, talin, and spectrin) was most clearly and consistently observed in v-Myc transformed cells (Figure 6A). To determine whether the proteolytic cleavage of FAK, talin, and spectrin in v-Myc transformed cells is specifically due to calpain activity, v-Myc transformed cells were treated with inhibitors against the calpains (ALLN), a specific proteasome inhibitor (lactacystin), and the broad caspase inhibitor (ZVAD) (Figure 6B). Proteolytic cleavage of FAK in v-Myc transformed cells was inhibited by both the calpain and the caspase inhibitors (Figure 6B). In contrast, talin and spectrin cleavage is only significantly blocked by the calpain inhibitor (Figure 6B). Previous studies indicate that FAK can be proteolytically cleaved by caspases during apoptotic cell death, resulting in proteolytic fragments similar in size to those generated by calpain cleavage [61,62]. Both calpain and caspase inhibitors suppressed FAK cleavage (Figure 6B), suggesting that calpains and caspases both cleave FAK in v-Myc transformed cells; alternatively, calpains may promote FAK cleavage indirectly by promoting apoptosis and caspase activation. However, our data argue against the second possibility as the caspase inhibitor, ZVAD, only partially suppresses FAK cleavage and is consistently less effective than the calpain inhibitor, ALLN (Figure 6B), suggesting that in the absence of caspase activity, calpain can still cleave FAK in v-Myc transformed cells. Consistent with the fact that talin has not been identified as a caspase substrate and caspase-mediated cleavage of α II spectrin results in the generation of a distinct 120-kDa fragment [60], calpain inhibitors, but not caspase inhibitors, suppressed talin and α II spectrin cleavage in v-Myc transformed cells. These results indicate that talin and spectrin cleavage in v-Myc transformed cells is mediated by calpain activity with no contribution from caspases.
The Motility of v-Src, v-Myc, v-Jun , v12k-Ras, but Not v-Fos Transformed Cells, Is Dependent on Calpain Activity
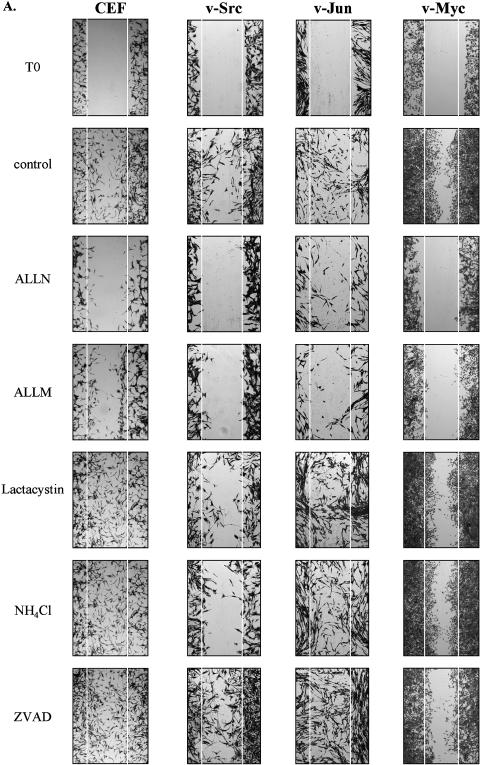
To determine whether modulated activities of calpain influenced the motility of cells transformed by different oncogenes, we performed wound repair assays in the presence of selective protease inhibitors and compared with untransformed control cells. The inhibitors used were calpains inhibitors (ALLN and ALLM), the specific proteasome inhibitor (lactacystin), an inhibitor of lysosomal cathepsins (ammonium chloride, NH4Cl), the cathepsin B inhibitor (CA-074-Me; results not shown), and the caspase inhibitor, ZVAD, which do not influence calpain activity in these cells (Figure 3). Only the calpain inhibitors, ALLN and ALLM, suppressed the motility of v-Src, v-Myc, and v-Jun transformed cells into the denuded area of the monolayer (Figure 7A). The calpain inhibitors also suppressed the motility of nontransformed CEFs into the wounded area (Figure 7A). These results indicate that the selective calpain inhibitors, ALLN and ALLM, impair cell migration and that calpain activity is required for the migration of both nontransformed and v-Src, v-Myc, and v-Jun (to a smaller extent) transformed cells. The calpain inhibitors also suppressed the movement of v12k-Ras transformed cells and their nontransformed Rat-1 counterparts into the wound (Figure 7B). In contrast to all other nontransformed and transformed cell types examined, migration of v-Fos transformed cells into the denuded area was not retarded by treatment with calpain inhibitors (Figure 7B). The migration of v-Myc and v-Fos transformed cells into the denuded area following treatment with the calpain inhibitor, ALLN, was quantified at sequential time points by microscopy of three defined areas of the wound (1, 2, and 3) (Figure 8, A–C). Cells suspected of floating into wound area were discounted; thus, only migration of cells from the wound periphery was quantified. These results further confirm that in contrast to v-Myc transformed cells, the motility of v-Fos transformed cells is not suppressed by the calpain inhibitor, ALLN (Figure 8C). This indicates that v-Fos transformed cells have acquired mechanisms of cell locomotion that are independent of calpain proteolytic activity and therefore distinct from their nontransformed counterparts and cells transformed by other oncoproteins.
