Mounting an appropriate immune response depends on the careful regulation of lymphocyte activation. To this end, lymphocytes require two independent signals to become fully activated. The first, an antigen-specific signal is sent via the unique antigen receptor: T cell receptor (TCR) on T cells or surface Ig on B cells. The second signal, termed costimulation, is independent of the antigen receptor and is critical to allow full activation, sustain cell proliferation, prevent anergy and/or apoptosis, induce differentiation to effector and memory status, and allow cell-cell cooperation. Costimulation is in turn regulated by the expression of inhibitory receptors upon lymphocyte activation. Here we will consider the growing list of costimulatory and inhibitory molecules, with emphasis on signaling events they initiate. Potentially complex patterns of regulation implied by the variety of receptor/ligand pairs and their differential expression patterns will also be discussed.
What is costimulation?
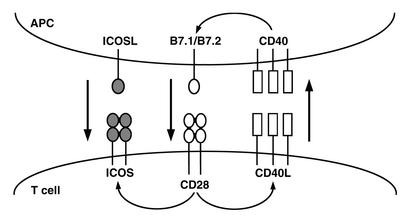
It has been recognized for over twenty years that signals in addition to those sent by the antigen receptor are required for full activation of a lymphocyte. Lymphocytes stimulated through the antigen receptor alone fail to produce cytokines, are unable to sustain proliferation, and often undergo apoptosis or become nonresponsive to subsequent stimulation. Early models favored soluble factors as the key transmitters of these signals, and numerous cytokines (IL-1, IL-2, and IL-4, among others) have been found to enhance the activation of both B and T cells. However, it has become clear that interactions between receptor/ligand pairs of cell surface molecules on the responder lymphocyte and an “accessory” cell — an antigen-presenting cell (APC) in the case of T cell activation, or a helper T cell for B cell activation — represent a critical event in the activation process, and it is this event that is generally referred to as costimulation. There is growing evidence for bidirectional communication between the cells, such that a T cell–B cell interaction can involve mutual costimulation and several levels of cross-talk, allowing very specific regulation of lymphocyte activation (Figure 1).
Figure 1.
Costimulation involves reciprocal and sequential signals between cells. A T cell–APC interaction begins when the T cell antigen receptor is stimulated by a specific peptide/MHC complex on the surface of the APC (not shown). Low constitutive levels of B7.1 and/or B7.2 on the APC activate CD28 on the T cell, inducing upregulation of CD40L. CD40L in turn binds to CD40 on the APC, enhancing B7.1/B7.2 expression and reinforcing the CD28/CD40 positive feedback loop. CD28 costimulation also induces T cell expression of ICOS, allowing a second level of costimulation by APC-expressed ICOSL. Other costimulatory and inhibitory molecules regulated by the initial costimulatory signals (not shown) can further shape the specific outcome of the interaction.
There still appears to be no general agreement on exactly how the term “costimulation” is defined. In some cases it is used broadly to mean nearly any interaction that enhances antigen receptor signaling, while in other cases it is more narrowly construed, meaning only signals that have no stimulatory capacity on their own, but whose synergism with the antigen receptor is required to allow full activation of a naive lymphocyte. In addition, the phrase “negative costimulation” has recently begun to be applied to inhibitory signaling events, further confusing the terminology. For this discussion, costimulation is defined as a signaling pathway that does more than simply augment antigen receptor–proximal activation events, but that intersects with antigen-specific signals synergistically to allow lymphocyte activation. Thus, a costimulatory molecule must initiate a positive signal without simply increasing TCR avidity (as might be the case for adhesion molecules) or enhancing recruitment of tyrosine kinases to the TCR complex (as is the case for the coreceptors CD4 and CD8).
Candidate costimulatory molecules
The first cell surface molecule shown to function as a costimulatory receptor was CD28 (1). Since the identification of CD28, the number of proposed costimulatory molecules has grown significantly (2, 3). Most receptors that satisfy the above definition can be divided into two classes based on sequence homologies. The first class contains the related CD28 and inducible costimulator (ICOS) molecules (3). CD28 and ICOS are both disulfide-linked homodimers that bind to distinct members of the B7 family of surface proteins. These appear to be the major costimulatory molecules for the activation of T cells, with naive and resting T cells using CD28 and activated and effector T cells using ICOS. Two other receptors, CTLA-4 and PD-1, share structural homology with this class and also bind B7 family members, but most evidence indicates that both are inhibitory in nature (see below) and thus fail to qualify as costimulatory. These proteins can be considered potential negative regulators of costimulation.
