Mutations that impair polypeptide folding commonly result in an unstable and hypofunctional gene product. Their phenotype reflects this loss of function, and the resulting genetic disorder is usually transmitted as a recessive trait. Common forms of cystic fibrosis, hemophilia, and familial hypercholesterolemia are examples of this genetic mechanism in action. A second class of mutations (so-called gain-of-function mutations) encodes proteins with new functions, whose associated disorders are typically transmitted as dominant traits. Some of these are “dominant negative” alleles whose encoded protein can interact with components of the cellular machinery normally accessed by the wild-type gene product. With varying degrees of specificity, these mutations affect the activities of the wild-type allele, for example by disrupting the assembly of a multisubunit complex. Recent observations suggest that gain-of-function mutations that affect protein folding can also impair cellular function by less specific mechanisms related to the ability of the mutant protein to challenge the folding capacity in specific cellular compartments. Such mutant proteins are hypothesized to act as proteotoxins and may play a role in important human diseases (1, 2). The paper by Oyadomari et al. (3) appearing in this issue of the JCI addresses important issues related to proteotoxicity in the endoplasmic reticulum (ER).
The Ins2C96Y mutation found in the Akita diabetic mouse precludes formation of an essential disulfide bond between insulin 2 chains and prevents proper folding and processing of this protein. The mutant, malfolded proinsulin-2 is retained in the pancreatic β cell ER (4), presumably by the quality control mechanisms that normally allow only properly folded proteins to exit the ER and progress in the secretory pathway (5). Mice carrying the Ins2C96Y mutation develop progressive diabetes mellitus, a phenotype that probably reflects more than merely loss of hormone production by the mutant Ins2 allele, as rodents have two insulin genes (Ins1 and Ins2) and even loss of both copies of Ins2 is fully compensated (6).
What, then, is the basis of the gain-of-function phenotype associated with the Ins2C96Y Akita mutation? Ins2C96Y mutant mice are born with normal-sized islets of Langerhans and a normal complement of insulin-producing β cells. Over time, however, they undergo a progressive loss of β cells. Apoptosis of these cells correlates with the development of diabetes mellitus. This aspect of the pathophysiology of the Akita mouse can be reproduced in vitro by expressing high levels of Ins2C96Y (but not wild-type Ins2) in the β cell line Min6 (3). It is likely, therefore, that expression of Ins2C96Y is toxic to islet cells and that loss of β cell mass plays a role in the development of hyperglycemia in the Akita mouse.
ER stress and cell death
Eukaryotic cells maintain equilibrium between the load of client proteins their ER must process and the capacity of the organelle to carry out this function. A threat to this equilibrium is referred to as ER stress and is counteracted by two distinct adaptations collectively referred to as the unfolded protein response (UPR) (7, 8). The first of these adaptations attenuates protein biosynthesis, to immediately relieve the load on the organelle. The second increases the synthesis of components of the machinery by which the ER processes client proteins (upregulating chaperones, glycosylation enzymes, oxidases, and other ER resident proteins). Several mediators of UPR signaling have been identified recently. Their activity is consistent with a model whereby the cell tries to defend a certain chaperone reserve. When this functional reserve is challenged, it appears, the cell senses ER stress and responds by activating the UPR (9, 10). Accordingly, the level of chaperones and other ER components is a measure of the level of ER stress the cell is under.
High levels of sustained ER stress can also lead to programmed cell death, and some of the mediators of this response have recently been identified. One, caspase-12, an ER-associated cell death effector, is specifically activated by proteolytic processing during ER stress. Another, the transcription factor CHOP, is strongly upregulated during ER stress. Mutations in either of the corresponding genes inhibit cell death caused by ER stress without otherwise affecting the UPR (11, 12). IRE1, one of the direct mediators of the UPR, activates Jun N-terminal kinase (JNK) and can also promote cell death (13, 14). It appears, therefore, that cell death during ER stress results from the activity of dedicated components.
It had been noted earlier that the islet cells in the Akita mice express higher levels of the ER chaperone BiP, a marker of ER stress (4). Now, Oyadomari and colleagues show that CHOP is also activated in the Akita mouse islets (3). Neither of these findings is particularly surprising, given the impact of the Ins2C96Y mutation on insulin 2 folding. Insulin is a major ER client protein in β cells, and one might imagine that expression of high levels of a mutant insulin protein could severely tax the folding capacity of the organelle and elicit ER stress. The crucial question in this system is whether ER stress contributes to β cell death and the development of diabetes mellitus. A positive answer could not be taken for granted, as ER stress is a physiological phenomenon in many cells (including β cells) and can be well compensated for by physiological adaptations (15). Furthermore, there are other plausible reasons for islet cell death and diabetes mellitus in the Akita mouse. For example, the mutant insulin may impair the processing or secretion of wild-type insulin, by specific dominant negative mechanisms.
