Abstract
Background: Conflicting data exist regarding the presence of magnesium (Mg) deficiency and the therapeutic efficacy of Mg in premenstrual syndrome or premenstrual dysphoric disorder (PMDD).
Methods: The % Mg retention was determined using 24-hour urinary Mg excretion and the total dose of Mg given intravenously. In women with (n = 17) and without (n = 14) prospectively diagnosed PMDD, several blood measures of Mg and mood were obtained before, immediately after, and the day following an intravenous Mg (.1 mmol/kg) loading dose. A positive mood response was seen under open conditions; as open Mg infusion improved mood, subsequent PMDD patients (n = 10) were randomized in a double-blind, placebo-controlled, crossover fashion.
Results: Patients (31.5%) and control subjects (27.5%) retained comparable mean percentages of Mg. Neither group differed in measures of mean Mg before, immediately after, or the day following Mg infusion. Although there was a time effect for all mood measures in the patient group (p <.01 for all), there was neither a treatment nor time-by-treatment effect.
Conclusions: Contrary to prior reports, we found no evidence of Mg deficiency in women with PMDD compared with control subjects. Furthermore, Mg was not superior to placebo in the mitigation of mood symptoms in women with PMDD.
Keywords: PMDD, magnesium, magnesium loading test
Premenstrual syndrome (PMS) is a cyclic disorder characterized by predictable mood and physical symptoms that occur in the late luteal phase of the menstrual cycle. The etiology of PMS remains unknown, as does the etiology of a severe form of PMS, premenstrual dysphoric disorder (PMDD). Abnormal magnesium (Mg) metabolism has been implicated in several neuropsychiatric disorders with prominent mood and physical symptoms (e.g., migraine, epilepsy, chronic pain; Bolte et al 2001; Tepper et al 2001; Tramér et al 2002; Weisleder et al 2002). Because PMS is associated with symptoms such as mood instability, fatigue, and fluid changes, Mg deficiency has also been implicated in the etiology of PMS. Magnesium deficiency has not yet been assessed in PMDD. (PMDD is defined in the appendix of DSM-IV of the American Psychiatric Association (1994) and represents a more symptomatically restricted and severe form of PMS. Nonetheless, many earlier studies of PMS identified women with luteal phase-confirmed affective symptoms associated with impairment, the essence of PMDD.)
Deficiencies in plasma (Posaci et al 1994), red blood cell (RBC; Abraham et al 1981; Rosenstein et al 1994; Sherwood et al 1986; Stewart et al 1986), and mononuclear blood cell (MBC;Rosenstein et al 1994) Mg concentrations have been observed in women with PMS relative to women without PMS. Additionally, two placebo-controlled, double-blind, prospective clinical trials of oral Mg supplementation in women with PMS found that Mg was superior to placebo in the alleviation of mood (Facchinetti et al 1991) and fluid retention symptoms (Walker et al 1998). One interpretation of these findings is that women with PMS are Mg deficient and that Mg replacement may be a treatment for some of the core symptoms of this disorder. Not all studies, however, have demonstrated either differences in Mg measures between women with PMS and control subjects or improvement in PMS symptoms in response to the administration of Mg (Cerin et al 1993; Mira et al 1988; Walker et al 2002). Consequently, the relevance of Mg in the pathophysiology, diagnosis, and treatment of PMS remains unclear.
The principal challenge in assessing the relevance of Mg in PMDD and other neuropsychiatric disorders is that tissue-specific and physiologically meaningful measurements of Mg are difficult to determine. Magnesium measures must be qualified according to the following three factors: body tissue distribution, intracellular versus extracellular location, and binding state (i.e., ionized Mg, protein-bound Mg, and Mg complexed to anions; Elin 1987;Figure 1). Despite the fact that less than 1% of total body Mg is present in the blood, almost all of the published literature on Mg in PMS reports blood element (i.e., serum, RBC, MBC) measures of Mg rather than Mg in solid tissues of relevance, such as the brain. Furthermore, although the ionized fraction of Mg (Mg++) is considered to be the biologically active form, it is total Mg (MgT) in the blood that has been reported in PMS studies. Whether blood measures of either MgT or Mg++ accurately reflect an individual’s total body Mg status has not been established (Elin 1987, 1988; Ryschon et al 1996).
Figure 1.
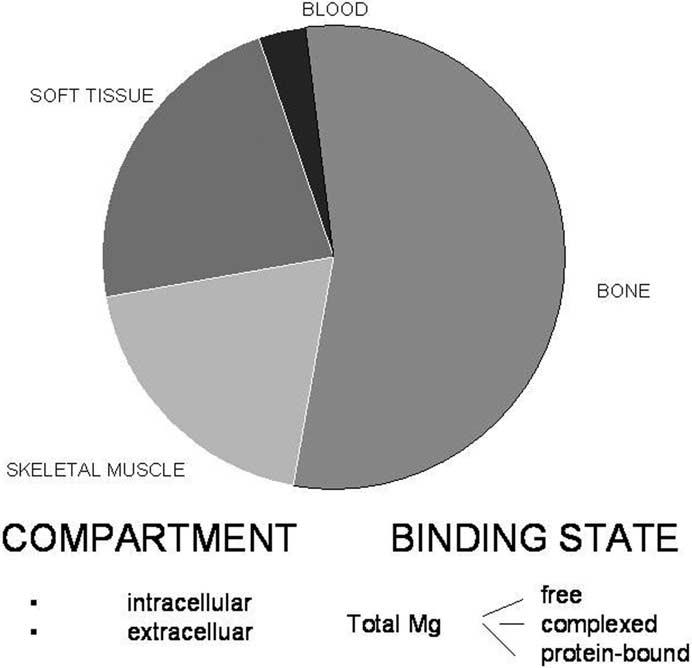
This figure shows the distribution of magnesium within the various divisions of the body. Within the divisions, there are intra- and extracellular compartments, as well as different binding states for magnesium. Data taken from Elin 1987.
