Abstract
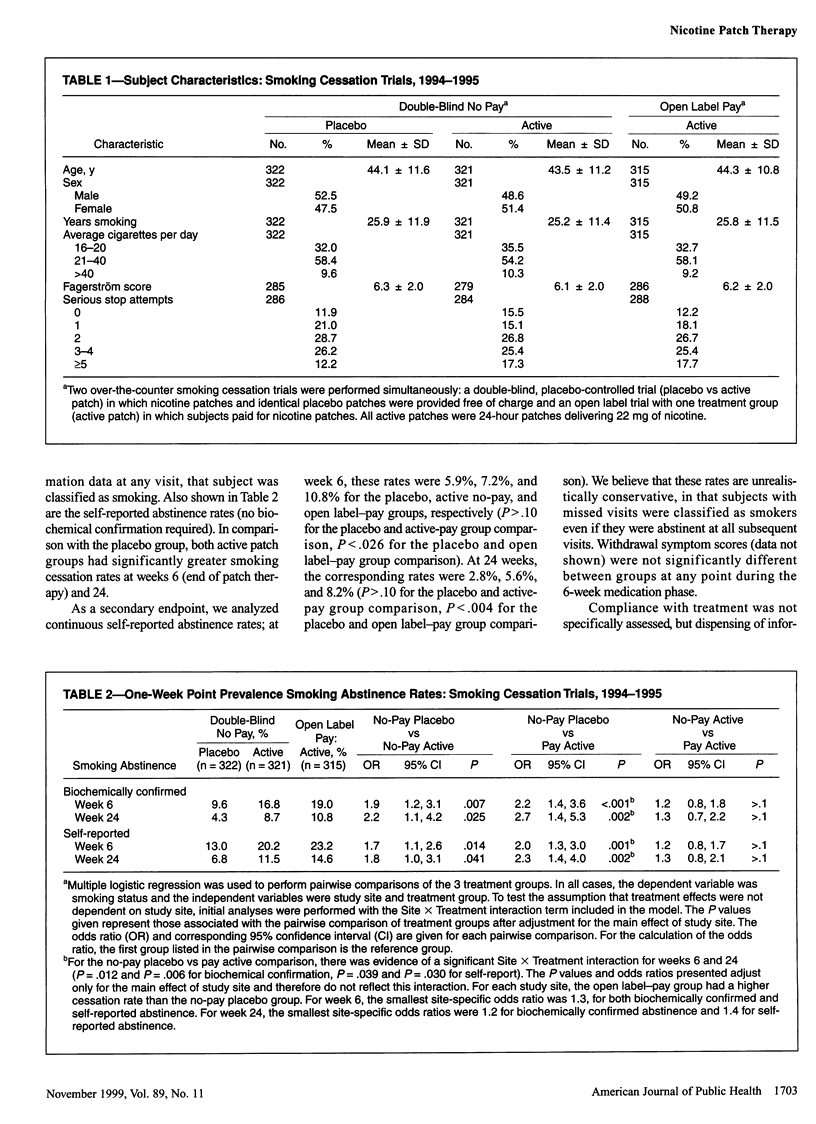
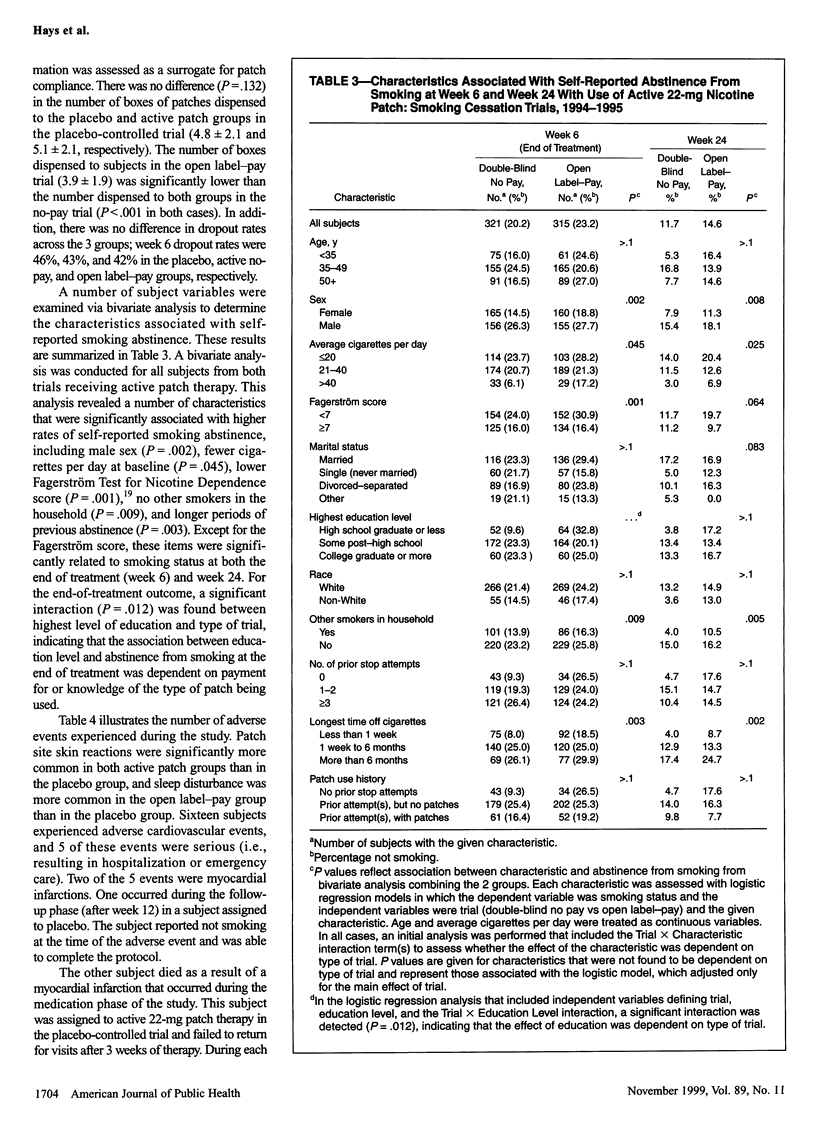
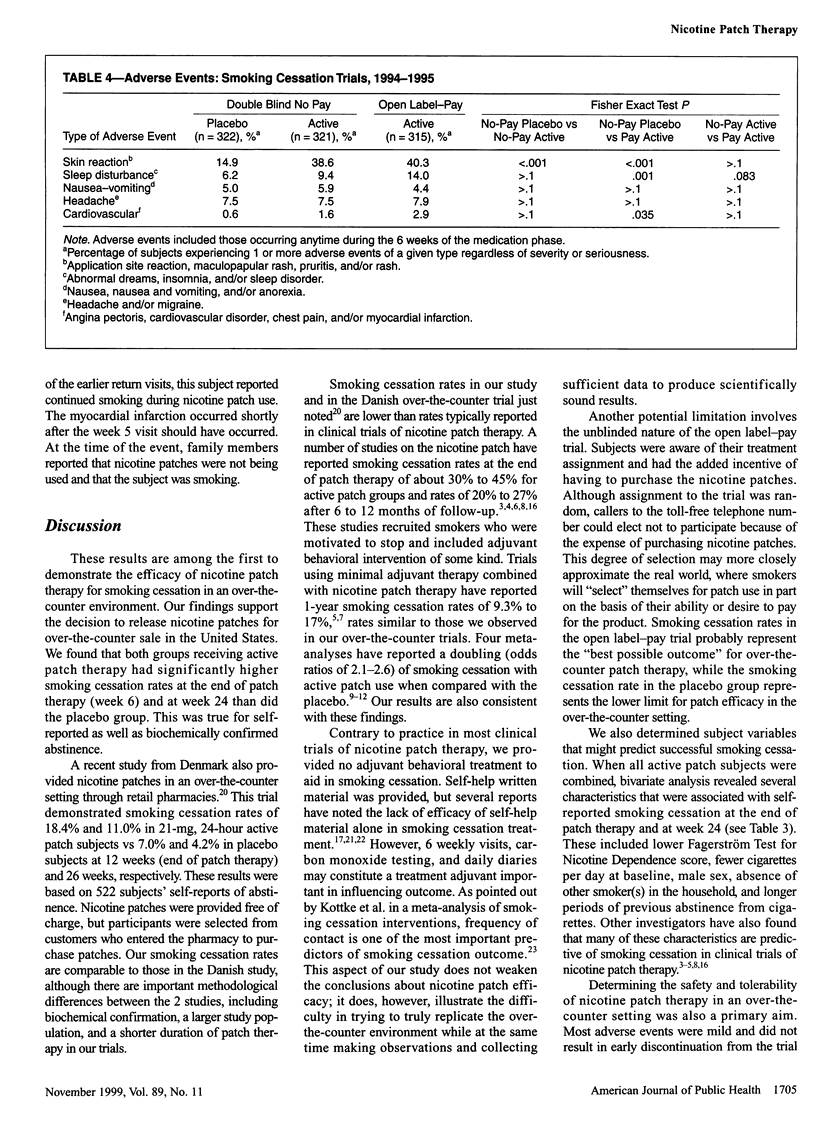
OBJECTIVES: The purpose of this study was to determine the efficacy and safety of the nicotine patch for smoking cessation in an over-the-counter environment. The years of study were 1994 to 1995. METHODS: Parallel 6-week trials were conducted: a placebo-controlled trial of no-cost 22-mg, 24-hour nicotine patch therapy and an open label trial of the same therapy with patches purchased by subjects. Participants (n = 958) were 18 years or older, had smoked at least 15 cigarettes daily for at least 6 months, and were enrolled at 3 study sites. The main outcome measure was self-reported smoking abstinence confirmed by expired carbon monoxide measurements. RESULTS: Smoking cessation rates in the placebo-controlled trial were 16.8% and 9.6% at week 6 and 8.7% and 4.3% at week 24 for the active patch and placebo groups, respectively. Smoking cessation rates in the open label-pay trial were 19.0% and 10.8% at weeks 6 and 24, respectively. A slight increase in adverse cardiovascular events was noted only in the open label-pay group in comparison with the placebo group. CONCLUSIONS: In an over-the-counter environment, the 22-mg, 24-hour nicotine patch is effective and safe for smoking cessation treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curry S. J., McBride C., Grothaus L. C., Louie D., Wagner E. H. A randomized trial of self-help materials, personalized feedback, and telephone counseling with nonvolunteer smokers. J Consult Clin Psychol. 1995 Dec;63(6):1005–1014. doi: 10.1037//0022-006x.63.6.1005. [DOI] [PubMed] [Google Scholar]
- Daughton D. M., Heatley S. A., Prendergast J. J., Causey D., Knowles M., Rolf C. N., Cheney R. A., Hatlelid K., Thompson A. B., Rennard S. I. Effect of transdermal nicotine delivery as an adjunct to low-intervention smoking cessation therapy. A randomized, placebo-controlled, double-blind study. Arch Intern Med. 1991 Apr;151(4):749–752. [PubMed] [Google Scholar]