Figure 7.
The motility of v-Src, v-Myc, v-Jun, v12k-Ras, but not v-Fos transformed cells, is dependent on calpain activity. (A) A wound was generated in a subconfluent monolayer of v-Src, v-Jun, and v-Myc transformed CEFs and nontransformed CEFs. Wound size was initially recorded at time 0 (T0), and cells were then further incubated for 12 hours in the absence (control) or presence of the calpain inhibitors, ALLN and ALLM; the proteasome inhibitor, lactacystin; the lysosomal cathepsin inhibitor, ammonium chloride (NH4Cl); and the caspase inhibitor, ZVAD. (B) Denuded wounds were also generated in subconfluent monolayers of v12k-Ras and nontransformed Rat-1 fibroblasts and v-Fos and nontransformed 208F fibroblasts. As above, wound size was recorded at time 0 and 12 hours following culture in the absence or presence of the panel of protease inhibitors described in (A). White lines represent the initial denuded area.
Figure 8.
Quantification of v-Myc and v-Fos transformed cell motility in presence of the calpain inhibitor, ALLN. A denuded wound area was generated in a subconfluent monolayer of (A) v-Myc transformed cells or (B) v-Fos transformed cells. Three defined areas (1, 2, and 3) along the wound were monitored 4, 8, and 12 hours following wound generation in cultures untreated or treated with ALLN (10 µM). White lines represent the remaining denuded area. (C) Data from (A) and (B) are expressed as the percentage of wound closure at 4, 8, and 12 hours following wound generation and represent mean values from three separate areas of wound in each sample.
Calpain Inhibitors Repress Anchorage-Independent Growth of v-Src, v-Jun, v-Myc, v12k-Ras, and v-Fos Transformed Cells
A hallmark of all transformed cells is their ability to grow in an anchorage-independent manner. Our previous studies indicate that calpain activity is required for anchorage-independent growth of v-Src transformed cells [30]. In contrast, previous studies on NIH 3T3 cells demonstrated that the calpain inhibitor, ALLN, promoted anchorage-independent growth [63]. Our data in this present study demonstrate that treatment with ALLN or ALLM significantly suppressed anchorage-independent growth of v-Src, v-Jun, and v-Myc transformed CEFs and also v12k-Ras transformed Rat-1 fibroblasts (Figure 9, A and B). ALLN and ALLM also slightly reduced anchorage-independent growth of v-Fos transformed rat fibroblasts. These data imply that calpain activity is required for optimal oncogene-induced anchorage-independent growth of the transformed cells examined in this study.
Figure 9.
Calpain inhibitors repress anchorage-independent growth of v-Src, v-Jun, v-Myc, v12k-Ras, and v-Fos transformed cells. (A) v-Src, v-Jun, v-Myc, v12k-Ras, and v-Fos transformed cells were cultured in soft agar in the absence (control) or presence of the calpain inhibitors (ALLN) or (ALLM). Phase pictures record colony formation 14 days following cell seeding. (B) Colony formation was quantified by counting the number of colonies per high-power field (magnification, x25).
Calpain Activity Contributes to v-Myc-Induced Apoptosis
One hallmark of Myc-induced transformation is high levels of apoptotic cell death [64]. Calpain activity has previously been shown to play a proapoptotic role by activation of caspases 3 and 12 [7,65] and cleavage of proapoptotic Bax and Bid proteins [66,67]. Conversely, calpain-mediated cleavage of p53 [68] and caspases 7 and 9 [69] suppresses apoptosis. Our data (Figures 1 and 6) suggest that elevated calpain activity in v-Myc transformed cells may cooperate with caspases to promote proteolytic cleavage of FAK. To demonstrate whether elevated calpain activity also contributes to v-Myc induced cell apoptosis, we monitored apoptosis in serum-starved v-Myc transformed cells treated with two separate calpain inhibitors, ALLN and ALLM, as well as proteasome, cathepsin, and caspase inhibitors. Apoptotic cell death was measured using an Annexin V FITC detection kit. Both ALLN and ALLM treatment reduced the levels of apoptotic cell death in v-Myc transformed cell cultures (Figure 10). As expected, the caspase inhibitor, ZVAD, also suppressed apoptosis, whereas the proteasome inhibitor, lactacystin, only had a mild suppressive effect on v-Myc induced apoptosis and the cathepsin inhibitors, ammonium chloride and CA-074-Me, had no effect on v-Myc-induced apoptosis (Figure 10).
Figure 10.