The second class of costimulatory receptors are members of the TNF receptor (TNFR) family. These include CD40, the major B cell costimulatory molecule, as well as OX-40, 4-1BB, CD30, and CD27. The ligands for these receptors are membrane-bound members of the TNF family. Recent reviews have discussed the costimulatory TNFR family members (2, 4), so we will focus on advances in understanding the CD28 family of costimulatory molecules.
CD28 signaling
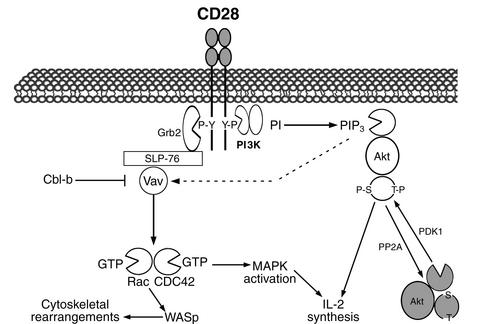
Studies in vitro and in vivo have shown that CD28 is the primary costimulatory molecule for naive T cells, although there appears to be differences between T cell subsets, with CD4+ (“helper”) cells more dependent than CD8+ (“cytotoxic”) cells on CD28 costimulation in vivo (4, 5). However, the signaling pathways downstream of CD28 have been difficult to characterize and have only recently begun to be elucidated (Figure 2). A key event in CD28 signaling appears to be the activation of the small Rho family GTPases Rac and CDC42. Rac and CDC42 activate p21-activated kinase, which may link them to the mitogen-activated protein kinase cascades and the subsequent induction of IL-2 synthesis. Rac and CDC42 are also important in CD28-mediated cytoskeletal rearrangements, through the action of the Wiscott-Aldrich syndrome protein (WASP) (6). However, there is debate regarding the pathways coupling CD28 ligation to these downstream signaling events, with phosphatidylinositol 3-kinase (PI3K), Akt, Vav, and cAMP all receiving recent attention.
Figure 2.
Pathways implicated in CD28 signaling. Cross-linking of CD28 induces tyrosine phosphorylation of the cytoplasmic tail, allowing interaction with Grb2 and PI3K (although both are portrayed interacting with a single CD28 dimer, it is unknown whether this is a physiological complex). Grb2 links via SLP-76 to the Rho family guanine-nucleotide exchange factor Vav, connecting CD28 to activation of Rac and CDC42. PI3K can signal by recruiting Akt. Negative regulation of Vav by Cbl-b and of Akt by PP2A opposes CD28 costimulatory signals.
PI3K
PI3K can associate with the cytoplasmic tail of CD28 in a tyrosine phosphorylation–dependent fashion. However, studies using pharmacological inhibitors of PI3K have provided evidence both for and against an essential role for PI3K in CD28 signaling (7). Studies using T cells transfected with CD28 mutants defective for PI3K binding have been similarly equivocal, and even the use of transgenic mice has been unable to resolve this conflict. Two laboratories have recently introduced mutant CD28 constructs unable to bind PI3K into CD28-deficient mice, with strikingly different results. Okkenhaug et al. reported that the mutant CD28 is indistinguishable from wild-type CD28 in supporting IL-2 synthesis, proliferation, B cell help, and in vivo response to viral infection. Due to a lack of induction of Akt and Bcl-xL, two downstream mediators of CD28 signal transduction, T cells from these animals display increased sensitivity to radiation-induced apoptosis (8). However, Harada et al. reported that T cells expressing the mutant CD28 are defective for IL-2 production and proliferation, especially at early time points, and fail to mediate graft-versus-host disease (9). It is unclear how such different results arose, since the two groups introduced the same mutant onto essentially identical genetic backgrounds, although they used different promoter/enhancer elements to control CD28 expression. In addition, it has been reported that T cells from mice lacking the p85α subunit of PI3K appear to function normally (10). Since the p85α subunit is believed to be required for CD28 binding to PI3K, these results also suggest that PI3K is not required for CD28 signaling. However, there may be some compensation from other p85 isoforms, which are upregulated in the p85α-deficient cells.