- Fiore M. C., Jorenby D. E., Baker T. B., Kenford S. L. Tobacco dependence and the nicotine patch. Clinical guidelines for effective use. JAMA. 1992 Nov 18;268(19):2687–2694. [PubMed] [Google Scholar]
- Fiore M. C., Smith S. S., Jorenby D. E., Baker T. B. The effectiveness of the nicotine patch for smoking cessation. A meta-analysis. JAMA. 1994 Jun 22;271(24):1940–1947. [PubMed] [Google Scholar]
- Gourlay S. The pros and cons of transdermal nicotine therapy. Med J Aust. 1994 Feb 7;160(3):152–159. [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991 Sep;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986 Mar;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hurt R. D., Dale L. C., Fredrickson P. A., Caldwell C. C., Lee G. A., Offord K. P., Lauger G. G., Marŭsić Z., Neese L. W., Lundberg T. G. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA. 1994 Feb 23;271(8):595–600. [PubMed] [Google Scholar]
- Joseph A. M., Norman S. M., Ferry L. H., Prochazka A. V., Westman E. C., Steele B. G., Sherman S. E., Cleveland M., Antonuccio D. O., Antonnucio D. O. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996 Dec 12;335(24):1792–1798. doi: 10.1056/NEJM199612123352402. [DOI] [PubMed] [Google Scholar]
- Kottke T. E., Battista R. N., DeFriese G. H., Brekke M. L. Attributes of successful smoking cessation interventions in medical practice. A meta-analysis of 39 controlled trials. JAMA. 1988 May 20;259(19):2883–2889. doi: 10.1001/jama.259.19.2883. [DOI] [PubMed] [Google Scholar]
- Li Wan Po A. Transdermal nicotine in smoking cessation. A meta-analysis. Eur J Clin Pharmacol. 1993;45(6):519–528. doi: 10.1007/BF00315308. [DOI] [PubMed] [Google Scholar]
