Abstract
The development of breast carcinomas involves a complex set of phenotypic alterations in breast epithelial cells and the surrounding microenvironment. While traditional transformation assays provide models for investigating certain aspects of the cellular processes associated with tumor initiation and progression, they do not model alterations in tissue architecture that are critically involved in tumor development. In this review, we provide examples of how three-dimensional (3D) cell culture models can be utilized to dissect the pathways involved in the development of mammary epithelial structures and to elucidate the mechanisms responsible for oncogene-induced phenotypic alterations in epithelial behavior and architecture. Many normal mammary epithelial cell lines undergo a stereotypic morphogenetic process when grown in the presence of exogenous matrix proteins. This 3D morphogenesis culminates in the formation of well-organized, polarized spheroids, and/or tubules that are highly reminiscent of normal glandular architecture. In contrast, transformed cell lines isolated from mammary tumors exhibit significant deviations from normal epithelial behavior in 3D culture. We describe the use of 3D models as a method for both reconstructing and deconstructing the cell biological and biochemical events involved in mammary neoplasia.
Keywords: mammary epithelial cells, breast cancer, morphogenesis, 3D cell culture, oncogenesis
Abbreviations used: 3D; three-dimensional; BARD-1, BRCA-1 associated ring domain; CDK, cyclin-dependent kinase; CGH, comparative genomic hybridization; CSF-1, colony-stimulating factor; CSF-1R, colony-stimulating factor receptor; DCIS, ductal carcinoma in situ; E7, human papilloma virus 16 E7 protein; ECM, extracellular matrix; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; EHS, Engelbreth-Holm-Swarm; EMT, epithelial-to-mesenchymal transition; ER, estrogen receptor; GAP, GTPase activating protein; GEF, guanine nucleotide exchange factor; HGF, hepatocyte growth factor; IGF, insulin-like growth factor; IL-1, interleukin-1; MAPK, mitogen-activated protein kinase; MEC, mammary epithelial cell; MMP, matrix metalloproteinase; MMTV, mouse mammary tumor virus; PI3K, phosphotidylinositol-3 kinase; PR, progesterone receptor; Rb, retinoblastoma protein; TGFβ, transforming growth factor beta; VEGF, vascular endothelial growth factor
FROM SOFT AGAR TO BASEMENT MEMBRANE GELS: MODELING MAMMARY TUMORIGENESIS IN VITRO
The mammary gland is a complex organ, comprised of multiple cell types and surrounded by a proteinaceous extracellular matrix (ECM). Mammary epithelial cells (MECs) represent the fundamental functional unit of the gland and comprise a polarized secretory network of bilayered hollow ducts and alveolar units. The epithelial tree is embedded in a collagen-rich matrix and develops specialized attachments to the surrounding basement membrane. Integration of the array of signals mediated by both cell–cell and cell–matrix interactions is required to regulate many aspects of cell behavior, including cell polarity, proliferation, adhesion, and survival. Importantly, such interactions are not only critical for normal development, but are also required for tissue homeostasis; aberrant interactions between the epithelium and its microenvironment can influence tumor progression (1).
Tumor development involves the acquisition of multiple genetic and epigenetic changes that cause aberrant loss or gain of function of cellular proteins. Collectively, such changes significantly alter tumor cell behavior. Hanahan and Weinberg have proposed that the following six alterations in cell physiology characterize malignant growth: 1) enhanced proliferative potential, 2) decreased sensitivity to apoptosis, 3) autonomous production of growth signals, 4) insensitivity to growth inhibitory signals, 5) angiogenic potential and 6) migratory and invasive capability (2). As the principal target of proliferative, morphogenetic, and hormonal signals, MECs are the cell type most commonly targeted for the alterations that drive these events. Unfortunately, the genetic heterogeneity of breast tumors has complicated the identification of specific lesions responsible for tumor-associated changes in epithelial behavior. Many genetic and epigenetic alterations have been catalogued in breast tumors using techniques such as fluorescent in situ hybridization, karyotyping, comparative genomic hybridization (CGH), DNA methylation analysis, and DNA sequencing (3–5). These studies reveal the wide spectrum of genetic and epigenetic changes present in mammary tumors and suggest that MEC transformation can proceed through a variety of yet uncharacterized pathways.
While hundreds of alterations have been detected in breast tumors, in most cases it is not clear whether the changes represent causal factors or simply bystander alterations accrued during neoplastic transformation (6). Moreover, an enhanced understanding of the mechanisms by which specific alterations alter MEC behavior is required to facilitate the design of novel therapeutic strategies. The roles of specific oncogenes or tumor suppressors in tumorigenesis have traditionally been examined using transformation assays, which monitor a single parameter, such as the ability to form foci on monolayers, to generate colonies in soft agar suspension, or to induce an epithelial-to-mesenchymal transition (EMT). However, such assays are an inadequate reflection of tumor physiology; furthermore, the extent to which specific oncogenes contribute to tumor development and progression is highly influenced by the matrix microenvironment. Thus, utilization of models that better recapitulate tissue architecture will improve our ability to interrogate regulators of mammary biology and tumorigenesis.
The advent of 3D cell culture models has allowed investigators to make significant progress toward characterization of the factors involved in the establishment and maintenance of epithelial architecture. Many aspects of the organization of glandular epithelial structures are recapitulated when primary mammary cells or established cell lines are exposed to a physiological exogenous matrix. Culture in the presence of pliable, laminin-rich extracellular matrix is sufficient to direct a cell line that grows as an unpolarized monolayer on tissue culture plastic to form tubular or alveolar structures highly reminiscent of structures found in vivo (7). Furthermore, treatment of murine MECs cultured on ECM with lactogenic hormones results in de novo milk production, illustrating that 3D culture can promote a level of differentiation not achieved by similar treatment of cells plated on a plastic substratum (8). It is clear that interaction with the ECM in vitro plays a critical role in the process of mammary differentiation and morphogenesis.
USE OF 3D CELL CULTURE TO MODEL EPITHELIAL ARCHITECTURE AND TRANSFORMATION
Cultured epithelial cells respond most appropriately to matrix proteins that mimic the composition of normal basement membrane, including among other components a high proportion of laminin and collagen. One commonly used mixture is that derived from the Engelbreth–Holm–Swarm (EHS) murine tumor, commercially available as MatrigelTM. Many MECs, including primary cells isolated from pregnant mice as well as immortalized human and murine cell lines, undergo a stereotypic morphogenetic program when cultured on EHS. A reproducible succession of cellular remodeling events culminate in the formation of polarized, growth-arrested hollow spheroids similar to those formed by other glandular epithelial cells in 3D culture, ensheathed in an organized basement membrane (9–11).