Questions concerning PI3K function in costimulation are not limited to CD28. The cytoplasmic tail of the inducible T cell costimulatory molecule ICOS also binds PI3K. Recent evidence suggests that PI3K is also important for costimulation of NK cells. Notably, the NK cell transmembrane protein DAP10 binds PI3K, and a receptor complex of DAP10 and the NKG2D molecule acts synergistically with other NK cell receptors to induce cytotoxicity and cytokine secretion in a PI3K-dependent fashion (11). Paradoxically, as discussed below, the T cell inhibitory receptor CTLA-4 also recruits PI3K, suggesting a potential negative function for PI3K. Thus, the role of PI3K in costimulation remains unresolved and offers an important area for continued investigation.
Akt
Akt (also called protein kinase B, or PKB) is a serine/threonine kinase that is recruited to the plasma membrane when its pleckstrin homology domain binds phospholipid products of PI3K. At the membrane, phosphorylation by the kinase phosphoinositide-dependent kinase 1 (PDK1) and a second kinase (which may be integrin-linked kinase) stimulates Akt (12), allowing it to phosphorylate downstream targets, such as the inhibitory kinase glycogen synthase kinase 3β, which is inactivated by Akt. Although Akt has been known for some time to protect cells against numerous apoptotic stimuli, its role in T cell activation has only recently begun to be appreciated. Antibody cross-linking of CD28 induces Akt phosphorylation and activation, even in the absence of signaling through the TCR complex–associated CD3 proteins (13, 14), and this activation is PI3K-dependent. Constitutively active Akt is able to substitute for CD28 signals and stimulate IL-2 production when introduced into mature CD28-deficient T cells (14). The downstream targets of Akt involved in IL-2 induction are unknown. Surprisingly, Akt does not enhance proliferation of CD28-deficient cells, despite restoration of IL-2 secretion. The expression of the IL-2Rα subunit (CD25), which is impaired in CD28-deficient T cells, has not been examined in mutant cells carrying the constitutive form of Akt. In any case, Akt appears to be important in mediating CD28 signals, but it requires cooperation of other pathways for full costimulation.
Vav
The proto-oncogene Vav acts as a guanine-nucleotide exchange factor for Rac and CDC42, allowing these molecules to switch from the inactive GDP-bound state to the active GTP-bound state (6). Signals downstream of both CD3 and CD28 can independently induce Vav phosphorylation and activation, but CD28 is significantly more potent and induces more sustained phosphorylation. Thus, Vav is a candidate for a direct link between CD28 and Rac/CDC42–controlled events. Vav interaction with CD28 is likely mediated by its participation in a multiprotein complex containing the adapter proteins SLP-76, LAT, and Grb2, as there is a Grb2-binding site in the cytoplasmic tail of CD28 (see Leo et al., this Perspective series, ref. 15). Vav may receive some CD28 signals via PI3K, since Vav activity is regulated in part by the binding of its pleckstrin homology domain to phospholipid products of PI3K activity (16).
Vav is also subject to negative regulation, and the RING-finger adapter protein Cbl-b appears to play a major role. T cells from Cbl-b–deficient mice hyperactivate Vav in response to TCR signals and do not require CD28 costimulation for activation in vitro or in vivo (17, 18). Thus, CD28 signals appear to be required to overcome Cbl-b inhibition of Vav activation. It is not known how Cbl-b regulates Vav activity, but Cbl-b functions as an E3 ubiquitin ligase (19), suggesting that Cbl-b may control the degradation of a Vav regulatory factor via the ubiquitin-proteasome pathway. However, CD28 still has costimulatory function in Cbl-b–deficient T cells (17, 18). Thus, as with Akt, regulation of Cbl-b/Vav is at most only a part of costimulatory signaling.
cAMP
The regulation of cAMP levels is critical for many processes in all cell types and is a feature of signal transduction from multiple receptors, including the TCR. However, elevation of cAMP levels in T lymphocytes is inhibitory, suggesting that inhibition or reversal of cAMP accumulation is required for T cell activation (20). CD28 costimulation induces expression of a cAMP phosphodiesterase, PDE7, leading to reduced cellular cAMP levels. Blocking PDE7 induction with an antisense oligonucleotide inhibits T cell proliferation, and this inhibition is reversed by addition of a cAMP analog that blocks downstream cAMP signaling (21). Inhibition by cAMP has been reported to affect nearly every pathway important for lymphocyte activation, allowing CD28 signaling to intersect a wide range of antigen receptor signals through a single effector. Interestingly, increased intracellular cAMP has also been implicated in the induction of T cell anergy, a nonresponsive state that occurs after T cells are stimulated through TCR/CD3 in the absence of costimulation (22, 23). Thus, in addition to providing positive signals, CD28 costimulation may also be critical for the elimination of TCR-initiated negative signals.