Calpain activity contributes to v-Myc-induced apoptosis. v-Myc transformed cells were incubated in serum-depleted media for 24 hours without treatment, or treatment with the calpain inhibitors, ALLN and ALLM; the proteasome inhibitor, lactacystin; the lysosomal cathepsin inhibitor, NH4Cl; the cathepsin B inhibitor, CA-074-Me; or the caspase inhibitor, ZVAD. Cells were washed and dual-stained with FITC-conjugated Annexin V and propidium iodide to detect apoptotic cells. (A) The proportion of viable (M1) and apoptotic (M2) cells in cultured populations was analyzed by flow cytometry. (B) Bar graph representing the percentage of apoptotic cells in v-Myc transformed cell cultures following treatment with various protease inhibitors.
Discussion
Previous studies present a conflicting role for the calpain family of proteolytic enzymes during oncogenic cell transformation. Antisense-mediated suppression of calpain 9 (formally nCL-4) promoted anchorage-independent growth of NIH 3T3 cells [25]. In addition, treatment with calpain inhibitors and overexpression of calpastatin also promoted anchorage-independent growth of NIH 3T3 cells [24,63]. These studies suggested that calpain activity suppresses cellular transformation. Curiously, in the second study by Hiwasa et al. [24], overexpression of calpain 4, the small regulatory domain required for both calpain 1 and calpain 2 activity [12], also promoted anchorage-independent growth of NIH 3T3, suggesting that calpain may also promote cellular transformation. Using a combination of approaches including calpain inhibitors, calpastatin overexpression, and calpain 4 KO cells, we previously demonstrated that calpain activity promotes morphological transformation and anchorage-independent growth of v-Src transformed fibroblasts [30]. Other studies suggest that calpain-mediated cleavage of IkBα also contributes to anchorage-independent growth of breast cancer cells [34] and, in SV40-transformed cells, calpain acquires new roles that promote invasion [32].
From the above studies, the precise role of calpain during cell transformation remains a controversial issue. The aim of this study was to address in some detail the requirement for calpain for different aspects of the transformed phenotype and to examine the regulation and role of calpain activity during cellular transformation induced by a number of well-characterized oncogenes that have previously been demonstrated to play important roles in cancer development. Using a synthetic fluorogenic substrate specific for calpain, we monitored total cellular levels of calpain activity in transformed cells. Our studies demonstrate that calpain activity is significantly upregulated in cells transformed by v-Src, v-Jun, v-Myc, v12k-Ras, and v-Fos when compared with matched nontransformed counterparts. The greatest increase in calpain activity was observed in v-Myc transformed cells, which was approximately 11-fold higher than levels found in their nontransformed counterparts. Although ERK/MAPK signalling is important for EGF-R-induced activation of calpain [33,45], our results indicate that in response to the specific oncogenes v12k-Ras and v-Myc, activation of calpain can occur independently of ERK/MAPK signalling. In contrast, elevated calpain activity in v-Src, v-Jun, and v-Fos transformed cells is strongly dependent on ERK/MAPK signalling. Thus, the influence that ERK/MAPK signalling has on regulating oncogene-induced calpain activation varies between different oncogenes. These results indicate that different modes of regulating calpain activity may operate in cells transformed by distinct oncogenes.
We have previously reported that increased protein synthesis of calpain 2 and calpain-mediated cleavage of calpastatin may promote calpain activity in v-Src transformed cells. In addition, caspase-mediated cleavage of calpastatin can promote calpain activity during cell apoptosis [70]. The calpain 2 gene contains an AP-1-binding motif, suggesting that calpain 2 may be transcriptionally regulated by Fos and Jun oncoproteins [71]. Our data indicate that calpain 2 protein levels are only slightly increased in v-Jun and v-Myc transformed cells but are not altered in v12k-Ras and v-Fos transformed cells (results not shown). Calpastatin protein levels are also not modulated in v-Jun and v-Myc transformed chick fibroblasts, and 110- and 70-kDa calpastatin isoforms are not subject to cleavage in v12k-Ras and v-Fos transformed cells (results not shown). These data indicate that in contrast to v-Src transformed cells, calpain-mediated degradation of calpastatin does not account for elevated calpain activity in cells transformed by other oncoproteins. Our data demonstrating that calpastatin protein levels are maintained in v-Myc transformed cells also rule out caspase-mediated cleavage of calpastatin as the mechanism responsible for the high levels of calpain activity in v-Myc transformed cells.
Several other regulatory mechanisms for controlling calpain activity have previously been described, including translocation to the plasma membrane and interaction with phospholipids [72,73], modulation of intracellular calcium levels [74], phosphorylation of calpastatin by PKC [75], phosphorylation of calpain mediated by PKA [76], and specific phosphorylation of calpain 2 on serine by ERK/MAPK [77]. To examine the phosphorylation status of calpain 2 following transformation, we immunoprecipitated calpain 2 from lysates extracted from nontransformed and transformed cells and then immunoblotted with an antiphosphoserine antibody. Our studies could not detect any serine phosphorylation of calpain 2 in either any of the nontransformed or transformed cells studied. However, this may be a result of the lack of sensitivity of the reagents used to detect serine phosphorylation. Further detailed studies will be required to determine whether any of the above mechanisms contributes to elevated calpain activity during oncogene-induced cell transformation.
