This Perspective series seeks to summarize the present concepts regarding the biological processes that mediate intrinsic and innate host defense against microbial invasion of the lung. From an evolutionary standpoint, the requirements for an extensive gas exchange region that comes in direct contact with inhaled particles and pathogens present a formidable challenge that has been countered by the layering and intersection of several intrinsic defense systems.
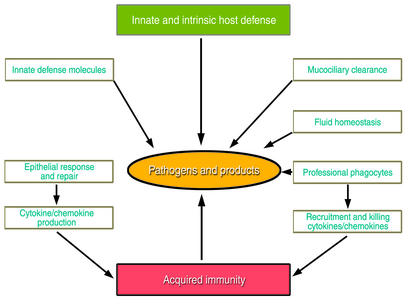
These systems, detailed in the accompanying articles, maintain pulmonary form and function required for ventilation by means of structural, mechanical, chemical, and cellular strategies, as well as innate and acquired host defenses. The epithelial barrier itself represents a first line of defense against pathogens, and its effectiveness is greatly enhanced by fluid homeostasis and mucociliary clearance. As discussed by Knowles and Boucher in this series, these mechanisms protect the epithelium and physically remove inhaled pathogens from the lung. Additional protection comes from polypeptide mediators of the innate host defense, such as the defensins and other antibiotic peptides reviewed by Ganz in this series, and the collectin family, considered by McCormack and Whitsett. Professional phagocytes also play their part in host defense in this tissue, in part by responding to molecules found on the surface of common pathogenic microbes. Finally, the cytokine and chemokine pathways that cooperate with host defense polypeptides to mediate initial host defenses by phagocytes also orchestrate tissue repair and subsequent acquired immune responses following infection, as discussed by Strieter et al. The interplay of these various mechanisms is depicted in Figure 1.
Figure 1.
The sterility of the respiratory tract is maintained by the concerted actions of the epithelial barrier, innate defense molecules, and responses of both the epithelium and professional phagocytes that remove pathogens and their products from the lung.
The concerted effects of mechanical, innate, and acquired host defense systems serve to recognize, localize, kill, and remove pathogens to maintain sterility of pulmonary tissues and the host. In general, pathogens are cleared from the lung without persistent or robust inflammation, thus protecting its inherent structure and maintaining its function following exposure to microbes. It is the failure of various arms of the host defense systems that allows local or systemic infection and destruction of lung tissue that are frequently the basis of morbidity and mortality from common lung diseases, whether related to genetic or environmental disease processes. Deficient or uncontrolled host defenses and inflammation underlie some of the most common pulmonary disorders — chronic obstructive pulmonary disease, cystic fibrosis, acute and chronic pneumonia, asthma, emphysema, and bronchopulmonary dysplasia.
Structure and function of the respiratory epithelium: form dictates function
The lung is a complex organ consisting of a series of branching tubules and alveoli that are highly vascularized to provide a large gas exchange surface. The respiratory tract is lined by endodermally derived epithelial cells that differentiate from the foregut endoderm. Commitment and proliferation of respiratory epithelial cells are dependent upon mesenchymal-epithelial interactions, mediated by a number of distinct and intersecting autocrine-paracrine pathways (e.g., bone morphogenetic protein–4 [BMP-4], FGF, sonic hedgehog [SHH]; see ref. 1 for review), which, in turn, regulate gene transcription to influence cell fate, proliferation, and function. In the lung, commitment and differentiation of the respiratory epithelium are mediated, at least in part, by the homeodomain protein NKx2.1 (also known as thyroid transcription factor-1 [TTF-1]), as well as transcription factors of the forkhead family (hepatocyte nuclear factor-3β [HNF-3β], HNF-3α, and hepatocyte factor homologue-4 [HFH-4]) and the zinc finger family (GATA-6) (reviewed in refs. 2–4). Lung tubules undergo stereotypic, dichotomous branching to form bronchi, bronchioles, and the peripheral airways and alveoli.