Characterization of the Program of In Vitro Alveolar Morphogenesis
Multiple studies have described the discrete steps involved in the morphogenesis of a single cell or small cluster of disorganized cells into structures closely resembling acini in vivo (Fig. 1). Upon seeding within an exogenous basement membrane, normal MECs proliferate to form small cell masses. Shortly thereafter, the structures develop an axis of apicobasal polarity, illustrated by the basal secretion of matrix components, the apical orientation of the Golgi, and the appropriate localization of junctional complexes (10,13). The structures subsequently become unresponsive to proliferative signals, and a bona fide lumen is formed by cavitation, involving the removal of centrally localized cells via multiple cell death processes (11,14,15). Finally, in cells with secretory activity, functional tight junction assembly precedes the secretion of milk proteins into the luminal space (10,16). Growth factors that are active in the mammary microenvironment in vivo, such as members of the epidermal growth factor (EGF) and insulin-like growth factor (IGF) families, are required for proliferation and morphogenesis (14). The specific growth factors employed often dictate the morphology of the structures that form; while EGF promotes spheroid formation, hepatocyte growth factor (HGF) can induce tubule formation (17).
Fig. 1.
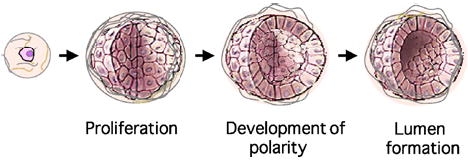
Modeling epithelial morphogenesis in basement membrane gels. Epithelial cells, such as the MCF-10A human mammary line, follow a reproducible morphogenetic program when seeded within an exogenous matrix. Single cells proliferate to form clonal cyst-like structures, which develop an axis of polarity and form hollow lumens, a process that involves death of the inner cells. Cartoon images of acini were modified from a figure in Ref. (12).
3D Cultures Reveal Transformation-Induced Changes in Mammary Epithelial Architecture
Many of these characteristic morphogenetic steps are compromised when transformed MEC lines are cultured in 3D. Pioneering work from Bissell and others has demonstrated that normal and tumorigenic mammary tissue can be readily distinguished when grown in a laminin-rich environment (18,19). The disorganized growth of transformed cell lines often involves a convergence of the following phenotypic changes: excessive proliferation/escape from growth suppression, loss of apicobasal polarity, compromised cell–cell adhesion, evidence of EMT, and formation of invasive and protrusive structures.
Bissell and colleagues have utilized the HMT-3522 series of mammary cell lines, which progress from the normal, nontransformed S1 cells to the pre-malignant S2 and tumorigenic T4-2 lines. Intriguingly, these cell lines are phenotypically indistinguishable when cultured on plastic; morphologic distinctions are revealed only when the cells are cultured in 3D. While S1 cells form well-organized acinar structures exhibiting apicobasal polarity and stable adherens junctions, T4-2 cells form highly disorganized and discohesive structures. In accordance with the hypothesis that 3D culture better recapitulates in vivo behavior, only the latter cell type is tumorigenic when transplanted in nude mice (20).
Basement membrane culture clearly illustrates the critical role of extracellular matrix (ECM) in directing morphogenetic processes that culminate in normal spheroid or tubule formation. Importantly, studies designed to define the cellular pathways responsible for the invasive character of several transformed lines also suggest that epithelial cell-matrix adhesion may either restrain or promote aberrant behavior. Overexpression of α2 integrin, an integrin subunit commonly downregulated in poorly differentiated mammary tumors, elicits reversion of the transformed phenotype of the α2-null Mm5MT murine MEC line (19). Whereas parental Mm5MT exhibit a disorganized, discohesive phenotype in 3D, reconstitution of α2 integrin promotes the formation of organized ductal structures. A similar analysis of the HMT-3522 progression series implicates aberrant integrin activation in the morphological alterations observed (21). Treatment of T4-2 structures with an inhibitory β1 integrin antibody causes a complete reversal of the transformed phenotype, resulting in proliferative arrest, the assembly of adherens junctions, and restoration of membrane polarity (22).
Along with studies establishing the importance of cell-matrix interactions for normal morphogenesis, related experiments have revealed an essential role for cell–cell adhesion in the regulation of mammary epithelial transformation. Loss of expression of the adherens junction protein E-cadherin is often observed during the progression of epithelial cancers and correlates with de-differentiation and tumor metastasis (23). Indeed, a hallmark feature of most lobular carcinomas is loss of functional E-cadherin (24). Reconstitution of E-cadherin expression in mammary tumor cell lines can revert the invasive behavior of these cells (22,25). These data suggest that the loss of adherens junction-mediated adhesion may be an important step in the progression of tumors to the invasive state. Furthermore, restoration of functional gap junctions by expression of connexin 26 or connexin 43 in the metastatic MDA-MB-435 line enables these cells to organize into spherical structures in 3D gels (26). This observation suggests that gap junctional communication between adjacent luminal cells may also be instrumental in the maintenance of epithelial architecture.
INDIVIDUAL ONCOGENES DIFFERENTIALLY PERTURB IN VITRO MAMMARY MORPHOGENESIS
The analysis of various human and murine MEC lines cultured in basement membrane has revealed several behavioral distinctions between normal and transformed cells (Table I). Unfortunately, the complexity of genetic alterations in the transformed lines has complicated the identification of individual signaling pathways responsible for the phenotypic alterations observed. To address this issue, several laboratories have now begun to utilize 3D culture of nontransformed MECs to assess the biological effects of the activation of specific oncogenes, individually or in defined combinations, thus extending investigators’ level of control over the system under observation.
Table I.
Representative Mammary Cell Lines Employed in 3D Culture Models
| Cell line | Origin | 3D culture behavior | Reference |
|---|---|---|---|
| S-1 | Human | Normal; polarized, growth-arrested acinar structures with hollow lumen | (18) |
| T4-2 | Human | Transformed in culture by long-term passage; loss of E-cadherin; disorganized | (18) |
| Eph4 | Mouse | Normal; branched tubes or acinar structures with hollow lumen | (27) |
| MCF-10A | Human | Normal; polarized, growth-arrested acinar structures with hollow lumen | (14) |
| MCF-102A | Human | Normal; acinar structures similar to, but somewhat larger than MCF-10A spheroids | (28) |
| MDA-MB-231 | Human | Breast carcinoma; E-cadherin negative; highly invasive, disorganized | (22) |
| MDA-MB-435 | Human | Breast carcinoma; E-cadherin negative; highly invasive, disorganized | (26) |
| MCF-7 | Human | Breast carcinoma; E-cadherin positive; estrogen receptor (ER) positive | (29) |
| Mm5MT | Mouse | MMTV-induced adenocarcinoma; disorganized, invasive | (19) |
| TAC-2 | Mouse | Normal; duct-like structures in collagen | (30) |
Hyperactivation of Transmembrane Receptors
Mammary morphogenesis requires an orchestrated response to numerous trophic factors that serve as activating ligands for membrane receptors (31). Elevated receptor activity resulting from altered expression or mutation can promote tumor development and progression. Three dimensional cell culture systems have been invaluable in revealing the complement of biological activities induced upon receptor activation. Moreover, while the consequences of activation of different transmembrane receptors may not be discernible in monolayer or soft agar assays, these effects are often quite distinct despite the activation of a similar spectrum of downstream pathways.