ICOS and ICOSL
Although CD28 is a key costimulatory molecule, it does not account for all costimulatory function in T cells. Mice lacking CD28 are still able to mount effective responses to some viruses (4), and CD28 deficiency actually enhances diabetes in the nonobese diabetic (NOD) mouse strain (24). Further, production of effector cytokines such as IFN-γ and IL-4 can be stimulated to normal levels by APCs lacking both B7.1 and B7.2 (25, 26). In addition, memory T cells are far less dependent than naive T cells on CD28/B7 costimulation (27). These results suggest that other costimulatory molecules can compensate for the absence of CD28 signaling.
The recently discovered ICOS molecule has attracted a great deal of attention as one such alternative costimulatory molecule. ICOS is structurally related to CD28 but does not detectably bind to B7.1 or B7.2. Instead, the ligand for ICOS (ICOSL) is a novel B7 family member that has been independently cloned by at least six different groups (3, 28). As with CD28, the cytoplasmic tail of ICOS is able to bind the PI3K p85 subunit and is associated with lipid kinase activity (29), although it is unknown whether this is important for ICOS signaling.
ICOS is either absent or expressed at very low levels on naive T cells, but it is upregulated upon stimulation (30), suggesting that its costimulatory role is more important for memory and effector T cells — precisely the cells that are known to have reduced dependence on CD28. Indeed, ICOS costimulation contributes to the production of the effector cytokines IFN-γ, TNF-α, IL-4, IL-5, and IL-10, but little IL-2, and studies with ICOS-deficient mice have shown a critical role for ICOS in T cell help for Ig class switching and germinal center formation (31–33). APCs show differential regulation of B7.1, B7.2, and ICOSL, which may allow specificity in eliciting responses from naive versus activated (or memory) T cells (34–36). Importantly, ICOSL expression can be induced in fibroblasts in culture, and nonlymphoid tissues in mice, by treatment with the inflammatory agents LPS and TNF-α, whereas CD28 ligands are not induced in these same tissues by similar treatment (34). Thus, inflamed peripheral tissues would be able to stimulate antigen-experienced T cells, but unable to stimulate naive T cells, due to the differential regulation of the ligands for CD28 and ICOS.
Negative regulation of costimulation
The requirement for costimulation allows lymphocyte activation to be strictly regulated by modulating the expression of either member (or both) of the costimulatory receptor/ligand pair. An additional level of regulation is achieved by the expression of inhibitory receptors that can initiate negative signals. The balance between such negative signals and positive signals from antigen receptor and costimulatory molecules apparently sets the threshold for lymphocyte activation. The interaction between costimulatory and inhibitory signals appears to be a critical aspect of the regulation of lymphocyte function.
Perhaps the best-studied inhibitory receptor is CTLA-4. CTLA-4 was the second member of the CD28 family to be identified. Like CD28, it binds to B7.1 and B7.2, although with significantly higher affinity. Resting T cells express little surface CTLA-4, but surface levels are increased upon activation, due to both redistribution of an intracellular pool and increased synthesis. CTLA-4 inhibits T cell proliferation and IL-2 synthesis in response to stimulation with anti-CD3 and anti-CD28 antibodies. Furthermore, preventing CTLA-4/B7 interactions enhances T cell responses in vitro and in vivo (37). These findings suggest a model in which the dynamic regulation of CTLA-4 and B7 levels is responsible for setting the activation threshold of T cells. Importantly, CD28 costimulation upregulates CTLA-4 expression, such that costimulation is a self-limiting process. CTLA-4 can also inhibit ICOS signaling (38).
CTLA-4 can interfere with T cell activation by both passive and active mechanisms. When CTLA-4 levels are high, and B7 levels limiting, the higher affinity of CTLA-4 for B7 allows it to outcompete CD28 for B7 binding, preventing CD28/B7 interactions (39). CTLA-4 can also engage inhibitory signal transduction machinery, including the phosphatases SHP-2 (40, 41) and PP2A (42), which may oppose the action of kinases downstream of CD3 and CD28. CTLA-4 ligation has been shown to inhibit activation of extracellular signal–regulated kinase (ERK) and Jun N-terminal kinase (43), both of which are at the ends of kinase cascades. CTLA-4–associated SHP-2 has been proposed to reduce activation-induced phosphorylation of LAT and TCRζ (41), although effects on TCR/CD3–proximal events are controversial (43). In addition, most evidence places SHP-2 in positive, rather than negative, signaling pathways (44). PP2A is able to dephosphorylate and deactivate Akt (45), suggesting that it may play a similar role in CTLA-4 inhibition of costimulatory signals. There is also growing evidence that CTLA-4 interacts with other inhibitory pathways, since mutation of the tyrosine residue critical for the SH2-mediated binding of SHP-2 and PP2A does not abolish CTLA-4 function (46–49). Intriguingly, CTLA-4 also interacts with PI3K, further complicating models of PI3K’s contribution to lymphocyte activation and inhibition (7).