To examine the role of ER stress in islet cell death and diabetes mellitus, Oyadomari and colleagues (3) bred the Akita mutation onto the CHOP knockout background. The CHOP gene is induced by ER stress through a signaling pathway that involves activation of the pancreas ER kinase (PERK) and phosphorylation of the translation initiation factor eIF2α. CHOP encodes a transcription factor that promotes programmed cell death (11, 16), and the authors reasoned that if ER stress–mediated cell death plays a role in the phenotype of the Akita mouse, a background mutation in CHOP could ameliorate its features. This is exactly what they observed: CHOP–/–;Ins2C96Y/WT mice had preserved islet cell mass, less β cell apoptosis, delayed onset of hyperglycemia, higher pancreatic insulin content, and greater body weight than CHOP–/+;Ins2C96Y/WT or CHOP+/+;Ins2C96Y/WT mice. Because CHOP activation is a nonspecific feature of ER stress, these results suggest that the mutant insulin 2 protein accumulating in the ER of the β cell exerts at least some of its effects as a nonspecific proteotoxin.
While CHOP is strongly activated by ER stress, other stress signals such as oxidative stress and amino acid deprivation that bypass the ER altogether can also induce CHOP gene expression (17, 18); therefore it is formally possible that the contribution of CHOP to death of the Akita β cells also reflects the activity of other stress pathways operating in these cells. However, the role of CHOP in promoting cell death in response to these other forms of stress remains unproven. It is also important to note that the CHOP mutation provided no measurable benefit to the severely affected homozygous Ins2C96Y/C96Y mice. This last observation suggests the existence of CHOP-independent ER stress–mediated cell death pathways or other unrelated mechanisms causing dysfunction of the homozygous mutant β cells.
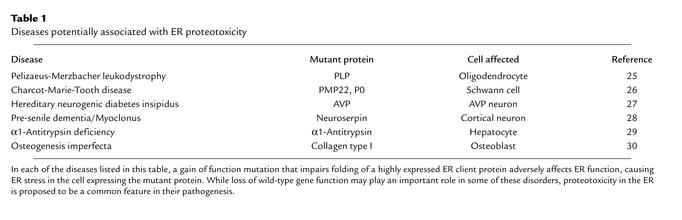
The aforementioned caveats aside, it is interesting to relate the observations described here to the possible role of nonspecific ER proteotoxicity in the pathogenesis of other genetic diseases and perhaps in other forms of diabetes mellitus. There are several human diseases caused by gain-of-function mutations affecting the folding of abundantly expressed ER client proteins (Table 1). Death or dysfunction of cells expressing the mutant protein goes a long way toward explaining the phenotype in these ER stress–associated genetic disorders, whereas interference of the mutant allele with the function of the wild-type one may be less important. In some cases, such as the PLP gene associated with Pelizaeus-Merzbacher leukodystrophy, or the PMP22 and P0 genes associated with Charcot-Marie-Tooth disease, the null state has a very different and sometimes weaker phenotype than does the dominant mutation affecting protein folding (19, 20). These findings are consistent with the idea that cell dysfunction or death by mechanisms related to those described here by Oyadomari and colleagues (3) might play a role in the pathogenesis of important human diseases.
Table 1.
Diseases potentially associated with ER proteotoxicity
Loss of β cell function plays an important role in the development of type 2 diabetes mellitus, but its causes remain unknown. Insulin biosynthesis is likely to result in a significant physiological load on the β cell. This load is certainly increased by insulin resistance, a universal feature of type 2 diabetes mellitus. The findings of Oyadomari and colleagues suggest that critical levels of ER stress can contribute to β cell death and the development of diabetes mellitus. The β cell may be especially sensitive to ER stress, as mice and humans lacking PERK, a critical component of the ER stress response, develop spontaneous diabetes mellitus at a very young age (reviewed in ref. 21). Together, these findings suggest that ER stress–mediated cell death or dysfunction may play a role in the development of common forms of diabetes mellitus. It may be possible to test this hypothesis by measuring the effect of CHOP or Caspase-12 mutations on the progressive diabetic phenotype of Db/Db (or Ob/Ob) C57BL/6KsJ mice. The latter provide good mouse models for the β cell attrition in human type 2 diabetes mellitus (22).