ErbB2
The consequences of activation of two members of the epidermal growth factor receptor (EGFR) family, ErbB1 and ErbB2, were distinguished using 3D cultures of the normal MEC line, MCF-10A (32). A synthetic dimerization strategy involving p75-ErbB-FKBP chimeras was used to selectively induce activated homodimers of either ErbB1 or ErbB2 in the context of a preformed acinus. While activation of ErbB1 has no effect on acinar architecture, ErbB2 dimerization relieves proliferative suppression, disrupts cell polarity, and leads to repopulation of the luminal cavity and the formation of multiacinar structures (32), (Fig. 2). These changes are reminiscent of the filled phenotype of comedotype ductal carcinoma in situ (DCIS) lesions, in which the ErbB2 locus is frequently amplified (33,34). Activated ErbB2 has also been reported to induce polarized, hollow branching tubules in collagen matrices (35). Such correlations lend substantial credence to the applicability to human mammary tumorigenesis of observations made using this model. Echoing a striking facet of the deconstructive studies discussed earlier, the changes elicited by ErbB2 activation are observed only in 3D culture, reinforcing the concept that certain phenotypes may only be manifest in the presence of matrix-derived morphogenetic cues.
Fig. 2.
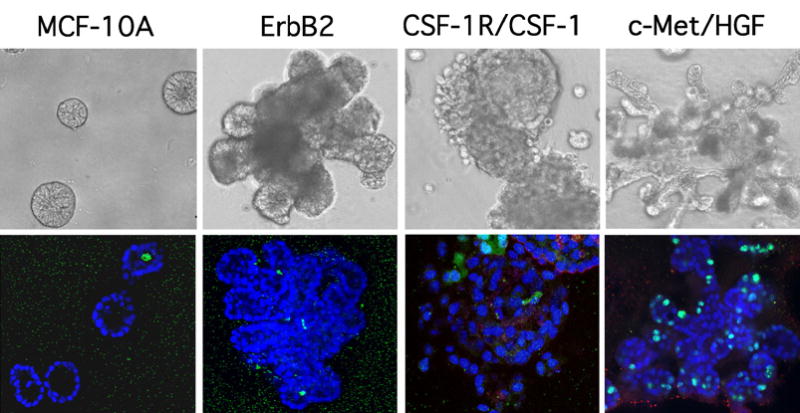
Distinct alterations in 3D morphogenesis result from hyperactivation of different RTKs. Phase and confocal immunofluorescence images of day 17 structures formed by parental MCF-10As or cells overexpressing ErbB2 (32), CSF-1R/CSF-1 (37), or c-Met/HGF (37) are shown. Structures were stained with DAPI (blue) and an antibody to the cleaved form of caspase-3 (green). The CSF-1R/CSF-1 confocal image was reproduced from ref. (37).
CSF-1R
In comparison to ErbB2 activation, co-overexpression of colony-stimulating factor receptor (CSF-1R) with its ligand (CSF-1), both of which are coordinately elevated in a significant proportion of mammary tumors, results in a much more severe disruption of MCF-10A acinar architecture (36). In addition to promoting excessive proliferation and aberrant survival, autocrine stimulation of CSF-1R leads to a striking and progressive disruption of cell–cell adhesion in these structures (37)(Fig. 2). Furthermore, E-cadherin is lost from the plasma membrane and accumulates in intracellular puncta, suggestive of the disruption of adherens junctions by an endocytic mechanism. These changes in adhesion culminate in the release of individual cells into the surrounding matrix, and provide a permissive background that enhances the activity of other oncogenes that promote migration and invasion. The adhesive defect is dependent upon the ability of CSF-1R to chronically activate Src family kinases; however, the relevant Src target(s) remain unknown. Interestingly, inducible activation of a v-src-ER fusion protein induces a similarly discohesive phenotype (M. Reginato, K. R. M. Shaw, and J. S. Brugge, unpublished results).
Met
An additional level of complexity to growth factor regulation of morphogenesis was revealed by co-overexpression of hepatocyte growth factor (HGF) and its receptor c-Met in 3D culture assays. HGF treatment of Eph4 murine MECs grown in 3D induces branching morphogenesis (27). Similarly, autocrine production of HGF in wild-type or c-Met-overexpressing MCF-10As elicits the formation of branched tubules. However, in contrast to the autocrine CSF-1R structures, both hollow lumen and functional adherens junctions are retained in these structures (37) (Fig. 2). Interestingly, while c-Met, CSF-1R, and ErbB2 each activate a similar array of downstream signaling molecules, including mitogen-activated protein kinase (MAPK), phosphotidylinositol-3 kinase (PI3K), and Src, and hyperactivation of each receptor enhances proliferation of MCF-10As, the phenotypic changes each receptor elicits in 3D culture are quite distinct. These differences suggest that while these receptors activate a related spectrum of the core signaling machinery, the activation of specific accessory pathways, possibly coupled with the ability to localize activated effector molecules to a unique set of targets, can cause dramatically distinct changes in MEC behavior.
Notch
Along with these receptor tyrosine kinases, the Notch family of transmembrane receptors also plays an important role in epithelial differentiation and morphogenesis. Notch4 was originally implicated in mammary tumorigenesis by the mapping of an insertion site of mouse mammary tumor virus (MMTV) proviral DNA to within the Notch4 locus (38). This insertion results in the expression of a truncated protein, Notch4(int-3), which is activated independently of extracellular ligand binding. MMTV-driven Notch4(int-3) causes excessive proliferation and inhibits ductal branching in the murine gland, eventually leading to the development of mammary carcinomas (39). To further characterize Notch4 activity, the consequences of its activation on the 3D morphogenesis of murine TAC-2 MECs was assessed (30). In addition to triggering excessive proliferation, Notch4(int-3) expression precludes normal cavitation of TAC-2 structures. Moreover, these cells also show signs of invasive activity. Collectively, these activities provide a basis for the tumorigenic effects of Notch4 activation in vivo.