Magnesium loading paradigms have been developed to provide more accurate and physiologically meaningful measures of total body Mg status (Caddell 1975; Danielson et al 1979; Dyckner and Wester 1982; Papazachariou et al 2000; Rasmussen et al 1988; Ryzen et al 1985; Thoren 1963). By collecting urine before and after the administration of a specified Mg load, states of Mg deficiency can be identified by measuring subsequent urinary Mg excretion and calculating how much of the administered Mg load was retained. Whereas people in Mg balance excrete essentially all of the administered Mg within 24 hours (Elin 1987), individuals with Mg deficiency retain a significant fraction (e.g., >30%) of the Mg load (Caddell 1985; Ryzen et al 1985).
In this study, we used an intravenous Mg loading test and serial blood measures (i.e., serum, RBC, MBC) of MgT to evaluate comprehensively the Mg status of women with and without prospectively diagnosed PMDD. (For accuracy, we have used the term PMS when this disorder, rather than the more recently described PMDD, was studied.) Our hypothesis was that women with PMDD would be Mg deficient, as reflected by greater Mg retention and lower intra- and extracellular MgT concentrations, relative to healthy women without PMDD. The principal out-come measure in this study was the percentage retention of an intravenously infused dose of magnesium sulfate (MgSO4). Concurrent serum, RBC, and MBC MgT measures were also obtained to verify whether women with PMDD have deficits in these measures relative to control subjects as previously reported. Additionally, we evaluated the potential mood effects of intravenously administered Mg in women with PMDD.
Methods and Materials
Participants
Seventeen women between the ages of 27 and 43 were diagnosed with PMDD according to the procedures described subsequently. Fourteen additional women, aged 20-37, were shown by the same methods not to have PMDD and served as a control group. Results of physical examinations and laboratory tests (including baseline serum MgT levels) were within normal limits in all subjects. This study was approved by the National Institute of Mental Health (NIMH) Institutional Review Board. Written informed consent was obtained from all women before participation in the study.
All patients and control subjects were self-referred to our PMS clinic in response to advertisements in local newspapers and hospital newsletters. No subject was taking psychoactive medications, hormonal preparations, or nonsteroidal antiinflammatory medications. Detailed diet histories were obtained, and women taking vitamins or mineral supplements were excluded from the study. Patients were free of current medical illness as well as current or recent (within the past 2 years) psychiatric illness as determined by the administration of a semistructured psychiatric diagnostic interview (Schedule for Affective Disorder and Schizophrenia Lifetime—modified; Spitzer et al 1979). All subjects completed daily visual analogue symptom rating scales for a screening period of at least 3 months before study entry. A woman was diagnosed with PMDD if she showed a greater than 30% increase (adjusted for range of the scale employed) in mean negative mood symptoms (e.g., anxiety, mood lability, irritability, and depression) in the week before menses compared with the week following the cessation of menses in at least two of three menstrual cycles (NIMH Premenstrual Syndrome Workshop Guidelines, 1983, unpublished). In addition to these operational criteria, women also met DSM-IV criteria for premenstrual dysphoric disorder (PMDD) applied retrospectively (American Psychiatric Association, 1994; i.e., at least 5 of 11 symptoms, asymptomatic in the follicular phase, and impaired function in the luteal phase). Control women in this study were free of current psychiatric and medical illness as well as past psychiatric illness, had no complaints of menstrual cycle-related mood symptoms, and had met these criteria in none of the three successive screening menstrual cycles.
Procedures
A protocol schematic for the Mg infusion procedure and data collection is presented in Figure 2. All infusions were performed in the luteal phase of the menstrual cycle between 8 and 12 days following the luteinizing hormone (LH) surge, determined by urine ovulation detection kits (Ovu Quick, Quidel, San Diego, California; ClearPlan Easy Unipath, Bedford, England). Subjects were given containers to collect their urine beginning 24 hours before each infusion for measurements of MgT, calcium (Ca), and creatinine.
Figure 2.
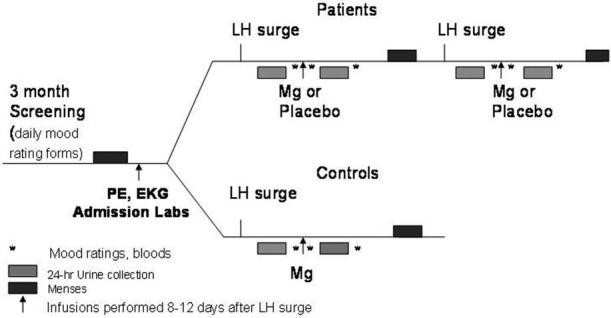
Flowchart for magnesium infusion study.
