Engagement of certain cell surface receptors by the their cognate ligands leads to the rapid induction of cell death by an intracellular suicide program known as apoptosis (1). The signaling pathways employed by these “death receptors” appear to involve the sequential activation of the caspase group of cysteine proteases in an ordered and hierarchical manner (2). A well-described cofactor in this process is FADD, a key adapter protein that mediates the recruitment of proximal caspases to the cell surface receptor (3). However, a recent report by Bannerman et al. in the JCI (4) reveals an intriguing additional function for FADD as a regulator of the transcription factor NF-κB.
FADD is best known as an intracellular factor that, upon ligand binding, is recruited to the cytoplasmic domain of Fas (also known as Apo-1 and CD95), a member of the TNF receptor (TNFR) superfamily (5, 6). Fas and FADD associate through a conserved motif known as the death domain (DD), which is found in both the Fas cytoplasmic tail and the FADD protein (7). In turn, FADD can recruit certain caspases, notably caspase-8, through a second element known as the death effector domain, present in FADD and in the prodomain of the caspase.
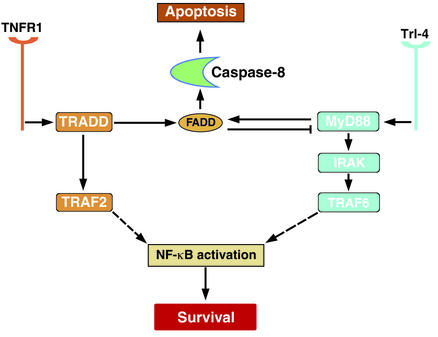
How might FADD control the apparently unrelated NF-κB pathway? A complex relationship exists between the control of apoptosis and NF-κB. Studies of signaling through the type 1 TNFR (TNFR1) have demonstrated the activation of both a proapoptotic pathway (similar to the Fas mechanism) and NF-κB (8); in this case activation of NF-κB appears to provide a prosurvival signal whose mechanism is still unclear but that is presumed to involve the transcriptional induction of various apoptotic suppressors (9). As reviewed recently by Budd (10), variations on this theme are numerous and may be cell type–specific. The existence of these two opposing signals may explain why many cells bearing TNF receptors do not undergo apoptosis upon TNF binding. Similarly, the proinflammatory molecule lipopolysaccharide (LPS) can act through the Toll-like receptor Tlr-4 to induce caspase and NF-κB activation. Some of the intracellular components involved in Tlr-4 signaling are different (the adapters MyD88, FADD, and the NF-κB–activating kinase IRAK are recruited to the Tlr-4 cytoplasmic domain in the latter pathway), but the paradigm is the same: caspases are activated, but so is NF-κB (11). The report by Bannerman et al. (4) shows that FADD can function in both pathways to activate apical caspases while at the same time suppressing NF-κB signaling (Figure 1). The precise manner in which FADD achieves this is still unclear. The authors speculate that through binding to MyD88, FADD might competitively displace IRAK from the complex. An alternative mechanism would be the direct binding of FADD to IRAK.
Figure 1.
Central components of the TNF and Tlr-4 signaling pathways. Recruitment and activation of FADD suppress Tlr-4– but not TNFR1-mediated signaling, presumably through an interaction with MyD88. See text for details.
As pointed out by Bannerman et al. (4), previous studies have found that FADD can act as a suppressor — and not, as in this report, an inducer — of NF-κB (12–14). One of the most significant experimental differences between these reports is the use by Bannerman et al. of lines containing stably overexpressed FADD, as well as lines derived from FADD-deficient mice. In contrast, the earlier studies employed transient overexpression of FADD. Differences in cell type and the nature of the stimulus may also have a profound influence. Indeed, Bannerman et al. found that, whereas FADD-deficient embryonic fibroblasts show heightened induction of NF-κB following LPS treatment, their response to TNF is normal (4).
Perhaps the most fascinating question arising from these studies concerns the physiologic advantage of coordinating caspase induction and NF-κB pathway suppression by FADD. Could the suppression of NF-κB by FADD determine the level of sensitivity to apoptosis? Given the ability of FADD to specifically block the induction of NF-κB by LPS, this is a possibility that should be fairly straightforward to test. Secondly, could the cell utilize this cross-talk mechanism to manage a more likely physiologic scenario, the simultaneous induction of more than one receptor pathway? For example, if LPS-mediated NF-κB induction is suppressed by FADD, would LPS stimulation alter the cellular responsiveness to, say, TNF? These types of studies might have great clinical significance, particularly in our understanding of the mechanism by which LPS-expressing Gram-negative bacteria induce septic shock. A tantalizing question raised by these studies is whether the effects seen by Bannerman et al. (4) could be regulated by neutralization of the TNF pathway. Thus, a more complete understanding of the signaling cascades induced by LPS may have great value in the treatment of septicemia.
Footnotes
See the related article beginning on page 419.
References
- 1.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 2.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 3.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 4.Bannerman DD, Tupper JC, Kelly JD, Winn RK, Harlan JM. The Fas-associated death domain protein suppresses activation of NF-κB by LPS and IL-1β. J Clin Invest. 2002;109:419–425. DOI:10.1172/JCI200214774. doi: 10.1172/JCI14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata S. Apoptosis: telling cells their time is up. Curr Biol. 1996;6:1241–1243. doi: 10.1016/s0960-9822(02)70706-9. [DOI] [PubMed] [Google Scholar]
- 6.Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- 7.Wallach D, et al. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 8.Hsu H, Shu H-B, Pan M-G, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis, while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 10.Budd RC. Death receptors couple to both cell proliferation and apoptosis. J Clin Invest. 2002;109:437–442. DOI:10.1172/JCI200215077. doi: 10.1172/JCI15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang G, Ghosh S. Toll-like receptor–mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhary PM, et al. Activation of the NF-κB pathway by caspase 8 and its homologs. Oncogene. 2000;19:4451–4460. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 13.Hu WH, Johnson H, Shu HB. Activation of NF-κB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838–10844. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 14.Schaub FJ, et al. Fas/FADD-mediated activation of a specific program of inflammatory gene expression in vascular smooth muscle cells. Nat Med. 2000;6:790–796. doi: 10.1038/77521. [DOI] [PubMed] [Google Scholar]