Cytoplasmic Signaling Effectors
Akt
Signals initiated by the activation of membrane receptors can be significantly amplified by their intra-cellular effector proteins. Elevated expression of specific downstream factors, such as the serine/threonine kinase Akt2, has also been demonstrated in mammary tumors, suggesting that such alterations may be sufficient to elicit at least a partial complement of the consequences of receptor hyperactivation (40). Correspondingly, inducible activation of a myristolated Akt-ER fusion protein in MCF-10As causes an increase in cell size and distortions in cell shape, as well as a limited enhancement of cell survival (41). Interestingly, unlike constitutively activated growth factor receptors, the ability of Akt to enhance proliferation of MCF-10A acini is limited to the period during which these cells normally proliferate. Acini expressing activated Akt remain unable to escape the controls that limit proliferation in later stage cultures. This observation may at least partially explain the inability of activated Akt variants to induce mammary hyperplasia in vivo (42,43). Interestingly, certain proliferative oncogenes, such as cyclin D1 or human papilloma virus E7 protein (E7), which alone are unable to promote EGF-independent proliferation, can cooperate with Akt to elicit acinar morphogenesis in the absence of growth factors (41). Furthermore, Akt was found to play a critical role in regulating proliferation of tumorigenic T4-2 cells in 3D culture (44).
Ras
The cyclic regulation of Ras family proteins is orchestrated by guanine nucleotide exchange factors (GEFs), which promote the exchange of GDP for GTP, in conjunction with GTPase activating proteins (GAPs), which enhance the intrinsic hydrolytic activity of these GTPases. In the GTP-bound state, Ras proteins can initiate a phosphorylation cascade that culminates in changes in cell proliferation, cell morphology, cell movement, differentiation, and survival. Oncogenic mutations in Ras impair its hydrolytic activity, maintaining the protein in an activated state. Consequently, downstream signaling pathways are constitutively activated, causing aberrant proliferation in vivo (35). Ras mutations have not been implicated in breast carcinomas; however, Ras overexpression is common in breast tumors (45). It is likely that elevated Ras levels contribute to hyperactivation of pathways initiated by upstream receptors. While activation of Ras in Eph4 murine MECs does not alter proliferation in 2D, it is sufficient to promote excessive proliferation in collagen gels (46).
Transcription Factors
AP-1 Family
The complete cascade of downstream events initiated by membrane receptor activation remains to be elucidated. However, as a significant complement of the changes induced by extracellular signals result from changes in gene transcription patterns, many laboratories have investigated the effects of overexpression of specific tumor-associated transcription factors on mammary morphogenesis. The transcriptional activity of multiple members of the AP-1 family is altered in breast carcinomas, and elevated expression of FRA-1 correlates with an aggressive tumor phenotype (47,48). AP-1 proteins are the nuclear targets of multiple signaling pathways, including those of the EGFR and fibroblast growth factor receptor families, and can significantly influence epithelial behavior. Inducible activation of c-Jun disrupts both polarity and cell–cell adhesion in Eph4 MECs, leading to the formation of disorganized cell aggregates (49). The reversible effects caused by c-Jun activation result in part from the inappropriate redistribution of membrane E-cadherin and the induction of growth factor expression. However, c-Jun activation is not sufficient to cause invasion into the underlying basement membrane, suggesting that additional genetic alterations are required for progression to the invasive state. In contrast, inducible c-Fos activation promotes an invasive, EMT-like phenotype through a mechanism involving loss of E-cadherin expression, nuclear translocation of β-catenin, and autocrine production of TGFβ (50).
Ets Family
Overexpression of members of the Ets family of transcription factors, which have been found to be upregulated in lung, prostate, colon, and breast tumors, has been shown to impact the organization of MECs in 3D culture (51). When overexpressed in MCF-12A cells, Ets-2 or ESX is sufficient to transform these cells, as assessed by colony formation in soft agar (28). Furthermore, when cells expressing ESX are grown in ECM, they form filled, disorganized structures, in contrast to the hollow, polarized spheres formed by vector control cells, and acquire the ability to invade into the surrounding matrix. Interestingly, overexpression of another family member, PEA3, induces branching morphogenesis in TAC-2 MECs, revealing a level of phenotypic specificity that suggests each factor may promote the transcription of a distinct set of target genes (52). Furthermore, according to a recent study, the transforming effects of ESE-1 involve a novel cytoplasmic function independent of its transcriptional activity, suggesting an additional level of complexity to the activities of Ets family proteins (53).
Cooperation Between Proliferative and Prosurvival Cues
The increase in proliferation resulting from ErbB2 activation raised the possibility that elevated proliferation alone might be sufficient to modify acinar architecture. However, studies of two other oncogenes, cyclin D1 and HPV E7, which induce hyperproliferation yet fail to disrupt lumen formation, provided evidence that additional activities are required for filling the luminal space. Cyclin D1, which promotes progression through the G1 phase of the cell cycle by mediating inactivation of the retinoblastoma protein (Rb) by cyclin-dependent kinases (CDK4/6), is amplified at the genomic level in approximately 15% of breast cancers, and overexpressed in over one-half of tumor samples (54,55). E7 promotes cell cycle progression in a similar fashion, via the CDK-independent inactivation of Rb family members and CDK inhibitors (56). MCF-10As expressing ectopic E7 or cyclin D1 remain EGF-dependent and form acini that, while somewhat larger in size, exhibit hollow lumens, normal cell–cell contacts, and appropriate localization of both apical and basal polarity markers (14). These results correspond with those of a previous study that examined the effects of E7 overexpression in primary human MECs (57). Interestingly, acinar lumens are maintained despite the fact that cells within cyclin D1 and E7 acini continue to proliferate after control cells succumb to growth suppression (Fig. 3). Notably, cells that proliferate into the center of acini undergo apoptosis, thus failing to populate the lumen. This result suggests that maintenance of acinar architecture may rely on the ability of increased cell death to counterbalance aberrant proliferation.
Fig. 3.
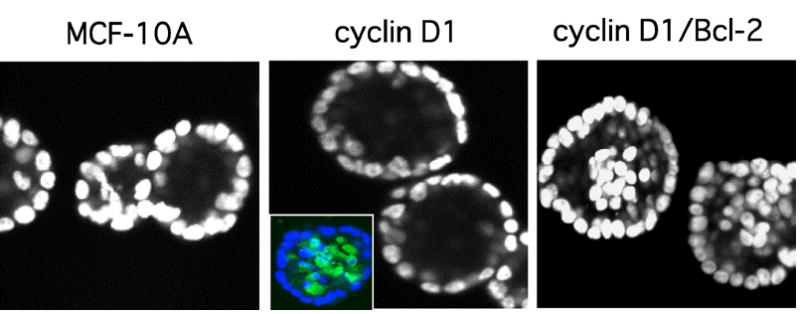
Cooperation between proliferative and survival signals results in filling of acinar lumen. Confocal sections of DAPI-stained day 20 structures formed by parental MCF-10As or cells overexpressing cyclin D1 or cyclin D1/Bcl-2 are shown. Inset, staining of cyclin D1 acini with DAPI (blue) and an antibody to the cleaved form of caspase-3 (green) reveals elevated levels of luminal apoptosis in these structures (13). The MCF-10A and cyclin D1 panels were reproduced from ref. (13).