Morphological transformation induced by oncogenes is often characterized by loss of focal adhesions, and actin stress fibers and many calpain substrates are focal adhesion proteins. We have previously shown that calpain inhibitors suppress v-Src-induced morphological transformation [4]. Here we show that calpain inhibitors promote cell spreading and the reappearance of large focal adhesions in v-Myc transformed cells (and also in v12k-Ras and v-Jun cells) reverting the morphological phenotype to that which is more similar to their nontransformed counterparts. In contrast, calpain inhibitors have no effect on the morphological transition of v-Fos transformed cells, which also includes the loss of focal adhesions and disruption of the actin cytoskeleton. To gain insights into how elevated calpain activity may contribute to oncogene-induced morphological transformation, we monitored the proteolytic cleavage of key substrates. In this study, we show for the first time that talin and α II spectrin are proteolytically cleaved by calpain in v-Myc transformed cells. Talin is an actin-binding protein that is also found at focal adhesion sites and links integrins to the actin cytoskeleton [78]. Calpain-mediated cleavage of talin has been proposed to contribute to both focal adhesion disassembly [12,58] and the clustering and activation of integrins [79]. Spectrins are also actin-binding proteins that are components of the membrane cytoskeleton; thus, calpain- mediated cleavage of α II spectrin has been proposed to play an important role during membrane skeleton remodelling [80]. Thus, calpain-mediated cleavage of talin and α II spectrin may contribute to focal adhesion remodelling and morphological transformation of v-Myc transformed cells. Calpain activity also contributes to FAK cleavage in v-Myc cells and to a smaller extent in v12k-Ras cells, and may also contribute to morphological changes in a similar manner to the role of calpain-mediated cleavage of FAK previously described in v-Src transformed cells [4,30]. Previous studies demonstrate that Src-induced phosphorylation of proteins such as cortactin, spectrin, and the NR2 subunits of NMDA receptors influence their ability to act as calpain substrates [14,80,81]. These studies indicate that calpain substrates in v-Src transformed cells may be different from substrates found in other cell types. Our data demonstrating that talin and a II spectrin cleavage is restricted to v12k-Ras and v-Myc transformed cells further indicate that calpain-mediated cleavage of specific substrates varies between transformed cells depending on the oncogene responsible for transformation. Thus, it is possible that calpain may play different roles in malignant transformation depending on the substrates that are targeted for calpain cleavage.
Treatment with inhibitors that block calpain proteolytic activity, but not other cellular proteolytic inhibitors, suppresses the migration of all the transformed cells studied with the exception of v-Fos transformed cells. Uniquely, v-Fos transformed cells have acquired mechanisms that are independent of calpain activity, which promote focal adhesion loss and cell motility. The elucidation of how focal adhesions and cell motility are influenced by the Fos oncoprotein may identify novel-selective therapeutic targets for tumor invasion where aberrant activation of AP-1 transcription factor complexes play a causative role. Calpain inhibition also suppressed the migration of nontransformed cells indicating that calpain activity in normal cells is required for cell motility. However, it remains possible that elevated calpain activity induced by v-Jun, v-Myc, v12k-Ras, and v-Src may contribute to an invasive phenotype of tumor cells in vivo.
Previous studies suggesting that calpain plays a negative role in permitting anchorage-independent growth have utilized the cell-permeable calpain inhibitor ALLN. ALLN was found to promote normal cell growth and anchorageindependent growth of NIH3T3 mouse fibroblasts [63]. However, ALLN did not significantly influence normal cell growth of Ras-transformed NIH 3T3 cells [24]. These studies indicate that oncogene-induced transformation of cells may alter the role of calpain activity in the normal proliferative response and in anchorage-independent growth. Our studies demonstrate that in contrast to these NIH 3T3 studies, anchorage-independent growth of v-Src, v-Jun, v-Myc, v12k-Ras, and v-Fos transformed cells is suppressed by treatment with the calpain inhibitors, ALLN and ALLM. These studies on the major oncogenic hallmark of transformation (anchorage-independent growth) indicate that calpain is an important and general mediator of the transformed cell phenotype.
Both v-Myc and c-Myc are potent inducers of apoptosis [82,83]. Myc-induced cell cycle progression has been proposed to sensitize transformed cells to apoptotic death; however, recent evidence indicates that c-Myc sensitizes cells to a wide range of proapoptotic stimuli that may not be directly linked to the cell cycle [83]. It has been proposed that the apoptotic function of Myc may have been developed as a safeguard to allow the elimination of Myc-transformed cells; however, in vivo, the apoptotic pathway may be suppressed due to the availability of survival factors. Understanding the mechanisms that mediate v-Myc-induced apoptosis has particular importance because of its potential role in tumor suppression. Conflicting roles for calpain activity in contributing to the promotion and suppression of apoptosis have been proposed [7,65–69]. In this study, we demonstrate that treatment with calpain inhibitors suppresses levels of apoptotic cell death in v-Myc transformed cell cultures, indicating that elevated calpain activity can be added to the list of contributors to Myc-induced apoptosis.