PD-1 is another inhibitory member of the CD28 family. As with the other CD28 family members, its ligands, PD-L1 (50) and PD-L2 (51), are related to the B7 proteins. PD-1 is expressed on activated B and T cells (52), and engagement of PD-1 has been shown to inhibit T cell proliferation and cytokine production in response to anti-CD3 and anti-CD28 antibody stimulation (50, 51). Further, mice deficient in PD-1 expression develop autoimmune disorders characterized by production of high titers of autoantibodies (53), indicating an important role in the regulation of autoreactive B cells. Unlike CTLA-4, the cytoplasmic tail of PD-1 contains a sequence known as the immunoreceptor tyrosine-based inhibition motif (ITIM; see Billadeau and Leibson, this Perspective series, ref. 54). The ITIM sequence is found in several classes of inhibitory receptors, including the killer inhibitory receptors found on NK cells and FcγRIIB on B cells, and functions by recruiting SH2-containing phosphatases (55). There appear to be receptor and cell type specificities in the identity of the phosphatase (SHP-1, SHP-2, or SHIP) recruited by the ITIM motif, and it is unknown which phosphatase is involved in PD-1 function, although, as with CTLA-4, there is some evidence for preferential activation of SHP-2 (50, 51).
On the horizon
Studies over the past several years have revealed a tremendous complexity in the cell-cell interactions regulating lymphocyte activation, with hints of even greater complexity yet. Genome-based searches will likely continue to uncover previously unknown costimulatory and inhibitory receptors and their respective ligands. The recently discovered B7 family member B7-H3 appears to engage a costimulatory molecule that does not correspond to any of the known CD28 family members (56), indicating the presence of at least one additional member. Also, generation of mice lacking both CD28 and CTLA-4 has produced evidence for a third B7.1/B7.2 receptor (57). The continued analysis of mice lacking one or more costimulatory receptors and/or ligands should help sort out the unique and overlapping roles of these molecules in the positive and negative regulation of immune responses. We expect that greater understanding of the interplay of the various costimulatory and inhibitory signals will lead to important insights into how lymphocyte differentiation is specified, effector cells are regulated, memory is generated, and tolerance is maintained. In addition, the newly identified receptor/ligand combinations provide targets for immune therapies, with potentially increased specificity. Likewise, elucidation of the specific signaling pathways engaged by these receptors will likely add to the options for therapeutic intervention in immune dysfunctions.
Acknowledgments
We would like to thank Sunit Talapatra, Wei-Xing Zong, and Jeffrey Rathmell for critical reading of this manuscript, and other members of the Thompson laboratory for helpful discussions.
References
- 1.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 2.Watts TH, DeBenedette MA. T cell co-stimulatory molecules other than CD28. Curr Opin Immunol. 1999;11:286–293. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 3.Mueller DL. T cells: a proliferation of costimulatory molecules. Curr Biol. 2000;10:R227–R230. doi: 10.1016/s0960-9822(00)00400-0. [DOI] [PubMed] [Google Scholar]
- 4.Whitmire JK, Ahmed R. Costimulation in antiviral immunity: differential requirements for CD4(+) and CD8(+) T cell responses. Curr Opin Immunol. 2000;12:448–455. doi: 10.1016/s0952-7915(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 5.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 6.Reif K, Cantrell DA. Networking Rho family GTPases in lymphocytes. Immunity. 1998;8:395–401. doi: 10.1016/s1074-7613(00)80545-2. [DOI] [PubMed] [Google Scholar]
- 7.Hutchcroft JE, Bierer BE. Signaling through CD28/CTLA-4 family receptors: puzzling participation of phosphatidylinositol-3 kinase. J Immunol. 1996;156:4071–4074. [PubMed] [Google Scholar]
- 8.Okkenhaug K, et al. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 9.Harada Y, et al. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797–3803. doi: 10.4049/jimmunol.166.6.3797. [DOI] [PubMed] [Google Scholar]
- 10.Fruman DA, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL. On guard: activating NK cell receptors. Nat Immunol. 2001;2:23–27. doi: 10.1038/83130. [DOI] [PubMed] [Google Scholar]
- 12.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 13.Parry RV, et al. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur J Immunol. 1997;27:2495–2501. doi: 10.1002/eji.1830271006. [DOI] [PubMed] [Google Scholar]