These results, as well as data from other laboratories implicating luminal apoptosis in acinar morphogenesis in vivo, suggested that proliferative signals may need to be coupled with a survival signal to induce luminal filling (58). Indeed, overexpression of cyclin D1 together with Bcl-2, a mitochondrial protein with potent anti-apoptotic activity, prevents cavitation of MCF-10A acinar structures (14) (Fig. 3). Although the combination of a proliferative stimulus and a survival signal is sufficient to prevent lumen formation, the structures do not progress beyond this phenotype, reminiscent of the early stages of malignancy. Specifically, the polarized organization of acini and the adhesive contacts of the outermost cell layer remained intact, presumably precluding progression to a more invasive state. It is evident from these results that cell death is not only a critical aspect of acinar morphogenesis, but also serves as an important antagonist of aberrant proliferation. Similarly, inhibition of apoptosis via increased expression of Bcl-2 appears to be capable of exacerbating the phenotypic changes induced by proliferative oncogenes in a variety of tumor types. However, inhibition of cell death is not on its own sufficient to dramatically influence mammary architecture (58,59).
Oncogene Synergy in the Genesis of Advanced Phenotypes
As suggested above, while studies examining the effects of individual oncogenes on MEC behavior and organization are likely to lead to a more thorough understanding of specific facets of cancer initiation and progression, a more accurate recapitulation of the complex genetic changes involved in tumori-genesis may require the simultaneous overexpression of multiple oncogenes. Several groups have initiated combinatorial studies in an attempt to better understand how different genes may interact to cause more advanced phenotypes.
As mentioned above, partial or transient loss of epithelial character is associated with carcinoma progression (60). Similarly, a transient conversion to a mesenchymal-like phenotype is required for branching morphogenesis during development and is mediated by members of the TGFβ family. While studies in monolayer culture have established TGFβ as a potent inhibitor of cell cycle progression, 3D cell culture has revealed divergent, concentration-dependent activities for TGFβ in mammary epithelial cells. Enhanced TGFβ signaling restrains glucocorticoid-induced branching morphogenesis, while limiting levels promote tubulogenesis (61). These data suggest that subtle changes in local TGFβ concentration might elicit dramatically different phenotypes. Moreover, when TGFβ is introduced in the context of an activated Ras/MAPK pathway, classical EMT ensues; this transition is stable even after these activating signals are removed (62). Correspondingly, when TGFβ is overexpressed in tandem with ErbB2, a potent Ras activator, the phenotypic alterations caused by ErbB2 activation are severely exacerbated. The two oncogenes cooperate to form poorly organized structures that exhibit continual remodeling of invasive protrusions (63–65) (Fig. 4). These results correlate with in vivo data suggesting that interaction of TGFβ signaling with additional pathways may be important for the progression to metastatic disease (66, 67).
Fig. 4.

Multiple oncogenes can cooperate to elicit an invasive phenotype. Phase images of day 12 structures formed by MCF-10As overexpressing ErbB2 or ErbB2/TGFβ. These images were reproduced from Ref. (64).
Tumor Suppressors
To date, few studies have examined the effects of altered expression of genes linked to familial breast cancer. However, a potential function for BRCA-1 in spheroid formation was explored by downregulation of a protein-interaction partner, the tumor suppressor BARD-1 (BRCA-1 associated ring domain). These studies were conducted in TAC-2 murine MECs, which form spheroids in response to hydrocortisone or tubules in the presence of HGF. Repression of BARD-1 using an antisense RNA strategy has no effect on tubulogenesis, but dramatically disrupts normal spheroid formation, resulting in cysts that are significantly smaller and fail to cavitate (68). Further studies will be required to determine whether these effects reflect a loss of the tumor suppressive function of BRCA-1 or rather represent a novel function of BARD-1.
EXPANDING UPON CURRENT 3D CULTURE SYSTEMS: THE NEXT GENERATION OF IN VITRO MODELS OF MAMMARY MORPHOGENESIS AND TRANSFORMATION
The remarkable similarities between the multiacinar phenotype elicited by activation of ErbB2 in MCF-10As in 3D culture and DCIS lesions associated with genetic amplification of the ErbB2 locus, as well as lesions induced by activation of Neu in the mouse mammary gland, suggest that this model has significant utility (32,69). Several additional studies have likewise revealed a correspondence between the in vitro and in vivo biological effects of various morphogenetic pathways, lending further credence to the capacity of 3D models for the identification and characterization of novel morphogens, oncogenes, and tumor suppressors (70,71). Nevertheless, several facets of current 3D cell culture models constrain how accurately these systems can model in vivo behavior. Despite the progress such homotypic models have facilitated, there remains a need for the development of complex culture systems that more closely mimic the in vivo environment.
Expanding the Availability of Primary MEC Lines that Retain In Vivo Character
Many immortalized MEC lines, including MCF-10A, do not represent a true luminal population, as they express markers of both luminal and myoepithelial cell lineages (72). Because of the hybrid nature of such lines, it is difficult to predict whether changes elicited by oncogene expression in vitro will appropriately reflect the events involved in mammary tumorigenesis in vivo. Definition of the cell culture conditions required for luminal cells to retain their unique ensemble of differentiated characteristics will represent an important advancement in the utility of 3D models. Another concern with immortalized MECs is the accumulation of genetic and epigenetic changes that accompany immortalization. For example, amplification of myc and inactivation of either p53 or p16 are commonly observed in immortalized mammary epithelial cells (73,74). Such documented changes, as well as additional uncharacterized alterations, may cooperate in unexpected ways with specific genes under study, thus complicating the interpretation of observed results.
Isolation of Mammary Stem Cells for Use in 3D Models
Recently, several investigators have been successful in propagating mammary cells that display stem cell characteristics (75,76). Human stem cell populations were shown to maintain the ability to functionally differentiate into multiple lineages, including ductal, alveolar and myoepithelial cells (75). These pluripotent cells could prove to be of great utility for the simultaneous study of the effects of various genes implicated in neoplastic transformation on multiple mammary cell types. However, this will first require the optimization of protocols for the isolation of stem cells and the maintenance of multilineage organotypic cultures.
Modeling Epithelial–Stromal Interactions
Importantly, the systems described above exclusively employ epithelial cells, and lack any stromal components. Given that mammary stroma, including fibroblasts, adipocytes, endothelial cells, and inflammatory cells, comprise over 80% of the cellular population of the in vivo gland, it is important to investigate how paracrine signals or cell–cell contact affects epithelial behavior using coculture of epithelial cells and their stromal counterparts. Preliminary studies of this nature have already established a critical role for multiple cell types in mammary epithelial morphogenesis and differentiation. For example, mammary myoepithelial cells impart critical polarizing signals when cultured jointly with luminal epithelial cells, likely via laminin-1 secretion (77). Moreover, when cocultured with mammary adipocytes, primary rat MECs exhibit enhanced differentiation, as measured by lipid and casein accumulation (78). Similarly, coculture with mammary fibroblasts promotes MEC alveolar morphogenesis, proliferation and differentiation (79).