In summary, this study demonstrates that elevated calpain proteolytic activity in cells transformed by particular oncogenes plays important roles in remodelling focal adhesion structures that contribute to cell motility and also in promoting anchorage-independent growth. Calpain plays a positive role in promoting morphological transformation and anchorage-independent growth of many transformed cells; however, the calpain substrates and mechanisms by which calpain achieves these effects vary depending on the oncoprotein responsible for transformation. Further studies are required to identify the key calpain substrates that are responsible for deregulated growth control and invasion of tumor cells. Once specific roles for calpain activity have been elucidated, targeting specific aspects of calpain activity may represent novel therapeutic treatments that can be administered to specific types of tumors depending on the oncogenic events responsible for carcinogenesis. In contrast to the majority of transformed cells studied, v-Fos induced morphological transformation and the motility of v-Fos transformed cells is independent of calpain activity, thus defining the unique mechanisms acquired by v-Fos transformed cells that promote cell motility and may identify further novel therapeutic targets for tumor invasion.
Acknowledgements
We thank the following for providing transformed cells: Brad Ozanne, Joe Winnie, Lynn McGarry (v-Fos), David Gillespie, Ann McLaren, Carolyn Wiltshire (v-Jun and v-Myc), Walter Kolch, and Clare Pollock (k-Ras) (all from Beatson Institute for Cancer Research). We also thank Linda Scott for help with manuscript preparation.
Abbreviations
- CEF
chicken embryo fibroblast
- EGF-R
epidermal growth factor receptor
- ERK
extracellular regulated kinase
- FAK
focal adhesion kinase
- ERK/MAPK
extracellular regulated kinase/mitogen-activated protein kinase
Footnotes
This work was funded by Cancer Research UK.
References
- 1.Emori Y, Kawasaki H, Imajoh S, Imahori K, Suzuki K. Endogenous inhibitor for calcium-dependent cysteine protease contains four internal repeats that could be responsible for its multiple reactive sites. Proc Natl Acad Sci USA. 1987;84:3590–3594. doi: 10.1073/pnas.84.11.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maki M, Bagci H, Hamaguchi K, Ueda M, Murachi T, Hatanaka M. Inhibition of calpain by a synthetic oligopeptide corresponding to an exon of the human calpastatin gene. J Biol Chem. 1989;264:18866–18869. [PubMed] [Google Scholar]
- 3.Kawasaki H, Emori Y, Imajoh-Ohmi S, Minami Y, Suzuki K. Identification and characterization of inhibitory sequences in four repeating domains of the endogenous inhibitor for calcium-dependent protease. J Biochem (Tokyo) 1989;106:274–281. doi: 10.1093/oxfordjournals.jbchem.a122844. [DOI] [PubMed] [Google Scholar]
- 4.Carragher NO, Fincham VJ, Riley D, Frame MC. Cleavage of focal adhesion kinase by different proteases during SRC-regulated transformation and apoptosis. Distinct roles for calpain and caspases. J Biol Chem. 2001;276:4270–4275. doi: 10.1074/jbc.M008972200. [DOI] [PubMed] [Google Scholar]
- 5.Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 6.Murray SS, Grisanti MS, Bentley GV, Kahn AJ, Urist MR, Murray EJ. The calpain-calpastatin system and cellular proliferation and differentiation in rodent osteoblastic cells. Exp Cell Res. 1997;233:297–309. doi: 10.1006/excr.1997.3550. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel YM, Lane MD. Mitotic clonal expansion during preadipocyte differentiation: calpain-mediated turnover of p27. J Biol Chem. 2000;275:17653–17660. doi: 10.1074/jbc.M910445199. [DOI] [PubMed] [Google Scholar]
- 9.Sorimachi H, Ishiura S, Suzuki K. Structure and physiological function of calpains. Biochem J. 1997;328:721–732. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckerle MC, Burridge K, DeMartino GN, Croall DE. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987;51:569–577. doi: 10.1016/0092-8674(87)90126-7. [DOI] [PubMed] [Google Scholar]
- 11.Bialkowska K, Kulkarni S, Du X, Goll DE, Saido TC, Fox JE. Evidence that beta3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active. J Cell Biol. 2000;151:685–696. doi: 10.1083/jcb.151.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, Huttenlocher A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt A, Kaverina I, Otey C, Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115:3415–3425. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Tandon NN, Greco NJ, Ni Y, Wang T, Zhan X. Proteolysis of platelet cortactin by calpain. J Biol Chem. 1997;272:19248–19252. doi: 10.1074/jbc.272.31.19248. [DOI] [PubMed] [Google Scholar]