- 14.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 15.Leo A, Wienands J, Baier G, Horejsi V, Schraven B. Adapters in lymphocyte signaling. J Clin Invest. 2002;109:301–309. DOI:10.1172/JCI200214942. doi: 10.1172/JCI14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantrell D. Lymphocyte signalling: a coordinating role for Vav? Curr Biol. 1998;8:R535–R538. doi: 10.1016/s0960-9822(07)00341-7. [DOI] [PubMed] [Google Scholar]
- 17.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 18.Chiang YJ, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 19.Joazeiro CA, et al. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 20.Kammer GM. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988;9:222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Yee C, Beavo JA. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283:848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- 22.Cone RE, Cochrane R, Lingenheld EG, Clark RB. Elevation of intracellular cyclic AMP induces an anergic-like state in Th1 clones. Cell Immunol. 1996;173:246–251. doi: 10.1006/cimm.1996.0274. [DOI] [PubMed] [Google Scholar]
- 23.Boussiotis VA, et al. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat Med. 2000;6:290–297. doi: 10.1038/73144. [DOI] [PubMed] [Google Scholar]
- 24.Lenschow DJ, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 25.Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998;161:2762–2771. [PubMed] [Google Scholar]
- 26.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 28.Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- 29.Coyle AJ, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 30.Hutloff A, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 31.Dong C, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 32.McAdam AJ, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 33.Tafuri A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 34.Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- 35.Yoshinaga SK, et al. Characterization of a new human B7-related protein: B7RP-1 is the ligand to the co-stimulatory protein ICOS. Int Immunol. 2000;12:1439–1447. doi: 10.1093/intimm/12.10.1439. [DOI] [PubMed] [Google Scholar]
- 36.Aicher A, et al. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- 37.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 38.Riley JL, et al. ICOS costimulation requires IL-2 and can be prevented by CTLA-4 engagement. J Immunol. 2001;166:4943–4948. doi: 10.4049/jimmunol.166.8.4943. [DOI] [PubMed] [Google Scholar]
- 39.Carreno BM, et al. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165:1352–1356. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- 40.Marengere LE, et al. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 41.Lee KM, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 42.Chuang E, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 43.Calvo CR, Amsen D, Kruisbeek AM. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and Jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor zeta and ZAP70. J Exp Med. 1997;186:1645–1653. doi: 10.1084/jem.186.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Vactor D, O’Reilly AM, Neel BG. Genetic analysis of protein tyrosine phosphatases. Curr Opin Genet Dev. 1998;8:112–126. doi: 10.1016/s0959-437x(98)80070-1. [DOI] [PubMed] [Google Scholar]
- 45.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 46.Nakaseko C, et al. Cytotoxic T lymphocyte antigen 4 (CTLA-4) engagement delivers an inhibitory signal through the membrane-proximal region in the absence of the tyrosine motif in the cytoplasmic tail. J Exp Med. 1999;190:765–774. doi: 10.1084/jem.190.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cinek T, Sadra A, Imboden JB. Cutting edge: tyrosine-independent transmission of inhibitory signals by CTLA-4. J Immunol. 2000;164:5–8. doi: 10.4049/jimmunol.164.1.5. [DOI] [PubMed] [Google Scholar]
- 48.Baroja ML, et al. The inhibitory function of CTLA-4 does not require its tyrosine phosphorylation. J Immunol. 2000;164:49–55. doi: 10.4049/jimmunol.164.1.49. [DOI] [PubMed] [Google Scholar]
- 49.Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 50.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latchman Y, et al. PD-L2 is a second ligand for PD-I and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 52.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 54.Billadeau DD, Leibson PJ. ITAMs versus ITIMs: striking a balance during cell regulation. J Clin Invest. 2002;109:161–168. DOI:10.1172/JCI200214843. doi: 10.1172/JCI14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vivier E, Daeron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 56.Chapoval AI, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 57.Mandelbrot DA, et al. B7-dependent T-cell costimulation in mice lacking CD28 and CTLA4. J Clin Invest. 2001;107:881–887. doi: 10.1172/JCI11710. [DOI] [PMC free article] [PubMed] [Google Scholar]