Evidence reviewed here for the possible role of ER stress–mediated programmed cell death and dysfunction in the pathogenesis of important human diseases suggests that the mediators of this response may be targets for therapeutic intervention. Enthusiasm for targeting the CHOP and Caspase-12 pathways should be tempered by the realization that mutations in either gene do not fully protect against cell death caused by ER stress. The phenotype of mutations that block CHOP’s upstream activators, PERK and phosphorylated eIF2α, reveals some of the complexities involved: PERK–/– cells and cells with mutations that prevent phosphorylation of PERK’s substrate, eIF2α, are unable to activate CHOP expression in response to ER stress (18, 23), yet both are dramatically hypersensitive to ER stress (15, 23, 24). PERK phosphorylation of eIF2α contributes substantially to both translational and transcriptional control in the UPR (15, 23). Therefore, the phenotype of the PERK and eIF2α mutations noted above demonstrates the important role of protective mechanisms against ER stress. These observations also suggest that augmenting these protective responses may be as important as blocking the known mediators of ER stress–induced cell death. In this broader context, the paper by Oyadomari and colleagues (3) should serve as a stimulus for further research into basic mechanisms of the ER stress response.
Footnotes
See the related article beginning on page 525.
References
- 1.Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 2.Carrell RW, Lomas DA. Conformational disease. Lancet. 1997;350:134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- 3.Oyadomari S, et al. Targeted disruption of the Chopgene delays endoplasmic reticulum stress–mediated diabetes. J Clin Invest. 2002;109:525–532. DOI:10.1172/JCI200214550. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 6.Leroux L, et al. Compensatory responses in mice carrying a null mutation for Ins1 or Ins2. Diabetes. 2001;50(Suppl. 1):S150–S153. doi: 10.2337/diabetes.50.2007.s150. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 8.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 9.Bertolotti A, Zhang Y, Hendershot L, Harding H, Ron D. Dynamic interaction of BiP and the ER stress transducers in the unfolded protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 10.Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress-response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 11.Zinszner H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa T, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 13.Urano F, et al. Coupling of stress in the endoplasmic reticulum to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 14.Wang XZ, et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harding H, et al. Diabetes mellitus and excocrine pancreatic dysfunction in Perk–/– mice reveals a role for translational control in survival of secretory cells. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 16.McCullough KD, et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fawcett TW, et al. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339:135–141. [PMC free article] [PubMed] [Google Scholar]
- 18.Harding H, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 19.Gow A, Lazzarini RA. A cellular mechanism governing the severity of Pelizaeus-Merzbacher disease. Nat Genet. 1996;13:422–428. doi: 10.1038/ng0896-422. [DOI] [PubMed] [Google Scholar]
- 20.Muller HW. Tetraspan myelin protein PMP22 and demyelinating peripheral neuropathies: new facts and hypotheses. Glia. 2000;29:182–185. doi: 10.1002/(sici)1098-1136(20000115)29:2<182::aid-glia12>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Sonenberg N, Newgard CB. Protein synthesis. The perks of balancing glucose. Science. 2001;293:818–819. doi: 10.1126/science.1062937. [DOI] [PubMed] [Google Scholar]
- 22.Baetens D, et al. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes. 1978;27:1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Scheuner D, et al. Translational control is required for the unfolded protein response and in-vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 24.Harding H, Zhang Y, Bertolotti A, Zeng H, Ron D. Perkis essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 25.Gow A, Southwood CM, Lazzarini RA. Disrupted proteolipid protein trafficking results in oligodendrocyte apoptosis in an animal model of Pelizaeus-Merzbacher disease. J Cell Biol. 1998;140:925–934. doi: 10.1083/jcb.140.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Urso D, Prior R, Greiner-Petter R, Gabreels-Festen AA, Muller HW. Overloaded endoplasmic reticulum-Golgi compartments, a possible pathomechanism of peripheral neuropathies caused by mutations of the peripheral myelin protein PMP22. J Neurosci. 1998;18:731–740. doi: 10.1523/JNEUROSCI.18-02-00731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito M, Jameson JL. Molecular basis of autosomal dominant neurohypophyseal diabetes insipidus. Cellular toxicity caused by the accumulation of mutant vasopressin precursors within the endoplasmic reticulum. J Clin Invest. 1997;99:1897–1905. doi: 10.1172/JCI119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RL, et al. Familial dementia caused by polymerization of mutant neuroserpin. Nature. 1999;401:376–379. doi: 10.1038/43894. [DOI] [PubMed] [Google Scholar]
- 29.Perlmutter DH. Alpha-1-antitrypsin deficiency. Semin Liver Dis. 1998;18:217–225. doi: 10.1055/s-2007-1007158. [DOI] [PubMed] [Google Scholar]
- 30.Lamande SR, Bateman JF. Procollagen folding and assembly: the role of endoplasmic reticulum enzymes and molecular chaperones. Semin Cell Dev Biol. 1999;10:455–464. doi: 10.1006/scdb.1999.0317. [DOI] [PubMed] [Google Scholar]