Initially, the lung is lined by an undifferentiated columnar epithelium, but as lung morphogenesis proceeds, the tubules are lined by an increasingly diverse population of respiratory epithelial cells that vary both spatially — along the cephalocaudal axis — and temporally. In the adult human lung, conducting airways are lined by a pseudostratified epithelium consisting of at least a dozen morphologically distinct epithelial cells, including squamous, ciliated, nonciliated bronchiolar (Clara cells), basal, intermediate, serous, goblet, and neuroepithelial cells, as well as alveolar type II and type I epithelial cells. This diversity of cell type accomplishes the various physiologic tasks that optimize mucociliary clearance, precise regulation of fluid homeostasis, and the synthesis and secretion of a myriad of such host defense proteins as lysozyme, defensins, surfactant proteins (SPs), and lactoferrin. The abundance and activity of the various cell types, which are strongly influenced by infection and other inflammatory stimuli, can themselves influence host defense function and respiratory cell proliferation and differentiation during repair. In the normal lung, the diverse repertoire of respiratory epithelial cells is maintained in their proper places and activation states, and they interact with professional phagocytes and the lymphoid components of the acquired immune system. In addition, an extensive system of tracheal-bronchial glands is lined by distinct epithelial cell types that produce mucous, fluid, and other host defense proteins critical for mucociliary activity that clears particles and pathogens from the lung. The generation and maintenance of this panoply of epithelial cell types provide structural framework required for host defense functions. Acute injury and chronic injury lead to epithelial cell dysplasia and metaplasia, as in the conducting airways in smokers, where squamous cell metaplasia and goblet cell hyperplasia are common. Epithelial defenses and mucociliary clearance are thus compromised, rendering the lung susceptible to injury by particles and pathogens.
Transcriptional control of lung formation and repair
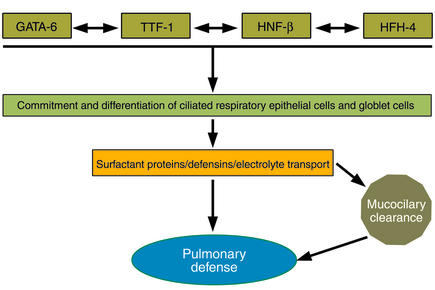
The formation, maintenance, and repair of the postnatal lung depend upon precisely regulated expression of many of the same transcription factors involved in formation of the lung earlier in lung morphogenesis. Three distinct families of transcription factors, including the homeodomain protein TTF-1, the zinc finger protein GATA-6, and a forkhead family member, HNF-3β (also known as Foxβ2), play critical roles in the differentiation of foregut endoderm to form the progenitors of the lung parenchyma (Figure 2). GATA-6 and HNF-3β are involved in formation of the endoderm per se (5–8), while TTF-1 is required for the generation of epithelial cells that form the lung periphery (9). Later in development, these same transcription factors regulate the transcription of host defense molecules, such as SP-A, SP-B, SP-C, and Clara cell secretory protein, modifiers of lung inflammation following infection or injury (10).
Figure 2.
Transcription proteins, including TTF-1, HNF, and GATA family members, mediate lung morphogenesis, epithelial cell differentiation, and gene expression, creating the diverse cells of the epithelial barrier and regulating intrinsic and innate host defense responses.
The precise temporal/spatial formation and function of the respiratory epithelium are controlled at the level of transcription, mediated by the con-certed actions of both tissue-specific and ubiqui-tous nuclear proteins. These nuclear transcription proteins influence differentiation of specific epithelial cell types and regulate the synthesis of the distinct host defense proteins elaborated by subsets of respiratory epithelial cells. For example, during development, HFH-4 (also known as Foxj1) is required for ciliated cell differentiation, expression of β-tubulin, and formation of cilia in conducting airways (11). Defects in ciliated cell function, as seen in ciliary dyskinesia and Kartagener syndrome, cause situs inversus and disturb mucociliary clearance, leading to chronic infection and destruction of lung tissues. While initially derived from common progenitors, the heterogeneous epithelial cell types lining the respiratory tract are generated under the direction of cell-cell, cell-matrix, autocrine-paracrine, and transcriptional pathways that are influenced by their microenvironments. Distinct respiratory epithelial cells synthesize diverse classes of host defense molecules that maintain pulmonary sterility. There is increasing evidence that the genetic pathways that initially direct lung morphogenesis during development are later employed to regulate the synthesis of host defense molecules and cellular responses to pathogens that, in turn, influence cell proliferation and tissue remodeling. Repair following injury, at least in part, recapitulates molecular and cellular processes mediating lung formation.