On the day of the study, subjects arrived at the hospital between 8 and 9 am and were placed on bed rest except to use the bathroom to collect urine. A 15-cc blood sample was drawn from all subjects for serum MgT, RBC MgT, and two measures of MBC MgT (MBC content [MBC-T] and concentration [MBC-C]). Several other baseline biochemical measures that have been implicated in the pathophysiology of PMS or associated with abnormal Mg metabolism were also assayed in study subjects, including thyroid stimulating hormone (TSH), free thyroxine (FT4), cortisol, follicle stimulating hormone (FSH), and luteinizing hormone (LH). Magnesium sulfate (MgSO4), at a dose of .1 mmol/kg body mass in 5% dextrose, was infused over 4 hours at a constant rate. We chose this paradigm because of reports that administration of this amount of Mg would not exceed the renal capacity to reabsorb Mg (Ryzen et al 1985).
Magnesium Assay
All Mg determinations were made using flame atomic absorption spectrophotometry (Video 22, Thermo Jarrel Ash, Waltham, Massachusetts). The MBCs were isolated using a Ficoll-Hypaque density gradient previously reported (Hosseini et al 1983). The MBC Mg content (Elin and Hosseini 1985) and concentration (Hosseini and Elin 1988) were performed as previously reported. We determined RBC Mg by an indirect method using a hematocrit measurement corrected for trapped plasma and diluted whole blood following lysis in distilled water (Archer et al 1972).
Blood pressure, pulse, and deep tendon reflexes were monitored every hour during the infusion to ensure that there were no clinical signs of hypermagnesemia (e.g., hypotonia, delayed deep tendon reflexes, bradycardia, and hypotension) during the study. At the end of the infusion, another 15-cc blood sample was drawn from each subject for repeat measurements of MgT (serum, RBC, MBC-T, and MBC-C). A second 24-hour urine collection was started at the beginning of the infusion for postinfusion measurements of MgT, Ca, and creatinine excretion. At the end of the second urine collection, a third 15-cc blood sample was drawn for MgT.
Because the principal purpose of this study was to assess Mg status in women with PMDD and healthy control subjects, the Mg infusion was initially administered under open-label condition; however, because Mg administration has been reported to alleviate symptoms of PMS, mood and behavioral ratings (described later) were obtained from all study participants throughout the study. After observing robust symptomatic improvement with the Mg infusion in the first six PMDD subjects studied under open-label conditions, the study design was modified to include a placebo infusion (5% dextrose in water) in a randomized, double-blind, crossover fashion (only for PMDD patients) as a control for the nonspecific mood effects of an infusion procedure. Additionally, these placebo infusions were used to ensure that any observed alterations in biological measures were attributable specifically to Mg infusion.
Mood and Behavioral Assessment
Subjects completed the following syndrome-based mood ratings before, immediately following, and the morning after each infusion: Beck Depression Inventory (BDI; Beck et al 1961), Spielberger State-Trait Anxiety Inventory (STAI; Spielberger et al 1970), Premenstrual Tension Scale Subjective (PMTS-S; Steiner et al 1980), and Premenstrual Tension Scale-Objective Rater (PMTS-O; Steiner et al 1980). Subjects completed 100-mm Visual Analogue Scales (VAS) of several PMS symptoms (i.e., irritability, sadness, tiredness, anxiety, self-esteem, mood stability, physical symptoms, and over all well-being), before, during (at time points 30, 90, 150, and 210 min), immediately after, and 24 hours after each infusion.
One of the initial six PMDD subjects studied under open Mg conditions was subsequently studied under placebo-controlled, double-blind conditions. For purposes of her baseline urine and blood measures, data from this subject’s first Mg infusion were used. For purposes of mood and behavioral assessment, data from the two subsequent double-blind infusions were used.
Statistical Analysis
Baseline data were obtained immediately before the first infusion (either open Mg infusion or double-blind infusion) and expressed as means ± SD. Baseline comparisons of biochemical measures between patients and control subjects were performed with the Student t test. Percent Mg retention was calculated by a previously specified manner (Ryzen et al 1985), using 24-hour urine collections, 24 hours prior and subsequent to the start of the Mg infusion. We used a previously determined upper limit of Mg retention, set at 27.5% (Gullestad et al 1994). Blood was sampled at three time points: preinfusion, postinfusion, and 24 hours after infusion. We measured mood ratings at each of these time points to examine the relationship between Mg retention, serum Mg measures, and mood. Mean percent Mg retention for control subjects and women with PMDD was analyzed with the Student t test. Repeated-measures analysis of variance was used to analyze changes in serial blood and urine measures as well as mood scale scores, with “diagnosis” (PMDD vs. control subjects) as the between subjects variable. As noted earlier, there were three levels of time (pre, post, and 24 hours after Mg infusion) for the blood samples and cross-sectional mood ratings and seven levels of time for the VAS (corresponding to the six time points on the day of the infusion and an additional time point 24 hours later). As VAS ratings were not obtained for seven subjects (all healthy control subjects) at the 24-hour time point, the repeated-measures ANOVA was calculated separately with and without the 24-hour time point. Post hoc comparisons for significant repeated-measures ANOVA were performed with Bonferroni t tests. Correlations between the various Mg measures and mood ratings were analyzed with the Spearman rank order correlation coefficient. Student t test was performed to determine whether there were differences between women who were Mg deficient and those who were not in the degree of symptom severity. Statistical analysis was performed using SYSTAT (2000 SPSS, Chicago, Illinois). To correct partially for multiple (> 10) comparisons, the alpha level was set at .01 for significant correlations.
Despite a prospectively confirmed diagnosis of PMDD, some women with PMDD may have episodic asymptomatic menstrual cycles. As inclusion of asymptomatic subjects might obscure biological differences between PMDD subjects and control subjects (Rosenstein et al 1996), statistical analyses of biochemical measures obtained in this study were performed with and without the inclusion of data from asymptomatic PMDD subjects. Data analyses of mood ratings in response to the infusions, however, included only PMDD subjects who were symptomatic at the time of the procedures.