Initial studies examining cocultures of MECs and inflammatory cells have also yielded substantial insight into the paracrine interactions between these two cell types. Interleukin-1 (IL-1) treatment of peritoneal macrophages induces the production of vascular endothelial growth factor (VEGF) in co-cultured DA/3 mammary tumor cells, suggesting a potential mechanism for the contribution of IL-1 to the metastatic spread of these cells in vivo (80). Furthermore, pericellular collagen proteolysis by BT-20 breast tumor spheroids cultured in 3D is enhanced by the inclusion of macrophages, which also exhibit intracellular degradation of collagen (81). The capacity of heterotypic communication to lead to a local modification of the ECM is further supported by the finding that coculture with MCF7 or T47D breast adenocarcinoma cells, but not normal MECs, enhances the synthesis of collagen and fibronectin by fibroblasts (82). These results highlight the important influence of the breast stroma on the mammary epithelial phenotype.
Challenges in the Optimization of Coculture Systems
While significant data support a role for cellular and acellular components of the mammary stroma in both development and tumorigenesis, significant technical obstacles must be overcome to establish effective systems for heterotypic 3D culture. Of utmost importance, culture conditions must be developed that allow each relevant cell type to maintain a fully differentiated phenotype. Furthermore, heterotypic assays can complicate analysis of results, and novel strategies are required to address such questions as the determination of the identity and origin of secreted factors and the isolation of cellular material (DNA, RNA or protein) from individual cell types. Once these challenges have been met, coculture studies should also prove useful for the investigation of the distinct contributions of normal versus tumor-associated stromal components to changes in epithelial cell behavior.
Modeling of Hormonal Responses
In addition to modeling the complex cellular and extracellular milieu, systems should be sought that incorporate the intricate hormonal signaling unique to the mammary gland. In vivo analysis of human and murine tumors indicates that the estrogen receptor (ER) and progesterone receptor (PR) status of a tumor are closely correlated with prognosis. However, few studies have explored the consequences of hormone receptor activation on MEC behavior and morphogenesis, largely due to the limited availability of MEC lines that are responsive to the principal mammary hormones. Coculture models have nonetheless begun to elucidate the paracrine effects of hormone stimulation of stromal cells. Estrogen treatment of breast fibroblasts enhances proliferation and induces tubulogenesis in MECs when cultured together in collagen gels, and these effects are dependent on stromal production of HGF (83). However, until hormone-dependent MEC lines are developed, an examination of the roles of epithelial ER and PR in tumorigenesis, either alone or in combination with other oncogenes, will remain elusive. In summary, the evolution of novel coculture techniques, as well as hormone responsive MEC lines, will significantly broaden the utility of ECM culture for the investigation of mammary tumorigenesis.
Despite the extensive advances in the study of mammary biology facilitated by the development of 3D models, experiments in animal models remain as the gold standard. In order to exploit the more tractable nature of in vitro experimentation within the in vivo environment, many investigators have utilized mammary gland reconstitution techniques. While transplantation of normal and transformed murine MECs has long been used to reconstitute the mammary epithelium, only recently has a strategy been developed that facilitates exploration of heterotypic interactions between cells of human origin. This novel technique involves the coinjection of a ‘humanized’ stromal component with human MECs, thus facilitating ductal outgrowth and morphogenesis in the recipient fat pad (84). Subsequent manipulation of the transplanted stromal cells revealed disparities in the behavior of ostensibly normal epithelial cells derived from different patients, reiterating the importance of evaluating the contributions of the stromal compartment to mammary morphogenesis and transformation. Such techniques can now be used in concert with studies in 3D coculture systems designed to identify putative oncogenic changes and their relevant target cell type. Collectively, these techniques will provide a powerful new spectrum of capabilities to the breast cancer research community.
SUMMARY
Recently, an increasing number of investigators have employed 3D cell culture techniques in an attempt to reconstruct discrete events associated with tumor initiation and progression, such as aberrant survival or compromised cell–cell adhesion. The introduction of individual genes associated with breast cancer into well-characterized MEC lines and the subsequent study of those cells in basement membrane culture has revealed dramatic distinctions in the phenotypic changes each ectopically expressed oncogene is able to promote. These include alterations in cell proliferation, survival, polarity, size, and adhesion, each observed in the context of multicellular mammary epithelial spheroids.
As evident from the results described above, the evolution of 3D culture models has galvanized the study of epithelial transformation. While we have been unable to comprehensively detail all such studies in this review, intriguing results have also been obtained from the 3D analysis of many additional factors implicated in mammary tumorigenesis, including but not limited to ephrins, NF-κB, and JAK/STATs. However, the studies we selected begin to reveal the broad array of phenotypes elicited by transformation of mammary epithelial cells and demonstrate how 3D culture can be effectively utilized to dissect the cell biological and biochemical changes involved. These novel techniques have enabled the characterization of phenotypes not amenable to study in monolayer culture, whose complexities are not revealed by conventional techniques such as growth in soft agar. By allowing investigators to merge traditional biochemical approaches, such as pharmacological inhibition of specific signaling molecules, with confocal immunofluorescence analysis of organized, gland-like structures, 3D models have facilitated a careful analysis of oncogene-induced alterations in greater mechanistic detail. Such studies have yielded significant insights into the mechanisms involved in the development and progression of breast cancer.
Acknowledgments
J.S.B. is supported by grants from the National Cancer Institute, the American Cancer Society, the Breast Cancer Research Foundation, the Department of Defense, and a gift from the Virginia and D.K. Ludwig Fund for Cancer Research to Harvard Medical School. C. N. Wrobel is supported by an HHMI Predoctoral Fellowship.