Shared pathways mediating lung morphogenesis, innate host defenses, and repair
The vertebrate lung is a relatively late evolutionary adaptation required for terrestrial survival, first appearing approximately 350–400 million years ago in the mid-Devonian fossil record. Of the estimated 40,000 distinct genes expressed by the human genome, relatively few are uniquely expressed in the lung, many of the molecular pathways controlling lung morphogenesis being borrowed from similar processes in other organs conserved among diverse phyla that were established long before the advent of the lung. Unique combinations of molecules mediating signaling and transcriptional pathways in many organs are used to form and maintain lung structure and function.
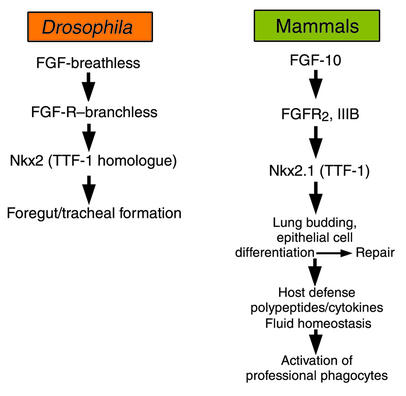
Signaling pathways involved in lung morphogenesis, lung defense, and repair are highly conserved. FGF receptor–, transcription factor–, cytokine-, and NF-κB–dependent pathways shared among diverse phyla participate in lung morphogenesis and repair. It is perhaps the unique combinatorial use of ancient signaling mechanisms that provides the molecular framework underlying lung formation and function. Indeed, the genes involved in generation and function of the lung have ancient antecedents, for example in Caenorhabditis elegans and Drosophila. FGF signaling via the Drosophila genes branchless and breathless (an FGF receptor [FGFR] and ligand, respectively) is required for formation of the tracheal system in the fly (12, 13), much as it is for lung formation in vertebrates (Figure 3). Likewise, FGF signaling medi-ates diverse functions of the respiratory epithelium. Deletion of either FGF-10 or FGFR signaling results in nearly complete lack of lung formation in the mouse (14, 15). Surprisingly, mutation in FGF-10 (11) or the FGFR2IIIb (16) also blocks formation of the limbs, FGF signaling mutants being both “lungless” and “limbless.” It may be significant that the FGF pathway determines the formation of two organs that have been critical for adaptation of vertebrates to terrestrial habitats; namely, breathing and tetrapod ambulation.
Figure 3.
FGF signaling in formation of the trachea and lung has been conserved from insects to vertebrates. FGFs influence proliferation, migration, and differentiation of respiratory epithelial cells, surfactant protein, and other host defense polypeptides that, in turn, orchestrate cytokine expression and the function of professional phagocytic cells mediating innate and acquired host defenses.
While FGF signaling is critical for lung formation, FGF family members also enhance epithelial cell proliferation and differentiation in the postnatal lung (17). Both FGF-7 and FGF-10 increased respiratory epithelial cell proliferation and enhanced expression of TTF-1 and its downstream targets, e.g., SP-A, -B, and -C, that are involved in innate defense and surfactant activity in the lung (18, 19). Furthermore, FGF stimulated production of cytokines and chemokines, recruiting and activating professional phagocytes. Likewise, FGF increased the expression of aquaporin V and C1-dependent fluid secretion to regulate fluid transport by the respiratory epithelium (18–20).