Results
PMDD patients were significantly older (mean age 37.4 years ± 4.4) than their control counterparts (mean age 28.6 years ± 6.4; Student t29 = 4.48, p < .01).
Baseline Biochemical Analyses
Mean baseline serum MgT concentrations were within the normal reference range in all subjects, and there were no significant differences between women with PMDD and healthy control subjects (.79 ± .09 mmol/L and .75 ± .10 mmol/L, respectively, reference range .75-1.00 mmol/L). Mean RBC MgT, MBC-C MgT, and MBC-T MgT measures also were not different between patients and healthy control subjects at baseline (Table 1). Similarly, there were no differences between patients and control subjects in baseline urinary Mg excretion or any other urinary measure (Table 2); however, the mean baseline 24-hour urinary magnesium output was below the lower limit of the reference range in both patients (2.14 ± .53 mmol/24 hour) and healthy control subjects (2.35 ± .60 mmol/24 hr; reference range 3.0 - 4.25 mmol/24 hour).
Table 1.
Mean (±SD) Magnesium Blood Measures at Pre, Post, and the Day after Receiving Mg Infusion
| PMDD Patients (n = 17)a | Control Subjects (n = 14) | |
|---|---|---|
| Serum MgTa(mmol/L) | ||
| Preinfusion | .79 (.09) | .75 (.10) |
| Postinfusion | 1.02 (.11) | 1.02 (.16) |
| Day after | .81 (.06) | .77 (.08) |
| RBC MgT (mmol/L) | ||
| Preinfusion | 2.04 (.33) | 1.87 (.24) |
| Postinfusion | 1.94 (.32) | 1.92 (.49) |
| Day after | 1.92 (.33) | 1.88 (.27) |
| MBC-C MgT (mmol/L) | ||
| Preinfusion | 13.9 (3.2) | 12.9 (2.4) |
| Postinfusion | 13.7 (2.8) | 13.8 (4.4) |
| Day after | 14.7 (4.0) | 13.8 (1.7) |
| MBC-T MgTc(fg/cell) | ||
| Preinfusion | 4.5 (1.3) | 4.2 (.72) |
| Postinfusion | 3.9 (.9) | 3.8 (.8) |
| Day after | 4.5 (1.3) | 4.7 (1.2) |
Samples for one PMDD subject were unavailable for post-infusion analysis.
Repeated-measures analysis of variance (ANOVA): significant time effect [F(2,56) = 112, p < .01] but no diagnosis-by-time effect.
Repeated-measures ANOVA: significant time effect [F(2,52) 6.1, p <.01] but no diagnosis-by-time effect.
Table 2.
Mean (± SD) 24-Hour Urinary Measures Before and After Magnesium (Mg) Infusion
| Mg t1 (Ref. Range 3.0-4.25 mmol/24 hr) | Mg t2 (mmol/24hr)a | Ca t1 (ref range Mg t2 (mmol/24 hr)a | Cr t1 (ref. range 1.25-6.25 mmol/24 hr) | |||
|---|---|---|---|---|---|---|
| Ca t2 (mmol/24 hr)b | .8-1.8 g/24 hr) | Cr t2 (g/24 hr) | ||||
| Patients | 2.14 (.53) | 4.22 (1.16) | 3.38 (1.88) | 3.64 (1.79) | 1.27 (.22) | 1.18 (.28) |
| HC | 2.35 (.60) | 4.74 (.84) | 3.35 (1.87) | 4.38 (1.35) | 1.33 (.22) | 1.34 (.26) |
Ca, calcium; Cr, creatinine; t1, starting 24 hours before Mg infusion; t2, starting at onset of Mg infusion; HC, healthy control subjects. Sample sizes ranged between 14 and 17.
Repeated-measures analysis of variance (ANOVA) significant time effect [F(1,28) 152, p< .01], but no time-by-diagnosis effects.
Repeated measures ANOVA: significant time effect [F(1,27) 5.5, p <.05], but no time-by-diagnosis effect.
None of the following baseline blood measures were found to be abnormal in study subjects or significantly different between patients and healthy control subjects: TSH, fT4, cortisol, FSH, and LH.
Biochemical Measures Following Magnesium Infusion
We found no statistically significant differences in (mean ± SD) urinary % Mg retention between patients (31.5 ± 25) and control subjects (27.5 ± 19; Figure 3). By the end of the 4-hour infusion of Mg, mean serum MgT had increased significantly in both women with PMDD and in healthy control subjects. Repeated-measures ANOVA results revealed a significant time effect [F(2,56) = 112, p < .001] for serum MgT, with concentrations peaking at the end of the infusion and returning to baseline levels by the next morning in all subjects. Post hoc testing revealed significant time effects for before and after infusion comparisons (t56 = 14.1, p < .01) and for after infusion versus day 2 comparisons (t56 = 12.7, p < .01). Neither diagnosis nor time-by-diagnosis effects were discovered, however. During the double-blind trial, no significant changes in serum Mg were observed after the placebo infusions conducted in women with PMDD. Thus, repeated-measures ANOVA results revealed a significant time by treatment effect for serum MgT in the women with PMDD [F(2,16) = 37.5, p < .01].
Figure 3.