References
- 1.Roskelley CD, Bissell MJ. The dominance of the microenvironment in breast and ovarian cancer. Semin Cancer Biol. 2002;12:97–104. doi: 10.1006/scbi.2001.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Albertson DG. Profiling breast cancer by array CGH. Breast Cancer Res Treat. 2003;78:289–98. doi: 10.1023/a:1023025506386. [DOI] [PubMed] [Google Scholar]
- 4.Kopelovich L, Crowell JA, Fay JR. The epigenome as a target for cancer chemoprevention. J Natl Cancer Inst. 2003;95:1747–57. doi: 10.1093/jnci/dig109. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell P. Genetic and cytogenetic analyses of breast cancer yield different perspectives of a complex disease. Breast Cancer Res Treat. 2003;78:347–57. doi: 10.1023/a:1023037925950. [DOI] [PubMed] [Google Scholar]
- 6.Coradini D, Daidone MG. Biomolecular prognostic factors in breast cancer. Curr Opin Obstet Gynecol. 2004;16:49–55. doi: 10.1097/00001703-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–28. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 8.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–55. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman MP, Kibbey MC, Letterio JJ, Kleinman HK. Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG) J Cell Sci. 1996;109(Pt 8):2013–21. doi: 10.1242/jcs.109.8.2013. [DOI] [PubMed] [Google Scholar]
- 10.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–35. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blatchford DR, Quarrie LH, Tonner E, McCarthy C, Flint DJ, Wilde CJ. Influence of microenvironment on mammary epithelial cell survival in primary culture. J Cell Physiol. 1999;181:304–11. doi: 10.1002/(SICI)1097-4652(199911)181:2<304::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Fridrich MJ. Studing cancer in 3 dimension. JAMA. 2003;290:1977–79. doi: 10.1001/jama.290.15.1977. [DOI] [PubMed] [Google Scholar]
- 13.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 14.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 15.Mills KR, Reginato M, Debnath J, Queenan B, Brugge JS. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc Natl Acad Sci USA. 2004;101:3438–43. doi: 10.1073/pnas.0400443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurley WL, Blatchford DR, Hendry KA, Wilde CJ. Extra-cellular matrix and mouse mammary cell function: Comparison of substrata in culture. In Vitro Cell Dev Biol Anim. 1994;30A:529–38. [PubMed] [Google Scholar]
- 17.Birchmeier W, Brinkmann V, Niemann C, Meiners S, DiCesare S, Naundorf H, et al. Role of HGF/SF and c-Met in morphogenesis and metastasis of epithelial cells. Ciba Found Symp. 1997;212:230–40. doi: 10.1002/9780470515457.ch15. discussion 40–6. [DOI] [PubMed] [Google Scholar]
- 18.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zutter MM, Santoro SA, Staatz WD, Tsung YL. Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci USA. 1995;92:7411–5. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin Cancer Biol. 1995;6:175–84. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–6. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, et al. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 24.Shackney SE, Silverman JF. Molecular evolutionary patterns in breast cancer. Adv Anat Pathol. 2003;10:278–90. doi: 10.1097/00125480-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Meiners S, Brinkmann V, Naundorf H, Birchmeier W. Role of morphogenetic factors in metastasis of mammary carcinoma cells. Oncogene. 1998;16:9–20. doi: 10.1038/sj.onc.1201486. [DOI] [PubMed] [Google Scholar]
- 26.Hirschi KK, Xu CE, Tsukamoto T, Sager R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 1996;7:861–70. [PubMed] [Google Scholar]
- 27.Niemann C, Brinkmann V, Spitzer E, Hartmann G, Sachs M, Naundorf H, et al. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998;143:533–45. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schedin PJ, Eckel-Mahan KL, McDaniel SM, Prescott JD, Brodsky KS, Tentler JJ, et al. ESX induces transformation and functional epithelial to mesenchymal transition in MCF-12A mammary epithelial cells. Oncogene. 2004;23:1766–79. doi: 10.1038/sj.onc.1207391. [DOI] [PubMed] [Google Scholar]
- 29.Villalobos M, Aranda M, Nunez MI, Becerra D, Olea N, Ruiz de Almodovar M, et al. Interaction between ionizing radiation, estrogens and antiestrogens in the modification of tumor microenvironment in estrogen dependent multicellular spheroids. Acta Oncol. 1995;34:413–7. doi: 10.3109/02841869509094000. [DOI] [PubMed] [Google Scholar]
- 30.Soriano JV, Uyttendaele H, Kitajewski J, Montesano R. Expression of an activated Notch4(int-3) oncoprotein disrupts morphogenesis and induces an invasive phenotype in mammary epithelial cells in vitro. Int J Cancer. 2000;86:652–9. doi: 10.1002/(sici)1097-0215(20000601)86:5<652::aid-ijc8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 31.Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia. 2002;7:17–38. doi: 10.1023/a:1015766322258. [DOI] [PubMed] [Google Scholar]
- 32.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–92. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–84. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 34.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–14. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 35.Janda E, Litos G, Grunert S, Downward J, Beug H. Oncogenic Ras/Her-2 mediate hyperproliferation of polarized epithelial cells in 3D cultures and rapid tumor growth via the PI3K pathway. Oncogene. 2002;21:5148–59. doi: 10.1038/sj.onc.1205661. [DOI] [PubMed] [Google Scholar]
- 36.Kacinski BM, Scata KA, Carter D, Yee LD, Sapi E, King BL, et al. FMS (CSF-1 receptor) and CSF-1 transcripts and protein are expressed by human breast carcinomas in vivo and in vitro. Oncogene. 1991;6:941–52. [PubMed] [Google Scholar]
- 37.Wrobel CN, Debnath J, Lin E, Beausoleil S, Roussel MF, Brugge JS. Autocrine CSF-1R activation promotes Src-dependent disruption of mammary epithelial architecture. J Cell Biol. 2004;165:263–73. doi: 10.1083/jcb.200309102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallahan D, Callahan R. The mouse mammary tumor associated gene INT3 is a unique member of the NOTCH gene family (NOTCH4) Oncogene. 1997;14:1883–90. doi: 10.1038/sj.onc.1201035. [DOI] [PubMed] [Google Scholar]
- 39.Jhappan C, Gallahan D, Stahle C, Chu E, Smith GH, Merlino G, et al. Expression of an activated Notch-related int-3 trans-gene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1992;6:345–55. doi: 10.1101/gad.6.3.345. [DOI] [PubMed] [Google Scholar]
- 40.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–5. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 41.Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol. 2003;163:315–26. doi: 10.1083/jcb.200304159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwertfeger KL, Richert MM, Anderson SM. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol Endocrinol. 2001;15:867–81. doi: 10.1210/mend.15.6.0663. [DOI] [PubMed] [Google Scholar]
- 43.Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 2004;64:3171–8. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol. 2004;164:603–12. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dati C, Muraca R, Tazartes O, Antoniotti S, Perroteau I, Giai M, et al. c-erbB-2 and ras expression levels in breast cancer are correlated and show a co-operative association with unfavorable clinical outcome. Int J Cancer. 1991;47:833–8. doi: 10.1002/ijc.2910470607. [DOI] [PubMed] [Google Scholar]