NF-κB: another intersection between lung morphogenesis and innate host defense
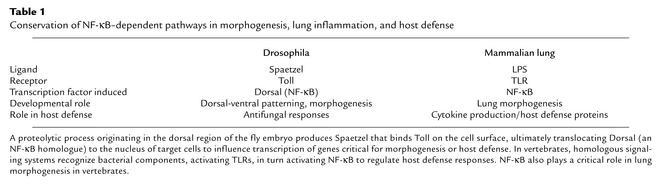
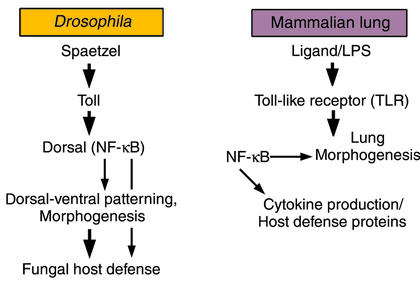
Transcriptional control of both acquired and innate host defense systems in the lung and other organs depends upon the nuclear translocation and activation of NF-κB in target cells (21). NF-κB family members include at least three polypeptides, P50, P65, and Rel-A. Like the FGF signaling pathway, NF-κB plays a dual role in lung morphogenesis and inflammatory responses to various pathogens. NF-κB is regulated by cell surface receptors of the Toll-like receptor (TLR) family. Activation of TLR/NF-κB is regulated, at least in part, by binding of pattern recognition molecules that present carbohydrate peptide or lipid components of pathogens (for example, endotoxin and proteoglycan) to professional phagocytes or epithelial cell surfaces (reviewed in ref. 22). In the lung, both SP-A and SP-D, members of the collectin family of polypeptides, bind promiscuously to complex carbohydrates, serving as nonself pattern recognition molecules, binding the surfaces and products of respiratory pathogens, presenting them for phagocytosis, and modulating subsequent inflammatory responses during infection (23). Furthermore, SP-A and SP-D interact with CD14/TLR to influence cellular responses to pathogens via transcriptional pathways activated by NF-κB translocation to the nucleus of responding cells (24, 25). Members of the TLR family and associated pathways were first recognized for their involvement in dorsal-ventral patterning during Drosophila development and in fungal host defense (26). TLRs and their homologues are critical mediators of innate defenses in plants and animals, activating gene transcription via NF-κB to enhance killing of microorganisms and the inflammatory response (Table 1; see also ref. 22). Thus, the ancient NF-κB pathway serves complex and critical roles in both morphogenesis and antimicrobial host defense.
Table 1.
Conservation of NF-κB–dependent pathways in morphogenesis, lung inflammation, and host defense
NF-κB and gene expression in lung morphogenesis and repair
Increased activity of NF-κB in embryonic lung mesenchyme inhibits growth and budding of the respiratory epithelium, while inhibition of NF-κB signaling enhances proliferation and budding of the respiratory epithelial cells in a process likely mediated by autocrine-paracrine mechanisms (27). While the mechanisms of action of NF-κB in the lung mesenchyme are not fully clarified, enhanced NF-κB activity blocks FGF-10 expression in the embryonic lung, suggesting that NF-κB and FGF signaling are linked during both lung and limb morphogenesis. NF-κB also regulates the expression of the water transport protein aquaporin V (28), various innate host defense proteins (defensins and mucins), and cytokines, and it activates professional phagocytes following pulmonary injury or infection.
Events in lung morphogenesis, at least in part, are recapitulated during inflammation and repair. As shown in Figure 4, the multicomponent innate defense response mollifies inflammation and initiates morphogenetic events that result in the orderly repair of the respiratory epithelium and underlying stroma. Indeed, FGF and NF-κB pathways are critically involved in lung repair. TTF-1 and its downstream targets are also increased during repair following severe lung injury (29). FGF protects respiratory epithelial cells during lung injury in vivo (30) and enhances expression of TTF-1 and its demonstrated targets, SP-A, SP-B, and SP-C, as well as fluid transport proteins (18–20). TTF-1 regulates epithelial cell differentiation and proliferation and influences the expression of innate host defense molecules. In turn, these molecules influence the activation state, oxidant production, and apoptotic activity of professional phagocytes that are involved in clearance of apopto-tic cells and pathogens from the lung (23).
Figure 4.
Formation and function of the lung are mediated by intrinsic and innate defense systems that are orchestrated by ancient transcriptional and signaling pathways that have allowed adaptation of vertebrates to air breathing.
The multiplicity and interaction of structural and cellular components of the intrinsic and innate host defenses of the lung are orchestrated temporally and spatially and are graded stochastically to prevent initial invasion, to limit the extent of injury, and to achieve rapid repair following pulmonary infection by respiratory pathogens. Detailed knowledge of these pathways should improve our understanding of the pathogenesis of common lung diseases and lead to new therapies based on knowledge of lung morphogenesis, repair, and innate host defense systems.