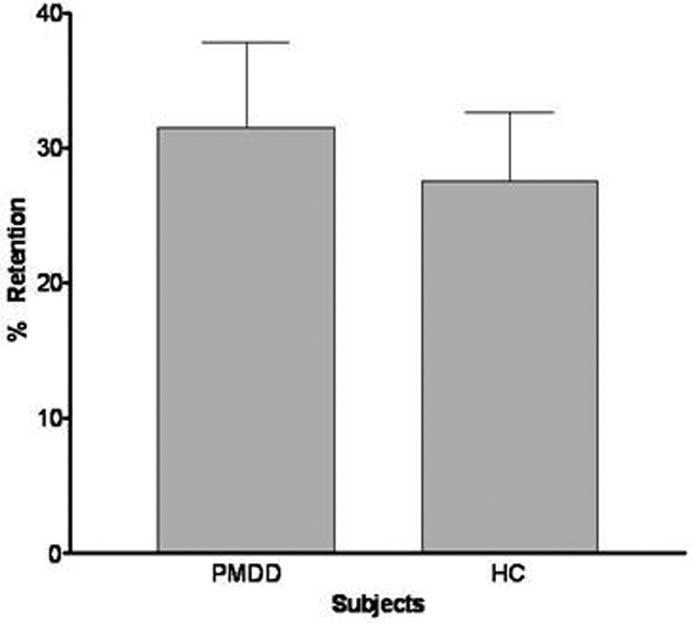
Mean magnesium retention in women with premenstrual dysphoric disorder (PMDD; n = 16) and healthy control subjects (HC; n = 14). No statistically significant differences were found in mean % Mg retention (± SEM) between women with PMDD and HC.
There were no significant time, diagnosis, or time-by-diagnosis effects in mean RBC and MBC-C MgT concentrations over the course of the study (preinfusion, postinfusion, and 24 hours postinfusion) as analyzed by repeated-measures ANOVA.
For MBC-T MgT, there was a significant time effect [F(2,52) = 6.1, p < .01], reflecting a decrease in MBC-T MgT postinfusion, but no diagnosis or time-by-diagnosis effects. Measures of MgT and Ca obtained in 24-hour urine collections before and after infusion of intravenous Mg (Table 2) showed a time effect [F(1,28) = 152, p < .01; F(1,27) = 5.5, p < .05, respectively], reflecting an increase in Mg and Ca excretion, but no diagnosis or time-by-diagnosis effects. Similarly, women with PMDD and control subjects did not differ in the urinary excretion of creatinine over time.
Of the 12 PMDD patients studied under double-blind conditions, 2 were asymptomatic at the time of Mg infusion. Hence all biochemical analyses were performed twice, first for all PMDD patients and again for only those PMDD patients who were symptomatic during the study cycle. Significant findings did not differ in the two analyses.
Mood and Behavioral Ratings
As expected, healthy control subjects initially had low scores on the BDI, STAI, PMTS-O, PMTS-S, and VAS. The intravenous administration of Mg was not associated with any mood changes in healthy control subjects.
In the open-label Mg infusions with the first six PMDD patients, we observed significant improvement in mean mood symptoms postinfusion as assessed by VAS (Figure 4; note that this figure shows only irritability; we found similar results with the other mood and physical symptoms). There was a significant time-by-diagnosis effect, with PMDD patients achieving substantial reductions in mood scores during and immediately after Mg infusion, compared with control subjects, whose mood scores did not change significantly (Figure 4). Post hoc Bonferroni t tests showed the significant improvement in symptoms to occur by 90 min and to continue throughout the Mg infusion and 24 hours after the end of the magnesium infusion. In addition, syndrome-based mood ratings revealed a significant time-by-diagnosis effect, with PMDD patients experiencing reductions in mood symptoms immediately after Mg infusion compared with control subjects, who remained asymptomatic throughout the study [BDI, F(1,18) = 28.7, p < .001; STAI, F(1,18) = 17.8, p < .001; PMTS-O, F(1,18) = 338, p < .001; PMTS-S, F(1,18) = 10.2, p < .01]. This significant effect was maintained after post hoc analyses with Bonferroni t tests [BDI, t (18) = 6.68, p < .01; STAI, t (18) = 5.59, p < .01; PMTS-O, t (18) = 22, p < .01; and PMTS-S, t (18) = 3.9, p < .01]. Under double-blind conditions in the 10 symptomatic PMDD patients receiving placebo and Mg infusions (Table 3), however, there was neither a treatment (Mg vs. placebo) nor time-by-treatment effect found for any of these mood and behavioral measures. In addition there were no statistically significant differences between women with (n = 6) and women without (n = 6) Mg deficiency in their symptom severity.
Figure 4.
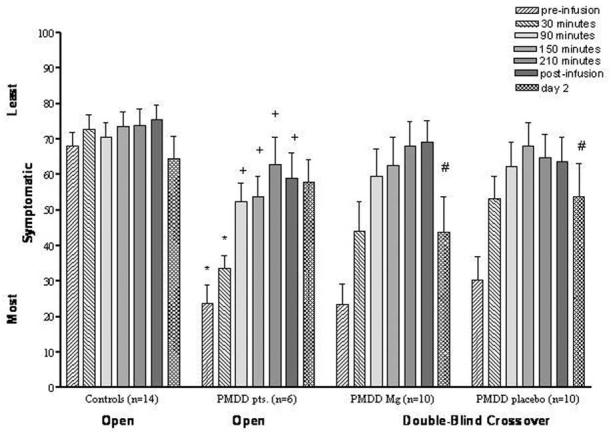
Improvement of irritability associated with magnesium (Mg) infusion during open and double-blind crossover trials. Open Mg trial: repeated-measures analysis of variance (ANOVA) of the first six time points revealed significant time-by-diagnosis effects [F(5,90) = 11.8, p < .001]. Bonferroni-adjusted post hoc: +compared with baseline [t(90) = 6.12- 8.12, p <.01]; *compared with control subjects [Bonferroni t(90) = 5.19 -5.89, p < .01]. Data for day 2 included only seven control subjects. Repeated-measures ANOVA with all time points for these seven control subjects and six patients replicated the findings with six time points in the larger group. Double-blind Mg trial: #significant time effect [F(6,54) = 10.7, p <.01] but not a significant effect of treatment or an interactive effect.