- 15.Hu RJ, Bennett V. In vitro proteolysis of brain spectrin by calpain I inhibits association of spectrin with ankyrin-independent membrane binding site(s) J Biol Chem. 1991;266:18200–18205. [PubMed] [Google Scholar]
- 16.Potter DA, Tirnauer JS, Janssen R, Croall DE, Hughes CN, Fiacco KA, Mier JW, Maki M, Herman IM. Calpain regulates actin remodeling during cell spreading. J Cell Biol. 1998;141:647–662. doi: 10.1083/jcb.141.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni S, Goll DE, Fox JE. Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J Biol Chem. 2002;277:24435–24441. doi: 10.1074/jbc.M203457200. [DOI] [PubMed] [Google Scholar]
- 18.Ek B, Heldin CH. Specific cleavage of the fibroblast receptor for platelet-derived growth factor by an endogenous Ca2+-dependent thiol protease. Eur J Biochem. 1986;155:409–413. doi: 10.1111/j.1432-1033.1986.tb09506.x. [DOI] [PubMed] [Google Scholar]
- 19.Hirai S, Kawasaki H, Yaniv M, Suzuki K. Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Lett. 1991;287:57–61. doi: 10.1016/0014-5793(91)80015-u. [DOI] [PubMed] [Google Scholar]
- 20.Carillo S, Pariat M, Steff AM, Roux P, Etienne-Julan M, Lorca T, Piechaczyk M. Differential sensitivity of FOS and JUN family members to calpains. Oncogene. 1994;9:1679–1689. [PubMed] [Google Scholar]
- 21.Oda A, Druker BJ, Ariyoshi H, Smith M, Salzman EW. pp60src is an endogenous substrate for calpain in human blood platelets. J Biol Chem. 1993;268:12603–12608. [PubMed] [Google Scholar]
- 22.Watanabe N, Vande Woude GF, Ikawa Y, Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989;342:505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- 23.Gregoriou M, Willis AC, Pearson MA, Crawford C. The calpain cleavage sites in the epidermal growth factor receptor kinase domain. Eur J Biochem. 1994;223:455–464. doi: 10.1111/j.1432-1033.1994.tb19013.x. [DOI] [PubMed] [Google Scholar]
- 24.Hiwasa T, Nakata M, Ohno S, Maki M, Suzuki K, Takiguchi M. Regulation of transformed state by calpastatin via PKCepsilon in NIH3T3 mouse fibroblasts. Biochem Biophys Res Commun. 2002;290:510–517. doi: 10.1006/bbrc.2001.6197. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Li L, Cohen SN. Antisense RNA-mediated deficiency of the calpain protease, nCL-4, in NIH3T3 cells is associated with neoplastic transformation and tumorigenesis. J Biol Chem. 2000;275:31093–31098. doi: 10.1074/jbc.M005451200. [DOI] [PubMed] [Google Scholar]
- 26.Kubbutat MH, Vousden KH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol. 1997;17:460–468. doi: 10.1128/mcb.17.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura Y, Saya H, Nakao M. Calpain-dependent proteolysis of NF2 protein: involvement in schwannomas and meningiomas. Neuropathology. 2000;20:153–160. doi: 10.1046/j.1440-1789.2000.00326.x. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto S, Seufzer BJ, Shumway SD. Novel IkappaB alpha proteolytic pathway in WEHI231 immature B cells. Mol Cell Biol. 1998;18:19–29. doi: 10.1128/mcb.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang JS, Lee SJ, Choi YH, Nguyen PM, Lee J, Hwang SG, Wu ML, Takano E, Maki M, Henkart PA. Posttranslational regulation of the retinoblastoma gene family member p107 by calpain protease. Oncogene. 1999;18:1789–1796. doi: 10.1038/sj.onc.1202497. [DOI] [PubMed] [Google Scholar]
- 30.Carragher NO, Westhoff MA, Riley D, Potter DA, Dutt P, Elce JS, Greer PA, Frame MC. v-Src-induced modulation of the calpain-calpastatin proteolytic system regulates transformation. Mol Cell Biol. 2002;22:257–269. doi: 10.1128/MCB.22.1.257-269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carragher NO, Frame MC. Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol. 2002;34:1539–1543. doi: 10.1016/s1357-2725(02)00069-9. [DOI] [PubMed] [Google Scholar]
- 32.Postovit LM, Dutt P, Dourdin N, Park M, Greer PA, Graham CH, Elce JS. Calpain is required for MMP-2 and u-PA expression in SV40 large T-antigen-immortalized cells. Biochem Biophys Res Commun. 2002;297:294–301. doi: 10.1016/s0006-291x(02)02187-3. [DOI] [PubMed] [Google Scholar]
- 33.Glading A, Chang P, Lauffenburger DA, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- 34.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62:6770–6778. [PubMed] [Google Scholar]
- 35.Mamoune A, Luo JH, Lauffenburger DA, Wells A. Calpain-2 as a target for limiting prostate cancer invasion. Cancer Res. 2003;63:4632–4640. [PubMed] [Google Scholar]