- Orleans C. T., Resch N., Noll E., Keintz M. K., Rimer B. K., Brown T. V., Snedden T. M. Use of transdermal nicotine in a state-level prescription plan for the elderly. A first look at 'real-world' patch users. JAMA. 1994 Feb 23;271(8):601–607. [PubMed] [Google Scholar]
- Pierce J. P., Gilpin E., Farkas A. J. Nicotine patch use in the general population: results from the 1993 California Tobacco Survey. J Natl Cancer Inst. 1995 Jan 18;87(2):87–93. doi: 10.1093/jnci/87.2.87. [DOI] [PubMed] [Google Scholar]
- Russell M. A., Stapleton J. A., Feyerabend C., Wiseman S. M., Gustavsson G., Sawe U., Connor P. Targeting heavy smokers in general practice: randomised controlled trial of transdermal nicotine patches. BMJ. 1993 May 15;306(6888):1308–1312. doi: 10.1136/bmj.306.6888.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs D. P., Säwe U., Leischow S. J. Effectiveness of a 16-hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Arch Intern Med. 1993 Aug 23;153(16):1881–1890. [PubMed] [Google Scholar]
- Silagy C., Mant D., Fowler G., Lodge M. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet. 1994 Jan 15;343(8890):139–142. doi: 10.1016/s0140-6736(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Sønderskov J., Olsen J., Sabroe S., Meillier L., Overvad K. Nicotine patches in smoking cessation: a randomized trial among over-the-counter customers in Denmark. Am J Epidemiol. 1997 Feb 15;145(4):309–318. doi: 10.1093/oxfordjournals.aje.a009107. [DOI] [PubMed] [Google Scholar]
- Tønnesen P., Nørregaard J., Simonsen K., Säwe U. A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med. 1991 Aug 1;325(5):311–315. doi: 10.1056/NEJM199108013250503. [DOI] [PubMed] [Google Scholar]
- Westman E. C., Levin E. D., Rose J. E. The nicotine patch in smoking cessation. A randomized trial with telephone counseling. Arch Intern Med. 1993 Aug 23;153(16):1917–1923. [PubMed] [Google Scholar]