Table 3.
Mood Measures in Symptomatic PMDD Women Pre, Post, and the Day After Receiving Magnesium and Placebo (Mean±SD)
| Magnesium (n = 10) |
Placebo (n = 10) |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | Day 2 | Pre | Post | Day 2 | |
| BDIa | 15.7 (6.3) | 8.6 (6.4) | 10.2 (9.2) | 16.3 (8.6) | 8.1 (4.7) | 9.4 (8.8) |
| STAIa | 52.1 (8.4) | 36.5 (9.1) | 43 (11.8) | 47.9 (13.6) | 36.9 (4.3) | 38.2 (12.1) |
| PMTS-Sa | 21.6 (5.4) | 13.5 (7.6) | 15.3 (9.0) | 21.0 (5.5) | 15.3 (7.5) | 13.5 (9.5) |
| PMTS-Oa | 19.1 (5.5) | 7.8 (3.7) | 12.8 (9.0) | 20.0 (5.6) | 10.6 (8.2) | 11.5 (10.0) |
BDI, Beck Depression Inventory; STAI, Spielberger State-Trait Anxiety Inventory; PMTS-S, Premenstrual Tension Scale Self-Rater; PMTS-O, Premenstrual Tension Scale-Objective Rater.
Repeated-measures analysis of variance: significant time effect but not time-by-treatment effect.
Correlations
We observed no significant correlations between the percent retention of Mg and symptom improvement (as measured by the BDI, STAI, PMTS-O, PMTS-S, or VAS) immediately after Mg infusion or the day after the infusion. There were no correlations between VAS symptoms and serum Mg or the percent Mg retention after corrections for multiple comparisons.
Discussion
This study was designed to evaluate comprehensively Mg status in women with PMDD because several studies have suggested possible etiologic and therapeutic roles for Mg in this population. We were unable to confirm previous reports of decreased RBC or MBC Mg levels in women with PMS. Neither serum Mg nor any of the three cellular measures of Mg differed in patients and control subjects. Furthermore, employing our primary outcome measure, an integrated measure of total body Mg status (i.e., Mg retention) and, therefore, a more accurate measure of putative Mg depletion, we again found no difference between patients and control subjects. It is of interest that the mean levels of Mg retention were elevated in both patients and control subjects, suggesting a mild degree of Mg depletion in both groups. Although the mean of the patients (31.5% retention) exceeded the normal threshold of 27.5%, the mean of the control subjects was exactly at the upper limit of normal (27.5% retention) and did not differ from that of the patients. Thus, our data failed to support the hypothesis that Mg depletion causes PMDD. Furthermore, comparison of the six women with PMDD who were not Mg deficient with those who exceeded the threshold for Mg deficiency showed no differences in symptom severity, again challenging the relevance of Mg status in women with PMS.
A number of studies have examined Mg and PMS, with each study confining itself to one or two measures of Mg. Ours is the first study not only to use all four cellular measures of Mg but also to determine the overall state of Mg depletion through use of the Mg retention test. Thus, although the literature has supported an interest in the role of Mg in PMS and differences observed across studies cannot be fully explained, our baseline and—more important—Mg retention findings strongly argue against PMDD as a magnesium deficiency disorder.
Despite the absence of evidence of Mg deficiency as a cause of PMDD, our initial experience with Mg loading suggested a dramatic therapeutic effect. Robust reductions in all four symptom rating scales were observed in PMDD patients (but not control subjects) by the end of the infusion and were still present 24 hours later. This surprising finding led to a change in study design to test the therapeutic efficacy of Mg loading under double-blind, placebo-controlled conditions. Under these conditions, Mg showed no therapeutic superiority to placebo; patients showed dramatic improvement in symptoms following both infusions (see Figure 4). Although unexpected, these findings are arguably the most interesting generated in this study and serve as a sobering caveat. Certainly, results from open therapeutic trials of Mg cannot be used for inferring the existence of Mg deficiency in PMDD. More generally, however, given the striking placebo response observed in our study, one should use the results of an open therapeutic trial as the basis for concluding the presence of therapeutic efficacy only with the greatest of trepidation.
In contrast to the changes in mood seen during Mg infusion, cellular Mg measures showed little effect: RBC Mg, MBC-C, and MBC-T did not significantly increase; in fact, a small but significant decrease in MBC-T occurred. In previous studies with hypomagnesemic patients, Mg replacement either increased or did not significantly change MBC-T (Reinhart et al 1990; Sacks et al 1997). In the latter study by Sacks et al, patients with .5-.75 mmol/kg (equivalent to 1.0-1.5 mEq/kg) Mg replacement, a much higher dose than that used in our study, did not have a significant change in their MBC Mg content. Hence, our failure to observe increases in cellular Mg after the Mg infusion is not surprising.