- 36.Braun C, Engel M, Seifert M, Theisinger B, Seitz G, Zang KD, Welter C. Expression of calpain I messenger RNA in human renal cell carcinoma: correlation with lymph node metastasis and histological type. Int J Cancer. 1999;84:6–9. doi: 10.1002/(sici)1097-0215(19990219)84:1<6::aid-ijc2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Shiba E, Kambayashi JI, Sakon M, Kawasaki T, Kobayashi T, Koyama E, Yayoi E, Takatsuka Y, Takai SI. Ca2+-dependent neutral protease (calpain) activity in breast cancer tissue and estrogen receptor status. Breast Cancer. 1996;3:13–17. doi: 10.1007/BF02966957. [DOI] [PubMed] [Google Scholar]
- 38.Wang XD, Rosales JL, Magliocco A, Gnanakumar R, Lee KY. Cyclin E in breast tumors is cleaved into its low molecular weight forms by calpain. Oncogene. 2003;22:769–774. doi: 10.1038/sj.onc.1206166. [DOI] [PubMed] [Google Scholar]
- 39.Witkowski JM, Zmuda-Trzebiatowska E, Swiercz JM, Cichorek M, Ciepluch H, Lewandowski K, Bryl E, Hellmann A. Modulation of the activity of calcium-activated neutral proteases (calpains) in chronic lymphocytic leukemia (B-CLL) cells. Blood. 2002;100:1802–1809. doi: 10.1182/blood-2001-11-0073. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa Y, Mukai H, Hino F, Asada K, Kato I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000;91:459–463. doi: 10.1111/j.1349-7006.2000.tb00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fincham VJ, Wyke JA, Frame MC. v-Src-induced degradation of focal adhesion kinase during morphological transformation of chicken embryo fibroblasts. Oncogene. 1995;10:2247–2252. (published erratum appears in Oncogene 1995; 11(10), 2185) [PubMed] [Google Scholar]
- 42.Welham MJ, Wyke JA. A single point mutation has pleiotropic effects on pp60v-src function. J Virol. 1988;62:1898–1906. doi: 10.1128/jvi.62.6.1898-1906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardner AM, Vaillancourt RR, Johnson GL. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase by G protein and tyrosine kinase oncoproteins. J Biol Chem. 1993;268:17896–17901. [PubMed] [Google Scholar]
- 44.Giehl K, Skripczynski B, Mansard A, Menke A, Gierschik P. Growth factor-dependent activation of the Ras-Raf-MEK-MAPK pathway in the human pancreatic carcinoma cell line PANC-1 carrying activated K-ras: implications for cell proliferation and cell migration. Oncogene. 2000;19:2930–2942. doi: 10.1038/sj.onc.1203612. [DOI] [PubMed] [Google Scholar]
- 45.Glading A, Uberall F, Keyse SM, Lauffenburger DA, Wells A. Membrane proximal ERK signaling is required for M-calpain activation downstream of epidermal growth factor receptor signaling. J Biol Chem. 2001;276:23341–23348. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki T, Kishi M, Saito M, Tanaka T, Higuchi N, Kominami E, Katunuma N, Murachi T. Inhibitory effect of di- and tripeptidyl aldehydes on calpains and cathepsins. J Enzyme Inhib. 1990;3:195–201. doi: 10.3109/14756369009035837. [DOI] [PubMed] [Google Scholar]
- 47.Sarin A, Clerici M, Blatt SP, Hendrix CW, Shearer GM, Henkart PA. Inhibition of activation-induced programmed cell death and restoration of defective immune responses of HIV+ donors by cysteine protease inhibitors. J Immunol. 1994;153:862–872. [PubMed] [Google Scholar]
- 48.Squier MK, Miller AC, Malkinson AM, Cohen JJ. Calpain activation in apoptosis. J Cell Physiol. 1994;159:229–237. doi: 10.1002/jcp.1041590206. [DOI] [PubMed] [Google Scholar]
- 49.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, chreiber SL. Inhibition of proteasome activities and subunit-specific aminoterminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 50.Seglen PO, Grinde B, Solheim AE. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979;95:215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sever N, Filipic M, Brzin J, Lah TT. Effect of cysteine proteinase inhibitors on murine B16 melanoma cell invasion in vitro. Biol Chem. 2002;383:839–842. doi: 10.1515/BC.2002.088. [DOI] [PubMed] [Google Scholar]
- 53.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 54.Burridge K, Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 1983;3:405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- 55.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 56.Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- 57.Cooray P, Yuan Y, Schoenwaelder SM, Mitchell CA, Salem HH, Jackson SP. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem J. 1996;318:41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol. 1999;147:619–630. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi M, Suzuki H, Kawashima S, Saido TC, Inomata M. The behavior of calpain-generated N- and C-terminal fragments of talin in integrin-mediated signaling pathways. Arch Biochem Biophys. 1999;371:133–141. doi: 10.1006/abbi.1999.1427. [DOI] [PubMed] [Google Scholar]