- 46.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Gene Dev. 1996;10:2462–77. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 47.Zajchowski DA, Bartholdi MF, Gong Y, Webster L, Liu HL, Munishkin A, et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 2001;61:5168–78. [PubMed] [Google Scholar]
- 48.Gee JM, Barroso AF, Ellis IO, Robertson JF, Nicholson RI. Biological and clinical associations of c-jun activation in human breast cancer. Int J Cancer. 2000;89:177–86. doi: 10.1002/(sici)1097-0215(20000320)89:2<177::aid-ijc13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 49.Fialka I, Schwarz H, Reichmann E, Oft M, Busslinger M, Beug H. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J Cell Biol. 1996;132:1115–32. doi: 10.1083/jcb.132.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eger A, Stockinger A, Park J, Langkopf E, Mikula M, Gotzmann J, et al. beta-Catenin and TGFbeta signalling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene. 2004;23:2672–80. doi: 10.1038/sj.onc.1207416. [DOI] [PubMed] [Google Scholar]
- 51.Feldman RJ, Sementchenko VI, Watson DK. The epithelial-specific Ets factors occupy a unique position in defining epithelial proliferation, differentiation and carcinogenesis. Anticancer Res. 2003;23:2125–31. [PubMed] [Google Scholar]
- 52.Chotteau-Lelievre A, Montesano R, Soriano J, Soulie P, Desbiens X, de Launoit Y. PEA3 transcription factors are expressed in tissues undergoing branching morphogenesis and promote formation of duct-like structures by mammary epithelial cells in vitro. Dev Biol. 2003;259:241–57. doi: 10.1016/s0012-1606(03)00182-9. [DOI] [PubMed] [Google Scholar]
- 53.Prescott JD, Koto KS, Singh M, Gutierrez-Hartmann A. The ETS transcription factor ESE-1 transforms MCF-12A human mammary epithelial cells via a novel cytoplasmic mechanism. Mol Cell Biol. 2004;24:5548–64. doi: 10.1128/MCB.24.12.5548-5564.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–61. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- 55.Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–7. [PubMed] [Google Scholar]
- 56.Munger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–98. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 57.Spancake KM, Anderson CB, Weaver VM, Matsunami N, Bissell MJ, White RL. E7-transduced human breast epithelial cells show partial differentiation in three-dimensional culture. Cancer Res. 1999;59:6042–5. [PubMed] [Google Scholar]
- 58.Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, et al. Apoptosis in the terminal endbud of the murine mammary gland: A mechanism of ductal morphogenesis. Development. 1996;122:4013–22. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- 59.Jager R, Herzer U, Schenkel J, Weiher H. Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene. 1997;15:1787–95. doi: 10.1038/sj.onc.1201353. [DOI] [PubMed] [Google Scholar]
- 60.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–6. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Soriano JV, Orci L, Montesano R. TGF-beta1 induces morphogenesis of branching cords by cloned mammary epithelial cells at subpicomolar concentrations. Biochem Biophys Res Commun. 1996;220:879–85. doi: 10.1006/bbrc.1996.0499. [DOI] [PubMed] [Google Scholar]
- 62.Jechlinger M, Grunert S, Beug H. Mechanisms in epithelial plasticity and metastasis: insights from 3D cultures and expression profiling. J Mammary Gland Biol Neoplasia. 2002;7:415–32. doi: 10.1023/a:1024090116451. [DOI] [PubMed] [Google Scholar]
- 63.Seton-Rogers SE, Brugge JS. ErbB2 and TGF-beta: A cooperative role in mammary tumor progression? Cell Cycle. 2004;3:597–600. [PubMed] [Google Scholar]
- 64.Seton-Rogers SE, Lu Y, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK, et al. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci U S A. 2004;101:1257–62. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ueda Y, Wang S, Dumont N, Yi JY, Koh Y, Arteaga CL. Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor beta-induced cell motility. J Biol Chem. 2004;279:24505–13. doi: 10.1074/jbc.M400081200. [DOI] [PubMed] [Google Scholar]
- 66.Muraoka RS, Koh Y, Roebuck LR, Sanders ME, Brantley-Sieders D, Gorska AE, et al. Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor beta1. Mol Cell Biol. 2003;23:8691–703. doi: 10.1128/MCB.23.23.8691-8703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA. 2003;100:8430–5. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Irminger-Finger I, Soriano JV, Vaudan G, Montesano R, Sappino AP. In vitro repression of Brca1-associated RING domain gene, Bard1, induces phenotypic changes in mammary epithelial cells. J Cell Biol. 1998;143:1329–39. doi: 10.1083/jcb.143.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–61. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 70.Rosfjord EC, Dickson RB. Growth factors, apoptosis, and survival of mammary epithelial cells. J Mammary Gland Biol Neoplasia. 1999;4:229–37. doi: 10.1023/a:1018789527533. [DOI] [PubMed] [Google Scholar]
- 71.Schmeichel KL, Weaver VM, Bissell MJ. Structural cues from the tissue microenvironment are essential determinants of the human mammary epithelial cell phenotype. J Mammary Gland Biol Neoplasia. 1998;3:201–13. doi: 10.1023/a:1018751124382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 73.Yaswen P, Stampfer MR. Epigenetic changes accompanying human mammary epithelial cell immortalization. J Mammary Gland Biol Neoplasia. 2001;6:223–34. doi: 10.1023/a:1011364925259. [DOI] [PubMed] [Google Scholar]
- 74.Yaswen P, Stampfer MR. Molecular changes accompanying senescence and immortalization of cultured human mammary epithelial cells. Int J Biochem Cell Biol. 2002;34:1382–94. doi: 10.1016/s1357-2725(02)00047-x. [DOI] [PubMed] [Google Scholar]
- 75.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Gene Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zangani D, Darcy KM, Masso-Welch PA, Bellamy ES, Desole MS, Ip MM. Multiple differentiation pathways of rat mammary stromal cells in vitro: acquisition of a fibroblast, adipocyte or endothelial phenotype is dependent on hormonal and extracellular matrix stimulation. Differentiation. 1999;64:91–101. doi: 10.1046/j.1432-0436.1999.6420091.x. [DOI] [PubMed] [Google Scholar]
- 79.Darcy KM, Zangani D, Shea-Eaton W, Shoemaker SF, Lee PP, Mead LH, et al. Mammary fibroblasts stimulate growth, alveolar morphogenesis, and functional differentiation of normal rat mammary epithelial cells. In Vitro Cell Dev Biol Anim. 2000;36:578–92. doi: 10.1007/BF02577526. [DOI] [PubMed] [Google Scholar]
- 80.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sameni M, Dosescu J, Moin K, Sloane BF. Functional imaging of proteolysis: Stromal and inflammatory cells increase tumor proteolysis. Mol Imag. 2003;2:159–75. doi: 10.1162/15353500200303136. [DOI] [PubMed] [Google Scholar]
- 82.Noel A, Munaut C, Boulvain A, Calberg-Bacq CM, Lambert CA, Nusgens B, et al. Modulation of collagen and fibronectin synthesis in fibroblasts by normal and malignant cells. J Cell Biochem. 1992;48:150–61. doi: 10.1002/jcb.240480207. [DOI] [PubMed] [Google Scholar]
- 83.Zhang HZ, Bennett JM, Smith KT, Sunil N, Haslam SZ. Estrogen mediates mammary epithelial cell proliferation in serum-free culture indirectly via mammary stroma-derived hepatocyte growth factor. Endocrinology. 2002;143:3427–34. doi: 10.1210/en.2002-220007. [DOI] [PubMed] [Google Scholar]
- 84.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]