There are several possible explanations for our negative finding. First, women with PMDD were significantly older than their normal volunteer counterparts; however, previous evidence suggests that serum Mg levels and percentage Mg retention are fairly stable across age groups (Gullestad et al 1994; Lowenstein and Stanton 1986). Consequently, our failure to find significant differences in Mg parameters between PMDD patients and healthy volunteers cannot be attributed to differences in age. Nonetheless, it is possible that some of the younger control subjects could later develop PMDD, which might have obscured a difference between patients and control subjects. Second, it is possible that despite using a paradigm that should not overload the renal reabsorption mechanism for Mg, the .1 mmol/kg of infused Mg may have overwhelmed the kidney’s ability to reabsorb Mg. This, in turn, could obscure a difference in retention between the healthy control subjects and patients with PMDD. Third, the small sample sizes in this study increase the likelihood of a Type II error. Based on our findings, however, one would need an exceptionally large sample to demonstrate as significant the differences in measures of Mg that we observed between women with PMDD and healthy control subjects. Fourth, limitations in interpreting these findings include the observation that normal volunteers had high levels of Mg retention. Although our normal volunteers did not have psychiatric illness or PMDD by prospective ratings, an inadvertent selection bias may have included control women with relative Mg deficiency, thus potentially obscuring a diagnostic difference between the two groups. Fifth, results from our infusion study might differ from those of a chronic oral treatment trial. Yet even if it were beneficial, this study would suggest the mechanism of action would be different from solely Mg deficiency. These alternative explanations notwithstanding, our data suggest, contrary to prior reports from our group and others, that women with PMDD are not Mg deficient relative to control women. Although there were no differences between the groups in Mg retention, it is noteworthy, as mentioned earlier, that both groups in the study were at the upper limits of the normal retention. Whether this reflects local or national dietary patterns resulting in an increased prevalence of Mg deficiency is unknown. In addition, there was a high variability and high degree of overlap of percent Mg retention between the two groups. Hence, although highly overlapping percentages of Mg retention were observed between patient and control groups, we cannot rule out the possibility that women with PMDD may be more susceptible than control subjects to mood symptoms in the context of Mg deficiency. We can state unequivocally, however, that even relative Mg deficiency is neither necessary nor sufficient for the expression of PMDD.
Acknowledgments
We are grateful to Merry Danaceau, R.N., for her help with the conduct of the study.
References
- Abraham GE, Lubran MM. Serum and red cell magnesium levels in patients with premenstrual tension. Am J Clin Nutr. 1981;34:2364–2366. doi: 10.1093/ajcn/34.11.2364. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed Author; Washington, DC: 1994. [Google Scholar]
- Archer WH, Emerson RI, Reusch CS. Intra and extracellular fluid magnesium by atomic absorption spectroscopy. Clin Biochem. 1972;5:159–161. doi: 10.1016/s0009-9120(72)80025-0. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bolte AC, van Geijn HP, Dekker GA. Management and monitoring of severe preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2001;96:8–20. doi: 10.1016/s0301-2115(00)00383-3. [DOI] [PubMed] [Google Scholar]
- Caddell JL. The magnesium load test: I. A design for infants. Clin Pediatr (Phila) 1975;14:449, 457, 518–459. doi: 10.1177/000992287501400504. [DOI] [PubMed] [Google Scholar]
- Caddell JL. Parenteral magnesium (Mg) retention test. J Am Coll Nutr. 1985;4:331. [Google Scholar]
- Cerin A, Collins A, Landgren B, Eneroth P. Hormonal and biochemical profiles of premenstrual syndrome: Treatment with essential fatty acids. Acta Obstet Gynecol Scand. 1993;72:337–343. doi: 10.3109/00016349309021108. [DOI] [PubMed] [Google Scholar]
- Danielson BG, Johansson G, Ljunghall S. Magnesium metabolism in healthy subjects. Scand J Urol Nephrol Suppl. 1979;51:49–73. [PubMed] [Google Scholar]
- Dyckner T, Wester PO. Magnesium deficiency— guidelines for diagnosis and substitution therapy. Acta Med Scand Suppl. 1982;661:37–41. doi: 10.1111/j.0954-6820.1982.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Elin RJ. Assessment of magnesium status. Clin Chem. 1987;33:1965–1970. [PubMed] [Google Scholar]
- Elin RJ. Status of the determination of magnesium in mononuclear blood cells in humans. Magnesium. 1988;7:300–305. [PubMed] [Google Scholar]
- Elin RJ, Hosseini JM. Magnesium content of mononuclear blood cells. Clin Chem. 1985;31:377–380. [PubMed] [Google Scholar]
- Facchinetti F, Borella P, Sances G, Fioroni L, Nappi RE, Genazzani AR. Oral magnesium successfully relieves premenstrual mood changes. Obstet Gynecol. 1991;78:177–181. [PubMed] [Google Scholar]