- 60.Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319:683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056–26061. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]
- 62.Levkau B, Herren B, Koyama H, Ross R, Raines EW. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J Exp Med. 1998;187:579–586. doi: 10.1084/jem.187.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hiwasa T, Sawada T, Sakiyama S. Cysteine proteinase inhibitors and ras gene products share the same biological activities including transforming activity toward NIH3T3 mouse fibroblasts and the differentiation-inducing activity toward PC12 rat pheochromocytoma cells. Carcinogenesis. 1990;11:75–80. doi: 10.1093/carcin/11.1.75. [DOI] [PubMed] [Google Scholar]
- 64.Petropoulos CJ, Givol I, Hughes SH. Comparative analysis of the structure and function of the chicken c-myc and v-myc genes: v-myc is a more potent inducer of cell proliferation and apoptosis than c-myc. Oncogene. 1996;12:2611–2621. [PubMed] [Google Scholar]
- 65.McCollum AT, Nasr P, Estus S. Calpain activates caspase-3 during UV-induced neuronal death but only calpain is necessary for death. J Neurochem. 2002;2:1208–1220. doi: 10.1046/j.1471-4159.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- 66.Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Exp Cell Res. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]
- 67.Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, Shoshan MC. Calpain-mediated Bid cleavage and calpainindependent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atencio IA, Ramachandra M, Shabram P, Demers GW. Calpaininhibitor 1 activates p53-dependent apoptosis in tumor cell lines. Cell Growth Differ. 2000;11:247–253. [PubMed] [Google Scholar]
- 69.Chua BT, Guo K, Li P. Direct cleavage by the calciumactivated protease calpain can lead to inactivation of caspases. J Biol Chem. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]
- 70.Porn-Ares MI, Samali A, Orrenius S. Cleavage of the calpain inhibitor, calpastatin, during apoptosis. Cell Death Differ. 1998;5:1028–1033. doi: 10.1038/sj.cdd.4400424. [DOI] [PubMed] [Google Scholar]
- 71.Zawaski K, Gruebele A, Kaplan D, Reddy S, Mortensen A, Novak RF. Evidence for enhanced expression of c-fos, cjun, and the Ca(2+)-activated neutral protease in rat liver following carbon tetrachloride administration. Biochem Biophys Res Commun. 1993;197:585–590. doi: 10.1006/bbrc.1993.2519. [DOI] [PubMed] [Google Scholar]
- 72.Pontremoli S, Melloni E, Sparatore B, Salamino F, Michetti M, Sacco O, Horecker BL. Role of phospholipids in the activation of the Ca2+-dependent neutral proteinase of human erythrocytes. Biochem Biophys Res Commun. 1985;129:389–395. doi: 10.1016/0006-291x(85)90163-9. [DOI] [PubMed] [Google Scholar]
- 73.Arthur JS, Crawford C. Investigation of the interaction of mcalpain with phospholipids: calpain-phospholipid interactions. Biochim Biophys Acta. 1996;1293:201–206. doi: 10.1016/0167-4838(95)00243-x. [DOI] [PubMed] [Google Scholar]
- 74.Small GW, Chou TY, Dang CV, Orlowski RZ. Evidence for involvement of calpain in c-Myc proteolysis in vivo. Arch Biochem Biophys. 2002;400:151–161. doi: 10.1016/S0003-9861(02)00005-X. [DOI] [PubMed] [Google Scholar]
- 75.Averna M, De Tullio R, Salamino F, Melloni E, Pontremoli S. Phosphorylation of rat brain calpastatins by protein kinase C. FEBS Lett. 1999;450:13–16. doi: 10.1016/s0014-5793(99)00461-5. [DOI] [PubMed] [Google Scholar]
- 76.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 78.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 79.Yan B, Calderwood DA, Yaspan B, Ginsberg MH. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J Biol Chem. 2001;276:28164–28170. doi: 10.1074/jbc.M104161200. [DOI] [PubMed] [Google Scholar]
- 80.Nicolas G, Fournier CM, Galand C, Malbert-Colas L, Bournier O, Kroviarski Y, Bourgeois M, Camonis JH, Dhermy D, Grandchamp B. Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol Cell Biol. 2002;22:3527–3536. doi: 10.1128/MCB.22.10.3527-3536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bi R, Rong Y, Bernard A, Khrestchatisky M, Baudry M. Src-mediated tyrosine phosphorylation of NR2 subunits of N-methyl- D-aspartate receptors protects from calpain-mediated truncation of their C-terminal domains. J Biol Chem. 2000;275:26477–26483. doi: 10.1074/jbc.M003763200. [DOI] [PubMed] [Google Scholar]
- 82.Lee CM, Reddy EP. The v-myc oncogene. Oncogene. 1999;18:2997–3003. doi: 10.1038/sj.onc.1202786. [DOI] [PubMed] [Google Scholar]
- 83.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Natl Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]