References
- 1.Hogan BLM. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 2.Perl AKT, Whitsett JA. Molecular mechanisms controlling lung morphogenesis. Clin Genet. 1999;56:14–27. doi: 10.1034/j.1399-0004.1999.560103.x. [DOI] [PubMed] [Google Scholar]
- 3.Costa RH, Kalinichenko VV, Lim L. Transcription factors in mouse lung development and function. Am J Physiol. 2001;280:L823–L838. doi: 10.1152/ajplung.2001.280.5.L823. [DOI] [PubMed] [Google Scholar]
- 4.Warburton D, et al. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 5.Ang SL, Rossant J. HNF-3β is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 6.Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 7.Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- 8.Keijzer R, et al. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development. 2001;128:503–511. doi: 10.1242/dev.128.4.503. [DOI] [PubMed] [Google Scholar]
- 9.Kimura S, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 10.Bohinski RJ, DiLauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brody SL, Yan XH, Wuerffel MK, Song S-K, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland D, Samakoulis C, Krasnow MA. Branchless encodes a Drosophila Fgf homolog that controls tracheal migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- 13.Glazer L, Shilo BZ. The Drosophila FGF-R homolog is expressed in the embryonic tracheal system and appears to be required for directed tracheal cell extension. Genes Dev. 1991;5:697–705. doi: 10.1101/gad.5.4.697. [DOI] [PubMed] [Google Scholar]
- 14.Peters K, et al. Targeted expression of a dominant negative FGF receptor blocks branching morphogenesis and epithelial differentiation of the mouse lung. EMBO J. 1994;13:3296–3301. doi: 10.1002/j.1460-2075.1994.tb06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min H, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajihosseini MK, Wilson S, De Moerlooze L, Dickson C. A splicing switch and gain-of-function mutation in FgfR2-IIIc hemizygotes causes Apert/Pfeiffer-syndrome-like phenotypes. Proc Natl Acad Sci USA. 2001;98:3855–3860. doi: 10.1073/pnas.071586898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulich TR, et al. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest. 1994;93:1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 19.Clark JC, et al. FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am J Physiol. 2001;280:L705–L715. doi: 10.1152/ajplung.2001.280.4.L705. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, et al. Keratinocyte growth factor stimulates CFTR-independent fluid secretion in the fetal lung in vitro. Am J Physiol. 1996;271:L987–L994. doi: 10.1152/ajplung.1996.271.6.L987. [DOI] [PubMed] [Google Scholar]
- 21.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann JA, Kafatos FC, Janeway CA, Ezkowitz RAB. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 23.LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect. 2001;3:161–166. doi: 10.1016/s1286-4579(00)01363-0. [DOI] [PubMed] [Google Scholar]
- 24.Sano H, et al. Surfactant proteins A and D bind CD14 by different mechanisms. J Biol Chem. 2000;275:22442–22451. doi: 10.1074/jbc.M001107200. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-κB and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol. 2001;166:7514–7519. doi: 10.4049/jimmunol.166.12.7514. [DOI] [PubMed] [Google Scholar]
- 26.Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactuscontrols the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 27.Muraoka RS, Bushdid PB, Brantley DM, Yull FE, Kerr LD. Mesenchymal expression of nuclear factor-kappaB inhibits epithelial growth and branching in the embryonic chick lung. Dev Biol. 2000;225:322–338. doi: 10.1006/dbio.2000.9824. [DOI] [PubMed] [Google Scholar]
- 28.Borok Z, et al. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol. 1998;18:554–561. doi: 10.1165/ajrcmb.18.4.2838. [DOI] [PubMed] [Google Scholar]
- 29.Stahlman MT, Gray ME, Whitsett JA. Expression of thyroid transcription factor-1 (TTF-1) in fetal and neonatal human lung. J Histochem Cytochem. 1996;44:673–678. doi: 10.1177/44.7.8675988. [DOI] [PubMed] [Google Scholar]
- 30.Panos RJ, Bak PM, Simonet WS, Rubin JS, Smith LJ. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest. 1995;96:2026–2033. doi: 10.1172/JCI118250. [DOI] [PMC free article] [PubMed] [Google Scholar]