- Gullestad L, Midtvedt K, Dolva LO, Norseth J, Kjekshus J. The magnesium loading test: Reference values in healthy subjects. Scand J Clin Lab Invest. 1994;54:23–31. doi: 10.3109/00365519409086506. [DOI] [PubMed] [Google Scholar]
- Hosseini JM, Elin RJ. A method for determining the magnesium concentration of mononuclear blood cells. Trace Elem Med. 1988;5:47–51. [Google Scholar]
- Hosseini JM, Johnson E, Elin RJ. Comparison of two separation techniques for the determination of blood mononuclear cell magnesium content. J Am Coll Nutr. 1983;4:361–368. doi: 10.1080/07315724.1983.10719933. [DOI] [PubMed] [Google Scholar]
- Lowenstein FW, Stanton MF. Serum magnesium levels in the United States, 1971-1974. J Am Coll Nutr. 1986;5:399–414. doi: 10.1080/07315724.1986.10720143. [DOI] [PubMed] [Google Scholar]
- Mira M, Stewart PM, Abraham SF. Vitamin and trace element status in premenstrual syndrome. Am J Clin Nutr. 1988;47:636–641. doi: 10.1093/ajcn/47.4.636. [DOI] [PubMed] [Google Scholar]
- Papazachariou IM, Martinez-Isla A, Efthimiou E, Williamson RCN, Girgis SI. Magnesium deficiency in patients with chronic pancreatitis identified by an intravenous loading test. Clin Chim Acta. 2000;302:145–154. doi: 10.1016/s0009-8981(00)00363-6. [DOI] [PubMed] [Google Scholar]
- Posaci C, Erten O, Uren A, Acar B. Plasma cooper, zinc and magnesium levels in patients with premenstrual tension syndrome. Acta Obstet Gynecol Scand. 1994;73:452–455. doi: 10.3109/00016349409013429. [DOI] [PubMed] [Google Scholar]
- Rasmussen HS, McNair P, Goransson L, Balslov S, Larsen OG, Aurup P. Magnesium deficiency in patients with ischemic heart disease with and without acute myocardial infarction uncovered by an intravenous loading test. Arch Intern Med. 1988;148:329–332. [PubMed] [Google Scholar]
- Reinhart RA, Fananapazir L, Cannon RO, III, Hosseini JM, Elin RJ. Effect of intravenous magnesium sulfate on blood magnesium parameters. Magnesium Trace Elements. 1990;9:191–197. [PubMed] [Google Scholar]
- Rosenstein DL, Elin RJ, Hosseini JM, Grover G, Rubinow DR. Magnesium measures across the menstrual cycle in premenstrual syndrome. Biol Psychiatry. 1994;35:557–561. doi: 10.1016/0006-3223(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Rosenstein DL, Kalogeras KT, Kalafut M, Malley J, Rubinow DR. Peripheral measures of arginine vasopressin, atrial natriuretic peptide and adrenocorticotropic hormone in premenstrual syndrome. Psychoneuroendocrinology. 1996;21:347–359. doi: 10.1016/0306-4530(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Ryschon TW, Rosenstein DL, Rubinow DR, Niemela JE, Elin RJ, Balaban RS. Relationship between skeletal muscle intracellular ionized magnesium and measurements of blood magnesium. J Lab Clin Med. 1996;127:207–213. doi: 10.1016/s0022-2143(96)90080-3. [DOI] [PubMed] [Google Scholar]
- Ryzen E, Elbaum N, Singer FR, Rude RK. Parenteral magnesium tolerance testing in the evaluation of magnesium deficiency. Magnesium. 1985;4:137–147. [PubMed] [Google Scholar]
- Sacks GS, Brown RO, Dickerson RN, Bhattacharya S, Lee PD, Mowatt-Larssen C, et al. Mononuclear blood cell magnesium content and serum magnesium concentration in critically ill hypomagnesemic patients after replacement therapy. Nutrition. 1997;13:303–308. [PubMed] [Google Scholar]
- Sherwood RA, Rocks BF, Stewart A, Saxton RS. Magnesium and the premenstrual syndrome. Ann Clin Biochem. 1986;23:667–670. doi: 10.1177/000456328602300607. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R. STAI manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia—Lifetime Version. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1979. [Google Scholar]
- Steiner M, Haskett RF, Carroll BJ. Premenstrual tension syndrome: The development of research diagnostic criteria and new rating scales. Acta Psychiatr Scand. 1980;62:177–190. doi: 10.1111/j.1600-0447.1980.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Stewart A, Howard J. Magnesium and potassium deficiencies in women with pre-menstrual syndrome. Magnesium Bull. 1986;8:314–316. [Google Scholar]
- Tepper SJ, Rapoport A, Sheftell F. The pathophysiology of migraine. Neurologist. 2001;7:279–286. doi: 10.1097/00127893-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Thoren L. Magnesium deficiency in gastrointestinal fluid lost. Acta Chir Scand. 1963;306(suppl):1–65. [PubMed] [Google Scholar]
- Tramér MR, Glynn CJ. Magnesium bier's block for treatment of chronic limb pain: A randomised, double-blind, cross-over study. Pain. 2002;99:235–241. doi: 10.1016/s0304-3959(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Walker AF, De Souza MC, Marakis G, Robinson PA, Morris AP, Bolland KM. Unexpected benefit of sorbitol placebo in Mg intervention study of premenstrual symptoms: implications for choice of placebo in RCTs. Med Hypotheses. 2002;58:213–220. doi: 10.1054/mehy.2001.1407. [DOI] [PubMed] [Google Scholar]
- Walker AF, De Souza MC, Vickers MF, Abeyasekera S, Collins ML, Trinca LA. Magnesium supplementation alleviates premenstrual symptoms of fluid retention. J Womens Health. 1998;7:1157–1165. doi: 10.1089/jwh.1998.7.1157. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Tobin JA, Kerrigan JF, III, Bodensteiner JB. Hypomagnesemic seizures: Case report and presumed pathophysiology. J Child Neurol. 2002;17:59–61. doi: 10.1177/088307380201700117. [DOI] [PubMed] [Google Scholar]


